Kallikrein-4 (KLK4) is a serine protease that is an attractive target for prostate cancer therapeutics. Here, the first crystal structure of catalytically active KLK4 in an uninhibited state is reported. The 1.64 Å resolution structure shows a canonical fold, but uses a nonstandard rotamer in a specificity-determining residue. This model will prove useful in understanding the subtle structural changes that are induced upon the binding of a ligand, and thus in the future structure-guided design of KLK4 inhibitors.
Keywords: kallikrein-related peptidase 4, KLK4, apo structure, unliganded
Abstract
Kallikrein 4 (KLK4) is a serine protease that is predominantly expressed in the prostate and is overexpressed in prostate cancer. As such, it has gained attention as an attractive target for prostate cancer therapeutics. Currently, only liganded structures of KLK4 exist in the Protein Data Bank. Until now, inferences about the subtle structural changes in KLK4 upon ligand binding have been made by comparison to other liganded forms, rather than to an apo form. In this study, an inhibitor-free form of KLK4 was crystallized. The crystals obtained belonged to space group P1, contained four molecules in the asymmetric unit and diffracted to 1.64 Å resolution. Interestingly, a nonstandard rotamer of the specificity-determining residue Asp189 was observed in all chains. This model will provide a useful unliganded structure for the future structure-guided design of KLK4 inhibitors.
1. Introduction
Kallikrein-related peptidase 4 (KLK4) is a secreted serine protease under androgenic regulation (Nelson et al., 1999 ▸; Yousef et al., 1999 ▸). It is normally expressed in the prostate, where it plays a role in a protease cascade leading to semen liquefaction (Prassas et al., 2015 ▸). Significantly, overexpression of KLK4 in the prostate is associated with both androgen-dependent and androgen-independent prostate cancers (Nelson et al., 1999 ▸; Yousef et al., 1999 ▸; Ramsay et al., 2008 ▸). As a result, KLK4 has come into view as an emerging target for prostate cancer therapeutics (Prassas et al., 2015 ▸).
As a serine protease target, standard-mechanism inhibitors have been developed against KLK4. By grafting optimal substrates into cyclopeptide inhibitor scaffolds, potent, selective inhibitors against KLK4 have been designed (Swedberg et al., 2009 ▸, 2011 ▸, 2018 ▸). Subsequent crystallographic studies have explored the engineered interactions between these inhibitors and KLK4 (Riley et al., 2016 ▸). In the prostate, the proteolytic activity of KLK4 is suppressed by a millimolar concentration of Zn2+ ions. These Zn2+ ions bind at an allosteric site between the 70–80 loop and the N-terminal strand, and disrupt the active site of the protease (Debela, Magdolen, Grimminger et al., 2006 ▸). Further structural perturbations at the active site were echoed in a crystal of KLK4 bound to Ni2+ (Riley et al., 2016 ▸).
Understanding the subtle structural changes induced upon the binding of a ligand is strongly aided by comparison to an uninhibited structure. Currently, all eight structures of KLK4 in the Protein Data Bank (PDB) are complexed with a known inhibitor. Specifically, structures exist for KLK4 bound to SFTI-1 analogues at the active site (PDB entries 4k1e, 4k8y, 4kel and 6o21; Riley et al., 2016 ▸, 2019 ▸); bound to Ni2+ at the active site (PDB entry 4kga; Riley et al., 2016 ▸); and bound to a p-aminobenzamidine inhibitor at the active site and Ni2+, Zn2+ and Co2+ at an allosteric regulatory site, respectively (PDB entries 2bdg, 2bdh and 2bdi; Debela, Magdolen, Grimminger et al., 2006 ▸). The metal ions bind to the allosteric site between His25 on the N-terminal strand and Glu77 within the 70–80 loop, disrupting the active site of the protease and explaining why the proteolytic activity of KLK4 is suppressed by a millimolar concentration of Zn2+ ions (Debela, Magdolen, Grimminger et al., 2006 ▸).
To aid both rational, structure-guided design of new inhibitors and future analyses of KLK4 inhibition, in this study we sought to crystallize and solve the structure of inhibitor-free KLK4.
