Abstract
Purpose of review
To describe how countries in Latin America and the Caribbean are (or are not) meeting 2016 WHO guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, that is, their progress on the adoption of ‘Treat All’ and of preexposure prophylaxis (PrEP) as an additional prevention tool for people at substantial risk of HIV infection.
Recent findings
The HIV epidemic in the region continues largely concentrated in vulnerable populations with MSM and transgender women (TGW) suffering the highest burden. On treatment, the challenges of late initiation as well as suboptimal adherence persist. On prevention, recent studies on PrEP willingness in key populations show promising results, meanwhile PrEP implementation projects as well as actual PrEP adoption by national health systems is expanding. A glimpse of real-world PrEP uptake is shown through Brazil's first-year experience of offering PrEP in multiple cities in all regions of the country.
Summary
In conclusion, accomplishments have been made though challenges for fully addressing the HIV epidemic persist. The impact of both treatment and PrEP will be limited by the availability and prompt use of all services, including HIV testing.
Keywords: epidemiology, HIV/AIDS, Latin America, preexposure prophylaxis, treatment as prevention
INTRODUCTION
According to the UNAIDS and WHO reports, at the end of 2017, an estimated 1.8 (1.5–2.3) million people were living with the HIV in Latin America with 61% (43–79%) of this population on antiretroviral therapy (ART) [1,2]. In lines with the 2016 WHO Guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, many countries in the Americas have strengthened their HIV treatment and prevention services. In this review, we highlight how Latin American countries are (or are not) meeting the treatment recommendations with respect to ‘Treat All’ (ART for all persons living with HIV regardless of clinical stage or CD4 cell count), and how they have managed the introduction of preexposure prophylaxis (PrEP) as an additional prevention tool for people at substantial risk of HIV infection. After a quick review on the current epidemiology of the region focusing on data from recent years, we address HIV treatment and highlight the progress that has been achieved with improved ART coverage but also challenges that continue to be critical: late treatment initiation and suboptimal adherence. Following, we address prevention and elaborate on PrEP and its improvements since the 2016 review by Ravasi et al.[3] highlighting how the roll-out of PrEP in the region is slowly increasing with some Latin American countries already providing PrEP through their public health systems. Finally, using Brazil as a case study, we provide actual PrEP uptake numbers for 2018, the program's first year of existence.
Box 1.

no caption available
EPIDEMIOLOGY
To date, the HIV epidemic in Latin America remains concentrated on large urban centers and in vulnerable populations with MSM and transgender women (TGW) suffering the highest burden. Overall regional numbers show that HIV prevalence surpasses 10% in these populations with no decrease in the number of new HIV infections over the last 15 years (MSM accounting for 41% of new HIV infections in 2017) [1]. Despite the lack of progress with regards to new HIV infections, ART coverage has led to a decrease in AIDS-related mortality in the region [4,5].
Epidemiologic studies from Latin American countries published in the past 5 years (searches were performed in Medline and Lilacs, a regional search engine) confirm these region-wide findings. The baseline assessment of cohort participants, MSM and transwomen seeking HIV or sexually transmitted infection (STI) testing and/or treatment, recruited from 2013 to 2014 in Lima, Peru (the PICASSO cohort), found that 30.1 and 33.7% were infected with HIV, respectively [6]. A study from Guatemala evaluated trends in HIV prevalence from 2005 to 2012 using cross-sectional data from STI clinics of over 4000 participants and found that prevalence among MSM was higher (8.2%) than among high-risk heterosexual men (4.1%) and female sex workers (2.1%) [7▪]. A few studies from Brazil, published in 2017/2018, estimated HIV prevalence in multiple key populations, all found to be at least 10 times higher compared with the general population: 5% prevalence among female sex workers [8], 14% among MSM [9▪], 31% among transwomen [10▪]. Additionally, one study among 37 000 17-to-22-year old men (Brazilian army conscripts), observed an HIV prevalence of 0.12% overall which was 14-fold higher among those who reported sex with men [11]. Studies from Mexico have shown the same pattern with a nationally representative survey conducted in 24 cities estimating HIV prevalence at 16.9% (15.6–18.3%) among MSM [12] whereas other studies assessing TGW observed even higher prevalence: 22% in Tijuana, a border city in Mexico [13], and 20–64% in Mexico city [14].
