Summary
Hypothalamic Agrp neurons regulate food ingestion in adult mice. Whether these neurons are functional before animals start to ingest food is unknown. Here, we studied the functional ontogeny of Agrp neurons during breastfeeding using postnatal day 10 mice. In contrast to adult mice, we show that isolation from the nursing nest, not milk deprivation or ingestion, activated Agrp neurons. Non-nutritive suckling and warm temperatures blunted this effect. Using in vivo fiber photometry, neonatal Agrp neurons showed a rapid increase in activity upon isolation from the nest, an effect rapidly diminished following reunion with littermates. Neonates unable to release GABA from Agrp neurons expressed blunted emission of isolation-induced ultrasonic vocalizations. Chemogenetic overactivation of these neurons further increased emission of these ultrasonic vocalizations, but not milk ingestion. We uncovered important functional properties of hypothalamic Agrp neurons during mouse development, suggesting these neurons facilitate offspring-to-caregiver bonding.
In Brief (eTOC Blurb)
Hypothalamic AgRP neurons play a role in offspring-to-caregiver bonding independent of their role in food ingestion.
Graphical Abstract
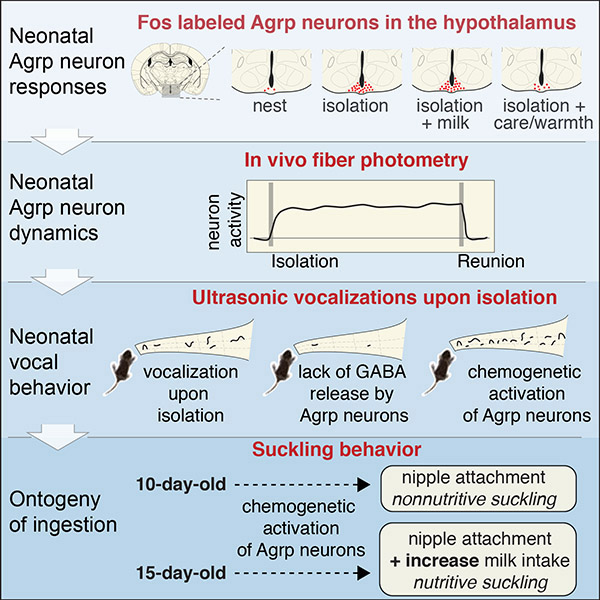
Introduction
Agouti-related peptide (Agrp) neurons in the arcuate nucleus of the hypothalamus serve as a central coordinator to regulate food intake. Ablation of these neurons leads to aphagia in adult mice (Gropp et al., 2005; Luquet et al., 2005), but not in neonates, suggesting that Agrp neurons do not contribute to ingestive behaviors early in development. In support of this view, Agrp neurons show delayed development in rodents (Nilsson et al., 2005; Padilla et al., 2010), when the final maturation of Agrp neuronal circuitry coincides with weaning (Grove and Smith, 2003). However, impairing development of Agrp neurons during the first postnatal week in mice has persistent consequences to metabolism and behavior (Dietrich et al., 2012; Joly-Amado et al., 2012), suggesting an unidentified function for Agrp neurons during early development.
Here, we assessed the functional ontogeny of Agrp neurons in early postnatal development of mice at postnatal day 10 (P10) and during the weaning period during postnatal days 15–21 (P15-P21).
Results
Isolation from the nursing nest, not nutrient intake, activates Agrp neurons in neonates
In adult mice, nutrient deprivation activates Agrp neurons (Hahn et al., 1998; Takahashi and Cone, 2005). So, we investigated the extent to which Agrp neurons respond to a lack of nutrients in neonatal mice. To test this, we isolated P10 mice from the nest for 90 minutes or 8 hours to prevent nutrient intake via milk ingestion (Figure 1A). Ninety minutes time from onset of separation maximizes Fos expression (Barros et al., 2015). Both periods of isolation increased Agrp neuronal activity, as indicated by the increased number of Fos positive Agrp neurons upon isolation (nest: 3.14 ± 0.96 cells, n = 9; isolation/90 min: 31.19 ± 1.89 cells, n = 10; isolation/8h: 46.19 ± 2.04 cells, n = 3; F2, 19 = 128.6, P < 10−11, one-way ANOVA; Figure 1B–D).
Figure 1: Fos labels Agrp neuron activation in P10 mice upon social isolation.
(A) P10 mice were socially isolated for 90 min or 8 h.
(B) Agrp neurons positive for Fos immunoreactivity (nest, n = 9; isolation/90 min, n = 10; isolation/8 h, n = 3).