2. Materials and methods
2.1. Macromolecule production
The previously reported pET12-proPSA-hK4 chimera plasmid (Takayama et al., 2001 ▸) encodes the peptidase domain of human KLK4 (Q9Y5K2-1), including the benign, nonsynonymous single-nucleotide polymophism His197Gln (VAR_028365). In this construct, the propeptide of KLK4 is replaced by the propeptide of KLK3, enabling auto-activation. This construct was transformed into Escherichia coli BL21(DE3) pLysS cells (Table 1 ▸), expressed into inclusion bodies and refolded and purified as described previously (Riley et al., 2016 ▸).
Table 1. Macromolecule-production information.
| Source organism | Homo sapiens |
| Expression vector | pET12-proPSA-hK4 (Takayama et al., 2001 ▸) |
| Expression host | E. coli BL21(DE3) pLysS |
| Complete amino-acid sequence of the construct produced† | MAPLILSR↓IINGEDCSPHSQPWQAALVMENELFCSGVLVHPQWVLSAAHCFQNSYTIGLGLHSLEADQEPGSQMVEASLSVRHPEYNRPLLANDLMLIKLDESVSESDTIRSISIASQCPTAGNSCLVSGWGLLANGRMPTVLQCVNVSVVSEEVCSKLYDPLYHPSMFCAGGGQDQKDSCNGDSGGPLICNGYLQGLVSFGKAPCGQVGVPGVYTNLCKFTEWIEKTVQAS |
The KLK3 propeptide is shown in italics and is autolytically cleaved at ↓ by KLK4.
2.2. Crystallization
The crystallization conditions for KLK4 in complex with SFTI-1 were used as a guide for crystallizing inhibitor-free KLK4. Hanging-drop vapour-diffusion crystal trays were set up manually using a 500 µl well volume and mixing 1 µl well buffer with 1 µl protein solution. Crystallization trials used 1 M lithium sulfate, 0.5 M sodium acetate and scanned 10–30%(v/v) PEG 8000, pH 4.5–6.0 and protein solution at 6, 9 and 12 mg ml−1. Crystals formed as plates after a week of incubation at 293 K using the conditions given in Table 2 ▸.
Table 2. Crystallization.
| Method | Vapour diffusion |
| Plate type | 24-well, hanging drop |
| Temperature (K) | 293 |
| Protein concentration (mg ml−1) | 9 |
| Buffer composition of protein solution | 50 mM Tris–HCl pH 7.5, 20 mM NaCl, 2 mM CaCl2 |
| Composition of reservoir solution | 0.2 M LiSO4, 0.1 M sodium acetate, 22% PEG 8000 pH 4.5 |
| Volume and ratio of drop | 2 µl (1 µl protein solution + 1 µl reservoir solution) |
| Volume of reservoir (µl) | 500 |
2.3. Data collection and processing
Crystals were transferred to a nylon loop (Hampton Research, California, USA) and cryoprotected by transferring them into mother liquor with 20%(v/v) glycerol before flash-cooling them in liquid nitrogen. Data were then collected at 100 K (nitrogen vapour stream) on the MX1 beamline at the Australian Synchrotron (Cowieson et al., 2015 ▸) using the Blu-Ice interface (McPhillips et al., 2002 ▸). Diffraction data were indexed and integrated with XDS (Kabsch, 2010 ▸) and scaled with AIMLESS (Winn et al., 2011 ▸). Statistics are shown in Table 3 ▸.
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | MX1, Australian Synchrotron |
| Wavelength (Å) | 0.9537 |
| Temperature (K) | 100 |
| Detector | ADSC Quantum 210r |
| Crystal-to-detector distance (mm) | 149.99 |
| Rotation range per image (°) | 0.5 |
| Total rotation range (°) | 360 |
| Exposure time per image (s) | 0.5 |
| Space group | P1 |
| a, b, c (Å) | 51.60, 65.72, 74.19 |
| α, β, γ (°) | 79.01, 72.91, 77.62 |
| Mosaicity (°) | 0.2 |
| Resolution range (Å) | 35.8–1.636 (1.695–1.636) |
| Total No. of reflections | 419222 (39545) |
| No. of unique reflections | 107669 (10360) |
| Completeness (%) | 96.78 (93.42) |
| Multiplicity | 3.9 (3.8) |
| 〈I/σ(I)〉 | 17.81 (2.09) |
| R r.i.m. | 0.07355 (0.7973) |
| Overall B factor from Wilson plot† (Å2) | 15.75 |
Ice ring in shell with resolution = 3.90 Å, I mean = 2918.85, Z-score = 4.05, completeness = 0.98, average completeness = 0.99.