HIV incidence rates estimates are much less available for the region. The iPrEx study, a randomized controlled efficacy trial of preexposure prophylaxis with co-formulated tenofovir disoproxil fumarate and emtricitabine included participants from 11 sites in six countries including Ecuador, Peru and Brazil from Latin America [15]. Its control arm provided valuable information regarding HIV incidence rates among 1248 MSM (87% male and 13% transgender male to female). HIV incidence rates per 100 person-years in the three Latin America countries were 6.5 (95% CI 4.1–10.5), 3.5 (95% CI 2.7–4.6), and 5.0 (95% CI 2.7–9.2) in Ecuador, Peru and Brazil, respectively [16]. Similar rates of HIV incidence among MSM are provided in review article on the global response to HIV with estimates varying from 2 in Guatemala and Panamá to 14 in Nicaragua, per 100 person-years [17].
TREATMENT
With successful ART, many people living with HIV (PLWH) around the globe are experiencing decreased AIDS-related morbidity and mortality, thus living longer [18,19]. It is expected that by 2020 all low-income and middle-income countries will have adopted WHO's ‘Treat All’ policy; however, as of mid-2018, a few countries in Latin America still apply immunologic criteria for ART treatment initiation (Guatemala, El Salvador, Nicaragua, and Colombia, though Guatemala and Nicaragua plan to implement Treat All ART recommendation in 2018–2020) [2]. As for ART monitoring, most Latin American countries have implemented routine viral load monitoring within their national policies [2]. Nevertheless, one analysis of the actual frequency of monitoring has shown it to occur less frequently than recommended: monitoring was performed once every 6 months for only 62% of cohort participants despite recommendations, which ranged from every 3–6 months depending on the country [20]. Despite the achievements, old challenges remain [21], we will focus on two: late treatment initiation and treatment adherence.
Late treatment initiation is a function of multiple, layered determinants with individual, interpersonal, social and structural factors playing an interdependent role. As such, immune status at ART initiation is a critical indicator in measuring how well programs are responding to the HIV epidemic [22]. A global analysis with over 379 000 adults (16 years or older) starting ART between 2002 and 2009 in a clinic participating in a multicohort collaboration spanning six continents (the International epidemiological Databases to Evaluate AIDS plus the ART Cohort Collaboration) showed that the median baseline CD4 cell count (defined as the nearest to the date of ART start with a window of 6 months before to 1 month after) in the seven Latin American countries included ranged, in women and men, respectively, from a low of 85 and 74 cells/μl in Peru to 208 and 186 cells/μl in Brazil [23].
A more recent analysis of the CCASAnet (the Caribbean, Central and South America network for HIV epidemiology) that included data from nine sites in seven countries (Argentina, Brazil, Chile, Haiti, Honduras, México and Perú) reported on the CD4 cell count of over 17 000 individuals starting treatment from 2003 to 2014. The results showed a significant trend toward increased probabilities of initiating ART with CD4 cell count greater than 200 cells/μl, though the model-adjusted probability of ART initiation with a CD4 of 200 or more only barely exceeded 0.5 in 2014 [24].
In Brazil, despite its universal health system that provides access to comprehensive prevention and care services including HIV testing, laboratory monitoring and antiretroviral treatment (including a ‘Treat All’ policy in place since 2014) late treatment initiation is persistent. A nation-wide analysis including over 115 000 participants who linked to care between 2004 and 2006 showed that 43.6% of participants were late presenters to care, defined as having, in their first contact with a healthcare facility, a CD4 cell count less than 200 cells/μl or an AIDS defining condition, or who died within 20 days of their first contact [25]. Another more recent analysis that included nation-wide data from 2006–2016 showed an improvement: 33% of those starting treatment had a CD4 cell count of less than 200 cells/μl, though only 20% started ART with CD4 cell count of at least 500 cells/μl [26]. In another analysis, not starting ART within 100 days of linkage to care was associated with (in addition to female sex, younger age, lower education) living in the North and Northeast and being from a municipality that is less densely populated or more economically disadvantaged (as measured by the social vulnerability index) [27▪▪].
The second persistent challenge is daily ART adherence. ART adherence is the best predictor of virologic suppression [28▪] and as such a basic requirement for improving HIV clinical outcomes, reducing morbidity and mortality of PLWH, and halting HIV transmission. A systematic review and meta-analysis of ART adherence in the Latin American region reported on the levels of adherence in the region [29]. It included 53 studies published between 2005 and 2016 with 22 603 PLHA in 25 countries from Latin America and Caribbean and reported an overall pooled adherence of 70% (95% CI: 63–76%). Interestingly, in subgroup analyses, it found higher pooled adherence estimates in countries classified as low or lower middle income as per World Bank definitions as well as in those low on human development index (HDI, a composite index measuring average achievement in three basic dimensions of human development – a long and healthy life, knowledge and a decent standard of living and, and gross national income that reflects the average income of a country's citizens). This analysis highlighted the barriers cited in the studies for meeting the daily requirements for ART adherence, which included: younger age, mental health problems (including depression), substance use in particular alcohol, low education, advanced HIV disease and high pill burden [29]. A national survey of self-reported adherence using WebAd-Q conducted in Brazil reported similar outcomes with 67.8% of the 2424 participants not missing any doses in the past 7 days [30]. Another more recent study showed higher (85.4%) adherence among those taking single-tablet first line regimens versus (82%) using multiple tablet regimens [31]. Though not much explored in these studies from Latin America, studies from other regions have highlighted the role of interpersonal factors, such as HIV stigma, social isolation, perceived discrimination [32], as well as structural factors, such as food insecurity, housing instability, ART regimen complexity [33], which likely play a role in the region as well and thus merit attention.
