SUMMARY
Traumatic brain injury (TBI) is one the most common human afflictions, contributing to long-term disability in survivors. Emerging data indicate that functional improvement or deterioration can occur years after TBI. In this regard, TBI is recognized as risk factor for late-life neurodegenerative disorders. TBI encompasses a heterogeneous disease process, in which diverse injury subtypes and multiple molecular mechanisms overlap. To develop precision medicine approaches where specific pathobiological processes are targeted by mechanistically appropriate therapies, techniques to identify and measure these subtypes are needed. Traumatic microvascular injury is a common but relatively understudied TBI endophenotype. In this review, we describe evidence of microvascular dysfunction in human and animal TBI, explore the role of vascular dysfunction in neurodegenerative disease, and discuss potential opportunities for vascular-directed therapies in ameliorating TBI-related neurodegeneration. We discuss the therapeutic potential of vascular-directed therapies in TBI and the use and limitations of preclinical models to explore these therapies.
Keywords: traumatic brain injury, cerebrovasculature, Alzheimer’s disease, neurodegenerative disease, blood-brain barrier, biomarker
INTRODUCTION
Traumatic brain injury (TBI) is a prevalent condition affecting all ages, races, and socioeconomic classes throughout the world. In the US alone, there are at least 2.8 million Emergency Department visits for TBIs annually, though many more TBIs likely go unreported (Taylor et al., 2017). The incidence of TBI is disproportionately higher in low and middle-income countries, where TBIs are the leading cause of death and disability in young adults (Maas et al., 2017). Because head injuries often affect young people in their most productive years, the cumulative loss of productivity is high compared to other injuries and illnesses (Max et al., 1991). Even mild TBI (mTBI), often termed concussion, which accounts for 80–90% of all TBIs, results in a tremendous societal and economic burden and accounts for up to 44% of the total lifetime costs of TBI (Max et al., 1991). As the population ages, TBIs from falls in older persons are becoming increasingly prevalent, and are associated with more morbidity and mortality due to coexisting medical illness, anticoagulant use, and slower recovery trajectories (Peters and Gardner, 2018). Public health initiatives focused on injury prevention through bike helmets, fall prevention, seat belt use, sport impact policy changes and other public safety measures have been very effective in reducing the incidence of and the mortality associated with severe TBI (Taylor et al., 2017). Clinical management has focused on controlling intracranial pressure and cerebral edema (Chesnut et al., 2012), oxygen deprivation (Okonkwo et al., 2017), or brain metabolic demand (Andrews et al., 2015) in cases of severe injury, though the effects of these interventions on clinical outcomes has been disappointing (Maas et al., 2017). Furthermore, there are no therapies that diminish the burden of disability resulting from mild TBI, which has historically been ignored by the health care system (Levin and Diaz-Arrastia, 2015).
TBI has traditionally been conceptualized as a primary injury event, caused by an initial mechanical impact, followed by secondary insults due to the molecular and cellular responses in reaction to the primary injury. Secondary injury can propagate a trauma-induced cascade in the surrounding brain tissue. This secondary injury, even in the most severe cases, was thought to extend only for a few weeks or at most a month, followed by a trajectory of recovery that was generally thought to be largely complete within months or at most, one year. Evidence accumulated over the past decade has led to a recognition that for many patients, the consequences of TBI continue to evolve long after the acute period of initial recovery (Wilson et al., 2017). Longitudinal studies have shown that outcomes following TBI are not fixed, as there can be improvement and/or deterioration in neurological functioning many years after injury (Corrigan and Hammond, 2013; Wilson et al., 2017). TBI can therefore be best conceptualized as a chronic health condition triggered by the injury which initiates long-lasting and still poorly understood downstream events that can impact brain functioning for decades (Corrigan and Hammond, 2013), having life-long effects on multiple health outcomes (Masel and DeWitt, 2010).
Given this complexity, there is an urgent need to better understand the specific pathophysiological mechanisms contributing to TBI-related dysfunction in the both acute and chronic phases of disease. In animal models, interventions aimed at molecular targets involved in secondary injury have been successful in limiting the extent of injury and improving neurologic recovery (Marklund et al., 2006; McIntosh et al., 1998). These results provide convincing proof of principle that effective therapeutic intervention is possible, but therapeutic efficacy has yet to be achieved in the human condition. In this review, we specifically focus on TBI-related injury of the cerebral microvasculature, discuss the relationship of vascular injury to chronic neurodegenerative sequelae of TBI, and highlight opportunities for preclinical and clinical studies to improve our understanding of this disease process and promote the development of effective therapies.
TRAUMATIC VASCULAR INJURY FOLLOWING TBI
The neurovascular unit
The brain is critically dependent on a steady blood supply that is acutely responsive to the constantly changing metabolic demands of the brain tissue. To accomplish this, the brain parenchyma is served by a vascular network of arteries, arterioles, capillaries, venules, and veins that runs approximately 400 miles in length (Sweeney et al., 2018). Several cell types distributed along this network act in concert to regulate cerebral blood flow, vascular permeability, and micronutrient supply. Collectively, these cells are termed the neurovascular unit (NVU) (Shlosberg et al., 2010). The NVU consists of the endothelial lining of the blood vessels, smooth muscle cells (at the artery/arteriole/venule levels), and pericytes (at the capillary level; Figure 1A), which help to regulate vascular tone, neurons, and perivascular astrocytes. The endothelial cells form a monolayer consisting of intercellular tight and adherens junctions to form the blood-brain barrier (BBB), 85% of which is provided by the capillary endothelium (Sweeney et al., 2018b). This BBB forms an exquisitely regulated barrier between the systemic vasculature and the brain parenchyma. All components of the NVU undergo continuous crosstalk under normal physiological conditions to form an integrated and keenly responsive system to changing cerebral and systemic factors. This process, termed neurovascular coupling, ensures consistent cerebral blood flow and micronutrient supply across the BBB as a function of neuronal activity.
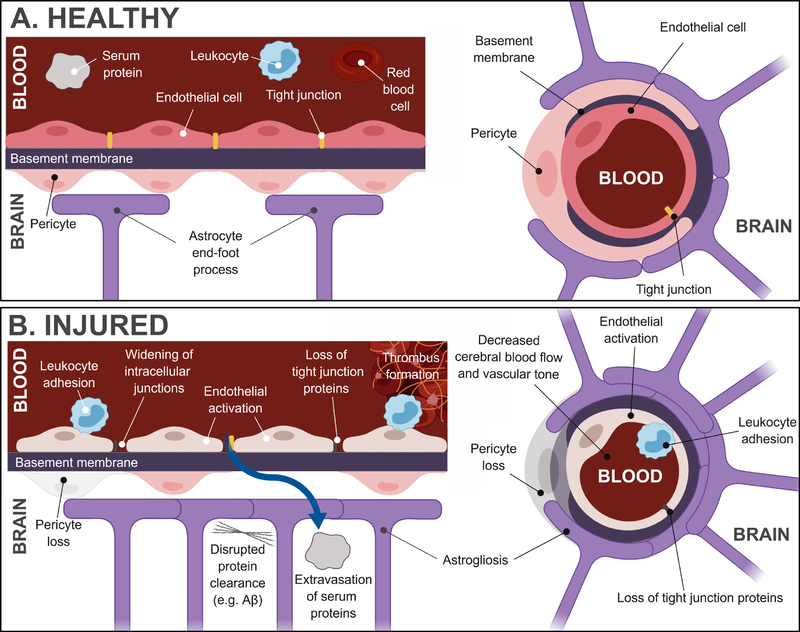
Figure 1: Healthy and injured neurovascular unit.
A. At the capillary level, the neurovascular unit is composed of endothelial cells, perivascular astrocytes, and pericytes. Smooth muscle cells are present at the artery/arteriole/venule levels (not shown). The endothelial cells form a monolayer consisting of intercellular tight junctions to form the blood-brain barrier. B. Following injury, a number of vascular changes have been reported in preclinical studies, including widening of intracellular junctions, loss of tight junction proteins – both of which contribute to extravasation of serum proteins into the parenchyma – astrogliosis, pericyte loss, endothelial activation, leukocyte adhesion, thrombus formation and disrupted protein clearance.
Following brain injury, these normal patterns of communication among the elements of the NVU can be severely altered (Figure 1B). Disturbed NVU functioning leads to inappropriate changes in cerebral blood flow in response to the altered metabolic demands of the injured brain. Dysfunction of the BBB disrupts the extracellular environment due to protein and electrolyte leakage and can trigger other downstream processes, like microglia activation and recruitment (Shlosberg et al., 2010; Zlokovic, 2011). Emerging evidence, as we discuss below, indicates that these disruptions last far longer than previously assumed and may contribute to ongoing neuropathology long after the primary injury.
Evidence from human patients
Clinical evidence indicates that microvascular dysfunction extends across the spectrum of TBI-related injury. Histologically-detected ischemic damage is seen in nearly 60% of fatal TBI without evidence of large vessel occlusions (Graham and Adams, 1971). In moderate-severe TBI, vasospasm of larger cerebral arteries can precipitate cerebral ischemia (Martin et al., 2009), but more universally trauma-induced vascular injury occurs at the arteriole/capillary level (Bouma and Muizelaar, 1992). Rodriguez-Baeza and colleagues created vascular corrosion casts to examine the cerebral microcirculation in patients who died following severe head trauma (Rodríguez-Baeza et al., 2003). They found that arterioles and capillaries in the middle and deep cortical vascular zones showed extensive injury characterized by sunken endothelial surfaces, longitudinal folds in the vessel wall, decreased lumen diameter, and corrugations, indicating a separation between the endothelium and smooth muscle cells and a disrupted BBB. Notably, larger pial and subpial vessels were histologically normal. In nonfatal head injury, cerebral intravascular microthromboses seem to be a near universal feature; Stein et al. observed intravascular thromboses in arterioles and venules in rodent, pig, and human TBI specimens (Stein et al., 2002). Multifocal BBB disruption has been observed in American football players who have sustained subconcussive injuries (but not clinical signs of concussion) using the same dynamic contrast enhanced MRI technique utilized in studies of BBB disruption in AD (Weissberg et al., 2014). At the endothelial level, endothelin-1, a peptide that acts as a vasoconstrictor, is upregulated following TBI (Chatfield et al., 2011; Maier et al., 2007; Salonia et al., 2010) and activates vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and other inflammatory pathways (Koyama et al., 2007, 2012). These molecules, in turn, contribute to further BBB disruption.