(C) Multiple comparisons test of the difference between means (95% confidence intervals).
(D) Representative images of immunohistochemistry for HA (cyan, labels Agrp neurons in AgrpRpl22-HA mice), Fos (magenta), and overlap. Scale bars represent 50 μm.
(E) Effect of isolation (90 min) on milk intake upon reunion with the dam and litter for an additional 90 min.
(F) Delta body weight as a measure of milk intake during 90 min in P10 mice (control, n = 12; reunion, n = 12).
(G) Plasma corticosterone levels in P10 mice after 90 min isolation (control, n = 7; reunion, n = 11). Boxplot represents median, 1st/3rd quartiles, and min/max values.
(H) Effect of exposure to predator odor (mT) during 90 min isolation in P10 mice.
(I) Quantification of Agrp neurons positive for Fos (nest, n = 2; isolation, n = 4; isolation/mT, n = 5).
(J) Multiple comparisons test of the difference between means (95% confidence intervals).
(K) Effect of milk infusion (mouse or bovine) in the activation of Agrp neurons in isolated P10 mice.
(L) Infused milk was labeled with a blue dye to confirm ingestion. Post-mortem images of the stomach of P10 mice.
(M) Quantification of Agrp neurons positive for Fos (nest, n = 5; isolation with no infusion, n = 12; isolation with infusion of mouse milk, n = 8; isolation with infusion of cow milk, n = 8).
(N) Multiple comparisons test of the difference between means (95% confidence intervals). Symbols represent mean ± SEM; red denotates statistically significant differences.
(O) Representative images of immunohistochemistry for HA (cyan, labeling Agrp neurons in AgrpRpl22-HA mice), Fos (magenta), and overlap. Scale bars represent 50 μm.
(P) Effect of artificial rearing mice on Fos upon isolation. P7 mice were fostered with a non-lactating dam, while hand-fed every 3–4 h for 3 days. At P10, all mice were hand-fed and separated in two groups - reunited with the foster dam or isolated for 90 min.
(Q) Quantification of Agrp neurons positive for Fos (foster dam, n = 6; isolation, n = 8).
(R) Representative images of immunohistochemistry for HA (cyan, labeling Agrp neurons in AgrpRpl22-HA mice), Fos (magenta), and overlap. Scale bars represent 50 μm.
In B, F, I, M, and Q bars indicate mean ± SEM. Circles represent individual values. In C and J, symbols represent mean ± SEM. Red denotes statistically significant differences.
Because ninety minutes significantly increased Fos positive Agrp neurons, we tested whether this period of isolation also stimulated milk intake. We measured milk intake by calculating the change in body weight in animals that remained in the nest for ninety minutes compared to animals isolated for ninety minutes followed by re-introduction to the nest for an additional ninety minutes period (Figure 1E). In this protocol, we did not observe significant differences in the body weight changes between the two experimental conditions (nest: 62.1 ± 15.4 mg, n = 12; reunion: 76.2 ± 14.4, n = 12; t22 = 0.65, P = 0.51, unpaired t-test; Figure 1F). Thus, activation of Agrp neurons after ninety minutes of isolation from the nest in P10 mice is not significant to increase milk intake. Moreover, the increased activation of Agrp neurons do not seem to arise from a generalized stress response, as corticosterone levels did not increase after isolation (nest: 9.96 ± 1.85 ng/mL, n = 7; isolation: 12.40 ± 1.85, n = 11; t16 = 0.88, P = 0.39, unpaired t-test; Figure 1G) and testing pups in the presence of a predator odor for 90 minutes did not alter Fos labeling in Agrp neurons (nest: 1.19 ± 0.92, n = 2; isolation: 23.82 ± 4.78, n = 4; isolation/predator odor: 22.71 ± 1.59, n = 5; F2, 8 = 9.73, P = 0.007, one-way ANOVA; Figure 1H–J).
To evaluate whether the activation of Agrp neurons upon isolation from nest is due to caloric deprivation, we isolated P10 mice while orally infusing bovine or mouse derived milk (Figure 1K). We confirmed milk ingestion by adding a colored dye to the milk to verify its presence in the stomach (Figure 1L). Surprisingly, our results showed no significant difference between the activation of Agrp neurons between the groups that received an oral infusion of milk and the control group that received a passive oral probe with no milk infusion (nest: 1.51 ± 0.65, n = 5; isolation/no infusion: 27.88 ± 2.39, n = 12; isolation/mouse milk: 30.73 ± 1.93, n = 8; isolation/bovine milk: 24.24 ± 3.14, n = 8; F3, 29 = 19.38, P < 10−6, one-way ANOVA; Figure 1M–O).
