Abstract
Background and objective
Neoadjuvant chemoradiation therapy (NCRT) followed by radical resection has been a common practice for patients with locally advanced rectal cancer. This study aimed to analyse the association of tumor differentiation and prognosis in rectal-cancer patients undergoing NCRT.
Methods
Patients with locally advanced, non-mucinous rectal cancer who underwent NCRT followed by radical resection between 2007 and 2017 were identified from an electronic health record system at the Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Multivariable logistic regression and multivariate Cox regression were performed to analyse the association of response to NCRT and survival with clinicopathological characteristics of all these patients.
Results
We identified 325 patients (241 males and 84 females; mean age, 54.4 ± 11.2 years) who underwent NCRT followed by radical resection, including 26 (8.0%) with poorly-differentiated rectal cancer, 182 (56.0%) with moderately-differentiated cancer and 117 (36.0%) with well differentiated cancer. Propensity score matching analysis and multivariable logistic regression analysis results showed that tumor differentiation was significantly associated with response to NCRT. In the poor differentiation and non-poor differentiation groups, the 3-year overall survival (OS) rates were 74.6 and 93.5%, respectively, whereas the 3-year local recurrence rates were 18.6 and 3.7%, respectively. Multivariable Cox regression analyses revealed that poor differentiation was an independent risk factor for local recurrence and OS.
Conclusions
Among the patients with locally advanced, non-mucinous rectal cancer, the patients with poorly-differentiated cancer who underwent NCRT had a worse response to NCRT and poorer prognosis than those with moderately- and well-differentiated diseases.
Keywords: Rectal cancer, neoadjuvant chemoradiation therapy, prognosis
Introduction
Neoadjuvant chemoradiation therapy (NCRT) followed by radical resection has been a common treatment of patients with locally advanced rectal cancer. NCRT contributes to tumor pathological regression and reduces the local recurrence rate by approximately 50% in rectal cancer [1]. However, when compared with surgery alone [2] or post-operative chemoradiation therapy [3], NCRT failed to improve overall survival (OS) rates. Pathological complete response (pCR), which indicates a much more favorable prognosis, was only observed in 15–27% of patients undergoing NCRT [4, 5]. Response to NCRT was associated with multiple risk factors of rectal-cancer patients, including clinical characteristics [6], gene mutation [7, 8] and biological behavior [9–11].
Histology appears to be an excellent predictor for response to NCRT and prognosis in cancer patients. Response to NCRT in patients with mucinous rectal cancer was much poorer than that in patients with non-mucinous rectal cancer [9, 11, 12]. Differentiation in the diagnostic biopsies may help to predict the tumor response to NCRT [13]. When compared with patients with poorly- and moderately-differentiated rectal cancer, those with well-differentiated rectal cancer had a higher percentage of pCR [14]. Poor differentiation was a risk factor for progression-free survival of patients treated with NCRT [15]. In this study, we retrospectively analysed the data of patients with non-mucinous rectal cancer to explore the association between tumor differentiation and prognosis.
Patients and methods
Patients
The study was approved by the Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). The patients who were diagnosed with locally advanced non-mucinous rectal cancer and underwent NCRT followed by radical resection between 2007 and 2017 were included in this study. The exclusion criteria were as follows: (i) patients with stage IV disease, (ii) patients with mucinous rectal cancer, (iii) patients with multiple primary tumors or recurrent cancer, (iv) patients with familiar adenomatous polyposis (FAP) or inflammatory bowel disease (IBD) and (v) patients with unavailable data.
Treatment and follow-up
All patients received at least one cycle of chemotherapy [including FOLFOX (leucovorin, fluorouracil and oxaliplatin), de Gramont (leucovorin and fluorouracil) or XELOX (capecitabine and oxaliplatin)] plus two to four cycles of radiotherapy (46.0–50.4 Gy delivered in 23–25 fractions) before surgery. Tumor regression grade (TRG) is a predictor of therapeutic response in rectal-cancer patients treated with NCRT followed by radical resection [16]. pCR was defined as the lack of any signs of cancer in tissue samples after NCRT. Magnetic resonance imaging (MRI) or computed tomography (CT) examination was performed before NCRT. The information of chemotherapy and radiotherapy, surgery and post-operative pathological diagnosis was also collected. The rectal cancer was categorized as poorly-differentiated cancer (PDC), moderately-differentiated (MDC) or well-differentiated cancer (WDC) based on post-operative pathological diagnosis.