PREVENTION
Curbing the HIV epidemic in Latin America will require the adoption of targeted prevention programs, such as a combination prevention program that includes PrEP. Currently, a vast body of evidence supports the use of PrEP to prevent HIV infection particularly in populations at substantial risk of HIV acquisition. A 2016 meta-analysis of 18 PrEP-related studies conducted in high-risk populations demonstrated that PrEP reduces HIV infection risk in MSM, TGW, injection drug users, and other high-risk populations [34]. Another, more recent, meta-analysis included 13 randomized trials with over 15 000 participants and demonstrated that PrEP use does not increase risk of grade 3/4 adverse events [35]. New strategies including novel oral agents (tenofovir alafanamide), long-acting injectables (cabotegravir), vaginal rings and broadly neutralizing monoclonal antibodies are in development as PrEP agents. In addition, new drug delivery systems, such as implants and transdermal devices are promising strategies that are being developed for HIV prevention use. These advances will expand PrEP options and as such offer preventive technologies that will appeal to a wide variety of individuals and to their changing needs over the life span.
Nevertheless, effective PrEP implementation depends on many measures including the approval of tenofovir/emtricitabine for prevention, the inclusion of PrEP in the national prevention policies and guidelines, the conduct of demonstration projects and implementation research [36▪]. Furthermore, given the concerns about PrEP's costs and its impact within already tight budgets of national HIV treatment and prevention programs, studies showing whether and for whom PrEP has favorable return on investment also support PrEP implementation. Notably, except for Argentina, Brazil, Chile, Colombia, Mexico and Uruguay, HIV programs in the regions depend on donor funding, which threatens their sustainability [1].
Is preexposure prophylaxis cost-effective in the region?
The assessment on the clinical and economic value of PrEP for a particular setting or region must address the specifics of the HIV epidemic. The long-term clinical benefits, costs and the cost-effectiveness of PrEP have been evaluated in modeling studies set to represent specific countries from Latin America. The first analysis, published in 2012, used a mathematical model to represent the HIV epidemic among MSM and transwomen in Lima, Peru [37]. This analysis highlighted the many factors that could drive PrEP's economic assessment, from prioritization among those at highest risk to adherence. The results showed that even the highest estimated cost per disability adjusted life-year averted was below Peru's gross domestic product (GDP) per capita, assuming PrEP use by 20% of MSM and TGW over 10 years [37]. Another analysis that evaluated PrEP use in the United States and Peru considered main and casual partnerships and found that targeted strategies could increase PrEP's efficiency: providing PrEP to men with 1.5–7 contacts per month could lead to a 30% reduction in new infections over 10 years [38].
Similarly, an analysis from Brazil found that PrEP provision to MSM/TGW at substantial risk (defined as any of the following behaviors in the prior 12 months: condomless anal sex with ≥2 partners, ≥2 episodes of anal sex with an HIV-infected partner, or history of STD diagnosis) would substantially reduce 5-year and lifetime risk of HIV infection [39]. In this analysis, PrEP adherence and uptake was assumed as per demonstration studies [40] and it was found that PrEP led to a cost per life-year saved that was well below Brazil's GDP per capita. Moreover, PrEP would remain cost-effective unless PrEP drug costs exceeded $1200/year ($100/month). In contrast, it was found that the PrEP strategy was no longer cost-effective if HIV incidence was less than 0.4 per 100 PY, which is consistent with the recommendation of PrEP to those at substantial risk with MSM populations from around the globe meeting such criteria [17].
Finally, the most recent analysis assessed PrEP within an HIV combination prevention framework for TGW sex workers from Lima, Peru, using a deterministic model that detailed the transmission dynamics of HIV among TGW sex workers, their stable partners and their clients [41]. In their base cases analysis, through the various interventions of increased condom use with clients (15% relative increase) and stable partners (10% relative increase), expansion of ART coverage to people with CD4 cell count 500 cells/μl or less, extending ART to people with CD4 cell count at least 500 cells/μl, and 15% PrEP coverage with generic drugs would avert between 4 and 47% of new infections within a 10-year time frame while implementing all these interventions could achieve a 50% reduction in incidence. Importantly, authors found that these investments in HIV combination prevention would be cost-effective even under stringent criteria.