Microvascular injury sequelae are not limited to the acute period following the primary injury. Hay et al. showed that 47% of brains examined from long-term TBI survivors (1–47 years post-injury) exhibited histological evidence of multifocal BBB breakdown, as demonstrated by fibrinogen and immunoglobulin G immunostaining, in perivascular and parenchymal gray matter (Hay et al., 2015). Tomkins et al. used contrast-enhanced MRI to evaluate subjects with relatively mild head injuries (GCS>13) assessed in the subacute (1 week)- chronic (3 months) period after injury (Tomkins et al., 2011). They found that those subjects who developed post-traumatic epilepsy were more likely to have BBB disturbance than those who had a history of TBI but did not have epilepsy (82% vs. 25%). In 4 subjects, BBB disruption was evident using this MRI technique 1.5–11 years after injury. Doherty et al. examined a case of chronic traumatic encephalopathy (CTE) using immunohistochemical techniques to examine the expression of the BBB tight junction proteins claudin-1 and zona occludens-1 (Doherty et al., 2016). Perivascular p-tau was present at sites with evidence of decreased tight junction protein expression. Fibrinogen and immunoglobulin G extravasation, indicative of BBB dysfunction, was also evident at these sites. Similarly, Tagge et al. found focal cortical lesions with perivascular accumulation of immunoglobulin G in 4 cases of mild concussive injury examined in the subacute-chronic period after injury (Tagge et al., 2018). These findings are consistent with extravasation and accumulation of serum proteins at sites of focal microvascular injury. BBB disruption in the acute period after severe brain injury, determined by a cerebrospinal fluid (CSF)/plasma protein ratio > 0.007, may correlate with worse long-term outcome (Ho et al., 2014), though the prognostic significance across the TBI spectrum needs to be further explored.
In addition to these changes at the BBB, cerebral blood flow (CBF) has been extensively studied after TBI in humans, mostly in the acute period within a few days of injury (Furuya et al., 2003; Menon, 2006) although several studies have examined CBF weeks to years after TBI (Kim et al., 2010). There is a consistent body of literature in humans indicating that deficits in CBF are common after TBI, including repetitive mTBI (Bonne et al., 2003). Studies using single photon emission computed tomography (SPECT) to measure regional CBF in patients with chronic TBI (Barkai et al., 2004; Bonne et al., 2003; Lewine et al., 2007) have consistently found regions of hypoperfusion in a subset of symptomatic TBI subjects (Raji et al., 2014). SPECT perfusion changes significantly correlated with neuropsychological or neurological deficits. Studies using Xenon-CT report similar findings (Lewine et al., 2007). In addition to nuclear medicine studies, advanced MRI techniques have also been helpful in evaluating traumatic microvascular injury. Arterial Spin Labelling (ASL) reveals alterations in global and regional resting CBF in TBI patients of all severities. Kim et al. showed that patients with chronic moderate-to-severe TBI have reduced global CBF, as well as decreased regional perfusion in the thalamus, posterior cingulate cortex, and frontal cortex (Kim et al., 2010). Regions with decreased resting CBF also had altered task-related activation during an ASL fMRI working memory paradigm in chronically injured subjects. Regional relative CBF can also be measured with perfusion-weighted imaging (Bartnik-Olson et al., 2014). The exact causes of these alterations in CBF following TBI are unclear. Decreased CBF may result from a lower metabolic demand from injured tissue, resulting in an appropriately matched reduction in blood flow. However, studies using fluorodeoxyglucose positron emission tomography (FDG-PET) have suggested that glucose metabolism is disrupted following TBI, but can be increased, decreased, or unchanged in ways that do not clearly correlate with structural abnormalities (Ito et al., 2016; Yamaki et al., 2018).
The studies above describe alterations in resting CBF following TBI. In addition, cerebrovascular reserve, or the ability of the cerebral vasculature to react to vasodilatory or vasoconstrictive stimuli, termed cerebrovascular reactivity (CVR), can be altered after TBI. Breath holding (resulting in induced hypercapnia), hyperventilation, CO2 inhalation, or acetazolamide administration can be used in conjunction with non-invasive imaging techniques to assess CVR (Kassner and Roberts, 2004). Transcranial Doppler (TCD) ultrasound, near infra-red spectroscopy (NIRS) and magnetic resonance imaging (MRI) have been the most popular methods to examine post-TBI CVR in recent studies. TCD offers the advantage of very high temporal resolution but suffers from poor spatial resolution. A large prospective study of 299 acute TBI patients assessed the incidence of cerebral vasospasm via TCD (Oertel et al., 2005). Nearly half of the patients met at least one TCD criterion for vasospasm. After the acute stage, studies of professional boxers exposed to repetitive mild TBI showed that CVR was reduced in the subacute period when measured with both TCD and NIRS particularly in boxers who had experienced the highest mTBI exposures (Bailey et al., 2013). In the boxers, lower CVR measurements correlate with worse neurocognitive dysfunction and were inversely correlated with head injury exposure. A recent meta-analysis identified three studies examining sport-related concussion and CVR via TCD in 42 athletes (primarily boxers and hockey players) and 33 healthy controls (Gardner et al., 2015). All three studies found decreased CVR in the acute period after a mTBI.
NIRS has been used to study CVR after TBI as well as other neurologic conditions (Lee et al., 2009; Rodriguez Merzagora et al., 2014; Zweifel et al. 2010). Changes in CBF and associated changes in tissue concentrations of oxy- and deoxy-hemoglobin can be measured by NIRS. Like TCD, NIRS offers a very high temporal resolution, and while spatial resolution is superior to TCD, it is not as high as MRI. NIRS allows for reliable CVR measurements over time during dynamic challenges that are independent of hemoglobin concentration, skull thickness and extracranial circulation (Kainerstorfer et al., 2015). NIRS has been used to assess the CVR index in 37 acute TBI patients in the intensive care unit (Diedler et al., 2011). NIRS has also been carried out in the chronic stage of TBI. In chronic moderate and severe TBI patients (mean 18 years after TBI), CVR remained significantly reduced compared to 15 age-sex matched controls (Rodriguez Merzagora et al., 2014), indicating that CVR disruption is a long-lasting deficit in at least a subset of patients. Notably, CVR abnormalities corresponded to cognitive performance.
CVR can also be measured with MRI coupled with a hypercapnia challenge (Amyot et al., 2017; Mutch et al., 2016a, 2016b). CVR is more sensitive than CBF for discriminating between injured and uninjured participants. Even in the absence of frank structural or CBF abnormality, impaired response to induced hypercapnia (end-tidal CO2 = 50 mmHg) can be observed years after even relatively minor injuries (Amyot et al., 2017; Haber et al., 2018), indicating that cerebrovascular reserve is chronically impaired in some patients following TBI. It should be noted that, while these responses are abnormal in TBI subjects compared to healthy, uninjured control subjects, they do not address whether the brain tissue served by these vessels experiences metabolic distress in the form of impaired oxygen and glucose delivery.
Evidence from preclinical animal models
Animal models of head injury have furthered our understanding of the vascular sequelae of TBI. Animal models allow studies of TBI-related vascular pathology using tools that are not practical or ethical in human patients. In animal models, BBB extravasation is typically studied histologically. Common methods include immunostaining for endogenous markers such as immunoglobulin G (IgG) and fibrin, or fluorescent detection of exogenous Evans blue or sodium fluorescein dyes (Qin et al., 2017; Yao et al., 2015; Zhiyuan et al., 2016). Additional tools include calculation of brain water content for evidence of edema (wet/dry weight ratio), vascular density through immunohistochemistry, or biochemical changes in tight junction protein or mRNA expression in whole, half, or micro-dissected brain lysates (Alluri et al., 2016; Blixt et al., 2015). Emerging functional assessments focus on CBF and intracranial pressure, and can be measured by ultrasonogram (Clevenger et al., 2015) and telemetry (Kawoos et al., 2016) respectively. As this field advances, a focus on simple, translational biomarkers to assess cerebrovascular impairment that can be applied clinically as well as in animal models are highly desirable.
Common vascular phenotypes following rodent and porcine fluid percussion injury (FPI), controlled cortical impact (CCI), blast, and other closed head injury (CHI) models include increased albumin permeability (Bhowmick et al., 2019; Johnson et al., 2018a; Qin et al., 2017; Yao et al., 2015; Zhiyuan et al., 2016), increased leukocyte adhesion to the vascular endothelium (Li et al., 2017; Schwarzmaier et al., 2013), decreased expression of tight junction proteins (Alluri et al., 2016; Bhowmick et al., 2019; Blixt et al., 2015), widening of intracellular junctions (Sangiorgi et al., 2013), acute pericyte loss (Washington et al., 2018), and disrupted cross-talk between pericytes and endothelial cells (Bhowmick et al., 2019). CBF decreases after rotational injury and FPI (Clevenger et al., 2015; Wang et al., 2016), whereas intracranial pressure has been reported to increase following blast injury (Kawoos et al., 2016) and CCI (Moon-Massat et al., 2017). Both phenomena are hypothesized to result from endothelial disruption and dysfunction that subsequently affects the homeostatic regulation of vascular tone (Villalba et al., 2017).
Injury-induced decreases in CBF and microvascular circulation can result in microthrombi formation (Schwarzmaier et al., 2016) and tissue hypoxia (Bragin et al., 2017). Compared to sham animals, animals exposed to CCI had a greater incidence of stationary leukocyte-platelet aggregates in parenchymal capillaries (Schwarzmaier et al., 2016). Microthrombi aggregation can lead to secondary injuries including hematoma formation and edema. Beyond local changes, vasogenic edema can also cause gross structural changes akin to those seen in humans after TBI, such as hemispheric expansion and midline shift, after CCI (Liu et al., 2017) or CHI (Blixt et al., 2015). Models of milder head impacts, designed to simulate concussive injuries, have shown similar disturbances of the microvasculature, including endothelial irregularities, dysmorphic capillaries, and disruption of pericytes and perivascular astrocytes at the ultrastructural level (Tagge et al., 2018). Thus, vascular pathology following TBI in animal models can be substantial, depending on injury type and severity, much like their human analogues, though exactly how the post-injury cerebrovascular phenotype varies based on the mechanism of injury induction is not yet known.