For all patients, follow-up was scheduled for surveillance every 3 months after the surgery for the first year, every 6 months for the next 2 years and every year thereafter. Response to NCRT was defined as down-staging of either T or N category (without any progression of T or N category) after NCRT.
Statistical analysis
The results of descriptive data for all factors are presented as the means and standard deviations for continuous variables or frequencies and percentages for categorical factors. Variance analysis or t-test was used for continuous factors and Chi-square test or Fisher’s exact probabilities test was used for categorical variables to compare the basic characteristics and the response to NCRT among groups.
Propensity score matching (PSM) was used to analyse the association of differentiation and response. In PSM and survival analyses, the patients with moderately-differentiated and well-differentiated rectal cancer were combined as the non-poor difference (PD) group, whereas those with poorly-differentiated rectal cancer were defined as the PD group. The PD group and non-PD group were matched using propensity scores with a ratio of 1:4 by adjusting for age, sex, hemoglobin level, body mass index (BMI), serum total protein, serum albumin, T and N categories before NCRT and tumor location. Univariate and multivariable logistic regression analyses were performed to validate the PSM results. Univariate and multivariable Cox regression analyses were conducted to investigate the risk factors of local recurrence and OS. Except for differentiation, only the variables with statistical significance at the level of 0.05 in univariate analyses were included in multivariable models. The Kaplan–Meier analysis was performed to compare the OS rate and local recurrence rate between the PD and non-PD groups. A P-value of <0.05 was considered statistically significant. All analyses were performed using R version 3.2.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of patients
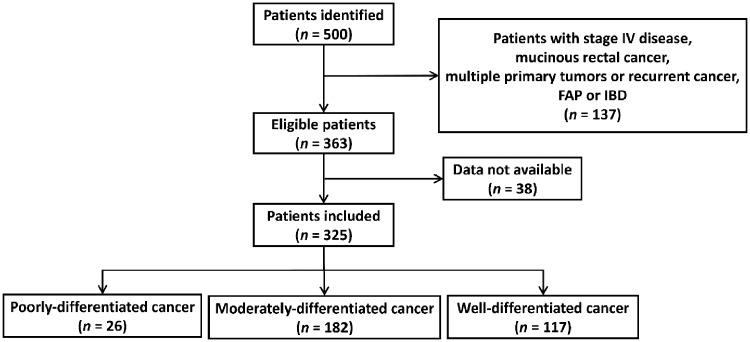
The patient-identification process is shown in Figure 1. A total of 500 patients with non-mucinous rectal cancer undergoing chemoradiation followed by surgery between 2007 and 2017 were identified from the electronic health record system at the Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). The final cohort included 325 patients, including 26, 182 and 117 patients with poorly-, moderately- and well-differentiated rectal cancers, respectively.
Figure 1.
. The flow chart of the patient-identification process. Data source: the electronic health record system at the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. FAP, familial adenomatous polyposis; IBD, inflammatory bowel disease.
Comparison of demographic and clinicopathological characteristics
Demographic characteristics among groups are shown in Table 1. The mean age of the patients was 54.4 ± 11.2 years. The patients with poorly-differentiated rectal cancer were younger than those with moderately- and well-differentiated diseases (46.0 ± 10.2 vs 54.6 ± 10.8 vs 55.9 ± 11.1 years, P < 0.001). A higher proportion of females and higher clinical T and N category were observed in the PD group than the non-PD group. Lower rectal cancer occurred in 76.9, 60.5 and 69.3% of poorly-, moderately- and well-differentiated rectal cancers, respectively. Of note, 26.9% of pathological N2 cases were found to be poorly-differentiated rectal cancer, whereas 2.8 and 2.6% were moderately- and well-differentiated rectal cancers, respectively.
Table 1.
