Abstract
Objective
Daily remote foot temperature monitoring is an evidence-based preventive practice for patients at risk for diabetic foot complications. Unfortunately, the conventional approach requires comparison of temperatures between contralaterally matched anatomy, limiting practice in high-risk cohorts such as patients with a wound to one foot and those with proximal lower extremity amputation (LEA). We developed and assessed a novel approach for monitoring of a single foot for the prevention and early detection of diabetic foot complications. The purpose of this study was to assess the sensitivity, specificity, and lead time associated with unilateral diabetic foot temperature monitoring.
Research design and methods
We used comparisons among ipsilateral foot temperatures and between foot temperatures and ambient temperature as a marker of inflammation. We analyzed data collected from a 129-participant longitudinal study to evaluate the predictive accuracy of our approach. To evaluate classification accuracy, we constructed a receiver operator characteristic curve and reported sensitivity, specificity, and lead time for four different monitoring settings.
Results
Using this approach, monitoring a single foot was found to predict 91% of impending non-acute plantar foot ulcers on average 41 days before clinical presentation with a resultant mean 4.2 alerts per participant-year. By adjusting the threshold temperature setting, the specificity could be increased to 78% with corresponding reduced sensitivity of 53%, lead time of 33 days, and 2.2 alerts per participant-year.
Conclusions
Given the high incidence of subsequent diabetic foot complications to the sound foot in patients with a history of proximal LEA and patients being treated for a wound, practice of daily temperature monitoring of a single foot has the potential to significantly improve outcomes and reduce resource utilization in this challenging high-risk population.
Keywords: prevention, remote monitoring, foot ulceration
Significance of this study.
What is already known about this subject?
Daily remote foot temperature monitoring is an evidence-based preventive practice for patients at risk for diabetic foot complications but requires comparison of temperatures between contralaterally matched anatomy, limiting its use in high-risk cohorts such as patients with a wound to one foot and those with proximal lower extremity amputation (LEA).
What are the new findings?
We present an empirical approach for unilateral once-daily foot temperature monitoring which was found to predict 91% of impending non-acute plantar foot ulcers on average 41 days before clinical presentation in data from a recent 129 participant, multicenter study of patients in remission from prior diabetic foot ulceration.
How might these results change the focus of research or clinical practice?
Given the high incidence of subsequent diabetic foot complications in patients with a history of proximal LEA and patients being treated for a wound to one foot, practice of daily temperature monitoring in these populations has the potential to significantly reduce morbidity, mortality, and resource utilization.
Introduction
Diabetic foot ulcers (DFU) are a devastating, limb-threatening, and costly complication.1–3 Prognosis following a DFU is worse than most forms of cancer, with 1-year, 2-year, and 5-year mortality rates for patients with a DFU at 19%, 31%, and 71%, respectively.4 DFU are the primary antecedent of diabetes-related lower extremity amputations (LEA), the incidence of which has increased by 50% between 2009 and 2015 after years of decline.5 A recent systematic review by Chan and colleagues estimated mean annual costs in the year following a DFU to be $44 200 for a public payer,6 of which two-thirds are attributable to inpatient care.7 Given this staggering human and economic burden, preventing DFU has long been a principal goal of care for patients with diabetes.8
Fortunately, more than 75% of DFU can be avoided through provision of comprehensive preventive foot care.9 Once-daily foot temperature monitoring is an effective, recommended, and emerging practice for reducing incidence of diabetic foot complications. It has been included in multiple clinical practice guidelines10–12 and is supported by evidence from three randomized controlled trials,13–15 which showed relative reductions in DFU incidence ranging from 61% to 85%. A recent comparative effectiveness review prepared for the US Health and Human Services Agency for Healthcare Research and Quality16 concludes that ‘home monitoring of foot skin temperature is effective for reducing foot ulcer incidence and recurrence’.
The objective of once-daily foot temperature monitoring is to identify impending inflammatory foot conditions such as DFU, infection, and acute Charcot neuroarthropathy episodes. After detecting inflammation, the care team has the opportunity to either work with the patient to prevent a wound or similar poor outcome or to begin treatment earlier than would be possible otherwise, both with the goal of minimizing cost and complications. Therapies in response to detected inflammation are low risk and inexpensive. Interventions typically include reduced ambulation, frequent and proper foot and footwear examination, and prompt treatment of any pre-ulcerative lesions on the foot such as calluses.