Next, we tested the hypothesis that Agrp neuron activity in P10 mice increases upon separation in anticipation of future nutrient deprivation. To this end, we investigated the extent to which isolation from the nest activates Agrp neurons when dissociating milk intake and the nest. P7 mice were housed with a foster non-lactating dam and were manually fed milk by an investigator (Figure 1P). All neonates quickly developed locomotor activity towards the investigator at feeding onset, which suggests a learned association that the milk source was outside the home nest. We then assessed activation of Agrp neurons at P10 at either isolation or return to the home cage with the foster dam for a period of ninety minutes after all pups were previously fed with equal volumes of milk. Similar to our previous experiments, isolation from the home nest strongly increased the number of Fos positive Agrp neurons compared to mice that returned to the home cage (foster dam: 15.59 ± 3.26, n = 6; isolation: 64.39 ± 3.94, n = 8; t12 = 9.06, P < 10−5, unpaired t-test; Figure 1Q–R). Taken together, these results suggest that activation of Agrp neurons in P10 mice following isolation from the nest does not require milk ingestion or anticipation of a future lack of milk ingestion, which stands in contrast to adult mice.
Non-nutritive suckling and thermal support blunt Agrp neuron activation in neonates
Our next goal was to evaluate the relative importance of different components of the nest environment and mother-infant interaction that contribute to Agrp neuron activation in P10 mice after isolation from the nest. In the nursing nest, pups receive care from the dam. An important feature of maternal care is neonatal attachment to the mother’s nipple and suckling. So, we investigated the extent to which suckling alters the activation of Agrp neurons. We fostered P10 mice with non-lactating dams, non-lactating dams with protruded nipples, and lactating dams (Figure 2A). In all cases, foster dams promptly retrieved the pups and placed them in the nest. All foster dams displayed maternal behaviors, such as grooming/licking and arched-back ‘nursing’ of pups, as expected. Interestingly, all foster dams blunted the activation of Agrp neurons in P10 mice compared to isolated pups (nest: 2.10 ± 0.21 cells, n = 6; isolation: 26.47 ± 1.57 cells, n = 6; non-lactating foster dam: 16.59 ± 1.64 cells, n = 7; non-lactating foster dam with protruded nipples: 8.66 ± 1.26 cells, n = 6; lactating foster dam: 10.29 ± 0.65 cells, n = 4; F4, 24 = 50.29, P < 10−10, one-way ANOVA; Figure 2B–C). Attachment of pups to the foster dam’s nipples further decreased the number of Fos labeled Agrp neurons compared to pups placed with a foster dam with non-protruded nipples to prevent nipple attachment (Figure 2B–C). The effect of nipple attachment was irrespective of milk availability, as the expression of Fos in pups showed a similar magnitude when placed with lactating and non-lactating foster dams with protruded nipples (Figure 2B–C). Overall, activation of Agrp neurons in P10 mice is blunted by non-nutritive suckling, an important component of maternal care, and is not further reduced by availability of milk in the dam’s nipples.
Figure 2: Warm temperatures blunt activation of Agrp neurons in P10 mice.
(A) Study design: P10 mice were either fostered with non-lactating dams, with non-lactating dams with protruded nipples, or with lactating dams for 90 min. A control group was not manipulated (nest) and a second group was isolated (isolation).
(B) Quantification of Agrp neurons positive for Fos (nest, n = 6; isolation, n = 6; non-lactating foster dam, n = 7; non-lactating foster dam with protruded nipples, n = 6; lactating foster dam, n = 4).
(C) Multiple comparisons test of the difference between means (95% confidence intervals).
(D) Thermo-photography of the nursing nest. Lactating dam is on the top of P10 offspring. Nest temperature is approximately 34–36°C.
(E) Offspring raised at room temperature (RT) were isolated either at room temperature, at thermoneutrality (TN, in a climate chamber set to 35°C), or at room temperature with a thermal support (TS) irradiating heat from underneath the cage (≈35°C).
(F) Quantification of Agrp neurons positive for Fos (nest/RT, n = 5; isolation/RT, n = 7; isolation/TN, n = 10; isolation/TS, n = 8).
(G) Multiple comparisons test of the difference between means (95% confidence intervals).
(H) Offspring raised at thermoneutrality (climate chambers at 35°C) were isolated for 90 min at RT or TN.
(I) Quantification of Agrp neurons positive for Fos (nest/TN, n = 2; isolation/RT, n = 3; isolation/TN, n = 3).
(J) Multiple comparisons test of the difference between means (95% confidence intervals). In B, F, and I, bars represent mean ± SEM, Round symbols represent individual values. In C, G, and J, symbols represent mean ± SEM. Red denotes statistically significant differences.