Clinicopathological characteristics of 325 patients with rectal cancer
| Clinicopathological feature | Total (n = 325) | Differentiation level of rectal cancer |
P-value | ||
|---|---|---|---|---|---|
| Poor differentiation (n = 26) | Moderate differentiation (n = 182) | Well differentiated (n = 117) | |||
| Age, mean ± SD (years) | 46.0 ± 10.2 | 54.6 ± 10.8 | 55.9 ± 11.1 | <0.001 | |
| Sex, n (%) | 0.430 | ||||
| Male | 241 (74.2) | 18 (69.2) | 140 (77.8) | 83 (70.9) | |
| Female | 84 (25.8) | 8 (30.8) | 42 (22.2) | 34 (29.1) | |
| T category before NCRT, n (%) | 0.622 | ||||
| T2 | 21 (6.5) | 2 (7.7) | 10 (5.5) | 9 (7.7) | |
| T3 | 234 (72.0) | 16 (61.5) | 133 (73.1) | 85 (72.6) | |
| T4 | 70 (21.5) | 8 (30.8) | 39 (21.4) | 23 (19.7) | |
| N category before NCRT, n (%) | 0.638 | ||||
| N0 | 71 (21.8) | 5 (19.2) | 44 (24.2) | 22 (18.8) | |
| N1 | 126 (38.8) | 9 (34.6) | 66 (36.3) | 51 (43.6) | |
| N2 | 128 (39.4) | 12 (46.2) | 72 (39.5) | 44 (37.6) | |
| Interval between NCRT and surgery, mean ± SD (days)a | 48.5 ± 26.4 | 53.6 ± 45.3 | 58.5 ± 45.0 | 0.494 | |
| Tumor location, n (%) | 0.409 | ||||
| Upper rectum | 32 (9.9) | 1 (3.9) | 21 (11.5) | 10 (8.5) | |
| Middle rectum | 82 (25.2) | 5 (19.2) | 51 (28.0) | 26 (22.2) | |
| Low rectum | 211 (64.9) | 20 (76.9) | 110 (60.5) | 81 (69.3) | |
| Surgical procedure, n (%) | 0.196 | ||||
| Open | 56 (17.2) | 8 (30.8) | 31 (17.0) | 17 (14.5) | |
| Hand-assisted | 12 (3.7) | 0 | 5 (2.7) | 7 (6.0) | |
| Laparoscopic | 257 (79.1) | 18 (69.2) | 146 (80.3) | 93 (79.5) | |
| Distance to the anal verge, mean ± SD (cm) | 3.9 ± 2.2 | 4.8 ± 2.6 | 5.0 ± 2.7 | 0.209 | |
| CRM status, n (%) | 0.062 | ||||
| Positive | 2 (0.6) | 1 (4) | 0 | 1 (0.9) | |
| Negative | 315 (99.4) | 24 (96) | 179 (100) | 112 (99.1) | |
| Number of lymph nodes dissected, mean ± SD | 8.8 ± 4.1 | 9.7 ± 5.4 | 9.5 ± 5.1 | 0.700 | |
| Pathological T category | 0.803 | ||||
| T0–T2 | 141 (43.4) | 11 (42.3) | 80 (44.0) | 50 (42.7) | |
| T3 | 171 (52.6) | 13 (50) | 96 (52.7) | 62 (53.0) | |
| T4 | 13 (4.0) | 2 (7.7) | 6 (3.3) | 5 (4.3) | |
| Pathological N category | <0.001 | ||||
| N0 | 278 (85.5) | 19 (73.1) | 155 (85.2) | 104 (88.9) | |
| N1 | 32 (9.9) | 0 (0) | 22 (12.1) | 10 (8.5) | |
| N2 | 15 (4.6) | 7 (26.9) | 5 (3.7) | 3 (2.6) | |
| TRGa | 0.147 | ||||
| 0 | 82 (26.1) | 12 (46.2) | 44 (24.2) | 26 (22.2) | |
| 1 | 99 (31.4) | 7 (26.9) | 52 (28.6) | 40 (34.2) | |
| 2–3 | 134 (42.5) | 7 (26.9) | 80 (44.0) | 47 (40.2) | |
| Down-staging of T category | 0.871 | ||||
| Yes | 149 (45.8) | 14 (53.8) | 80 (44.0) | 55 (47.0) | |
| No | 176 (54.2) | 12 (46.2) | 102 (56.0) | 62 (53.0) | |
| Down-staging of N category | 0.053 | ||||
| Yes | 225 (69.2) | 14 (53.8) | 122 (67.0) | 89 (76.1) | |
| No | 100 (30.8) | 12 (46.2) | 60 (33.0) | 28 (23.9) | |
| Response | 0.119 | ||||
| Yes | 263 (81.9) | 17 (65.4) | 149 (81.9) | 97 (82.9) | |
| No | 62 (19.1) | 9 (34.6) | 33 (18.1) | 20 (17.1) | |
| pCR | 0.276 | ||||
| Yes | 62 (19.1) | 8 (30.8) | 32 (17.6) | 22 (18.8) | |
| No | 263 (80.9) | 18 (69.2) | 150 (82.4) | 95 (81.2) | |
SD, standard deviation; NCRT, neoadjuvant chemoradiation; CRM, circumferential resection margin; TRG, tumor regression grade; pCR, pathological complete regression.