Traditionally, large temperature differences between one site and the corresponding contralateral site (temperature asymmetry) have been used to indicate elevated risk for impending foot complications. The most common approach13–15 initiates preventive care when a patient is observed with asymmetry exceeding 2.2°C (4 °F) for at least two consecutive days between any of the following six contralaterally matched plantar ‘keypoint’ locations: the hallux, first, third, and fifth metatarsal heads, midfoot, and heel. A recent investigation17 assessed the predictive accuracy of remote temperature monitoring over a range of asymmetry threshold temperatures. Using a remote temperature monitoring mat to implement the 2.2°C asymmetry approach, the investigators predicted 97% of all non-acute plantar DFU on average 35 days before clinical presentation with a specificity of 43%.
The contralateral foot is an excellent control for foot temperature monitoring because it eliminates the confounding effects of ambient temperature, which is a known covariate of foot temperature in patients with diabetic polyneuropathy.18–21 It also compensates for known physiological fluctuations in body temperature (such as circadian rhythms20 22 23), systemic inflammatory responses such as fever, and transient temperature changes from intermittent physical activity and footwear.
Unfortunately, many at-risk patients are unable to measure temperatures on both feet due to history of high-level amputation or due to ongoing treatment of an unhealed wound which necessitates dressings, casting, or other therapeutic footwear that should not be removed. Additionally, patients who have suffered LEA are at high risk for complications to the contralateral foot.24–31 These studies show that as many as 55% of patients with a history of LEA suffer subsequent amputation of the contralateral limb within 5 years. For these patients, the value of prevention is significant because the risk factors for diabetic foot complications affect both limbs. Furthermore, history of amputation or ongoing treatment of an existing wound may result in gait deviation and elevated pressure to the sound foot, additionally predisposing the patient to complications.
Thus, inability to assess the risk of a single foot represents an enormous limitation of conventional foot temperature monitoring given the high risk to the sound foot for those with a history of proximal LEA and those being treated for a DFU. We therefore aimed to validate an approach for identifying foot inflammation and other early warning signs of diabetic foot complications with remote foot temperature monitoring for patients with only one foot available for evaluation. We hypothesized that other temperatures, such as ambient temperature or ipsilateral foot temperatures, could serve as a comparator for identifying plantar foot inflammation in the absence of a contralateral foot.
Research design and methods
We addressed our aim through secondary analysis of existing data from a 2017 prospective, multicenter, cohort study (ClinicalTrials.gov Identifier NCT02647346).17 This study’s primary objective was to assess the accuracy of conventional asymmetry-based remote temperature monitoring for predicting plantar DFU.
These investigators followed 129 participants for a maximum duration of 34 weeks each or until the participant became lost-to-followup or withdrew consent. Each study participant was required to have diabetes mellitus and history of a previously healed plantar DFU. Study exclusion criteria included unhealed plantar DFU, active Charcot foot disease, severe peripheral arterial disease (ankle–brachial index less than 0.5 in the absence of palpable pulses), and history of proximal LEA.
All participants received standard medical and preventive diabetic foot care, including appropriate footwear, instructions to continue daily foot inspections, and instructions to contact the principal investigator on discovering any wound. Participants who developed a DFU were not withdrawn from the study, allowing assessment of multiple DFU to a single participant as independent events. Participants who developed a plantar DFU during participation were instructed to discontinue use of the study device until epithelialization of the wound, at which point the participant was encouraged to resume use.
The study device, shown in figure 1, was a daily remote temperature monitoring foot mat (Remote Temperature Monitoring System; Podimetrics, Somerville, MA, USA). It has been certified as a ‘high-traction’ product by the National Floor Safety Institute and is designed to minimize the risk of trips, slips, and falls. It can be used either sitting or standing. It is legally marketed in the USA as a class I medical device (510(k) designation K150557) and has been cleared for its intended use of ‘periodic evaluation of the temperature over the soles of the feet for signs of inflammation’. The device is indicated for patients at risk for inflammatory foot diseases. It may be used with the sound foot by patients with a history of any-level LEA and by patients being treated for a DFU to one limb.
Figure 1.