The nursing nest provides critical thermal insulation, which reduces heat loss from neonates that have not fully developed homeostatic mechanisms for thermoregulation. Dams contribute to thermal insulation by building a nest and skin-to-skin contact with pups (Figure 2D). To test the effects of thermal insulation on the activation of Agrp neurons, we isolated P10 mice at room temperature or at a thermoneutral temperature (≈35°C; Figure 2E). Because temperature exchanges in the nest occur by skin-to-skin contact, we included an additional control group, in which we provided thermal support by irradiating heat (≈35°C) from underneath (Figure 2E). Ninety minutes of isolation at thermoneutrality or with thermal support strongly suppressed activation of Agrp neurons in P10 mice as assayed by Fos labeling compared with pups isolated at room temperature (nest: 0.77 ± 0.39 cells, n = 5; isolation/RT: 22.19 ± 2.53 cells, n = 7; isolation/TN: 5.90 ± 0.86 cells, n = 10; isolation/TS: 2.75 ± 0.51 cells, n = 8; F3, 26 = 47.31, P = 10−10, one-way ANOVA; Figure 2F–G). Thus, Agrp neurons in P10 mice respond to the withdrawal of thermal insulation when isolated from the nursing nest. This factor holds primary importance for the responses of these neurons to isolation.
Next, we tested the extent to which the response of Agrp neurons in P10 mice to the withdrawal of thermal insulation was dependent on prior experience with drops in ambient temperature. To prevent mice from experiencing ambient temperatures lower than nest temperatures, we repeated the experiments in animals born and raised in a thermoneutral environment (Figure 2H). P10 mice raised at thermoneutrality showed increased Fos labeled Agrp neurons when isolated at room temperature but not at thermoneutrality (nest/TN: 0.62 ± 0.33 cells, n = 2; isolation/RT: 27.94 ± 3.13 cells, n = 3; isolation/TN: 6.16 ± 1.54 cells, n = 3; F2, 5 = 37.91, P = 0.001, one-way ANOVA; Figure 2I–J). Thus, the response of Agrp neurons to withdrawal of thermal insulation in P10 mice does not require previous experiences with drops in ambient temperature.
Neonatal Agrp neurons undergo rapid activity changes
In the previous experiments, we could not elucidate the temporal dynamics of physiological activation of Agrp neurons. For example, Agrp neurons after isolation (Figure 3A) could slowly increase their activity similar to a homeostat (Figure 3B). Alternatively, these neurons could rapidly respond to isolation (Figure 3C) similar to an alarm/reflexive system. A third alternative suggests that Agrp neurons could show delayed activation (Figure 3D), suggesting a thresholding mechanism triggers these neurons in neonates.
Figure 3: Rapid dynamics of Agrp neuronal activity in mice during early development.
(A) Experimental model of isolation and reunion in neonates, which activates Agrp neurons after 90 min. Three theoretical models of activity changes of these neurons are illustrated in B-D.
(B) Model ‘A’: activity of Agrp neurons gradually increases during the 90 min isolation.
(C) Model ‘B’: activity of Agrp neurons rapidly increases upon isolation.
(D) Model ‘C’: activity of Agrp neurons increases in isolation after a delay.
(E) In newborn AgrpCre/Cre mice, an adeno-associated virus was injected in the arcuate nucleus of the hypothalamus to express jGCaMP7s in Agrp neurons (AAV-CAG-Flex-jGCaMP7s.
(F) Preweaning mouse connected to an optic fiber and its dam.
(G) Expression of jGCaMP7s in Agrp neurons of a P14 mouse.
(H) Z score of Agrp neuronal activity in P13–14 mice. Baseline was recorded for 1 min, then pups were isolated for 10 min. Subsequently, pups were reunited with the litter. Plot represents mean ± SEM (n = 7 animals).
(I) Heat plot representing individual responses to isolation/reunion.
To better understand the natural activity dynamics of Agrp neurons early in postnatal development, we injected an adeno-associated virus encoding jGCaMP7s in a Cre-dependent manner in newborn AgrpCre/Cre mice (Figure 3E–G) (Dana et al., 2018). We then used fiber photometry to measure calcium transients originating from Agrp neurons upon isolation-reunion in P13–14 pups (Figure 3A). We found that pup isolation from the nest increased activity of Agrp neurons that occurred within seconds (Figure 3H–I) and persisted throughout the isolation period (10 minutes). After this initial separation, reunion of the isolated animal with the litter immediately decreased the activity of Agrp neurons (Figure 3H–I). The suppression of Agrp neuronal activity was robust and rapid, normalizing the detected signal to pre-isolation levels in less than 30 seconds (Figure 3H–I). All animals tested showed this response to isolation-reunion, suggesting a general model in which Agrp neurons in neonates rapidly respond to disruptions in the nest conditions (Figure 3C).