The data of 10 patients were missed and only 315 patients have complete information here.
The response to NCRT
A total of 263 patients (74.7%) achieved response after NCRT. The response rate of poorly-differentiated rectal cancer (65.4%) was lower than those of moderately- and well-differentiated rectal cancers (81.9 and 82.9%, respectively). Poorly-differentiated rectal cancer seemed to achieve a more favorable TRG and higher pCR rate than other groups; however, the differences were not significant between these groups in terms of response, TRG or pCR rate (Table 1).
Propensity score analyses were performed to investigate the association of differentiation and response. Based on propensity scores, 104 patients in the non-PD group were matched to 26 patients in the PD group. The baseline characteristics and clinical outcome of these two groups are listed in Table 2. Univariate analysis results showed that patients in both groups were demographically homogeneous and that the PD group had a lower response rate than did the non-PD group (P = 0.020). However, no significant difference was found in the pCR rate or TRG between the two groups (Table 2).
Table 2.
Baseline clinicopathological characteristics and outcomes before and after matching on the propensity score
| Variable | Before matching (n = 325) |
P-value | After matching (n = 130) |
P-value | ||
|---|---|---|---|---|---|---|
| PD group (n = 26) | Non-PD group (n = 299) | PD group (n = 26) | Non-PD group (n = 104) | |||
| Age, mean ± SD (years) | 46.0 ± 10.2 | 55.1 ± 11.0 | <0.001 | 46.0 ± 10.2 | 47.0 ± 9.8 | 0.662 |
| Hemoglobin, mean ± SD (g/L) | 107.9 ± 30.9 | 118.4 ± 17.3 | 0.102 | 107.9 ± 30.9 | 114.3 ± 19.6 | 0.588 |
| Serum total protein, mean ± SD (g/L) | 64.1 ± 11.0 | 68.5 ± 6.3 | 0.007 | 64.1 ± 11.0 | 66.7 ± 6.9 | 0.337 |
| Serum albumin, mean ± SD (g/L) | 41.1 ± 4.3 | 41.1 ± 4.1 | 0.575 | 41.1 ± 4.3 | 41.2 ± 4.4 | 0.884 |
| CEA, mean ± SD (ng/mL) | 2.5 ± 2.1 | 3.9 ± 10.2 | 0.251 | 2.5 ± 2.1 | 2.2 ± 1.6 | 0.337 |
| Sex, n (%) | 0.716 | 0.999 | ||||
| Female | 8 (30.8) | 76 (25.4) | 8 (30.8) | 31 (29.8) | ||
| Male | 18 (69.2) | 223 (74.6) | 18 (69.2) | 73 (70.2) | ||
| BMI, n (%) | 0.293 | 0.962 | ||||
| <18.5 or ≥24 | 7 (26.9) | 118 (39.5) | 7 (26.9) | 31 (29.8) | ||
| 18.5–23.9 | 19 (73.1) | 181 (60.5) | 19 (73.1) | 73 (70.2) | ||
| T category before NCRT, n (%) | 0.324 | 0.814 | ||||
| T2 | 2 (7.7) | 19 (6.4) | 2 (7.7) | 6 (5.8) | ||
| T3 | 16 (61.5) | 218 (72.9) | 16 (61.5) | 69 (66.3) | ||
| T4 | 8 (20.8) | 62 (20.7) | 8 (30.8) | 29 (27.9) | ||
| N category before NCRT, n (%) | 0.762 | 0.641 | ||||
| N0 | 5 (19.2) | 66 (22.1) | 5 (19.2) | 20 (19.2) | ||
| N1 | 9 (34.6) | 117 (39.1) | 9 (34.6) | 27 (26.0) | ||
| N2 | 12 (46.2) | 116 (38.8) | 12 (46.2) | 57 (54.8) | ||
| Surgical procedure, n (%) | 0.167 | 0.210 | ||||
| Open | 8 (30.8) | 48 (16.1) | 8 (30.8) | 18 (17.3) | ||