Preexposure prophylaxis: from awareness to implementation
The results cited above suggest PrEP's favorable cost-effectiveness profile, which to be achieved, depends, ultimately, on uptake and adherence to PrEP by those at substantial risk. To pave the way to uptake, studies form the region have assessed awareness and willingness to use PrEP in key populations. A 2016 online survey conducted using geosocial networking smartphone apps in 10 Brazilian state capitals reported that 58% (2932/5065) were aware of PrEP, willingness to use daily PrEP was reported by 52% (2653/5065) [42]. In another online survey, conducted in Brazil, Mexico and Peru with almost 20 000 participants, PrEP awareness was shown higher in Brazil (69%) and Mexico (64%) than in Peru (47%) [43]. Recent studies also show promising results among TGW with one study from Brazil reporting 76% willingness to use PrEP [44▪] and another from Argentina reporting 89% (301/337) willingness [45].
Actual implementation of PrEP has been assessed in some settings through the conduction of demonstration and implementation projects. The first demonstration project of the region was launched in Brazil in 2015 including sites initially in Rio de Janeiro and São Paulo and later in Manaus and Porto Alegre [40]. ‘PrEP Brasil’ was a prospective, multicentre, open-label project, that reported, among other things, on PrEP delivery, uptake and adherence among MSM and TGW at substantial risk of HIV infection. Of the 1270 individuals assessed for participation, 738 (58%) were eligible of which 450 (60.9%) decided to uptake PrEP. Adherence, measured at 4 and 48 weeks was high with 78 and 74% having dried blood spot measurements of drug levels consistent with the taking of four or more pills per week [46▪▪]. Results from week 96 continue to show promising results with 281 (62%) and 277 (61%) participants retained and persisting on PrEP for 2 years [47]. Though TGW were also targeted for inclusion in PrEP Brasil, only 25 were included in the study. To address PrEP uptake, engagement and persistence specifically among TGW, another demonstration project, PrEParadas, was launched. Overall, 318 transwomen were assessed for participation, 271 were potentially eligible and 130 were offered PrEP leading to an uptake of 48% [48].
PrEP demonstration projects and implementation in Latin American countries has expanded since 2016 [3] according to data from WHO. One multicountry implementation project is IMPREP. Funded by UNITAID, it is a joint collaboration between the Oswaldo Cruz Foundation and Ministries of Health from Brazil, Peru and Mexico. It aims to provide same day PrEP to 7500 individuals at substantial risk of HIV acquisition in the three countries. As of December 2018 (Table 1), demonstration projects are ongoing in five countries: Brazil, Peru, Mexico, Dominican Republic and Jamaica, whereas four are in the planning phase (Argentina, Guatemala, Colombia, Paraguay). PrEP has been formally implemented in four countries (Bahamas, Barbados, Brazil and Cuba) with these countries and Haiti having PrEP policies in place. Chile, Dominican Republic, Guatemala and Paraguay are planning the implementation of a PrEP public policy.
Table 1.
Latin American countries that have either implemented a demonstration project or have made preexposure prophylaxis available through a national policy as of December 2018
| Country | Guidelines | Demonstration project | National policy | Private sector |
| Argentina | Drafted | Planning | – | Yesa |
| Bahamas (The) | Available | – | Yes | – |
| Barbados | Available | – | Yes | Yesa |
| Bolivia | – | Planning | – | – |
| Brazil | Available | Implemented | Yes | – |
| Chile | Available | Planning | Planning | Yesa |
| Colombia | – | Planning | – | – |
| Cuba | Available | – | Yes | – |
| Dominican Republic | Drafted | Implemented | Planning | Yesb |
| Guatemala | Available | Planning | Planning | Yesb |
| Haiti | Available | Planning | Yes | – |
| Jamaica | – | Planning | – | – |
| Mexico | Available | Implemented | – | – |
| Paraguay | – | Planning | Planning | Yesb |
| Peru | Available | Implemented | – | Yesa |
Source: WHO.
aThrough private physicians.
bThrough nongovernmental organizations.
Brazil as a case study of preexposure prophylaxis uptake in the real-world
So far, we have reviewed the results from relatively small scale, local, projects. It is expected that actual implementation of PrEP within the health system of any given country will suffer additional challenges. Using Brazil as a case study, we report on the program's first year of existence. In December 2017, national guidelines detailing when, how and to whom to offer PrEP within the national health system were published. During this first year, 56 cites have provided PrEP in primary care facilities to adults (18 years or older) at substantial risk of HIV acquisition. Among those who self-identify as men, report sex with other men, and meet eligibility criteria, a total of 6523 have started PrEP. Number on PrEP varied substantially by city with a mean of 117 MSM per city (median 12, interquartile range 3–101).