Animal studies have reported increased expression of matrix metalloproteinase-9 (MMP-9) post-CCI (Alluri et al., 2016; Shi et al., 2015; Tao et al., 2017; Washington et al., 2018). Further, compared to wild-type controls, MMP-9 knockout mice displayed attenuated vascular permeability measured by extravasation of exogenous fluorescein isothiocyanate labelled bovine serum albumin (FITC-BSA) following CCI (Muradashvili et al., 2016), which correlated with improved behavioral performance on cognitive tasks. Suppression of MMP-9 also mitigated long term gross tissue damage following CCI (Shi et al., 2015). Inhibition of claudin-5, a tight junction protein important for small molecule transport across the BBB, using a short interfering RNA approach increased water transport across the BBB leading to cerebral edema, suggesting that claudin-5 may be an attractive target for acute management of TBI-related BBB dysfunction (Campbell et al., 2012). Inhibition of endothelin-1 receptors decreased expression of VEGF and MMPs and decreased brain edema in a FPI mouse model (Michinaga et al., 2018), similar to the human condition. Aquaporins are also an integral part of vascular homeostasis and are postulated to have a role in water influx and efflux. Genetic deletion of aquaporin-4 (AQP-4) prevented cytotoxic edema and reduced intracranial pressure after CCI, but did not affect extravasation outcomes or CSF tracer uptake (Smith et al., 2017; Yao et al., 2015). Notably, AQP-4 expression is altered in human AD and animal AD models and may play a role in Aβ clearance and degradation (Yang et al., 2016). It is important to note, however, that the mechanisms by which CSF and interstitial fluid flow and perivascular drainage pathways govern the clearance of brain waste products, before and after TBI, is not fully understood.
TBI-RELATED NEURODEGENERATION
Neurodegenerative disease following TBI was first described in professional boxers in the 1920’s who sustained repeated head trauma (Martland, 1928). Termed dementia pugilistica, it was appreciated that a history of head impacts is associated with the development of AD and other dementias (Barnes et al., 2018; Gardner et al., 2014a), PD (Gardner et al., 2018), and/or amyotrophic lateral sclerosis (Sweeney et al., 2018) later in life. Though far from universal (Weiner et al., 2017), the possibility of these long-term consequences are understandably of great concern to patients and their families. Epidemiological studies aimed at better understanding this relationship have been mixed. It has long been recognized that moderate and severe TBI in early and mid-life is associated with an increased risk of late-life dementia, with relative risks (RRs) in the order of 2.5–5.0 (Fleminger et al., 2003; Guo et al., 2000; Plassman et al., 2000). Several prospective observational studies failed to establish a relationship between mTBI, which is a far more common injury, and later-life dementia (DamsO’Connor et al., 2013; Mehta et al., 1999; Weiner et al., 2017). More recently, careful epidemiologic studies indicate that mTBI is also associated with increased risk of dementia, with RRs in the order of 1.3–2.0 (Gardner et al., 2014; Nordström et al., 2014; Wang et al., 2012). RRs for those who sustain multiple mild TBIs approach those associated with a single severe TBI (Nordström et al., 2014). Additional, detailed prospective studies will be critical to better understand the patient and injury characteristics that associate with later neurodegeneration.
It is often asserted that TBI-associated dementia is similar to Alzheimer’s disease (AD) (Fleminger et al., 2003), which is the most common type of dementia in the general population. However, prior studies on the etiology of dementia associated with TBI have used chart reviews or clinical interviews for dementia ascertainment, which are recognized to have a low specificity (Kukull et al., 1990). No prior study of TBI-associated dementia has used pathologic confirmation of the dementia subtype, which is recognized as the gold standard. Furthermore, no prior studies have used modern neurodiagnostic tools such as neuroimaging or biomarker assays in CSF, serum, or plasma, which are recognized to provide refinements over the clinical diagnosis alone (Jack et al., 2011; Walhovd et al., 2010). Despite the substantial social burden imposed by the long-term neurodegeneration after TBI, very little is known about the pathophysiology involved, there are no biomarkers that are prognostic for identifying individuals at risk for progressive neurodegeneration, and as a consequence, there are no effective therapies to prevent or slow this process. It is unclear whether brain trauma in early or mid-life leads to an acceleration and higher risk of AD-type pathology, or whether TBI-associated dementia is a distinct pathologic entity.
Much recent attention has focused on the neurodegenerative sequelae of those exposed to mild repetitive subconcussive injuries, such as those sustained as a result of multiple sport-related impacts similar to those diagnosed with dementia pugilistica in Martland’s original case series (Changa et al., 2018). Now called CTE, the diagnosis can only be made upon neuropathological examination after death. While there has been much lay media attention on the clinical features of CTE, which can include memory impairments, personality changes, and neuropsychiatric disturbances, the clinical criteria have not been rigorously defined. The importance of accurately portraying the diagnostic challenges of CTE has recently been highlighted (Stewart et al., 2019). The neuropathology of CTE is similarly complex, consisting of deposits of tau and amyloid-β (Aβ), neuronal and axonal loss, and gliosis. The most consistent neuropathological characteristic of CTE neuropathology is accumulation of phosphorylated tau protein (p-tau) in perivascular regions and at sulcal depths (McKee et al., 2016). Although Aβ pathology can be observed following TBI, tau pathology is prominent both in CTE and in long-term survivors after a single moderate-severe TBI. Aβ plaques can be seen after acute TBI (Gentleman et al., 1997; Graham et al., 1995), but are more rarely seen in long-term survivors and, when present, are more likely to be in a mature fibrillary form (Johnson et al., 2012). Emerging preclinical evidence suggests that this variability in pathology may be influenced by the age at injury and underlying genetic factors, and most intriguingly, may influence post-TBI behaviors (Cheng et al., 2018, 2019).
VASCULAR DYSFUNCTION AND NEURODEGENERATION
Given that vascular dysfunction is prevalent and persistent following mild-severe TBI and neurodegeneration is a late consequence following some TBIs, it stands to reason that the two may be mechanistically linked. To date, much of what we know about vascular dysfunction and neurodegeneration has been described in the context of AD, where there is mounting evidence that vascular dysfunction plays a role in pathogenesis (Snyder et al., 2015; Sweeney et al., 2019a). Patients with AD and related dementias frequently exhibit alterations in the brain microcirculation, including decreased capillary density, fibrohyalinosis of microvessel walls, loss of tight and adherens junctions, and increased BBB permeability (Farkas et al., 2006; Zlokovic, 2011). The neuropathological hallmarks of AD include Aβ-containing plaques and neurofibrillary tangles composed of hyperphosphorylated tau protein. The two-hit vascular hypothesis of AD proposes that blood vessel and BBB disruption are the initiating events leading to Aβ deposition and accumulation in the brain (Sweeney et al., 2018). Recent evidence suggests that alterations in BBB permeability can be detected at the earliest stages of cognitive decline. Changes in the CSF pericyte soluble platelet-derived growth factor (PDGF) receptor-β marker could be detected before changes in the AD biomarkers Aβ and tau were evident in subjects with very mild cognitive dysfunction (Nation et al., 2019). Observational data suggest that treatment of vascular risk factors like hypertension and hyperlipidemia are associated with a decreased risk of AD, but rigorous, prospective studies are lacking (Valenti et al., 2014). There are several different (and not mutually exclusive) ways in which vascular dysfunction contributes to neurodegenerative pathology, including hypoperfusion/ischemia, BBB breakdown, and endothelial dysfunction (Zlokovic, 2011).
Hypoperfusion/Ischemia
Neurovascular coupling, where CBF is regulated to precisely match neuronal activity and cellular metabolism in the surrounding brain tissue, is particularly relevant. For neurovascular coupling to occur properly, vascular smooth muscle cells and pericytes, at the arteriole and capillary levels respectively, modulate vessel diameter to regulate blood flow to neurons (Hill et al., 2015; Iadecola, 2004; Kisler et al., 2017; Yemisci et al., 2009). Mild decreases in CBF affect neuronal protein synthesis and synaptic plasticity. More marked decreases in CBF decrease ATP generation, disturbs neuronal electrolyte balances, and impairs action potential propagation, resulting in severe neuronal dysfunction. When CBF is decreased by >80%, neuronal cell death ensues (Zlokovic, 2011). Reductions in CBF have been observed in individuals at high risk of AD even before the onset of cognitive decline (Ruitenberg et al., 2005). In animal models, hypoperfusion can induce AD-like neuropathological changes, including accumulation of Aβ and hyperphosphorylated tau, and cause CBF reductions. The mechanisms and sequalae by which cerebral blood flow reductions contribute to neuropathology are beginning to be understood, including hypoxia inducible factor-1a (HIF1a)-dependent increases in Aβ production as well as alterations in transporters required for Aβ clearance (Zhang et al., 2007). Interestingly, prior to the onset of cognitive impairment and significant cortical Aβ deposition, microvascular pathologies in the form of microaneurysms, have been detected in 4–5 months old APP/PS1 mice, a preclinical model of AD (Kelly et al., 2017). In a pharmacologically induced-model of PD, acute, sub-acute, and chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) exposure led to decreased vessel intensity, length and number within the nigrostriatal pathway (Sarkar et al., 2014). These vascular phenotypes in particular may be intimately related to detectable changes in CBF, suggesting a general vulnerability of the cerebrovasculature in preclinical models of neurodegeneration. Even in the absence of frank CBF changes, there is also evidence of impaired CVR in AD (den Abeelen et al., 2014; Yezhuvath et al., 2012), reflecting an impairment in cerebrovascular reserve. Thus, even when absolute CBF is not impaired at baseline, the vascular response may not be appropriate when physiologically stressed (Len et al., 2011; Lynch et al., 2016).