| Hand-assisted | 0 | 12 (4.0) | 0 | 5 (4.8) | ||
| Laparoscopic | 18 (69.2) | 239 (79.9) | 18 (69.2) | 81 (77.9) | ||
| Location, n (%) | 0.436 | 0.924 | ||||
| Low rectum | 20 (76.9) | 191 (63.9) | 20 (76.9) | 75 (72.1) | ||
| Middle rectum | 5 (19.2) | 77 (25.8) | 5 (19.2) | 23 (22.2) | ||
| Upper rectum | 1 (3.9) | 31 (10.3) | 1 (3.9) | 6 (5.8) | ||
| pCR, n (%) | 0.122 | 0.650 | ||||
| No | 18 (69.2) | 245 (81.9) | 18 (69.2) | 79 (76.0) | ||
| Yes | 8 (30.8) | 54 (18.1) | 8 (30.8) | 25 (24.0) | ||
| TRG, n (%) | 0.045 | 0.180 | ||||
| 0 | 12 (46.2) | 70 (23.4) | 12 (46.2) | 29 (27.9) | ||
| 1 | 7 (26.9) | 92 (30.8) | 7 (26.9) | 32 (30.8) | ||
| 2–3 | 7 (26.9) | 127 (42.5) | 7 (26.9) | 43 (41.3) | ||
| Response, n (%) | 0.063 | 0.020 | ||||
| No | 9 (34.6) | 53 (17.7) | 9 (34.6) | 14 (13.5) | ||
| Yes | 17 (65.4) | 246 (82.3) | 17 (65.4) | 90 (86.5) | ||
| Down-staging of T category, n (%) | 1.000 | 0.623 | ||||
| No | 12 (46.2) | 135 (45.2) | 12 (46.2) | 40 (38.5) | ||
| Yes | 14 (53.8) | 164 (54.8) | 14 (53.8) | 64 (61.5) | ||
| Down-staging of N category, n (%) | 0.121 | 0.046 | ||||
| No | 12 (46.2) | 88 (29.4) | 12 (46.2) | 25 (24.0) | ||
| Yes | 14 (53.8) | 211 (70.6) | 14 (53.8) | 79 (76.0) | ||
PD, poor differentiation; SD, standard deviation; CEA, carcinoembryonic antigen; BMI, body mass index; NCRT, neoadjuvant chemoradiation therapy; pCR, pathological complete response; TRG, tumor regression grade.
The association of differentiation and response
Univariate and multivariable logistic regression analyses were performed to further assess the risk factors associated with response to NCRT. After adjusting for risk factors, including sex, differentiated stage, T and N categories before NCRT, hemoglobin, serum total protein and serum albumin, multivariable analysis results showed that poor differentiation was an independent risk factor for response to NCRT [odds ratio (OR), 5.11; 95% confidence interval (CI), 1.72–15.0 and OR, 4.97; 95% CI, 1.62–15.2 for moderately- and well-differentiated rectal cancers, respectively, when compared with poor-differentiated rectal cancer] (Table 3).
Table 3.
Factors associated with response in 325 patients with rectal cancer
| Factors | No. of patients (%) | Response |
||||
|---|---|---|---|---|---|---|
| Response rate (%) | OR (95% CI) | OR (95% CI) | ||||
| Univariate regression | P-value | Multivariate regression | P-value | |||
| Age, mean ± SD (years) | 54.4 ± 11.2 | 1.00 (0.98, 1.03) | 0.626 | |||
| Sex, n (%) | ||||||
| Male | 241 | 187 (77.6%) | Reference | Reference | ||
| Female | 84 | 76 (90.5%) | 2.74 (1.31, 6.48) | 0.012 | 2.41 (1.02, 6.43) | 0.058 |
| Differentiation level, n (%) | ||||||
| PD | 26 | 17 (65.4%) | Reference | Reference | ||
| MD | 182 | 149 (81.7%) | 2.39 (0.94, 5.73) | 0.055 | 5.11 (1.72, 15.0) | 0.003 |
| WD | 117 | 97 (82.9%) | 2.57 (0.97, 6.51) | 0.049 | 4.97 (1.62, 15.2) | 0.005 |