To give a sense of the actual uptake, we updated previous calculations of PrEP demand among MSM [49] using more recent nationally representative demographic and surveillance data. We focused on the 12 cities (from the 5 regions) included in the second nation-wide respondent-driven sampling study funded by Department of Surveillance, Prevention and Control of STI, HIV/AIDS and Viral Hepatitis of the Ministry of Health [9▪] in addition to two cities that were able to provide PrEP to more than 100 individuals.
The number of men aged 18–64 years residing in each of the 14 cities were gathered from the Brazilian Institute of Geography and Statistics (IBGE [50]). A nation-wide survey of sexual practices and behavior was then used to infer the fraction of these men who report sex with other men during their lifetime [3.5%, 95% confidence interval (CI) 2.9–4.3%] [51]. From this, we removed those who would already be HIV-infected using the nation-wide results on MSM reporting sexual activity in the prior 12 months among which HIV prevalence with on-site rapid testing (positivity confirmed by a second test) ranged from 5.9% (3.5–9.6) in Brasilia to 24.8% (18.5–32.4) in São Paulo [9▪]. Finally, among the uninfected MSM, we inferred those meeting eligibility criteria (condomless anal sex in the prior 6 months) [52] and who would be willing to uptake PrEP [40].
Figure 1 shows PrEP demand and uptake by city in the year 2018. Demand ranged from 1059 MSM (717–1559) in Florianopolis to 22 386 (14 218–34 475) in São Paulo. Across the country, uptake ranged from 6.9% (292) in Manaus (North), to 3.7% (113) in Recife (Northeast), 8.0% (1087) in Rio de Janeiro (Southeast), 12.8% (276) in Curitiba (South), and 3.2% (215) in Brasília. The highest uptakes were in Florianopolis (260, 24.6%), Porto Alegre (408, 12.8%), and São Paulo (2244, 10.0%). The lowest occurred in Belem (15, 0.5%) and Campo Grande (0). The observed wide range of PrEP uptake among the cities mirrors the heterogeneity reported for many other indicators, from behavior including sexual and substance use [11,52], to HIV testing [53], HIV prevalence [9▪], laboratory performance [54], and linkage to care, the treatment cascade and mortality [25,26,27▪▪,55]. A possible spatial clustering of these indicators and their interactions should be explored in future studies.
FIGURE 1.
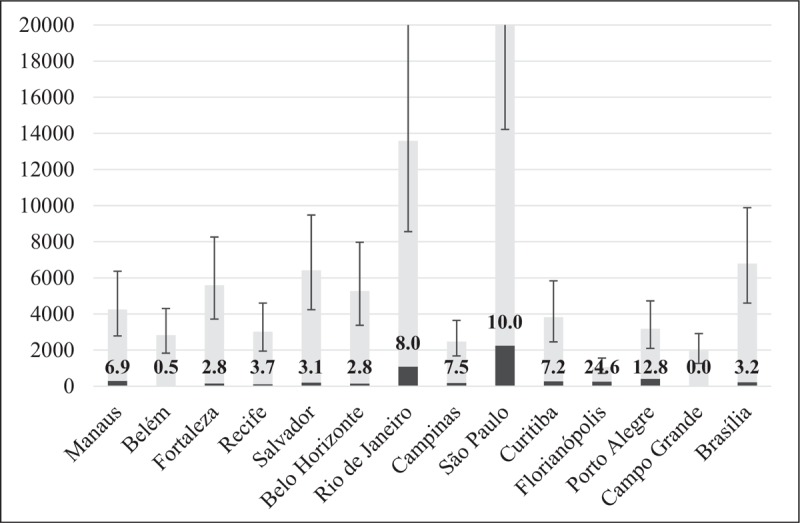
Preexposure prophylaxis MSM demand (bars with ranges) and uptake (dark gray) in 2018 for 14 Brazilian cities, 12 of which were chosen by Department of Surveillance, Prevention and Control of STI, HIV/AIDS and Viral Hepatitis of the Ministry of Health for the second nation-wide respondent-driven sampling study that estimated HIV prevalence and behavioral practices related to HIV risk, and two other cities that were able to provide PrEP to more than 100 MSM (Florianopolis and Campinas). The percentage uptake is given by the numbers within the bars. Footnote: y-axis upper limit was set to 20 000 to increase clarity. Numbers not shown include demand for Rio de Janeiro 13590 (8562-20702) and São Paulo 22386 (14218-34475). PrEP, preexposure prophylaxis.