As cannabinoids similarly regulate vascular tone, mechanistic studies have explored the role of the cannabinoid receptors CB1 and CB2 in this process, including elucidating the role of a potential atypical endothelial cannabinoid receptor (Bondarenko, 2014). Cannabinoid receptor activation causes vascular relaxation through direct inhibition of smooth muscle contractility as well as stimulating the release of endothelium-derived vasodilators. Under stress conditions, however, cannabinoid receptor activation can reduce CBF. Amenta and colleagues found that mice with genetic deletion of CB2 had increased BBB extravasation, as demonstrated by sodium fluorescein uptake (Amenta et al., 2014). While little human data are available, expression of CB1 and CB2 is altered in AD brains (Aso and Ferrer, 2016; Solas et al., 2013).
Blood-brain barrier dysfunction
Hypoperfusion also causes the release of reactive oxygen species that can damage the vascular endothelium and disrupt the BBB (Carvalho et al., 2009; Fernández-Checa et al., 2010). Like CBF changes, BBB dysfunction has been shown in early stages of AD as well as other neurodegenerative disorders. Montagne et al. used MRI-based methods to examine BBB leakage and found increased BBB permeability in the hippocampus and dentate gyrus during normal aging (Montagne et al., 2015). BBB disruption was associated with pericyte injury, as determined by biomarker measurements, indicating dysfunction at the level of the capillaries. Further studies have shown that BBB breakdown, as determined using dynamic contrast enhanced MRI, is evident in patients with clinically determined mild cognitive impairment (Nation et al., 2019) and early AD (van de Haar et al., 2016). BBB breakdown has also been observed in PD, most notably in vascular PD, where vascular lesions in the striatum as well as lacunar infarcts are characteristic (Benamer and Grosset, 2009; Bertrand et al., 2008). In amyotrophic lateral sclerosis, characterized by upper motor neuron degeneration in the spinal cord, there is a similar degradation between the spinal cord and its blood supply, suggesting that BBB breakdown is common in the pathophysiology of these various neurodegenerative disorders (Engelhardt and Appel, 1990).
The mechanisms that underlie BBB dysfunction in neurodegenerative diseases are not fully understood. Expression levels of many tight junction and related proteins decrease in several neurodegenerative conditions (Yamazaki and Kanekiyo, 2017). These changes may be related to dysfunction and loss of pericytes (Bell et al., 2012), which express many of the proteins required for tight junction formation, in the aging and injured brain (Bhowmick et al., 2019). MMP activity, responsible for degrading many tight and adherens junction proteins, is increased in many of these disorders and may play important roles in BBB breakdown (Rosenberg, 2009). Decreased synthesis/increased breakdown of these proteins makes the BBB “leaky”, allowing previously excluded proteins and other circulating factors to enter the brain parenchyma, leading to an accumulation of toxic serum proteins and/or reactive cascades in the brain, potentiating further neurovascular damage, brain edema, and neuronal toxicity. A recent study using FPI to induce TBI in mice (Bhowmick et al., 2019) demonstrated that injury to pericytes causing decreased PDGF receptor-β dependent signaling was associated with enhanced expression of AQP-4 in the perivascular region, down-regulation of basement membrane and tight junction proteins, and increased BBB permeability, as well as changes in the serum biomarkers S100B and neuron specific enolase. These studies suggest that pharmacologic approaches to maintaining pericyte-endothelium integrity following TBI represent an attractive therapeutic strategy.
Proper functioning of the BBB is required not only to protect the brain from peripheral toxins but also for appropriate removal of proteins and other substances from the brain parenchyma (Sweeney et al., 2019b). In animal models and humans with AD, Aβ clearance through the BBB is disrupted, promoting the aggregation of amyloid deposits within the brain parenchyma and, in particular, within the vessel walls of the cerebrovasculature, a phenomenon known as cerebral amyloid angiopathy (CAA) (Mawuenyega et al., 2010; Pflanzner et al., 2010; Shibata et al., 2000). Apolipoprotein E (apoE) is the principal lipid carrier in the brain and modifies Aβ accumulation in AD (Kim et al., 2011) in part through its actions on the cerebrovasculature (Liu et al., 2009; Sagare et al., 2012; Zhao et al., 2018). There are 3 isoforms of apoE (apoE2, apoE3 and apoE4), with apoE4 being associated with impaired Aβ metabolism and increased AD risk (Roses et al., 1996). Recently, Robert et al. used a tissue engineering approach to show that apoE4 is less efficient at promoting Aβ transport across a synthetic blood vessel when compared to other apoE isoforms (Robert et al., 2017), supporting a mechanism by which apoE4 increases susceptibility to neurodegenerative disease.
Endothelial dysfunction
Endothelial cells may contribute in other ways to neurodegenerative pathology in addition to BBB breakdown. When cerebral microvessels were isolated from the brains of autopsy specimens with AD, but not age-matched control brains, they were found to release higher levels of the inflammatory mediators interleukin (IL)-1B, IL-6, and monocyte chemoattractant protein-1 (Grammas and Ovase, 2001) as well as VEGF, MMP-9 and angiopoietin-2 (Thirumangalakudi et al., 2006). Endothelial cells derived from AD microvessels synthesize thrombin, a protein with known neurotoxic effects (Yin et al., 2010). High levels of thrombin were detected in the blood vessel walls from these AD subjects, but not controls, and could also be detected in the CSF. In 2 transgenic mouse AD models, the cerebrovasculature was found to express thrombin, tumor necrosis factor-α, IL-6, MMP-9, and IL-1β. When this so-called “vascular activation” phenotype was targeted pharmacologically using sunitinib, a multikinase inhibitor targeting VEGF and PDGF receptors, expression of these molecules was blunted and cognitive behaviors improved in these animals (Grammas et al., 2014). In vitro, sunitinib blocked endothelial cell release of MMP-9, TNF-α and Aβ, suggesting its effects are mediated at the level of the endothelium.
LOOKING TO THE FUTURE: VASCULAR DIRECTED THERAPEUTICS FOR TBI
While direct links between traumatic injury of the cerebral vasculature and neurodegenerative disease in humans have not yet been established, the evidence presented above suggests the hypothesis that TBI-related neurodegeneration may be, at least in part, a consequence of chronic microvasculopathy, akin to vascular dementia and the vascular contributions to dementia (Iadecola, 2004; Sweeney et al., 2019a). Together, these clinical and preclinical observations suggest that therapies targeting the cerebral microvasculature are promising areas of investigation in TBI-related neurodegenerative disorders.
Animal studies have been widely used to provide target validation and proof-of-concept efficacy data for TBI therapeutics and there are a number of promising options that influence the microvasculature (Figure 2). Pre-treatment with aspirin, a non-steroidal anti-inflammatory drug, inhibited thrombosis of parenchymal vessels in a subdural hemorrhage injury model, preventing secondary injury due to hypoperfusion and ischemia (Wang et al., 2018). Activated protein C (APC), a serine protease that has anti-inflammatory, anti-thrombotic, and neurodegenerative effects, has been shown to promote angiogenesis and improve outcomes in a CCI model of TBI (Petraglia et al., 2010). Recently, 3K3A-APC, an agonist of the APC receptor protease activated receptor-, was shown to decrease hemorrhage rates when administered following human ischemic stroke treated with intravascular thrombolytic agents or mechanical thrombectomy (Lyden et al., 2019). These data suggest that this agent may prove efficacious and safe for the treatment of TBI-related injury.
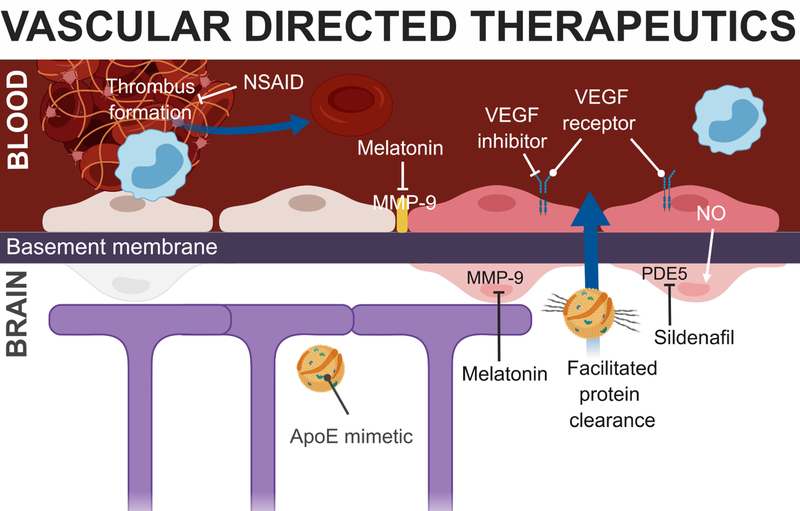
Figure 2: Potential vascular-directed therapeutics for TMVI.
Several therapies have shown promise in improving cerebrovascular function after TBI (see text), a number of which have known targets in the capillary, shown here. Acute inhibition of MMP-9 diminished the loss of tight junction proteins and pericytes after CCI (Alluri et al., 2016). VEGF inhibition prevented FPI-induced endothelial activation (Gao et al., 2015). Administration of an apoE mimetic facilitated protein clearance and reduced BBB disruption after CCI (Qin et al., 2017). NSAID pre-treatment has been shown to inhibit thrombosis of parenchymal vessels in a subdural hemorrhage injury model (Wang et al., 2018). Phosphodiesterase-5 inhibition using sildenafil improves cerebrovascular reactivity in humans with chronic TBI-related injuries (Kenney et al., 2018).
A number of agents have been found in impact BBB permeability after TBI. Co-treatment with lithium and valproic acid, clinically-approved therapies for the treatment of mood disorders and epileptic seizures, have shown promise in attenuating BBB damage measured by IgG extravasation and also improving long-term functional recovery after CCI (Yu et al., 2013). Other methods of ameliorating TBI-induced pathophysiology include inhibiting endogenous MMP-9, VEGF and endocannabinoid degradation pathways. Acute MMP-9 inhibition, with the use of melatonin, was found to protect against CCI-induced BBB permeability and edema (Alluri et al., 2016). VEGF inhibitor treatment lowered vascular permeability to albumin and upregulated tight junction proteins following FPI (Gao et al., 2015). Additionally, inhibiting endocannabinoid degradation, namely for 2-arachidonoyl glycerol and N-arachidonoylethanolamine, protected BBB integrity and rescued behavioral phenotypes after FPI (Katz et al., 2015).