2.4. Structure solution and refinement
The structure of inhibitor-free KLK4 was solved by molecular replacement with Phaser (McCoy et al., 2007 ▸) using PDB entry 4k8y (Riley et al., 2016 ▸) as a search model after removing solvent molecules and ligands. Models were then built in Coot (Emsley et al., 2010 ▸) and refined using PHENIX (Adams et al., 2010 ▸). Statistics for the structure refinement are presented in Table 4 ▸. Atomic coordinates and structure factors have been deposited in the Protein Data Bank with PDB accession code 6nvb and raw diffraction images are available at https://store.erc.monash.edu/experiment/view/9862/.
Table 4. Structure refinement.
Values in parentheses are for the outer shell.
| Resolution range (Å) | 35.8–1.636 (1.695–1.636) |
| Completeness (%) | 96.78 (93.42) |
| No. of reflections, working set | 102290 (9875) |
| No. of reflections, test set | 5369 (485) |
| Final R cryst | 0.1541 (0.2494) |
| Final R free | 0.1803 (0.2700) |
| Cruickshank DPI | 0.117 |
| No. of non-H atoms | |
| Total | 7845 |
| Protein | 6725 |
| Ligand | 46 |
| Water | 1074 |
| R.m.s. deviations | |
| Bonds (Å) | 0.007 |
| Angles (°) | 1.22 |
| Average B factors (Å2) | |
| Overall | 21.54 |
| Protein | 19.61 |
| Ramachandran plot | |
| Most favoured (%) | 98.53 |
| Allowed (%) | 1.47 |
3. Results and discussion
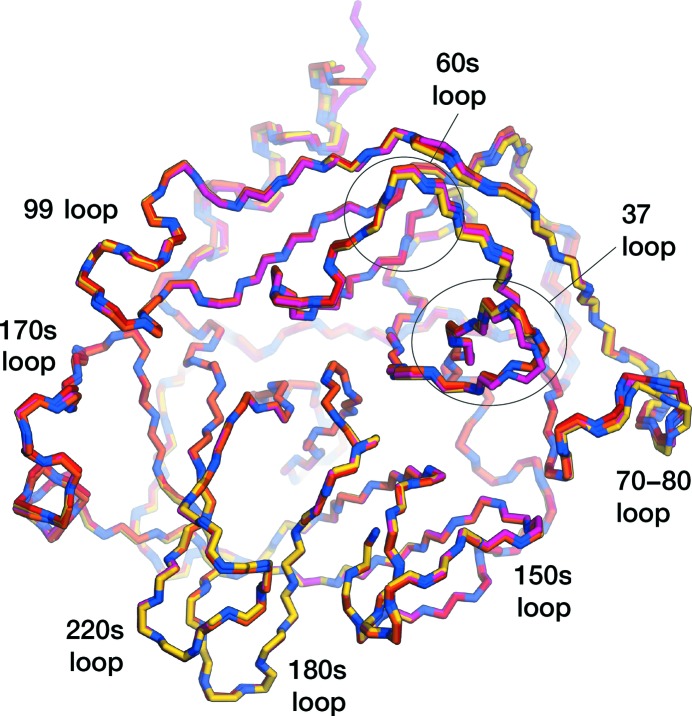
In this study, the structure of an inhibitor-free form of KLK4 was successfully solved to 1.64 Å resolution, enabling the modelling of all side chains. The KLK4 protein was overexpressed in E. coli, purified from inclusion bodies using a previously described refolding protocol (Riley et al., 2016 ▸) and crystallized. The crystals obtained belonged to space group P1, contained four molecules in the asymmetric unit and diffracted to 1.64 Å resolution. The structure of inhibitor-free KLK4 was determined and refined at a resolution of 1.64 Å, with an R work of 15.4% and an R free of 18.0% (Table 4 ▸). The structure showed the typical serine protease fold for KLK4, with an r.m.s.d. of 0.37 Å over 222 Cα atoms compared with PDB entry 4k8y (Riley et al., 2016 ▸). Within the inhibitor-free structure all chains show good structural agreement, with all-atom r.m.s.d.s within the range 0.72–0.83 Å and Cα r.m.s.d.s of between 0.27 and 0.37 Å (Fig. 1 ▸). The largest differences between chains occur within the 30s loop and the 70–80 loop, reflecting the dynamic nature of these regions, as was noted in molecular-dynamics simulations of KLK4 (Riley et al., 2016 ▸), although all chains of inhibitor-free KLK4 also displayed strong, continuous density for the backbone of the 70–80 loop and side chains could be modelled.