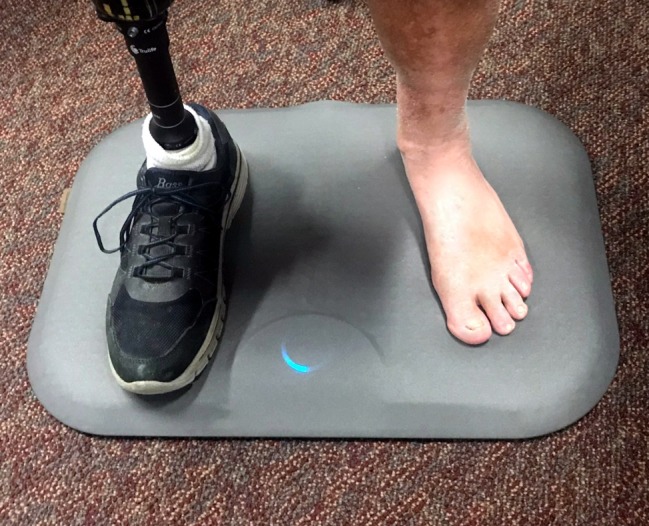
The study device is a daily-use telemedicine mat with integrated thermometric sensors and secure transmission of data via cellular network for analysis and triage.
Analysis plan
Given the study inclusion and exclusion criteria, data from patients with proximal LEA and patients with a bandaged foot are not available to derive a model for remote temperature monitoring in a single foot. To approximate the data that would be available for patients with proximal LEA or a bandaged foot, we evaluated the temperatures and outcomes to each foot from the study separately.
In the absence of contralateral foot temperatures to serve as a comparator, we evaluated alternative variables based on availability in our reference data and review of the literature. Several investigators have reported that foot temperature in otherwise healthy patients with diabetic polyneuropathy is highly correlated with ambient temperature.18–21 Additionally, large differences between foot temperature and ambient temperature in patients with diabetes are associated with peripheral neuropathy32–34 and peripheral arterial disease,34 35 both of which are known antecedents of DFU and LEA. Thus, foot temperature exceeding ambient temperature by more than typical may reflect elevated risk for DFU. Additionally, we considered comparisons of temperatures within the same foot. Recent research suggests that foot temperature patterns in diabetic feet (both with36–38 and without18 39 clinical signs of inflammation) coincide with plantar angiosomes, a concept from plastic surgery which segments the body into volumes of tissue vascularized by a common set of arteries. Inflammation due to repetitive stresses on the foot tissue is often isolated to the affected location and surrounding vascular tree. Thus, the temperatures in one angiosome can be used as a comparator for those in another.
To characterize interangiosomal variations in foot temperature, we use the difference between the maximum foot temperature and minimum foot temperature among the six locations traditionally used for remote temperature monitoring, which we refer to as the ‘ipsilateral temperature range’ (ITR). To characterize foot temperature relative to ambient, we calculated the difference between the median foot temperature at these six locations and the average temperature of sensors on the study device on which the participant was not standing (a measurement of ambient temperature). We refer to this difference as the ‘ambient temperature difference’ (ATD). To evaluate the evolution of a patient’s risk, we evaluated ITR against a single setting at several sensitivity levels. We evaluated our model with settings applied over three different durations (one, two, and three consecutive observations) and selected the duration with the best classification accuracy.
To maintain consistency with previous research on remote temperature monitoring, we have chosen to characterize the spatial distribution of foot temperatures at the following six keypoint locations: the hallux, first, third, and fifth metatarsal heads, midfoot, and heel. Based on the high prognostic accuracy reported for asymmetry-based remote temperature monitoring,17 it is clear that these locations sample the angiosomes of the feet with sufficient spatial resolution to detect localized inflammation.
Consistent with the approach employed previously with these data,17 we chose to report our false-positive and false-negative rates over a 2-month period. Reporting these statistics over a 2-month interval allows for a clinically meaningful and consistent interpretation of the results commensurate with a hypothesized duration between office visits for a high-risk patient. Another benefit of this approach is that it implicitly weights the outcomes for each participant by the quantity of data collected for that participant, naturally handling participants with censored data due to developing a clinical contraindication or discontinuing participation in the study. This approach also allows for unambiguous treatment of participants who suffered multiple DFU events during the study, whereas the more traditional approach of aggregating data on a per-participant basis would under-report the potential impact for those patients at highest risk. Finally, using 2-month intervals for reporting better ensures causality between the temperatures measured and the development of any subsequent DFU given the long duration of follow-up.
We considered those cases in which the approach indicated an impending DFU prior to the participant developing a DFU in the same 2-month interval as true positives. False-positive cases were those in which the analysis indicated an impending DFU that did not subsequently occur over the following 2 months. To evaluate classification accuracy, we constructed a receiver operator characteristic (ROC) curve that defined the prediction’s sensitivity and specificity.
Results
We compared two categories of feet: those with known inflammation due to DFU and those without. A total of 53 DFU occurred to 37 participants. Of these 37 participants, 8 suffered DFU to both feet during the trial, and 29 had unilateral DFU.