Most, but not all, studies have shown an association between poor outcome after TBI and APOE4 carrier status in humans (Lawrence et al., 2015). While the exact mechanisms underlying this difference is not known, different apoE isoforms distinctly affect BBB recovery, where human targeted replacement apoE3 mice displayed faster recovery after TBI compared to their apoE4 counterparts (Washington et al., 2018). Relative to wild-type controls, apoE-deficient mice also displayed greater axonal damage and impaired cognitive recovery after mild, repetitive closed head injury (Namjoshi et al., 2013). In mice that express high levels of human amyloid precursor protein, a model of AD pathology, exposed to CCI, expression of human apoE4 was associated with Aβ deposits, visualized by Thioflavin S staining, in the dentate gyrus, an effect not observed in mice expressing human apoE3 or deficient in apoE (Hartman et al., 2002). Aβ deposition occurred at a much younger age than in the same mouse model that was not exposed to TBI. As apoE4 impairs Aβ clearance through the cerebrovasculature (Liu et al., 2013), these data suggest that apoE may influence neurodegenerative pathology through a vascular mechanism, a phenomenon exacerbated by TBI. The apoE mimetic peptide, COG1410, reduced BBB disruption and cerebral glucose uptake at acute time-points after CCI (Qin et al., 2017).
Studies using exogenous administration of endothelial progenitor and mesenchymal stem cells important for blood vessel formation and maintenance have shown remarkable promise with respect to increasing microvascular density following FPI (Guo et al., 2017; Huang et al., 2013), decreasing BBB extravasation following FPI and CCI (Huang et al., 2013; Menge et al., 2014), and decreasing water content following FPI (Huang et al., 2013). Nikolian et al. found that increases in endothelial permeability following moderate-severe TBI in a swine model could be attenuated using endothelial-protective resuscitation strategies that promote barrier integrity (Nikolian et al., 2018). Similarly, one small retrospective study showed an association between marijuana use, which activates the cannabinoid system and can impact vascular tone, and improved outcome after TBI (Nguyen et al., 2014). While preliminary, these data raise the possibility that these vascular-directed therapies may serve as a therapeutic target that could prevent or limit secondary injury following TBI. It should be noted, however, that most of these trials have focused on treatment in the acute setting; whether these interventions will be durable over a longer time course or effective if instituted in the chronic phase after injury remains to be studied.
While a number of recent studies have shown preliminary translational promise, we must remember that equally compelling TBI therapies, similarly based on sound preclinical observations, have not translated to effective intervention for the human condition (Stein, 2015). The consensus of several expert panels convened to improve TBI-related clinical trials have emphasized the urgent need for TBI imaging and molecular biomarkers that facilitate (1) the identification of subgroups of TBI patients with specific brain pathologies who can be selected for targeted therapeutic trials, (2) confirmation that the therapy is engaging the proposed molecular target, (3) measurement of pharmacodynamics and therapeutic efficacy (Diaz-Arrastia et al., 2014; Saatman et al., 2008). In this regard, biomarkers of BBB breakdown and other vascular pathology are also being developed. The most common biofluid biomarker of BBB breakdown is the CSF:serum albumin ratio which is altered in mild cognitive impairment, Alzheimer’s disease, and severe TBI (Saw et al., 2014), but not clearly altered in milder forms of TBI (Papa et al., 2015). Soluble PDGF receptor-β is shed from pericytes and can be used to distinguish pericyte injury from injury to brain endothelial or vascular smooth muscle cells (Sagare et al., 2015). Development of markers for these other vascular cell types will be important, as will determining if biofluid levels of soluble PDGF receptor-β correlate with TBI-related neurodegeneration, as was recently demonstrated for CSF levels in those with mild cognitive impairment (Nation et al., 2019). We also need to develop and leverage imaging biomarkers that can assess vascular damage and BBB permeability, using available techniques like NIRS, ASL, and CVR. Additional studies are required to assess longitudinal changes in these imaging markers so that they can be used to identify patients with vascular dysfunction and monitor their response to vascular-directed therapeutics. The validation and implementation of such biomarkers will be absolutely critical to make studies of long-term outcomes practically feasible.
Another reason behind the lack of translational success may lie in the difficulty of modelling traumatic vascular injury in a rodent or porcine model. Rodents in particular possess a more robust and resilient cerebrovasculature than humans, a hypothesized consequence of differing circulating lipoproteins that affect vascular health. Specifically, as wild-type mice do not express cholesteryl ester transfer protein, they have far higher levels of vasoprotective high density lipoproteins compared to humans, and are therefore resistant to atherosclerosis (Marotti et al., 1993). Further, the method of inducing head injury is an equally important consideration. Though no single animal model has been able to capture the entire spectrum of vascular outcomes of human TBI, rotational platforms are showing promise (Clevenger et al., 2015; Johnson et al., 2018b). From a biomechanical perspective, few models are able to capture the head kinematics that contribute to TBI-induced tissue deformation. We speculate that impact-acceleration and rotation are key biomechanical features that would increase the likelihood of a pre-clinical model being sufficient to reproduce the complex vascular pathophysiology following TBI.
CONCLUSIONS
Preclinical models of both TBI and neurodegenerative disease should be leveraged to explore molecular mechanisms and genetic contributions to traumatic vascular dysfunction that cannot be easily explored in studies of human patients, with a focus on mechanisms that can be therapeutically targeted. Continued work to develop imaging and biochemical biomarkers that are specific to TBI-related vascular injury and can be measured reliably in both preclinical models and in human patients will be critical so that we can use these directed therapies in appropriately injured patients, monitor vascular-specific therapeutic responses, and track recovery. Imaging and physiological monitoring tools are available to measure CBF, vascular reactivity, brain oxygen delivery, and BBB integrity in humans (Citerio et al., 2015). Recently, similar tools have been described that can be used in mouse models using advanced two-photon microscopy and intrinsic optical signal imaging to assess hemodynamic responses both regionally and individual capillaries/arterioles, regional brain tissue oxygenation, and the ability of oxygen supply the metabolic demands of the brain tissue (Kisler et al., 2018). Using these and similar tools in preclinical models will be critical to translating basic science observations into efficacious human interventions. Using what we know and are continuing to learn from neurodegenerative diseases, we will hopefully be able to lessen the vascular consequences of TBI.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health/National Institute of Neurological Diseases and Stroke (DKS: K23 NS104239, RD-A: U01 NS086090, U01 NS099046) and the US Department of Defense (RD-A: W81XWH-14-2-0176). AB is supported by the University of British Columbia Four Year Doctoral Fellowship. CLW receives operating support from the Canadian Traumatic Brain Injury Research Consortium Seed Grant and the Weston Brain Institute Transformational Award (TR10-0070).