Figure 1.
Overlay of the N—Cα—C backbone traces of all chains in the apo KLK4 structure. Chain A is in red, chain B is in orange, chain C is in yellow and chain D is in pink, with N atoms coloured blue.
In the inhibited KLK4 crystal structures in the PDB, Asp189 occupies a t0 rotamer, pointing towards a P1 residue (here, we adopt the Coot/MolProbity rotamer nomenclature detailed in Lovell et al., 2000 ▸). In our inhibitor-free structure, Asp189 occupies a drastically different rotamer to all other KLK4 structures (Fig. 2 ▸). A glycerol molecule, used as a cryoprotectant in this experiment, could be modelled in the S1 pocket which occludes the t0 rotamer (Fig. 3 ▸). Asp189 in all chains of our inhibitor-free structure makes a hydrogen bond to Gly185 N and instead adopts an m-80 rotamer (m-20 is the most commonly observed rotamer of Asp). The chemical environment within the deep S1 pockets is somewhat distinct from that close to bulk solvent, and the pK a values of residues can differ substantially from the expected values (for Asp, this is 3.8). In general, where there is a cationic ligand/side chain present in the S1 pocket, the pK a of Asp189 is predicted by PROPKA3.0 (Olsson et al., 2011 ▸) to be around 3.6, supporting the expected deprotonation of Asp189. However, in structures in which the S1 pocket is empty, the pK a of Asp189 is predicted to be between 5.8 and 6.8. On the basis of this prediction, the crystallization buffer at pH 4.5 and the observed rotamer, we might tentatively infer that our crystal structure has captured a protonated Asp189 that promotes this alternative conformation.
Figure 2.
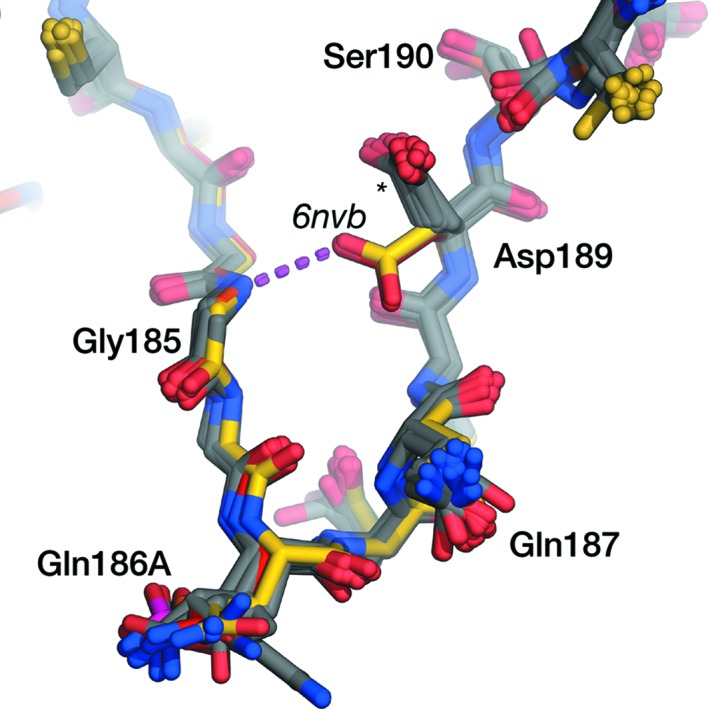
Overlay of crystal structures of the KLK4 S1 pocket. From PDB entry 6nvb, chain A is in red, chain B is in orange, chain C is in yellow and chain D is in pink. All other structures of KLK4 in the PDB are shown in grey. Asp189 in all chains of the apo KLK4 structure makes a hydrogen bond (magenta broken line) to Gly185 N and occupies an m-80 rotamer; in all other structures (marked with a star) Asp189 occupies a t0 rotamer, pointing upwards towards the P1 residue of a ligand.