The predictive accuracy of our ITR model, characterized by the specificity at 90% sensitivity, was maximized with a duration of one observation. Interestingly, despite the weak correlation between ATD and DFU incidence, we found that eliminating ATD from our model and relying solely on ITR resulted in nearly a 15% relative reduction in specificity at high sensitivity. We hypothesize that this is due to inflammation which manifests across all four plantar angiosomes of the foot, thus resulting in low ITR but large ATD. We therefore included ATD as a disjunctive term (ie, an ‘OR’ condition) in our model and computed the ROC over a range of ITR at a constant ATD settings associated with high specificity to minimize false positives and allow detection of spatially uniform inflammation. We set this ATD setting to coincide with the 95% of observed ATD in the data, which corresponds to approximately 10.5°C.
We considered four ITR settings and a single ATD threshold. These settings span sensitivity and specificity ranges that we believe may find use in clinical practice. The temperature settings are available from the authors on reasonable request, consistent with this journal’s ‘Tier 2’ data sharing policy. At ITR setting 2, the system correctly identified 91% of non-acute plantar DFU with an average lead time of 41 days with a false-positive rate of 54%. Extrapolating over a year by assuming the true-positive and false-positive rates are constant and equal to those observed during the 34-week trial, we would expect 4.2 notifications per participant per year at this setting. Using the more specific ITR three setting reduces sensitivity to 80% but improves the false-positive rate to 41%. These data are illustrated in table 1.
Table 1.
Accuracy of diabetic foot ulcer prediction across four ipsilateral temperature range settings at a constant ambient temperature difference of 10.5°C
| Setting | 1 | 2 | 3 | 4 |
| Sensitivity | 97% | 91% | 80% | 53% |
| Specificity | 33% | 46% | 59% | 78% |
| Lead time (days) | 40±18 | 41±18 | 42±16 | 33±18 |
| Positive predictive value | 15% | 17% | 18% | 22% |
| Negative predictive value | 99% | 98% | 96% | 93% |
| Alerts (per participant-year) | 4.9 | 4.2 | 3.7 | 2.2 |
Discussion
The results of this study suggest that a telemedicine mat can be used to detect inflammation in patients at-risk for DFU recurrence who have only a single foot available for monitoring. While the most commonly used protocol relies on contralateral temperature differences and thus cannot be used in patients with proximal LEA or those being treated for a wound to one foot, the approach presented herein relies on comparisons among ipsilateral foot temperatures and between foot temperature and ambient temperature.
We completed a secondary analysis of existing data to derive this approach for unilateral once-daily remote temperature monitoring. Although classification accuracy with temperature asymmetry is better than the unilateral monitoring approach presented herein, our approach currently represents the only alternative for remote temperature monitoring of a single foot. At ITR setting 2, the sensitivity is 91% and the lead time is 41 days in our data.
Overall, the burden to the patient, caregivers, and providers is expected to be low, especially relative to the potential benefits of once-daily temperature monitoring in these high-risk populations. At ITR setting 2, 4.2 alerts per participant-year are expected. As noted by Crisologo and Lavery in a recent translational medicine review,40 ‘the potential to arrest re-ulceration is worth the perceived inconvenience to the patient’ associated with increased diligence. Additionally, a recent investigation41 suggests that the false-positive rate reported by Frykberg and colleagues may be artificially elevated due to a lack of meaningful clinical intervention on detection of inflammation. This research reports that in a commercial setting, only 1.4 alerts/patient-year were observed, compared with 3.1 alerts/patient-year reported by Frykberg and colleagues. Thus, it is possible that the alert burden associated with unilateral foot temperature monitoring in a commercial setting is similarly lower than what has been reported in this present effort.
Despite concerted limb salvage efforts, incidence of DFU and LEA remains alarmingly high in high-risk cohorts.4 17 42 Promisingly, some of the more advanced and efficacious recommendations for DFU prevention, such as daily remote foot temperature monitoring, are now finding more widespread use.43 44 These preventive foot care practices represent an enormous opportunity to improve outcomes and reduce resource utilization. Unfortunately, one of the most effective preventive foot care practices, daily remote temperature monitoring, was previously limited in use to patients with two limbs available for measurement, excluding patients with proximal LEA or a wounded foot that is bandaged or casted.