| T category before NCRT, n (%) | ||||||
| T2 | 21 | 15 (57.7%) | Reference | Reference | ||
| T3 | 234 | 185 (79.1%) | 1.51 (0.51, 3.93) | 0.418 | 0.77 (0.23, 2.34) | 0.652 |
| T4 | 70 | 63 (90.0%) | 3.60 (1.03, 12.5) | 0.041 | 1.42 (0.34, 5.81) | 0.623 |
| N category before NCRT, n (%) | ||||||
| N0 | 71 | 39 (54.9%) | Reference | Reference | ||
| N1 | 126 | 108 (85.7%) | 4.92 (2.51, 9.92) | <0.001 | 4.73 (2.32, 9.94) | <0.001 |
| N2 | 128 | 116 (90.6%) | 7.93 (3.81, 17.5) | <0.001 | 7.89 (3.51, 19.0) | <0.001 |
| Interval between NCRT and surgery, mean ± SD (days) | (n = 315) | 52.7 ± 35.1 | 0.99 (0.98, 1.00) | 0.076 | ||
| Hemoglobin (g/L) | 116.1 ± 19.6 | 0.97 (0.96, 0.99) | 0.004 | 0.99 (0.97, 1.01) | 0.342 | |
| WBC (× 109/L) | ||||||
| 4–10 | 191 | 156 (81.7%) | 1.12 (0.64, 1.96) | 0.680 | ||
| <4 or >10 | 134 | 107 (79.8%) | Reference | |||
| BMI (kg/m2) | ||||||
| 18.5–23.9 | 200 | 167 (83.5%) | 1.52 (0.87, 2.67) | 0.136 | ||
| <18.5 or ≥24 | 125 | 96 (76.8%) | Reference | |||
| Serum total protein (g/L) | (n = 324) | 67.8 ± 6.9 | 0.95 (0.90, 0.99) | 0.019 | 0.95 (0.89, 1.01) | 0.080 |
| Serum albumin (g/L) | (n = 324) | 40.9 ± 4.0 | 0.91 (0.84, 0.98) | 0.011 | 1.00 (0.90, 1.10) | 0.939 |
| CEA (ng/mL) | (n = 323) | |||||
| 0–5 | 276 | 224 (81.2%) | 1.16 (0.52, 2.41) | 0.695 | ||
| >5 | 47 | 37 (78.7%) | Reference | |||
| Tumor location, n (%) | ||||||
| Upper rectum | 32 | 25 (78.1%) | Reference | |||
| Middle rectum | 82 | 70 (85.4%) | 1.64 (0.55, 4.54) | 0.354 | ||
| Low rectum | 211 | 168 (79.6%) | 1.09 (0.41, 2.58) | 0.845 | ||
OR, odds ratio; CI, confidence interval; SD, standard deviation; PD, poor differentiation; MD, medium differentiation; WD, well differentiated; NCRT, neoadjuvant chemoradiation therapy; WBC, white blood cell; BMI, body mass index; CEA, carcinoembryonic antigen.
Comparison of clinical outcomes
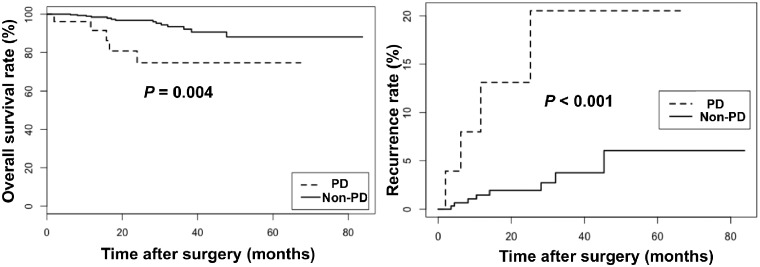
The median duration of follow-up was 24.8 months. The 3-year OS rates were 74.6 and 93.5% in the PD and non-PD groups, respectively (Figure 2). The 3-year local recurrence rates were 18.6 and 3.7% in the PD and non-PD groups, respectively (Figure 2). The results of multivariable Cox analysis revealed that PD was an independent risk factor for local recurrence (HR, 5.61; 95% CI, 1.64–19.11, P = 0.006) and OS (HR, 7.47, 95% CI, 2.18–25.5, P = 0.001) in rectal-cancer patients undergoing NCRT. However, a positive circumferential resection margin was also found to be an independent risk factor for OS (HR, 20.7, 95% CI, 1.83–235.00, P = 0.014) but not for local recurrence (Table 4).
Figure 2.
Kaplan–Meier curves of overall survival rate (A) and local recurrence rate (B) in differentiation groups. The PD group means poorly-differentiated cancer group and the non-PD group includes moderately- and well-differentiated cancer groups.
Table 4.