Neonatal Agrp neurons modulate the emission of ultrasonic vocalizations
In most neonatal mammals, including mice, disruptions in the nest condition leads to infant vocalization (Hofer, 1994). In mice and rats, neonates emit ultrasonic vocalizations (USVs) when separated from the dam (Noirot, 1966, 1968; Zippelius and Schleidt, 1956) (Figure 4A–B). We investigated whether activation of Agrp neurons in neonates upon isolation from the nest could modulate emission of USVs.
Figure 4: Agrp neurons in P10 mice modulate emission of ultrasonic vocalizations via GABA release.
(A) Experimental model of isolation in P10 mice at room temperature (RT) or at thermoneutral conditions (TN; climate chamber set at 35°C).
(B) Representative spectrogram of ultrasonic vocalizations (USVs) in P10 mice recorded in isolation.
(C) Number of USVs during isolation at RT or TN and control group recorded in the nest.
(D) Total number of USVs during recording from (C).
(E) Multiple comparisons test of the difference between means (95% confidence intervals).
(F) The total number of vocalizations upon isolation (10 minutes) in Npy+/+ (n = 12), NpyKO/+ (n = 17), and NpyKO/KO (n = 7) P10 mice.
(G) Similar to F, but using P10 mice knockout for Vgat specifically in Agrp neurons (AgrpVgat-KO) and their littermate controls (control, n = 10; AgrpVgat-KO, n = 14).
(H) Raster plots, ticks represent USVs.
(I-K) Violin plots representing the distribution of USV characteristics in control (n = 3,427 USVs) and AgrpVgat-KO mice (n = 786 USVs) in (I) duration, (J) mean frequency of the main component, and (K) the bandwidth.
(L-V) Types of USVs labeled in this study. Each panel represents the spectrogram of one type of vocal call.
(X) Distribution of absolute counts of each vocal call type.
(Y) Similar to X, but vocal types normalized to total counts. In X-Y, multiple t tests with P values corrected for multiple comparisons using the Holm-Sidak method.
In C, symbols represent mean ± SEM. In D, F, G, X, and Y, bars represent mean ± SEM. In E, symbols represent mean ± 95% CI. In I, J, and K, data distribution is plotted. In D, F, and G, symbols represent individual data. Statistically significant P values displayed in the panels.
First, we confirmed that isolation from the nest induced vocal behavior in P10 mice (Figure 4A). We then investigated whether isolation at thermoneutrality would influence USVs, since these conditions blunt activation of Agrp neurons upon isolation (Figure 2). Analysis of vocal behavior showed a rapid increase in USV emission upon isolation from the nest (Figure 4B–E), an effect that blunted at thermoneutrality (nest, n = 3; isolated/RT, n = 16; isolated/TN, n = 4; F2,20 = 18.08, P < 10−4, one-way ANOVA; Figure 4C–E). Thus, vocal behavior dynamics in neonatal mice follows the dynamics of Agrp neuron activation upon isolation in these experimental conditions.
To test the extent to which Agrp neurons contribute to vocal behavior of P10 mice upon isolation, we tested animals lacking the transmitters released by Agrp neurons (NPY and GABA) (Hahn et al., 1998; Horvath et al., 1997). We recorded the emission of USVs in NpyKO and AgrpVgat-KO mice and their littermate controls following isolation in P10 mice. Animals lacking NPY exhibited a similar number of USVs after isolation compared to controls (control: 343.4 ± 56.8 USVs, n = 12; NpyKO/+: 384.5 ± 69.3 USVs, n = 17; NpyKO/KO: 348.0 ± 91.7 USVs, n = 7; P = 0.74, KW test; Figure 4F). In contrast, AgrpVgat-KO mice had a significant decrease in emission of USVs upon isolation (control: 321.0 ± 50.4 USVs, n = 10; AgrpVgat-KO: 57.7 ± 14.6 USVs, n = 14; U = 2, P < 10−5, Mann-Whitney Test; Figure 4G–H).