Footnotes
DECLARATION OF INTERESTS
Dr. Diaz-Arrastia is a member of the Professional Advisory Board of Neural Analytics, Inc., and he also holds an equity position in that company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alluri H, Wilson RL, Shaji CA, Wiggins-Dohlvik K, Patel S, Liu Y, Peng X, Beeram MR, Davis ML, Huang JH, et al. (2016). Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS One 11, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta PS, Jallo JI, Tuma RF, Hooper DC, and Elliott MB (2014). Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J Neuroinflammation 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyot F, Kenney K, Moore C, Haber M, Turtzo LC, Shenouda C, Silverman E, Gong Y, Qu B-X, Harburg L, et al. (2017). Imaging of cerebrovascular function in chronic traumatic brain injury. J Neurotrauma 35, 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PJD, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JKJ, Murray GD, and Eurotherm3235 Trial Collaborators (2015). Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med 373, 2403–2412. [DOI] [PubMed] [Google Scholar]
- Aso E, and Ferrer I (2016). CB2 cannabinoid receptor as potential target against Alzheimer’s Disease. Front Neurosci 10, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Jones DW, Sinnott A, Brugniaux JV, New KJ, Hodson D, Marley CJ, Smirl JD, Ogoh S, and Ainslie PN (2013). Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci 124, 177–189. [DOI] [PubMed] [Google Scholar]
- Barkai G, Goshen E, Tzila Zwas S, Dolberg OT, Pick CG, Bonne O, and Schreiber S (2004). Acetazolamide-enhanced neuroSPECT scan reveals functional impairment after minimal traumatic brain injury not otherwise discernible. Psychiatry Res 132, 279–283. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Seal KH, Byers AL, Gardner RC, and Yaffe K (2018). Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 75, 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, and Ashwal S (2014). Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. J Neurotrauma 31, 1497–1506. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al. (2012). Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamer HTS, and Grosset DG (2009). Vascular parkinsonism: a clinical review. Eur Neurol 61, 11–15. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Lewandowska E, Stepień T, Szpak GM, Pasennik E, and Modzelewska J (2008). Amyloid angiopathy in idiopathic Parkinson’s disease. Immunohistochemical and ultrastructural study. Folia Neuropathol 46, 255–270. [PubMed] [Google Scholar]
- Bhowmick S, D’Mello V, Caruso D, Wallerstein A, and Abdul-Muneer PM (2019). Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp Neurol 317, 260–270. [DOI] [PubMed] [Google Scholar]
- Blixt J, Svensson M, Gunnarson E, and Wanecek M (2015). Aquaporins and blood – brain barrier permeability in early edema development after traumatic brain injury. Brain Res 1611, 18–28. [DOI] [PubMed] [Google Scholar]
- Bondarenko AI (2014). Endothelial atypical cannabinoid receptor: do we have enough evidence? Br J Pharmacol 171, 5573–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O, Gilboa A, Louzoun Y, Kempf-Sherf O, Katz M, Fishman Y, BenNahum Z, Krausz Y, Bocher M, Lester H, et al. (2003). Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res 124, 141–152. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, and Muizelaar JP (1992). Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J Neurotrauma 9 Suppl 1, S333–48. [PubMed] [Google Scholar]
- Bragin DE, Peng Z, Bragina OA, Statom GL, Kameneva MV, and Nemoto EM (2017). Improvement of impaired cerebral microcirculation using rheological modulation by drag-reducing polymers. Adv Exp Med Biol 923, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang AS, Humphries MM, Nguyen ATH, Ozaki E, Keaney J, Blau CW, et al. (2012). Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun 3, 849. [DOI] [PubMed] [Google Scholar]
- Carvalho C, Correia SC, Santos RX, Cardoso S, Moreira PI, Clark TA, Zhu X, Smith MA, and Perry G (2009). Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J Bioenerg Biomembr 41, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changa AR, Vietrogoski RA, and Carmel PW (2018). Dr. Harrison Martland and the history of punch drunk syndrome. Brain 141, 318–321. [DOI] [PubMed] [Google Scholar]
- Chatfield DA, Brahmbhatt DH, Sharp T, Perkes IE, Outrim JG, and Menon DK (2011). Juguloarterial endothelin-1 gradients after severe traumatic brain Injury. Neurocrit Care 14, 55–60. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Stukas S, Martens KM, Namjoshi DR, Button EB, Wilkinson A, Bashir A, Robert J, Cripton PA, and Wellington CL (2018). Age at injury and genotype modify acute inflammatory and neurofilament-light responses to mild CHIMERA traumatic brain injury in wild-type and APP/PS1 mice. Exp Neurol 301, 26–38. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Martens KM, Bashir A, Cheung H, Stukas S, Gibbs E, Namjoshi DR, Button EB, Wilkinson A, Barron CJ, et al. (2019). CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice. Alzheimers Res Ther 11, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, Petroni G, Lujan S, Pridgeon J, Barber J, et al. (2012). A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 367, 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citerio G, Oddo M, and Taccone FS (2015). Recommendations for the use of multimodal monitoring in the neurointensive care unit. Curr Opin Crit Care 21, 113–119. [DOI] [PubMed] [Google Scholar]
- Clevenger AC, Kilbaugh T, and Margulies S (2015). Carotid artery blood flow decreases after rapid head rotation in piglets. J Neurotrauma 32, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, and Hammond FM (2013). Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil 94, 1199–1201. [DOI] [PubMed] [Google Scholar]
- Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, and Crane PK (2013). Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 84, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Abeelen ASSM, Lagro J, van Beek AHEA, and Claassen JAHR (2014). Impaired cerebral autoregulation and vasomotor reactivity in sporadic Alzheimer’s disease. Curr Alzheimer Res 11, 11–17. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Kochanek PM, Bergold P, Kenney K, Marx CE, Grimes CJB, Loh LY, Adam LGE, Oskvig D, Curley KC, et al. (2014). Pharmacotherapy of Traumatic Brain Injury: State of the Science and the Road Forward: Report of the Department of Defense Neurotrauma Pharmacology Workgroup. J Neurotrauma 31, 135–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedler J, Zweifel C, Budohoski KP, Kasprowicz M, Sorrentino E, Haubrich C, Brady KM, Czosnyka M, Pickard JD, and Smielewski P (2011). The limitations of near-infrared spectroscopy to assess cerebrovascular reactivity. Anesth Analg 113, 849–857. [DOI] [PubMed] [Google Scholar]
- Doherty CP, O’Keefe E, Wallace E, Loftus T, Keaney J, Kealy J, Humphries MM, Molloy MG, Meaney JF, Farrell M, et al. (2016). Blood-brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 75, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JI, and Appel SH (1990). IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol 47, 1210–1216. [DOI] [PubMed] [Google Scholar]
- Farkas E, de Vos RAI, Donka G, Jansen Steur EN, Mihály A, and Luiten PGM (2006). Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol 111, 150–157. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa JC, Fernández A, Morales A, Marí M, García-Ruiz C, and Colell A (2010). Oxidative stress and altered mitochondrial function in neurodegenerative diseases: lessons from mouse models. CNS Neurol Disord Drug Targets 9, 439–454. [DOI] [PubMed] [Google Scholar]
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, and Giora A (2003). Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya Y, Hlatky R, Valadka AB, Diaz P, and Robertson CS (2003). Comparison of cerebral blood flow in computed tomographic hypodense areas of the brain in head-injured patients. Neurosurgery 52, 340–345; discussion 345–6. [DOI] [PubMed] [Google Scholar]
- Gao W, Zhao Z, Yu G, and Zhou Z (2015). VEGI attenuates the inflammatory injury and disruption of blood – brain barrier partly by suppressing the TLR4 / NF- κ B signaling pathway in experimental traumatic brain injury. Brain Res 1622, 230–239. [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Tan CO, Ainslie PN, van Donkelaar P, Stanwell P, Levi CR, and Iverson GL (2015). Cerebrovascular reactivity assessed by transcranial Doppler ultrasound in sport-related concussion: a systematic review. Br J Sports Med 49, 1050–1055. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, and Yaffe K (2014a). Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol 71, 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, and Yaffe K (2018). Mild TBI and risk of Parkinson disease. Neurology 90, e1771–e1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Greenberg BD, Savage MJ, Noori M, Newman SJ, Roberts GW, Griffin WS, and Graham DI (1997). A-beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport 8, 1519–1522. [DOI] [PubMed] [Google Scholar]
- Graham DI, and Adams JH (1971). Ischaemic brain damage in fatal head injuries. Lancet 1, 265–266. [DOI] [PubMed] [Google Scholar]
- Graham DI, Gentleman SM, Lynch A, and Roberts GW (1995). Distribution of beta-amyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol 21, 27–34. [DOI] [PubMed] [Google Scholar]
- Grammas P, and Ovase R (2001). Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging 22, 837–842. [DOI] [PubMed] [Google Scholar]
- Grammas P, Martinez J, Sanchez A, Yin X, Riley J, Gay D, Desobry K, Tripathy D, Luo J, Evola M, et al. (2014). A new paradigm for the treatment of Alzheimer’s Disease: targeting vascular activation. J Alzheimer’s Dis 40, 619–630. [DOI] [PubMed] [Google Scholar]
- Guo X, Deng X, and Wei Y (2017). Homing of cultured endothelial progenitor cells and their effect on traumatic brain injury in rat model. Sci Rep 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, et al. (2000). Head injury and the risk of AD in the MIRAGE study. Neurology 54, 1316–1323. [DOI] [PubMed] [Google Scholar]
- van de Haar HJ, Burgmans S, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Hofman PAM, Verhey FRJ, and Backes WH (2016). Blood-brain barrier leakage in patients with early Alzheimer Disease. Radiology 281, 527–535. [DOI] [PubMed] [Google Scholar]
- Haber M, Amyot F, Kenney K, Meredith-Duliba T, Moore C, Silverman E, Podell J, Chou Y-Y, Pham DL, Butman J, et al. (2018). Vascular abnormalities within normal appearing tissue in chronic traumatic brain injury. J Neurotrauma 35: 2250–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Laurer H, Longhi L, Bales KR, Paul SM, Mcintosh TK, and Holtzman DM (2002). Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer ‘s Disease. J Neurosci 22, 10083–10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JR, Johnson VE, Young AMH, Smith DH, and Stewart W (2015). Bloodbrain barrier disruption Is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol 74, 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, and Grutzendler J (2015). Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KM, Honeybul S, Yip CB, and Silbert BI (2014). Prognostic significance of blood-brain barrier disruption in patients with severe nonpenetrating traumatic brain injury requiring decompressive craniectomy. J Neurosurg 121, 674–679. [DOI] [PubMed] [Google Scholar]
- Huang X-T, Zhang Y-Q, Li S-J, Li S-H, Tang Q, Wang Z-T, Dong J-F, and Zhang J-N (2013). Intracerebroventricular transplantation of ex vivo expanded endothelial colony-forming cells restores blood–brain barrier integrity and promotes angiogenesis of mice with traumatic brain injury. J Neurotrauma 30, 2080–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5, 347–360. [DOI] [PubMed] [Google Scholar]