Contralateral complications are common in people in remission from previous diabetic foot complications.24–31 In fact, more than 25% of all LEAs are re-amputations.45 A large number of all LEA suffered by patients with diabetes in many regions qualify as ‘proximal’ (Syme ankle disarticulation or more proximal) and result in loss of the entire plantar surface of one of the feet.46 47
Those being treated for a wound are also at elevated risk for diabetes-related complications to the sound foot. One potentially underappreciated aspect of DFU is that they are likely to recur at anatomical locations distinct from the primary occurrence. Major risk factors, which include peripheral neuropathy and peripheral arterial disease, affect the entirety of both extremities. It is thus crucial that the provision of care for the patient reflect the patient’s elevated risk in both limbs. Orneholm and colleagues48 reported that only 19% of DFU recur at the same location, with 43% occurring at another ipsilateral location and 38% occurring to the contralateral foot. Perhaps surprisingly, given the increased clinical attention during treatment, a recent peer-reviewed abstract49 suggests high incidence (0.41 DFU/ulcer-year) for those being treated for a previous unhealed DFU. High-quality preventive care is thus essential to increase ulcer-free days in this population, but because treatment of DFU often precludes assessment of temperature to the wounded foot due to bandages or accommodative footwear that cannot or should not be removed, these populations cannot benefit from traditional approaches to remote temperature monitoring.
An additional potential benefit of using the study device to monitor the sound foot in patients with a wounded foot is that the patient is able to establish a preventive routine before healing. Research strongly suggests that recurrence is most likely in the first months after healing,1 and beginning a routine of once-daily use of the study device during healing ensures the patient is monitored throughout the critical post-healing period.
Finally, this approach and its extensions may find use in other patient populations. For example, patients who develop bilateral DFU may not present with large temperature asymmetry and thus the traditional asymmetry monitoring approach would not detect any early warning signs of DFU. In the future, more sophisticated models of patient risk may incorporate insights from ITR and ATD to more accurately predict DFU.
This research has limitations that should be considered when interpreting it. Several of these limitations are inherited from the study that served as the source of our data.17 40 We are also limited by data availability, which is inherent to any secondary analysis of existing data. The data from the prior study were not chosen for our aim and therefore may be suboptimal for our purposes. For example, other temperatures, such as those from the dorsal foot or leg, were not available and may be more useful as a comparator for remote temperature monitoring than ITR and ATD.
Another important limitation related to data availability is that the study’s participants did not correspond to those for whom this model was built. Specifically, the study excluded patients with proximal LEA and unhealed wounds. Instead, we approximated the data that would be available from those with proximal LEA by considering each foot in the study independently. These patients may have precipitating risk factors not appropriately represented in our cohort (such as a higher prevalence of peripheral arterial disease). Alternatively, chronic changes in physiology may manifest as a result of these conditions that otherwise differentiates these patients from the prior study’s cohort.
Nonetheless, although it has not been thoroughly studied, there is no reason to suspect that having suffered a proximal LEA or being treated for a wound results in an altered inflammatory response to repetitive microtrauma in the contralateral foot. Furthermore, prior research17 has validated the use of remote temperature monitoring in patients who, while having not suffered proximal LEA, have nonetheless lost the vast majority of the plantar surface of one or both feet (eg, Chopart amputation). Related research50 suggests that remote temperature monitoring is perhaps more accurate in this subcohort of patients.
This study opens several avenues for future research. A prospective study in patients with tailored inclusion and exclusion criteria would allow independent validation of the monitoring approach presented herein without the limitations associated with a secondary analysis of existing data. Such a study could eliminate any remaining questions regarding a potentially altered inflammatory response in patients with high-level amputation history and patients being treated for a wound to one foot.
In addition to appropriate inclusion and exclusion criteria, such a prospective study could also improve the characterization of outcomes. Frykberg and colleagues considered only DFU as outcomes. However, other inflammatory foot conditions such as Charcot neuroarthropathy, pre-ulcerative lesions such as callus and blister, and foot infections may also be detected by the study device, which is Food and Drug Administration (FDA) cleared for the indication of ‘periodic evaluation of the temperature over the soles of the feet for signs of inflammation’. In this study, inflammation associated with any of these outcomes, all of which are clinically relevant, would have been deemed false positives due to this limitation in the study design. Any future prospective study should attempt to characterize the etiology of inflammation detected by the study device and report a false-positive rate reflecting whether detected inflammation was corroborated by clinically relevant findings on exam such as pre-ulcerative lesion or infection.