We further analyzed the spectro-temporal characteristics of 3,427 USVs from control mice and 786 USVs from AgrpVgat-KO mice. We characterized individual USVs by changes in spectro-temporal characteristics, such as duration, frequency, and bandwidth. Compared to control mice, USVs from AgrpVgat-KO mice decreased in duration of 9.5 ms (control: 37.84 ± 0.45 ms; AgrpVgat-KO: 28.31 ± 0.84 ms; D = 0.176, P = 10−15, KS test; Figure 4I), in mean frequency of 1.9 kHz (control: 82.20 ± 0.28 kHz; AgrpVgat-KO: 80.24 ± 0.55 kHz; D = 0.10, P = 10−15, KS test; Figure 4J), and in bandwidth of 5.6 kHz (control: 22.86 ± 0.35 kHz; AgrpVgat-KO: 17.20 ± 0.67 kHz; D = 0.17, P = 10−15, KS test; Figure 4K). We also found an overall decrease in the number of vocalizations across most USV categories (Figure 4L–X) (Grimsley et al., 2011). However, we observed an increase in the incidence of ‘short’ vocalizations, when analyzing the relative frequency of USV categories (Figure 4Y). This syllable represents the simplest form of vocalization by neonatal mice based on spectro-temporal characteristics (Figure 4L–V). Thus, lacking GABA release from Agrp neurons, P10 AgrpVgat-KO mice led to fewer and simpler USVs compared to control animals. Taken together, these findings indicate Agrp neurons are critically positioned to modulate the emission of USVs in neonatal mice.
Chemogenetic activation of Agrp neurons increases USV emission
We further tested whether chemogenetic activation of Agrp neurons could modulate emission of USV in isolated P10 pups using AgrpTrpv1 mice (Dietrich et al., 2015; Ruan et al., 2014)(Arenkiel et al., 2008; Guler et al., 2012) (Figure 5A and Supplementary Figure 1). Subcutaneous injection of capsaicin (10 mg/kg) in AgrpTrpv1 mice robustly activated Agrp neurons in young pups (Figure 5B; n = 5 mice per group; U = 0, P = 0.004, Mann-Whitney Test). Chemogenetic activation of Agrp neurons using the AgrpTrpv1 animal model induced a 61 % increase in USV emission in P10 mice (control: 686.7 ± 50.22 USVs, n = 32; AgrpTrpv1: 1040.0 ± 66.56 USVs, n = 24; t51 = 4.318, P < 0.0001; 2-tailed unpaired t-test; Figure 5C–E).
Figure 5: Activation of Agrp neurons in P10 mice increases USV emission without altering the dam’s behavior.
(A) Generation of AgrpTrpv1 mice.
(B) Fos in the arcuate nucleus of P15 AgrpTrpv1 mice upon injection of capsaicin (10 mg/kg, s.c.; n = 5 mice per group). Scale bar corresponds to 50 μm. Bars and symbols represent mean ± SEM.
(C) Raster plots show USV in isolated P10 controls (n = 32) and AgrpTrpv1 mice (n = 24). A tick represents a USV.
(D) Related to C, number of USVs (in 5 min bins).
(B) Related to C-D, total number of USVs in the 20 min after activating Agrp neurons in isolated pups.
(F-H) Violin plots representing the distribution of USV characteristics in control (n = 22,168 USVs) and AgrpTrpv1 mice (n = 24,971 USVs) in (F) duration, (G) mean frequency of the main component of the USV, and (H) the bandwidth.
(I) Distribution of absolute counts of each vocal call type.
(J) Similar to I, but vocal types normalized to total counts. In I-J, multiple t tests with P values corrected for multiple comparisons using the Holm-Sidak method.
(K) Distribution of the fold change (number of USVs from AgrpTrpv1 mice related to number of USVs from control mice) in each of the 60 output clusters analyzed by MUPET (see methods). Shown in blue are 6 clusters highly enriched in USVs from AgrpTrpv1 mice. Shown in green is 1 cluster enriched in USVs from controls.
(L) Related to K, images represent the 6 clusters enriched in USVs from AgrpTrpv1 mice.
(M) Related to K, image represents the cluster enriched in USVs from control mice.
(N) Maternal preference test (MPT).
(O) Representative tracking (in blue) of a dam in the preference stage.
(P) Preference index (in seconds) during MPT.
In D, symbols represent mean ± SEM. In B, I, and J, bars represent mean ± SEM. In F, G, and H, data distribution is plotted. In E and P, symbols represent individual data. In P, black lines indicate mean ± SEM. See also Figure S1.