- Ito K, Asano Y, Ikegame Y, and Shinoda J (2016). Differences in brain metabolic impairment between chronic mild/moderate TBI patients with and without visible brain lesions based on MRI. Biomed Res Int 2016, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ, Shaw LM, Bernstein MA, Petersen RC, Weiner MW, et al. (2011). Evidence for ordering of Alzheimer Disease biomarkers. Arch Neurol 68, 1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, and Smith DH (2012). Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol 22, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Weber MT, Xiao R, Cullen DK, Meaney DF, Stewart W, and Smith DH (2018a). Mechanical disruption of the blood–brain barrier following experimental concussion. Acta Neuropathol 135, 711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Weber MT, Xiao R, Cullen DK, Meaney DF, Stewart W, and Smith DH (2018b). Mechanical disruption of the blood – brain barrier following experimental concussion. Acta Neuropathol 135, 711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainerstorfer JM, Sassaroli A, Tgavalekos KT, and Fantini S (2015). Cerebral autoregulation in the microvasculature measured with near-infrared spectroscopy. J Cereb Blood Flow Metab 35, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner A, and Roberts TPL (2004). Beyond perfusion: cerebral vascular reactivity and assessment of microvascular permeability. Top Magn Reson Imaging 15, 58–65. [DOI] [PubMed] [Google Scholar]
- Katz PS, Sulzer JK, Impastato RA, Teng SX, Rogers EK, Molina PE, and Abstract (2015). Endocannabinoid degradation inhibition improves mild traumatic brain injury. J Neurotrauma 32, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawoos U, Gu M, Lankasky J, Mccarron RM, and Chavko M (2016). Effects of exposure to blast overpressure on intracranial pressure and blood-brain barrier permeability in a rat model. PLoS One 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Denver P, Satchell SC, Ackermann M, Konerding MA, and Mitchell CA (2017). Microvascular ultrastructural changes precede cognitive impairment in the murine APPswe/PS1dE9 model of Alzheimer’s disease. Angiogenesis 20, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K, Amyot F, Moore C, Haber M, Turtzo LC, Shenouda C, Silverman E, Gong Y, Qu B-X, Harburg L, et al. (2018). Phosphodiesterase-5 inhibition potentiates cerebrovascular reactivity in chronic traumatic brain injury. Ann Clin Transl Neurol 5, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Avants B, Europa E, Wang J, Slattery J, Gee JC, Coslett HB, and Detre JA (2010). Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion fMRI study. J Neurotrauma 27, 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, and Holtzman DM (2011). The role of apolipoprotein E in Alzheimer’s Disease. Neuron 63, 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, et al. (2017). Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 20, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Lazic D, Sweeney MD, Plunkett S, El Khatib M, Vinogradov SA, Boas DA, Sakadži S, and Zlokovic BV (2018). In vivo imaging and analysis of cerebrovascular hemodynamic responses and tissue oxygenation in the mouse brain. Nat Protoc 13, 1377–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Baba A, and Matsuda T (2007). Intracerebroventricular administration of an endothelin ETB receptor agonist increases expression of tissue inhibitor of matrix metalloproteinase-1 and −3 in rat brain. Neuroscience 147, 620–630. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Maebara Y, Hayashi M, Nagae R, Tokuyama S, and Michinaga S (2012). Endothelins reciprocally regulate VEGF-A and angiopoietin-1 production in cultured rat astrocytes: implications on astrocytic proliferation. Glia 60, 1954–1963. [DOI] [PubMed] [Google Scholar]
- Kukull WA, Larson EB, Reifler BV, Lampe TH, Yerby MS, and Hughes JP (1990). The validity of 3 clinical diagnostic criteria for Alzheimer’s disease. Neurology 40, 1364–1369. [DOI] [PubMed] [Google Scholar]
- Lawrence DW, Comper P, Hutchison MG, and Sharma B (2015). The role of apolipoprotein E episilon (ɛ)-4 allele on outcome following traumatic brain injury: A systematic review. Brain Inj 29, 1018–1031. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler RC, Shaffner DH, and Brady KM (2009). Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Len TK, Neary JP, Asmundson GJG, Goodman DG, Bjornson B, and Bhambhani YN (2011). Cerebrovascular reactivity impairment after sport-induced concussion. Med Sci Sport Exerc 43, 2241–2248. [DOI] [PubMed] [Google Scholar]
- Levin HS, and Diaz-Arrastia RR (2015). Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol 14, 506–517. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Davis JT, Bigler ED, Thoma R, Hill D, Funke M, Sloan JH, Hall S, and Orrison WW (2007). Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J Head Trauma Rehabil 22, 141–155. [DOI] [PubMed] [Google Scholar]
- Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, Holena DN, Steven R, Browne KD, Smith DH, et al. (2017). Enoxaparin ameliorates post–traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg 79, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Huaxi X, and Bu G (2009). Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, and Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xu Z, Yang G, Yang D, Ding J, Chen H, Yuan F, and Tian H (2017). Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacol Sin 38, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, Sawyer RN, Claassen J, Adeoye O, Song S, et al. (2019). Final results of the RHAPSODY trial: A multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol 85, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CE, Crynen G, Ferguson S, Mouzon B, Paris D, Ojo J, Leary P, Crawford F, and Bachmeier C (2016). Chronic cerebrovascular abnormalities in a mouse model of repetitive mild traumatic brain injury. Brain Inj 30, 1414–1427. [DOI] [PubMed] [Google Scholar]
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, et al. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16, 987–1048. [DOI] [PubMed] [Google Scholar]
- Maier B, Lehnert M, Laurer HL, and Marzi I (2007). Biphasic elevation in cerebrospinal fluid and plasma concentrations of endothelin 1 after traumatic brain injury in human patients. Shock 27, 610–614. [DOI] [PubMed] [Google Scholar]
- Marklund N, Bakshi A, Castelbuono DJ, Conte V, and McIntosh TK (2006). Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des 12, 1645–1680. [DOI] [PubMed] [Google Scholar]
- Marotti KR, Castle CK, Boyle TP, Lin AH, Murray RW, and Melchior GW (1993). Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature 364, 73–75. [DOI] [PubMed] [Google Scholar]
- Martin NA, Doberstein C, Alexander M, Khanna R, Benalzacar H, Alsina G, Zane C, McBride D, Kelly D, Hovda D, et al. (2009). Posttraumatic cerebral arterial spasm. J Neurotrauma 12, 897–901. [DOI] [PubMed] [Google Scholar]
- Martland HS (1928). Punch Drunk. J Am Med Assoc 91, 1103. [Google Scholar]
- Masel BE, and DeWitt DS (2010). Traumatic brain injury: a disease process, not an event. J Neurotrauma 27, 1529–1540. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, and Bateman RJ (2010). Decreased clearance of CNS-amyloid in Alzheimer’s Disease. Science 330, 1774–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max W, MacKenzie EJ, and Rice DP (1991). Head injuries: costs and consequences. J Head Inj Rehab 6, 76–91. [Google Scholar]
- McIntosh TK, Juhler M, and Wieloch T (1998). Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J Neurotrauma 15, 731–769. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Dirk Keene C, Litvan I, Perl DP, Stein TD, Vonsattel J-P, Stewart W, et al. (2016). The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, and Breteler MM (1999). Head trauma and risk of dementia and Alzheimer’s disease: the Rotterdam study. Neurology 53, 1959–1962. [DOI] [PubMed] [Google Scholar]
- Menge T, Zhao Y, Zhao J, Wataha K, Geber M, Letourneau P, Redell J, Shen L, Wang J, Peng Z, et al. (2014). Mesenchymal stem cells regulate blood brain barrier integrity in traumatic brain injury through production of the soluble factor TIMP3. Sci Transl Med 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon DK (2006). Brain ischaemia after traumatic brain injury: Lessons from 15O2 positron emission tomography. Curr Opin Crit Care 12, 85–89. [DOI] [PubMed] [Google Scholar]
- Michinaga S, Kimura A, Hatanaka S, Minami S, Asano A, Ikushima Y, Matsui S, Toriyama Y, Fujii M, and Koyama Y (2018). Delayed administration of BQ788, an ETB antagonist, after experimental traumatic brain injury promotes recovery of blood-brain barrier function and a reduction of cerebral edema in mice. J Neurotrauma 35, 1481–1494. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, et al. (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon-Massat P, Mullah SH, Abutarboush R, Saha BK, Pappas G, Haque A, Auker C, Mccarron RM, and Arnaud F (2017). Cerebral vasoactivity and oxygenation with oxygen carrier M101 in rats. J Neurotrauma 34, 2812–2822. [DOI] [PubMed] [Google Scholar]
- Muradashvili N, Benton RL, Saatman KE, Tyagi SC, and Lominadze D (2016). Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metab Brain Dis 30, 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch WAC, Ellis MJ, Ryner LN, Ruth Graham M, Dufault B, Gregson B, Hall T, Bunge M, Essig M, Fisher JA, et al. (2016a). Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J Neurosurg 125, 648–660. [DOI] [PubMed] [Google Scholar]
- Mutch WAC, Ellis MJ, Ryner LN, Morissette MP, Pries PJ, Dufault B, Essig M, Mikulis DJ, Duffin J, and Fisher JA (2016b). Longitudinal brain magnetic resonance imaging CO2 stress testing in individual adolescent sports-related concussion patients: a pilot study. Front Neurol 7, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namjoshi DR, Martin G, Donkin J, Wilkinson A, Stukas S, Fan J, Carr M, Tabarestani S, Wuerth K, Hancock REW, et al. (2013). The liver X receptor agonist GW3965 improves recovery from mild repetitive traumatic brain injury in mice partly through apolipoprotein E. PLoS One 8, e53529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation DA, Sweeney MD, Montagne A, Sagare AP, D’orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, et al. (2019). Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BM, Kim D, Bricker S, Bongard F, Neville A, Putnam B, Smith J, and Plurad D (2014). Effect of marijuana use on outcomes in traumatic brain injury. Am Surg 80, 979–983. [PubMed] [Google Scholar]
- Nikolian VC, Dekker SE, Bambakidis T, Higgins GA, Dennahy IS, Georgoff PE, Williams AM, Andjelkovic AV, and Alam HB (2018). Improvement of blood-brain barrier integrity in traumatic brain injury and hemorrhagic shock following treatment with valproic acid and fresh frozen plasma. Crit Care Med 46, 59–66. [DOI] [PubMed] [Google Scholar]
- Nordström P, Michaëlsson K, Gustafson Y, and Nordström A (2014). Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol 75, 374–381. [DOI] [PubMed] [Google Scholar]
- Oertel M, Boscardin WJ, Obrist WD, Glenn TC, McArthur DL, Gravori T, Lee JH, and Martin NA (2005). Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg 103, 812–824. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ, Andaluz N, Chesnut RM, Bullock MR, Grant GA, et al. (2017). Brain oxygen optimization in severe traumatic brain injury phase-II. Crit Care Med 45, 1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Ramia MM, Edwards D, Johnson BD, and Slobounov SM (2015). Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J Neurotrauma 32, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ME, and Gardner RC (2018). Traumatic brain injury in older adults: do we need a different approach? Concussion 3, CNC56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Marky AH, Walker C, Thiyagarajan M, and Zlokovic BV (2010). Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery 66, 165–171; discussion 171–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanzner T, Kuhlmann CR, and Pietrzik CU (2010). Blood-brain-barrier models for the investigation of transporter- and receptor-mediated amyloid-β clearance in Alzheimer’s disease. Curr Alzheimer Res 7, 578–590. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, et al. (2000). Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Qin X, You H, Cao F, Wu Y, Peng J, Pang J, Xu H, Chen Y, Chen L, Vitek MP, et al. (2017). Apolipoprotein E mimetic peptide increases cerebral glucose uptake by reducing blood – brainbarrier disruption after controlled cortical impact in Mice : an 18F-fluorodeoxyglucose PET/CT study. J Neurotrauma 34, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Tarzwell R, Pavel D, Schneider H, Uszler M, Thornton J, van Lierop M, Cohen P, Amen DG, and Henderson T (2014). Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: a systematic review. PLoS One 9, e91088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Button EB, Yuen B, Gilmour M, Kang K, Bahrabadi A, Stukas S, Zhao W, Kulic I, and Wellington CL (2017). Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating highdensity lipoproteins in bioengineered human vessels. Elife 6, E29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Baeza A, Reina-de la Torre F, Poca A, Martí M, and Garnacho A (2003). Morphological features in human cortical brain microvessels after head injury: A three-dimensional and immunocytochemical study. Anat Rec Part A Discov Mol Cell Evol Biol 273, 583–593. [DOI] [PubMed] [Google Scholar]