Furthermore, there is potential clinical value in assessing the progression and healing of DFU with remote temperature monitoring.51 Presumably, the same approaches that are used for identifying emergent inflammation and impending DFU may equally effective in monitoring the resolution of inflammation as DFU heal. However, additional research is warranted to validate accuracy and, if necessary, develop tailored approaches for monitoring healing. Remote temperature monitoring of wounds is complicated by the need to protect against contamination and infection. Currently, use of the study device to monitor a foot with an open wound is contraindicated, although future product development may eliminate this restriction while ensuring patient safety in accordance with FDA guidelines.
Finally, we attempted to maintain consistency with previous research to provide continuity and context for practitioners. Thus, we have chosen to extend existing approaches based on keypoints and simple point-to-point temperature comparisons. However, more sophisticated approaches may be employed in the future to build and validate higher accuracy models for predicting DFU in patients with a history of proximal LEA. While these models may prove more accurate, they will come at the expense of intelligibility to the practitioner, who will lose the ability to interpret and reason about the prediction. These models will likely also require specialized software to implement properly.
In summary, we have developed an empirical approach to remote temperature monitoring for one foot which was found to predict 91% of impending non-acute plantar foot ulcers on average 41 days before clinical presentation with a false-positive rate of 54% in our data. Given the high incidence of subsequent diabetic foot complications in patients with a history of proximal LEA and patients being treated for a wound, practice of daily temperature monitoring in these populations has the potential to significantly reduce morbidity, mortality, and resource utilization.
Acknowledgments
Nicole J Neff and Katherine A Wood contributed to the copyediting and assembly of this manuscript.
Footnotes
Contributors: LAL: concept and design, interpretation of results, and preparation and review of the manuscript. BJP: concept and design, analysis of data, interpretation of results, and preparation and review of the manuscript. DRL: concept and design, analysis of data, interpretation of results, and preparation and review of the manuscript. JDB: concept and design, interpretation of results, and preparation and review of the manuscript. GMR: concept and design, interpretation of results, and preparation and review of the manuscript. DGA: concept and design, interpretation of results, and preparation and review of the manuscript. All authors contributed in critically revising the manuscript and have given final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: BJP, DRL, and JDB are employees of Podimetrics, Inc., a private company which designed and manufactured the study device and provided financial support sole sponsor of the study on which the analyses presented herein are based. GMR is a consulting Medical Director at Podimetrics, Inc. LAL and DGA are members of the Scientific Advisory Board of Podimetrics, Inc.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–75. 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJM, Armstrong DG, Kirsner RS, et al. Diagnosis and management of diabetic foot complications. Arlington (VA): American Diabetes Association, 2019. [PubMed] [Google Scholar]
- 3.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. 10.1001/jama.293.2.217 [DOI] [PubMed] [Google Scholar]
- 4.Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications 2017;31:556–61. 10.1016/j.jdiacomp.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiss LS, Li Y, Hora I, et al. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 2019;42:50–4. 10.2337/dc18-1380 [DOI] [PubMed] [Google Scholar]
- 6.Chan B, Cadarette S, Wodchis W, et al. Cost-Of-Illness studies in chronic ulcers: a systematic review. J Wound Care 2017;26:S4–S14. 10.12968/jowc.2017.26.Sup4.S4 [DOI] [PubMed] [Google Scholar]
- 7.Rice JB, Desai U, Cummings AKG, et al. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2014;37:651–8. 10.2337/dc13-2176 [DOI] [PubMed] [Google Scholar]
- 8.Krans HJM, Porta M, Keen H. Diabetes care and research in Europe: the St. Vincent Declaration action programme: implementation document, 1992: 66. [Google Scholar]