We also analyzed the spectro-temporal characteristics of a total of 21,019 USVs from control mice and 24,976 vocalizations from AgrpTrpv1 mice. Upon activation of Agrp neurons, the USV duration decreased by 4 ms (control: 51.42 ± 0.23 ms; AgrpTrpv1: 47.09 ± 0.19 ms; D = 0.077, P = 10−15, KS test; Figure 5F), the mean frequency increased by 3 kHz (control: 77.50 ± 0.08 kHz; AgrpTrpv1: 80.96 ± 0.07 kHz; D = 0.12, P = 10−15, KS test; Figure 5G), and the bandwidth increased by 2 kHz (control: 27.31 ± 0.13 kHz; AgrpTrpv1: 29.84 ± 0.12 kHz; D = 0.07, P = 10−15, KS test; Figure 5H). The selected categories of USVs induced by activation of Agrp neurons (Figure 5I) showed a higher complexity compared to those suppressed in AgrpVgat- KO mice (Figure 4).
Interestingly, when we analyzed the relative frequency of USV categories, we found a selective increase in the frequency of ‘chevrons’ upon chemogenetic activation of Agrp neurons (Figure 5J and Figure 4N). To further corroborate these findings, we used a second software tool MUPET to classify vocalizations based on their shape (Van Segbroeck et al., 2017). We found six clusters of vocalizations that showed a more than 2.5-fold increase upon activation of Agrp neurons (Figure 5K–L). These clusters were very similar to each other, resembling our previous analysis (Figure 4G–H). We also found that activation of Agrp neurons suppressed vocalizations in cluster 39 (Figures 5K and 5M). The USVs in cluster 39 were simpler in shape, resembling vocalizations predominantly emitted by AgrpVgat-KO pups (Figure 4). In contrast to mice lacking GABA release by Agrp neurons, chemogenetic activation of these neurons stimulated USV emission at a higher rate and with higher complexity. Together, these results strongly suggest a model in which neonatal Agrp neurons are rapidly activated upon isolation from the nest, which modulates USV emission, presumably to attract the dam.
Chemogenetic activation of Agrp neurons in neonatal mice modulates the behavior of dams
Neonatal behavior is linked to maternal behavior, as the former can enhance and entrain the behavior of the latter. Based on our previous results, we expected that chemogenetic activation of Agrp neurons in the neonate would change the response of the dam towards the neonate. We devised a test to assess maternal responsiveness to P10 mice, the ‘maternal preference test’ (Figure 5N). In this test, maternal responsiveness was measured as the time of dam-pup interaction. We found maternal exploratory behavior was strongly skewed towards AgrpTrpv1 pups compared to controls (preference index - control: 44.96 ± 13.68 s, n = 25; AgrpTrpv1: 125.1 ± 21.74 s, n = 25; t24 = 2.69; P = 0.01, 2-tailed paired t test; Figure 5O–P). These results support the model in which neonatal Agrp neurons modulate vocal behaviors signaling the dam should return to the nest.
Chemogenetic activation of neonatal Agrp neurons increases odds for nipple attachment
In young pups, contact with the dam is critical for suckling behavior and ingestion of breast-milk. Thus, we next devised behavior experiments to test whether activation of Agrp neurons in pups would drive behaviors towards the dam, including exploratory activity, suckling, and milk intake. We eliminated active participation by the dam as the driver of these behaviors by examining the behavior of P10 mice towards anesthetized dams (Figure 6A). In anesthetized dams, milk ejection decreases considerably (Lincoln et al., 1973), so suckling under these conditions is considered non-nutritive. Indeed, we did not observe the stretching reflex in pups that suckled during our experiments, which is a pathognomonic sign of milk ejection and ingestion (Vorherr et al., 1967).
Figure 6: Agrp neuron activation and suckling behavior in P10 mice.
(A) Suckling behavior in mice.
(B-G) Quantification of suckling behavior in P10 mice tested with an anesthetized, non-lactating dam. In (B), proportion of mice displaying nipple attachment. In (C), total distance traveled. In (D), tracking of locomotor activity (bottom: starting point; top: anesthetized dam). In (E-G), data only considering mice that attached to nipples. In (E), number of nipple attachments. In (F), latency to the first attachment. In (G), total time attached to nipples.
(H) Suckling behavior using thermal support (≈35°C).
(I-N) Quantification of suckling behavior in P10 mice tested with an anesthetized, non-lactating dam (as displayed in (H)). In (I), proportion of mice that attached to the nipples. In (J), total distance traveled. In (K), tracking of locomotor activity (bottom: starting point; top: anesthetized dam). In (L), number of nipple attachments. In (M), latency to the first attachment. In (N), total time attached to nipples.
(O) Milk letdown prior to and after oxytocin injection in anesthetized dams.
(P-T) Quantification of suckling behavior in P10 mice tested with an anesthetized, lactating dam. In (P), proportion of P10 mice that attached to the nipples. In (Q), total distance traveled. In (R-T), data only reflect pups that attached to nipples. In (R), number of nipple attachments. In (S), latency to the first attachment. In (T), the total time attached to nipples.