Multivariate Cox analysis of the 325 rectal-cancer patients
| Variable | Recurrence |
P-value | Overall survival |
P-value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age | 1.04 | (0.99, 1.09) | 0.124 | |||
| BMI | 0.42 | (0.16, 1.12) | 0.081 | |||
| CEA | 0.37 | (0.12, 1.10) | 0.074 | |||
| Serum total protein | 0.31 | (0.09, 1.09) | 0.068 | 0.43 | (0.14, 1.28) | 0.129 |
| CRM | 20.70 | (1.83, 235.00) | 0.014 | |||
| Histology | ||||||
| Non-PD | Reference | Reference | ||||
| PD | 5.61 | (1.64, 19.11) | 0.006 | 7.47 | (2.18, 25.50) | 0.001 |
| N stage before NCRT | ||||||
| N0 | Reference | Reference | ||||
| N1 | 1.26 | (0.11, 14.18) | 0.852 | 1.58 | (0.29, 8.46) | 0.595 |
| N2 | 6.00 | (0.74, 48.10) | 0.091 | 3.97 | (0.83, 18.90) | 0.083 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; CEA, carcinoembryonic antigen; CRM, circumferential resection margin; PD, poor differentiation; NCRT, neoadjuvant chemoradiation therapy.
Discussion
The present study found that patients with poorly-differentiated rectal cancer had a worse response but comparable pCR and TRG to NCRT than the patients with moderately- and well-differentiated rectal cancers. Poor differentiation was an independent risk factor for local recurrence and OS in patients with locally advance rectal cancer who underwent NCRT.
The majority of previous studies used pCR as the outcome to assess the response to NCRT. However, existing evidence and our results both failed to show any association between differentiation and pCR [14, 17]. NCRT could benefit patients in other ways, including achieving the down-staging of T or N category and without progression. In a retrospective study with 96 patients, Qiu et al. [18] reported that the response and down-staging rates to NCRT in patients with poorly differentiated rectal cancer were only 52.2 and 30.4%, respectively, which were lower than those in the non-PDC group (78.9 and 54.9%). However, multivariable analysis results revealed that differentiation was not an independent factor for the response to NCRT [18]. Another retrospective study performed by Reggiani et al. [19] showed that patients with poorly-differentiated rectal cancer had poorer performance in down-staging of T category (P < 0.001) and TRG (P = 0.073) than those with moderately- and well-differentiated rectal cancers, which is consistent with the results of Garcia-Florex et al. [13]. The present study found that the non-PD group had a better response than did the PD group, indicating that NCRT could still benefit patients with moderately- and well-differentiated rectal cancers even when pCR did not occur. Further study under broader context and using a uniform protocol of NCRT should be conducted to confirm the association of tumor differentiation and the response to NCRT.
Previous reports presented that NCRT might improve the local control but not the OS rate of rectal-cancer patients [2, 3]. However, some characteristics of the cancer might lead to differences of the prognosis, including histology and molecular marker. Zitt et al. [20] revealed that tumor differentiation, operative procedure and down-staging were independent risk factors for OS. Cebrian et al. [21] found that decreased polo-like kinase 1 (Plk1) expression, accompanied with higher-grade differentiation, was associated with poor pathological response and a low disease-free survival rate in rectal-cancer patients undergoing NCRT. Based on the literature and present results, the conclusion could be drawn that patients with poorly-differentiated rectal cancer who underwent NCRT have a worse prognosis than those with moderately- and well-differentiated rectal cancers. The following reasons may contribute to this poor prognosis in patients with poorly-differentiated rectal cancer. First, poorly-differentiated rectal cancer was located nearer to the anal verge than moderately- and well-differentiated rectal cancers, increasing the complications of NCRT and the difficulty of surgery. Second, de-differentiated tumors are more often found to invade vascular and neural structures and transgress histological boundaries [22]. Finally, NCRT might not improve the OS rate of rectal-cancer patients and, generally, an immature tumor is more aggressive than the differentiated tumors [22].
This study is limited by its retrospective design, in which inevitable heterogeneity appeared among groups. However, we performed PSM and multivariable analyses to overcome the drawback of the design and obtain a convincing result. Though the patients who received at least one cycle of chemotherapy plus radiotherapy before surgery were included in this study, we lack details of the exact neoadjuvant chemotherapy regimen and their associated toxicity. Another limitation is that the sample size of the PD group (n = 26, 8%) was relatively small.
In conclusion, poor differentiation was associated with poor response to NCRT and prognosis in patients with locally advanced non-mucinous rectal cancer undergoing NCRT. These results may help clinicians to predict the prognosis of patients and develop more adaptive treatment strategies, reducing the extra suffering and financial burden of overtreatment.
Funding
This study was supported by the National Key Clinical Discipline, National Natural Science Foundation of China (No. 81570596 and No. 81770557) and Natural Science Foundation of Guangdong Province (No. E20160107201906268).