CONCLUSION
In conclusion, there are important challenges to be addressed in the region in order to achieve proposed HIV targets of 90-90-90 and/or HIV incidence reduction by 2030. Though the endorsement of ‘Treat All’ by all Latin American countries is paramount, the actual impact of such policy, as models have shown [56], will be limited by just how much testing is universally available and performed and how fast treatment can be initiated. On testing, low risk perception has been shown highly discordant to actual risk taking behavior [42,43], which certainly hinders its use. We need to expand the adoption of new testing platforms, such as self-testing in Latin America [2], local studies have shown its feasibility and acceptability [57,58]. On treatment, late treatment initiation and suboptimal adherence are significant challenges for which solutions are needed. Long-acting ART might help with the latter but the former will require a positive engagement with all services: testing, care and treatment. To that end, we highlight the importance of acknowledging and spreading the message that U = U (undetectable equals untransmissible) in the region [59]. For the individual, the knowledge of U = U empowers people living with HIV, improves adherence and decreases self-stigma. For the population, U = U can lead to decreased HIV transmission by reducing community viral load. Recent studies from the region have shown that the most prevalent reason for never testing for HIV is fear of being HIV infected [43]. Today, we have an opportunity to use testing in a scenario where there are valuable options for both groups, of those who test positive and negative. We need to take this opportunity forward.
Acknowledgements
None.
Financial support and sponsorship
P.M.L. and B.G. acknowledge funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.UNAIDS. Miles to go. 2018. [Google Scholar]
- 2.WHO. HIV treatment and care fact sheet. WHO HIV policy adoption and implementation status in countries. Available at: https://apps.who.int/iris/bitstream/handle/10665/259532/WHO-HIV-2017.58-eng.pdf 2018. [Google Scholar]
- 3.Ravasi G, Grinsztejn B, Baruch R, et al. Towards a fair consideration of PrEP as part of combination HIV prevention in Latin America. J Int AIDS Soc 2016; 19 7 Suppl 6:21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carriquiry G, Fink V, Koethe JR, et al. Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean. J Int AIDS Soc 2015; 18:20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinsztejn B, Luz PM, Pacheco AG, et al. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One 2013; 8:e59768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima N, Park H, Konda KA, et al. The PICASSO cohort: baseline characteristics of a cohort of men who have sex with men and male-to-female transgender women at high risk for syphilis infection in Lima, Peru. BMC Infect Dis 2017; 17:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Garcia JI, Sabido M, Nikiforov M, et al. The UALE project: a cross-sectional approach for trends in HIV/STI prevalence among key populations attending STI clinics in Guatemala. BMJ Open 2018; 8:e022632. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides trends in prevalence of HIV and other STIs over an 8-year period in Guatemala among clinic attendees that included female sex workers, men who have sex with men and high-risk heterosexuals.
- 8.Ferreira-Junior ODC, Guimaraes MDC, Damacena GN, et al. Prevalence estimates of HIV, syphilis, hepatitis B and C among female sex workers (FSW) in Brazil, 2008. Medicine (Baltimore) 2018; 97 Suppl 1:S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Kerr L, Kendall C, Guimaraes MDC, et al. HIV prevalence among men who have sex with men in Brazil: results of the 2nd national survey using respondent-driven sampling. Medicine (Baltimore) 2018; 97 1S Suppl 1:S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides updated HIV prevalence estimates among men who have sex with men in 12 cities from all regions of the country.
- 10▪.Grinsztejn B, Jalil EM, Monteiro L, et al. Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV 2017; 4:e169–e176. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study conducted in Rio de Janeiro, Brazil, describes sociodemographic, substance use, HIV/STD prevalence, and hormone use among transgender women using a respondent-driven sampling design.