Figure 3.
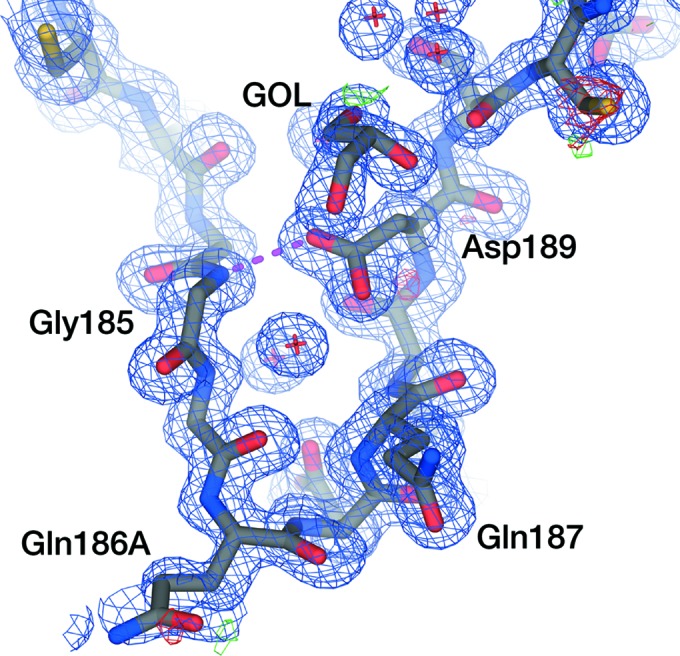
Electron density around Asp189 in the apo KLK4 S1 pocket. Chain B is shown as an exemplar. 2mF o − DF c density from a refined composite-omit map (Hodel et al., 1992 ▸) contoured at 1σ is shown as a blue mesh; mF o ± DF c density is shown as red and green mesh contoured at −3σ and +3σ, respectively. Only residues 180–194 of the S1 pocket are shown. Asp189 makes a hydrogen bond (magenta broken line) to Gly185 N and occupies an m-80 rotamer; the upwards t0 rotamer is occluded by the presence of glycerol (GOL) in the S1 pocket.
All substrate-specificity profiling experiments have found that Arg is the preferred P1 residue of KLK4, followed by Lys (Matsumura et al., 2005 ▸). This is expected as a result of Asp189 at the bottom of the S1 pocket. However, these profiling experiments also suggest that KLK4 may tolerate and be able to cleave after P1 Gly, Gln and Asn (Debela, Magdolen, Schechter et al., 2006 ▸) and P1 Tyr (Borgoño et al., 2007 ▸). The ability of Asp189 to adopt the observed rotamer may explain the ability of KLK4 to tolerate noncanonical, uncharged P1 residues. This structure will be useful to inform future structure-based drug design against KLK4.
Supplementary Material
PDB reference: apo KLK4, 6nvb
X-ray diffraction images of apo KLK4 crystals.: https://store.erc.monash.edu/experiment/view/9862/
Acknowledgments
This research was undertaken in part using the MX1 beamline at the Australian Synchrotron (CAP No. 11894d), which forms part of ANSTO.
References
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. [DOI] [PMC free article] [PubMed]
- Borgoño, C. A., Gavigan, J.-A., Alves, J., Bowles, B., Harris, J. L., Sotiropoulou, G. & Diamandis, E. P. (2007). Biol. Chem. 388, 1215–1225. [DOI] [PubMed]
- Cowieson, N. P., Aragao, D., Clift, M., Ericsson, D. J., Gee, C., Harrop, S. J., Mudie, N., Panjikar, S., Price, J. R., Riboldi-Tunnicliffe, A., Williamson, R. & Caradoc-Davies, T. (2015). J. Synchrotron Rad. 22, 187–190. [DOI] [PMC free article] [PubMed]
- Debela, M., Magdolen, V., Grimminger, V., Sommerhoff, C. P., Messerschmidt, A., Huber, R., Friedrich, R., Bode, W. & Goettig, P. (2006). J. Mol. Biol. 362, 1094–1107. [DOI] [PubMed]
- Debela, M., Magdolen, V., Schechter, N., Valachova, M., Lottspeich, F., Craik, C. S., Choe, Y., Bode, W. & Goettig, P. (2006). J. Biol. Chem. 281, 25678–25688. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Hodel, A., Kim, S.-H. & Brünger, A. T. (1992). Acta Cryst. A48, 851–858.