Acknowledgements
Q.H. contributed to the data collection, statistical analysis and manuscript drafting. H.Q., J.X., M.X., X.H. and Q.Y. contributed to the data interpretation. X.H., P.L. and L.L. contributed to the study design. All authors reviewed and approved the final manuscript.
Conflict of interest
The authors declared no financial conflict of interest.
References
- 1. Fleming FJ, Pahlman L, Monson JR.. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum 2011;54:901–12. [DOI] [PubMed] [Google Scholar]
- 2. Williamson JS, Jones HG, Davies M. et al. Outcomes in locally advanced rectal cancer with highly selective preoperative chemoradiotherapy. Br J Surg 2014;101:1290–8. [DOI] [PubMed] [Google Scholar]
- 3. Sauer R, Becker H, Hohenberger W. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- 4. Maas M, Nelemans PJ, Valentini V. et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. [DOI] [PubMed] [Google Scholar]
- 5. Kalyan A, Rozelle S, Benson A 3rd. Neoadjuvant treatment of rectal cancer: where are we now? Gastroenterol Rep (Oxf) 2016;4:206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon WH, Kim HJ, Kim CH. et al. Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res 2015;88:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bengala C, Bettelli S, Bertolini F. et al. Epidermal growth factor receptor gene copy number, K-ras mutation and pathological response to preoperative cetuximab, 5-FU and radiation therapy in locally advanced rectal cancer. Ann Oncol 2009;20:469–74. [DOI] [PubMed] [Google Scholar]
- 8. Cecchin E, Agostini M, Pucciarelli S. et al. Tumor response is predicted by patient genetic profile in rectal cancer patients treated with neo-adjuvant chemo-radiotherapy. Pharmacogenomics J 2011;11:214–26. [DOI] [PubMed] [Google Scholar]
- 9. Sengul N, Wexner SD, Woodhouse S. et al. Effects of radiotherapy on different histopathological types of rectal carcinoma. Colorect Dis 2006;8:283–8. [DOI] [PubMed] [Google Scholar]
- 10. Rogers AC, Gibbons D, Hanly AM. et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 2014;27:156–62. [DOI] [PubMed] [Google Scholar]
- 11. Simha V, Kapoor R, Gupta R. et al. Mucinous adenocarcinoma of the rectum: a poor candidate for neo-adjuvant chemoradiation? J Gastrointest Oncol 2014;5:276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oberholzer K, Menig M, Kreft A. et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys 2012;82:842–8. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Florez LJ, Gomez-Alvarez G, Frunza AM. et al. Predictive markers of response to neoadjuvant therapy in rectal cancer. J Surg Res 2015;194:120–6. [DOI] [PubMed] [Google Scholar]
- 14. Huh JW, Kim HR, Kim YJ.. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum 2013;56:698–703. [DOI] [PubMed] [Google Scholar]
- 15. Li Q, Peng Y, Wang LA. et al. The influence of neoadjuvant therapy for the prognosis in patients with rectal carcinoma: a retrospective study. Tumor Biol 2016;37:3441–9. [DOI] [PubMed] [Google Scholar]
- 16. Kim SH, Chang HJ, Kim DY. et al. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res Treat 2016;48:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng WG, Liang JW, Wang Z. et al. Clinical parameters predicting pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Chin J Cancer 2015;34:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu HZ, Wu B, Xiao Y. et al. Combination of differentiation and T stage can predict unresponsiveness to neoadjuvant therapy for rectal cancer. Colorectal Dis 2011;13:1353–60. [DOI] [PubMed] [Google Scholar]
- 19. Reggiani Bonetti L, Lionti S, Domati F. et al. Do pathological variables have prognostic significance in rectal adenocarcinoma treated with neoadjuvant chemoradiotherapy and surgery? WJG 2017;23:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zitt M, DeVries A, Thaler J. et al. Long-term surveillance of locally advanced rectal cancer patients with neoadjuvant chemoradiation and aggressive surgical treatment of recurrent disease: a consecutive single-centre experience. Int J Colorectal Dis 2015;30:1705–14. [DOI] [PubMed] [Google Scholar]
- 21. Cebrian A, Gomez Del Pulgar T, Fernandez-Acenero MJ. et al. Decreased PLK1 expression denotes therapy resistance and unfavourable disease-free survival in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Pathol Res Pract 2016;212:1133–7. [DOI] [PubMed] [Google Scholar]
- 22. Jogi A, Vaapil M, Johansson M. et al. Cancer cell differentiation heterogeneity and aggressive behavior in solid tumors. Ups J Med Sci 2012;117:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]