- 11.Sperhacke RD, da Motta LR, Kato SK, et al. HIV prevalence and sexual behavior among young male conscripts in the Brazilian army, 2016. Medicine (Baltimore) 2018; 97 1S Suppl 1:S25–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bautista-Arredondo S, Colchero MA, Romero M, et al. Is the HIV epidemic stable among MSM in Mexico? HIV prevalence and risk behavior results from a nationally representative survey among men who have sex with men. PLoS One 2013; 8:e72616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salas-Espinoza KJ, Menchaca-Diaz R, Patterson TL, et al. HIV prevalence and risk behaviors in male to female (MTF) transgender persons in Tijuana, Mexico. AIDS Behav 2017; 21:3271–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colchero MA, Cortes-Ortiz MA, Romero-Martinez M, et al. HIV prevalence, sociodemographic characteristics, and sexual behaviors among transwomen in Mexico City. Salud Publica Mex 2015; 57 Suppl 2:s99–s106. [DOI] [PubMed] [Google Scholar]
- 15.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchbinder SP, Glidden DV, Liu AY, et al. HIV preexposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis 2014; 14:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS 2013; 27:2665–2678. [DOI] [PubMed] [Google Scholar]
- 18.ARTCC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caro-Vega Y, Belaunzaran-Zamudio PF, Crabtree-Ramirez B, et al. Trends in proportion of older HIV-infected people in care in Latin America and the Caribbean: a growing challenge. Epidemiol Infect 2018; 146:1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belaunzaran-Zamudio PF, Caro-Vega YN, Shepherd BE, et al. CCASAnet. Monitoring of HIV treatment in seven countries in the WHO Region of the Americas. Bull World Health Organ 2015; 93:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastos FI, Caceres C, Galvao J, et al. AIDS in Latin America: assessing the current status of the epidemic and the ongoing response. Int J Epidemiol 2008; 37:729–737. [DOI] [PubMed] [Google Scholar]
- 22.Ford N, Mills EJ, Egger M. Editorial commentary: immunodeficiency at start of antiretroviral therapy: the persistent problem of late presentation to care. Clin Infect Dis 2015; 60:1128–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, et al. IeDea, Collaborations ARTC. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2014; 65:e8–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff MJ, Cortes CP, Mejia FA, et al. Caribbean, Central and South America network for HIV epidemiology (CCASAnet). Evaluating the care cascade after antiretroviral therapy initiation in Latin America. Int J STD AIDS 2018; 29:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grangeiro A, Escuder MM, Pereira JC. Late entry into HIV care: lessons from Brazil, 2003 to 2006. BMC Infect Dis 2012; 12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangal TD, Meireles MV, Pascom ARP, et al. Determinants of survival of people living with HIV/AIDS on antiretroviral therapy in Brazil 2006-2015. BMC Infect Dis 2019; 19:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪▪.Pascom ARP, Meireles MV, Benzaken AS. Sociodemographic determinants of attrition in the HIV continuum of care in Brazil, in 2016. Medicine (Baltimore) 2018; 97 1S Suppl 1:S69–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides a pooled ART adherence estimate for the entire region while also qualitatively discussing the main barriers to adherence.
- 28▪.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016; 95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an estimate of ART effectiveness in real-world national health system-based HIV care in Brazil of currently used single tablet regimens.
- 29.Costa JM, Torres TS, Coelho LE, Luz PM. Adherence to antiretroviral therapy for HIV/AIDS in Latin America and the Caribbean: Systematic review and meta-analysis. J Int AIDS Soc 2018; 21:e25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos MA, Guimaraes MDC, Helena ETS, et al. Monitoring self-reported adherence to antiretroviral therapy in public HIV care facilities in Brazil: A national cross-sectional study. Medicine (Baltimore) 2018; 97 1S Suppl 1:S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa JO, Ceccato M, Silveira MR, et al. Effectiveness of antiretroviral therapy in the single-tablet regimen era. Rev Saude Publica 2018; 52:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16 3 Suppl 2:18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida-Brasil CC, Moodie EEM, McLinden T, et al. Medication nonadherence, multitablet regimens, and food insecurity are key experiences in the pathway to incomplete HIV suppression. AIDS 2018; 32:1323–1332. [DOI] [PubMed] [Google Scholar]
- 34.Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30:1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilkington V, Hill A, Hughes S, et al. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad 2018; 4:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Caceres CF, Borquez A, Klausner JD, et al. Implementation of preexposure prophylaxis for human immunodeficiency virus infection: progress and emerging issues in research and policy. J Int AIDS Soc 2016; 19 7 Suppl 6:21108. [DOI] [PMC free article] [PubMed] [Google Scholar]; A model-based analysis that estimates PrEP's cost-effectiveness within the Brazilian health system provided to high-risk MSM.