- 9.Bus SA, van Netten JJ. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab Res Rev 2016;32(Suppl 1):195–200. 10.1002/dmrr.2738 [DOI] [PubMed] [Google Scholar]
- 10.Frykberg RG, Armstrong DG, Giurini J, et al. Diabetic foot disorders. A clinical practice guideline. for the American College of foot and ankle surgeons and the American College of foot and ankle Orthopedics and medicine. J Foot Ankle Surg 2000;(Suppl):1–60. [PubMed] [Google Scholar]
- 11.Bus SA, van Netten JJ, Lavery LA, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev 2016;32:16–24. 10.1002/dmrr.2696 [DOI] [PubMed] [Google Scholar]
- 12.Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Rep and Reg 2016;24:112–26. 10.1111/wrr.12391 [DOI] [PubMed] [Google Scholar]
- 13.Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care 2004;27:2642–7. 10.2337/diacare.27.11.2642 [DOI] [PubMed] [Google Scholar]
- 14.Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care 2007;30:14–20. 10.2337/dc06-1600 [DOI] [PubMed] [Google Scholar]
- 15.Armstrong DG, Holtz-Neiderer K, Wendel C, et al. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med 2007;120:1042–6. 10.1016/j.amjmed.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 16.M.s. S, Dy SM SM, Bennett WL M. Preventing complications and treating symptoms of diabetic peripheral neuropathy [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US), 2017. [PubMed] [Google Scholar]
- 17.Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a smart mat technology to predict development of diabetic plantar ulcers. Diabetes Care 2017;40:973–80. 10.2337/dc16-2294 [DOI] [PubMed] [Google Scholar]
- 18.Nagase T, Sanada H, Takehara K, et al. Variations of plantar thermographic patterns in normal controls and non-ulcer diabetic patients: novel classification using angiosome concept. Journal of Plastic, Reconstructive & Aesthetic Surgery 2011;64:860–6. 10.1016/j.bjps.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 19.Hasselberg MJ, McMahon J, Parker K. The validity, reliability, and utility of the iButton® for measurement of body temperature circadian rhythms in sleep/wake research. Sleep Med 2013;14:5–11. 10.1016/j.sleep.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Kang PB, Hoffman SN, Krimitsos E, et al. Ambulatory foot temperature measurement: a new technique in polyneuropathy evaluation. Muscle Nerve 2003;27:737–42. 10.1002/mus.10379 [DOI] [PubMed] [Google Scholar]
- 21.Rutkove SB. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve 2001;24:867–82. 10.1002/mus.1084 [DOI] [PubMed] [Google Scholar]
- 22.Rutkove SB, Chapman KM, Acosta JA, et al. Foot temperature in diabetic polyneuropathy: innocent bystander or unrecognized accomplice? Diabet Med 2005;22:231–8. 10.1111/j.1464-5491.2005.01486.x [DOI] [PubMed] [Google Scholar]
- 23.Nardin RA, Fogerson PM, Nie R, et al. Foot temperature in healthy individuals: effects of ambient temperature and age. J Am Podiatr Med Assoc 2010;100:258–64. [DOI] [PubMed] [Google Scholar]
- 24.Baddeley RM, Fulford JC. A trial of conservative amputations for lesions of the feet in diabetes mellitus. Br J Surg 1965;52:38–43. 10.1002/bjs.1800520109 [DOI] [PubMed] [Google Scholar]
- 25.Ebskov B, Josephsen P. Incidence of reamputation and death after gangrene of the lower extremity. Prosthet Orthot Int 1980;4:77–80. 10.3109/03093648009164567 [DOI] [PubMed] [Google Scholar]
- 26.Font-Jiménez I, Llaurado-Serra M, Roig-Garcia M, et al. Retrospective study of the evolution of the incidence of non-traumatic lower-extremity amputations (2007–2013) and risk factors of reamputation. Prim Care Diabetes 2016;10:434–41. 10.1016/j.pcd.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 27.Glaser JD, Bensley RP, Hurks R, et al. Fate of the contralateral limb after lower extremity amputation. J Vasc Surg 2013;58:1571–7. 10.1016/j.jvs.2013.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi Y, Satterfield K, Lee S, et al. Risk of Reamputation in diabetic patients stratified by limb and level of amputation: a 10-year observation. Diabetes Care 2006;29:566–70. 10.2337/diacare.29.03.06.dc05-1992 [DOI] [PubMed] [Google Scholar]
- 29.Kanade R, van Deursen R, Burton J, et al. Re-amputation occurrence in the diabetic population in South Wales, UK. Int Wound J 2007;4:344–52. 10.1111/j.1742-481X.2007.00313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frieden RA. The geriatric amputee. Phys Med Rehabil Clin N Am 2005;16:179–95. 10.1016/j.pmr.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Silbert S. Amputation of the lower extremity in diabetes mellitus: a follow-up study of 294 cases. Diabetes 1952;1:297–9. 10.2337/diab.1.4.297 [DOI] [PubMed] [Google Scholar]