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Lovell, S. C., Word, J. M., Richardson, J. S. & Richardson, D. C. (2000). Proteins, 40, 389–408. [PubMed]
- Matsumura, M., Bhatt, A. S., Andress, D., Clegg, N., Takayama, T. K., Craik, C. S. & Nelson, P. S. (2005). Prostate, 62, 1–13. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- McPhillips, T. M., McPhillips, S. E., Chiu, H.-J., Cohen, A. E., Deacon, A. M., Ellis, P. J., Garman, E., Gonzalez, A., Sauter, N. K., Phizackerley, R. P., Soltis, S. M. & Kuhn, P. (2002). J. Synchrotron Rad. 9, 401–406. [DOI] [PubMed]
- Nelson, P. S., Gan, L., Ferguson, C., Moss, P., Gelinas, R., Hood, L. & Wang, K. (1999). Proc. Natl Acad. Sci. USA, 96, 3114–3119. [DOI] [PMC free article] [PubMed]
- Olsson, M. H. M., Søndergaard, C. R., Rostkowski, M. & Jensen, J. H. (2011). J. Chem. Theory Comput. 7, 525–537. [DOI] [PubMed]
- Prassas, I., Eissa, A., Poda, G. & Diamandis, E. P. (2015). Nature Rev. Drug Discov. 14, 183–202. [DOI] [PubMed]
- Ramsay, A. J., Dong, Y., Hunt, M. L., Linn, M., Samaratunga, H., Clements, J. A. & Hooper, J. D. (2008). J. Biol. Chem. 283, 12293–12304. [DOI] [PubMed]
- Riley, B. T., Ilyichova, O., Costa, M. G. S., Porebski, B. T., de Veer, S. J., Swedberg, J. E., Kass, I., Harris, J. M., Hoke, D. E. & Buckle, A. M. (2016). Sci. Rep. 6, 35385. [DOI] [PMC free article] [PubMed]
- Riley, B. T., Ilyichova, O., de Veer, S. J., Swedberg, J. E., Wilson, E., Hoke, D. E., Harris, J. M. & Buckle, A. M. (2019). Biochemistry, 58, 2524–2533. [DOI] [PubMed]
- Swedberg, J. E., de Veer, S. J., Sit, K. C., Reboul, C. F., Buckle, A. M. & Harris, J. M. (2011). PLoS One, 6, e19302. [DOI] [PMC free article] [PubMed]
- Swedberg, J. E., Ghani, H. A., Harris, J. M., de Veer, S. J. & Craik, D. J. (2018). ACS Med. Chem. Lett. 9, 1258–1262. [DOI] [PMC free article] [PubMed]
- Swedberg, J. E., Nigon, L. V., Reid, J. C., de Veer, S. J., Walpole, C. M., Stephens, C. R., Walsh, T. P., Takayama, T. K., Hooper, J. D., Clements, J. A., Buckle, A. M. & Harris, J. M. (2009). Chem. Biol. 16, 633–643. [DOI] [PubMed]
- Takayama, T. K., McMullen, B. A., Nelson, P. S., Matsumura, M. & Fujikawa, K. (2001). Biochemistry, 40, 15341–15348. [DOI] [PubMed]
- Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., Keegan, R. M., Krissinel, E. B., Leslie, A. G. W., McCoy, A., McNicholas, S. J., Murshudov, G. N., Pannu, N. S., Potterton, E. A., Powell, H. R., Read, R. J., Vagin, A. & Wilson, K. S. (2011). Acta Cryst. D67, 235–242. [DOI] [PMC free article] [PubMed]
- Yousef, G. M., Obiezu, C. V., Luo, L.-Y., Black, M. H. & Diamandis, E. P. (1999). Cancer Res. 59, 4252–4256. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: apo KLK4, 6nvb
X-ray diffraction images of apo KLK4 crystals.: https://store.erc.monash.edu/experiment/view/9862/