- 37.Gomez GB, Borquez A, Caceres CF, et al. The potential impact of preexposure prophylaxis for HIV prevention among men who have sex with men and transwomen in Lima, Peru: a mathematical modelling study. PLoS Med 2012; 9:e1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnegie NB, Goodreau SM, Liu A, et al. Targeting preexposure prophylaxis among men who have sex with men in the United States and Peru: partnership types, contact rates, and sexual role. J Acquir Immune Defic Syndr 2015; 69:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luz PM, Osher B, Grinsztejn B, et al. The cost-effectiveness of HIV preexposure prophylaxis in men who have sex with men and transgender women at high risk of HIV infection in Brazil. J Int AIDS Soc 2018; 21:e25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoagland B, Moreira RI, De Boni RB, et al. High preexposure prophylaxis uptake and early adherence among men who have sex with men and transgender women at risk for HIV Infection: the PrEP Brasil demonstration project. J Int AIDS Soc 2017; 20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borquez A, Guanira JV, Revill P, et al. The impact and cost-effectiveness of combined HIV prevention scenarios among transgender women sex-workers in Lima, Peru: a mathematical modelling study. Lancet Public Health 2019; 4:e127–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres TS, De Boni RB, de Vasconcellos MT, et al. Awareness of prevention strategies and willingness to use preexposure prophylaxis in Brazilian men who have sex with men using apps for sexual encounters: online cross-sectional study. JMIR Public Health Surveill 2018; 4:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assasf R, Konda KA, Torres T, et al. Prep and PEP awareness among men who have sex with men (MSM) at higher risk for HIV in Brazil, Mexico, and Peru, 2018. CROI 2017 Seattle, WA, USA 2019 Poster # 975. 2019. [Google Scholar]
- 44▪.Jalil EM, Wilson EC, Velasque L, et al. PrEP for transwomen in Rio De Janeiro, Brazil: low awareness but high willingness and candidacy to take PrEP. HIV Research for Prevention 2016, Chicago [Internet]. 2016. [Google Scholar]; A poster at CROI 2019 addressing awareness and willingness to use PrEP in three countries of the region: Brazil, Peru, Mexico.
- 45.Zalazar V, Aristegui I, Kerr T, et al. High willingness to use HIV pre-exposure prophylaxis among transgender women in Argentina. Transgend Health 2016; 1:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪▪.Grinsztejn B, Hoagland B, Moreira RI, et al. Retention, engagement, and adherence to preexposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV 2018; 5:e136–e145. [DOI] [PubMed] [Google Scholar]; This article provides additional results on the PrEP Brasil demonstration project. It provides estimates of engagement in PrEP services as well as PrEP adherence at the end of 1 year.
- 47.Veloso VG, Torres T, Moreira RI, et al. Retention and persistence on PrEP for MSM and TGW: 96 weeks results of the PrEP Brasil demonstration study. Abstract in HIVR4P, Madrid. 2018. [Google Scholar]
- 48.Jalil EM, Torres T, Moreira RI, et al. IAS 2019, accepted oral presentation. Mexico City. PrEP uptake and early adherence among at HIV risk transgender women from Rio de Janeiro, Brazil. Results from the PrEParadas demonstration study. [Google Scholar]
- 49.Luz PM, Benzaken A, Alencar TM, et al. PrEP adopted by the Brazilian national health system: what is the size of the demand? Medicine (Baltimore) 2018; 97 1S Suppl 1:S75–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IBGE. Indicadores Demograficos e de Saude. Informacoes Sociais, Demograficas e Economicas. Instituto Brasileiro de Geografia e Estatistica. Available at: http://www.ibge.gov.br/2019. [Google Scholar]
- 51. Ministério da Saúde. Pesquisa de Conhecimentos, Atitudes e Práticas na População Brasileira. Brazilian Ministry of Health; 2013. [Google Scholar]
- 52.Guimaraes MDC, Kendall C, Magno L, et al. Brazilian HIV/MSM Surveillance Group. Comparing HIV risk-related behaviors between 2 RDS national samples of MSM in Brazil, 2009 and 2016. Medicine (Baltimore) 2018; 97 1S Suppl 1:S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brito AM, Kendall C, Kerr L, et al. Factors associated with low levels of HIV testing among men who have sex with men (MSM) in Brazil. PLoS One 2015; 10:e0130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaspar PC, Wohlke BLP, Brunialti MKC, et al. External quality assessment for CD4 + T-lymphocyte count test: performance of the Brazilian public health laboratories network. Medicine (Baltimore) 2018; 97 1S Suppl 1:S32–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grangeiro A, Escuder MM, Castilho EA. Magnitude and trend of the AIDS epidemic in Brazilian cities, from 2002 to 2006. Rev Saude Publica 2010; 44:430–440. [DOI] [PubMed] [Google Scholar]
- 56.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 57.De Boni RB, Lentini N, Santelli AC, et al. Self-testing, communication and information technology to promote HIV diagnosis among young gay and other men who have sex with men (MSM) in Brazil. J Int AIDS Soc 2018; 21 Suppl 5:e25116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk JE, Lippman SA, Grinsztejn B, et al. Acceptability and feasibility of HIV self-testing among men who have sex with men in Peru and Brazil. Int J STD AIDS 2016; 27:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calabrese SK, Mayer KH. Providers should discuss U=U with all patients living with HIV. Lancet HIV 2019; 6:e211–e213. [DOI] [PubMed] [Google Scholar]