- 32.Bagavathiappan S, Philip J, Jayakumar T, et al. Correlation between plantar foot temperature and diabetic neuropathy: a case study by using an infrared thermal imaging technique. J Diabetes Sci Technol 2010;4:1386–92. 10.1177/193229681000400613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papanas N, Papatheodorou K, Papazoglou D, et al. Foot temperature in type 2 diabetic patients with or without peripheral neuropathy. Exp Clin Endocrinol Diabetes 2009;117:44–7. 10.1055/s-2008-1081498 [DOI] [PubMed] [Google Scholar]
- 34.Bharara M, Cobb JE, Claremont DJ. Thermography and thermometry in the assessment of diabetic neuropathic foot: a case for Furthering the role of thermal techniques. Int J Low Extrem Wounds 2006;5:250–60. 10.1177/1534734606293481 [DOI] [PubMed] [Google Scholar]
- 35.Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care 2005;28:2206–10. 10.2337/diacare.28.9.2206 [DOI] [PubMed] [Google Scholar]
- 36.Clemens MW, Attinger CE. Angiosomes and wound care in the diabetic foot. Foot Ankle Clin 2010;15:439–64. 10.1016/j.fcl.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 37.Peregrina-Barreto H, Morales-Hernandez LA, Rangel-Magdaleno JJ, et al. Quantitative estimation of temperature variations in plantar angiosomes: a study case for diabetic foot. Comput Math Methods Med 2014;2014:1–10. 10.1155/2014/585306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peregrina-Barreto H, Morales-Hernandez LA, Rangel-Magdaleno JJ. Thermal image processing for quantitative determination of temperature variations in plantar angiosomes [Internet]. 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), 2013. [Google Scholar]
- 39.Bharara M, Schoess J, Armstrong DG. Coming events cast their shadows before: detecting inflammation in the acute diabetic foot and the foot in remission. Diabetes Metab Res Rev 2012;28:15–20. 10.1002/dmrr.2231 [DOI] [PubMed] [Google Scholar]
- 40.Crisologo PA, Lavery LA. Remote home monitoring to identify and prevent diabetic foot ulceration. Ann Transl Med 2017;5 10.21037/atm.2017.08.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong DG, Rothenberg GM, Petersen BJ, et al. Remote temperature monitoring with a telemedicine mat: from research to practice. International Symposium on the Diabetic Foot, 2019. [Google Scholar]
- 42.Franklin H, Rajan M, Tseng C-L, et al. Cost of lower-limb amputation in U.S. veterans with diabetes using health services data in fiscal years 2004 and 2010. J Rehabil Res Dev 2014;51:1325–30. 10.1682/JRRD.2013.11.0249 [DOI] [PubMed] [Google Scholar]
- 43.Begur P, Frykberg RG. Prevention of Lower Extremity Amputations. Podiatry Today [Internet]. Available: https://www.podimetrics.com/publications/Begur%202017%20Prevention%20of%20Lower%20Extremity%20Amputations.pdf [Accessed 26 Mar 2019].
- 44.Killeen AL, Walters JL. Remote temperature monitoring in diabetic foot ulcer detection. Wounds 2018;30:E44–8. [PubMed] [Google Scholar]
- 45.Sambamoorthi U, Tseng C-L, Rajan M, et al. Initial nontraumatic lower-extremity amputations among veterans with diabetes. Med Care 2006;44:779–87. 10.1097/01.mlr.0000218793.74558.0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayfield JA, Reiber GE, Maynard C, et al. Survival following lower-limb amputation in a veteran population. J Rehabil Res Dev 2001;38:341–5. [PubMed] [Google Scholar]
- 47.Wrobel JS, Robbins J, Armstrong DG. The high-low amputation ratio: a deeper insight into diabetic foot care? The Journal of Foot and Ankle Surgery 2006;45:375–9. 10.1053/j.jfas.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 48.Örneholm H, Apelqvist J, Larsson J, et al. Recurrent and other new foot ulcers after healed plantar forefoot diabetic ulcer. Wound Rep and Reg 2017;25:309–15. 10.1111/wrr.12522 [DOI] [PubMed] [Google Scholar]
- 49.Armstrong DG, Petersen BJ, Bloom JD, et al. Ulcer metastasis? location of recurrence for patients in diabetic foot remission. Symposium on Advanced Wound Care/WHS Spring 2019, San Antonio, TX, 2019. [Google Scholar]
- 50.Gordon I, Nouvong A, Najafi B, et al. Accuracy of daily foot temperature monitoring for patients with recently healed diabetic foot ulcers or history of amputation. Diabetes 2018;67(Supplement 1):114 10.2337/db18-114-OR [DOI] [Google Scholar]
- 51.Bharara M, Schoess J, Nouvong A, et al. Wound inflammatory index: a “proof of concept” study to assess wound healing trajectory. J Diabetes Sci Technol 2010;4:773–9. 10.1177/193229681000400402 [DOI] [PMC free article] [PubMed] [Google Scholar]