- Rodriguez Merzagora AC, Izzetoglu M, Onaral B, and Schultheis MT (2014). Verbal working memory impairments following traumatic brain injury: an fNIRS investigation. Brain Imaging Behav 8, 446–459. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA (2009). Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8, 205–216. [DOI] [PubMed] [Google Scholar]
- Roses AD, Einstein G, Gilbert J, Goedert M, Han SH, Huang D, Hulette C, Masliah E, Pericak-Vance MA, Saunders AM, et al. (1996). Morphological, biochemical, and genetic support for an apolipoprotein E effect on microtubular metabolism. Ann N Y Acad Sci 777, 146–157. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, and Breteler MMB (2005). Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57, 789–794. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Duhaime A-C, Bullock R, Maas AIR, Valadka A, Manley GT, and Workshop Scientific Team and Advisory Panel Members (2008). Classification of traumatic brain injury for targeted therapies. J Neurotrauma 25, 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, and Zlokovic BV (2012). Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer’s disease. Adv Alzheimer’s Dis 3, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare AP, Sweeney MD, Makshanoff J, and Zlokovic BV (2015). Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett 607, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia R, Empey PE, Poloyac SM, Wisniewski SR, Klamerus M, Ozawa H, Wagner AK, Ruppel R, Bell MJ, Feldman K, et al. (2010). Endothelin-1 is increased in cerebrospinal fluid and associated with unfavorable outcomes in children after severe traumatic brain injury. J Neurotrauma 27, 1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi S, Benedictis A De, Protasoni M, Manelli A, Reguzzoni M, Cividini A, Dell’orbo C, Tomei G, and Balbi S (2013). Early-stage microvascular alterations of a new model of controlled cortical traumatic brain injury: 3D morphological analysis using scanning electron microscopy and corrosion casting. J Neurosurg 118, 763–774. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Raymick J, Mann D, Bowyer J, Hanig J, Schmued L, Paule M, and Chigurupati S (2014). Neurovascular changes in acute, sub-acute and chronic mouse models of Parkinson’s Disease. Curr Neurovasc Res 11, 48–61. [DOI] [PubMed] [Google Scholar]
- Saw MM, Chamberlain J, Barr M, Morgan MPG, Burnett JR, and Ho KM (2014). Differential disruption of blood–brain barrier in severe traumatic brain injury. Neurocrit Care 20, 209–216. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier SM, Zimmermann R, Mcgarry NB, Trabold R, Kim S, and Plesnila N (2013). In vivo temporal and spatial profile of leukocyte adhesion and migration after experimental traumatic brain injury in mice. J Neuroinflammation 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmaier SM, Balbi M, Terpolilli NA, Kleinschnitz C, Gruber A, and Plesnila N (2016). The formation of microthrombi in parenchymal microvessels after traumatic brain injury Is independent of coagulation factor XI. J Neurotrauma 33, 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang H, Pu H, Shi Y, Zhang J, and Zhang W (2015). Ethyl pyruvate protects against blood-brain barrier damage and improves long-term neurological outcomes in a rat model of traumatic brain injury. CNS Neurosci Ther 21, 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, et al. (2000). Clearance of Alzheimer’s amyloid-β1–40 peptide from brain by LDL receptor–related protein-1 at the bloodbrain barrier. J Clin Invest 106, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, and Friedman A (2010). Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Yao X, Dix JA, Jin B-J, and Verkman AS (2017). Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, et al. (2015). Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s Dement 11, 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solas M, Francis PT, Franco R, and Ramirez MJ (2013). CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol Aging 34, 805–808. [DOI] [PubMed] [Google Scholar]
- Stein DG (2015). Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj 29, 1362–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SC, Chen X-H, Sinson GP, and Smith DH (2002). Intravascular coagulation: a major secondary insult in nonfatal traumatic brain injury. J Neurosurg 97, 1373–1377. [DOI] [PubMed] [Google Scholar]
- Stewart W, Allinson K, Al-Sarraj S, Bachmeier C, Barlow K, Belli A, Burns MP, Carson A, Crawford F, Dams-O’Connor K, et al. (2019). Primum non nocere: a call for balance when reporting on CTE. Lancet Neurol 18, 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, and Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 21 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, et al. (2019a). Vascular dysfunction—The disregarded partner of Alzheimer’s disease. Alzheimer’s Dement 15, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, and Zlokovic BV (2019b). Blood-brain barrier: from physiology to disease and back. Physiol Rev 99, 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang X-L, Wojnarowicz MW, Casey N, Lu H, Kokiko-Cochran ON, et al. (2018). Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Shi J, Hao S, Chen X, and Liu B (2017). Protective effects of calpain inhibition on neurovascular unit injury through downregulating nuclear factor-κB- related Inflammation during traumatic brain injury in mice. Chinese Med J ¦ 130, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, and Xu L (2017). Traumatic Brain Injury– Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ 66, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, and Grammas P (2006). Angiogenic proteins are expressed by brain blood vessels in Alzheimer’s disease. J Alzheimer’s Dis 10, 111–118. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, and Shelef I (2011). Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol 2011, 765923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti R, Pantoni L, and Markus HS (2014). Treatment of vascular risk factors in patients with a diagnosis of Alzheimer’s disease: a systematic review. BMC Med 12, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba N, Sackheim AM, Nunez IA, Hill-eubanks DC, Nelson MT, Wellman GC, and Freeman K (2017). Traumatic brain injury causes endothelial dysfunction in the systemic microcirculation through arginase-1– dependent uncoupling. J Neurotrauma 34, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brewer J, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jennings RG, Karow D, Dale AM, and Alzheimer’s Disease Neuroimaging Initiative (2010). Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer Disease. Am J Neuroradiol 31, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-K, Lin S-H, Sung P-S, Wu M-H, Hung K-W, Wang L-C, Huang C-Y, Lu K, Chen H-J, and Tsai K-J (2012). Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry 83, 1080–1085. [DOI] [PubMed] [Google Scholar]
- Wang H, Tsai J, Lee J, Huang S, Huang AP, Lin W, Hsieh S, and Wang K (2018). Direct visualization of microcirculation impairment after acute subdural hemorrhage in a novel animal model. J Neurosurg 129, 997–1007. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fan W, Cai Y, Wu Q, Mo L, and Huang Z (2016). Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids 48, 2169–2177. [DOI] [PubMed] [Google Scholar]
- Washington PM, Rodriguez OC, Sloley SS, Parsadanian M, Agbaegbu C, McCann MS, Burns MP, Stefos K, Main BS, Barton DJ, et al. (2018). Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury. Mol Neurodegener 13, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Crane PK, Montine TJ, Bennett DA, and Veitch DP (2017). Traumatic brain injury may not increase the risk of Alzheimer disease. Neurology 89, 1923–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg I, Veksler R, Kamintsky L, Saar-Ashkenazy R, Milikovsky DZ, Shelef I, and Friedman A (2014). Imaging blood-brain barrier dysfunction in football players. JAMA Neurol 71, 1453. [DOI] [PubMed] [Google Scholar]
- Wilson L, Dams-O ‘ K, Phd C, Diaz-Arrastia R, Wilson L, Stewart W, Dams-O ‘connor K, Diaz-Arrastia R, Horton L, Menon DK, et al. (2017). The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 16, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki T, Uchino Y, Henmi H, Kamezawa M, Hayakawa M, Uchida T, Ozaki Y, Onodera S, Oka N, Odaki M, et al. (2018). Increased brain glucose metabolism in chronic severe traumatic brain injury as determined by longitudinal 18F-FDG PET/CT. J Clin Neurosci 57, 20–25. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, and Kanekiyo T (2017). Blood-brain barrier dysfunction and the pathogenesis of alzheimer’s disease. Int J Mol Sci 18, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Huang X, Huang X, Mai H, Li J, Jiang T, Wang X, and Lü T (2016). Aquaporin-4 and Alzheimer’s Disease. J Alzheimers Dis 52, 391–402. [DOI] [PubMed] [Google Scholar]
- Yao X, Uchida K, Manley GT, and Verkman AS (2015). Mildly reduced brain wwelling and improved neurological outcome in aquaporin-4 knockout mice following controlled cortical impact brain injury. J Neurotrauma 32, 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, and Dalkara T (2009). Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 15, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Yezhuvath US, Uh J, Cheng Y, Martin-Cook K, Weiner M, Diaz-Arrastia R, van Osch M, and Lu H (2012). Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiol Aging 33, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Wright J, Wall T, and Grammas P (2010). Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer’s Disease. Am J Pathol 176, 1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wang Z, Tanaka M, Chiu C-T, Leeds P, Zhang Y, and Chuang D-M (2013). Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury. J Neurosurg 119, 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E, Miller T, Langlois JA, and Selassie AW (2008). Prevalence of longterm disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil 23, 394–400. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao F-F, Xu H, and Zhang Y (2007). Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem 282, 10873–10880. [DOI] [PubMed] [Google Scholar]
- Zhao N, Liu C, Qiao W, and Bu G (2018). Review Apolipoprotein E, receptors, and modulation of Alzheimer’s Disease. Biol Psychiatry 83, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiyuan Q, Qingyong L, Shengming H, and Hui M (2016). Protective effect of rhEPO on tight junctions of cerebral microvascular endothelial cells early following traumatic brain injury in rats. Brain Inj 30, 462–467. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel C, Castellani G, Czosnyka M, Helmy A, Manktelow A, Carrera E, Brady KM, Hutchinson PJA, Menon DK, Pickard JD, et al. (2010). Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma 27, 1951–1958. [DOI] [PubMed] [Google Scholar]