(U) Delta body weight (control, n = 8; AgrpTrpv1, n = 13; pups that did not attach, n = 12).
(V) Tukey-Kramer’s multiple comparisons test of the difference between means (95% confidence intervals from (U)), representing effect sizes.
In B, I, and P, statistical analysis was performed using the Chi-square test (2-tailed). In yellow, proportion of mice that attached to the dam’s nipples. In grey, mice that did not attach.
In C, E, F, G, J, L, M, N, Q, R, S, T, and U, violin plots represent the distribution of the data. Symbols represent individual values. Statistical analysis was performed using Mann-Whitney Test, with the exception of U. P values provided in the panels when statistically significant.
Activation of Agrp neurons in P10 mice increased the total number of pups that attached to the dam’s nipples (control: 9 out of 22; AgrpTrpv1: 13 out of 15; P = 0.005, Chi-square Test; Figure 6B) and increased the distance traveled in the testing chamber (control: 0.75 ± 0.14 m, n = 22; AgrpTrpv1: 1.20 ± 0.21 m, n = 15; U = 94, P = 0.027, Mann-Whitney Test; Figure 6C–D). We then compared nipple attachment behavior of P10 mice, excluding animals that did not attach to the dam’s nipples from post-hoc analysis. Chemogenetic activation of Agrp neurons did not change the frequency (control: 3.11 ± 1.23; AgrpTrpv1: 3.61 ± 0.83; U = 51, P = 0.63, Mann-Whitney Test; Figure 6E), latency (control: 279.9 ± 57.4 s; AgrpTrpv1: 485.4 ± 86.2 s; U = 35, P = 0.12, Mann-Whitney Test; Figure 6F), or the duration of nipple attachment (control: 719.4 ± 128.0 s; AgrpTrpv1: 529.8 ± 99.86 s; U = 39, P = 0.20, Mann-Whitney Test; Figure 6G). Thus, while chemogenetic activation of Agrp neurons in P10 mice increased the probability of attaching to the dam’s nipples, it did not change the observable microstructure of nipple attachment behavior.
Next, we investigated the influence of the environmental temperature on nipple attachment behavior (Figure 6H). Thermal support from underneath the testing chamber completely suppressed nipple attachment behavior in P10 control mice (0 out of 11 pups tested attached), while 4 out of 10 pups with activated Agrp neurons attached to the dam’s nipples (P = 0.019, Chi-square Test; Figure 6I). Interestingly, the arousal response of P10 mice upon activation of Agrp neurons was intact, as measured by the distance traveled during the test (control: 0.30 ± 0.07 m, n = 11; AgrpTrpv1: 1.03 ± 0.19 m, n = 10; U = 13, P = 0.002, Mann-Whitney Test; Figure 6J–K). When we analyzed the different components of nipple attachment behavior of the four P10 mice that successfully attached, we found the frequency of attachments was largely suppressed with mice only attaching once during the test (Figure 6L). The latency to attach (386.5 ± 49.62 s; Figure 6M) was similar to the other experimental conditions (Figures 6F and 6S). The duration animals remained attached to the nipples (787.9 ± 48.20 s; Figure 6N) was within the range of our other experiments (Figures 6G and 6T). We conclude providing warmth did not blunt the arousal response after activating Agrp neurons but did suppress nipple attachment behavior of P10 mice. Taken together, our behavioral experiments further support the importance of a thermal stimulus in neonates to modulate the functional properties of Agrp neurons.
We then assessed whether a lactating anaesthetized dam would alter attachment by P10 mice. Oxytocin triggers the milk ejection reflex following nipple stimulation by the pups during suckling (Lincoln and Paisley, 1982). To facilitate milk ejection, we injected a group of anesthetized dams with oxytocin immediately before testing each pup (Figure 6O) (Singh and Hofer, 1978; Vorherr et al., 1967). Similar to non-lactating dams, activation of Agrp neurons in P10 mice increased the number of mice that attached to the dam’s nipples (control: 8 out of 18; AgrpTrpv1: 13 out of 15; P = 0.012, Chi-square Test; Figure 6P). The total distance traveled during the test was not different between groups (control: 0.87 ± 0.22 m, n = 18; AgrpTrpv1: 1.13 ± 0.28 m, n = 15; U = 100.5, P = 0.21, Mann-Whitney Test; Figure 6Q). When comparing the behavior of animals that attached to the dam’s nipples, we did not find statistical differences in the frequency (control: 5.25 ± 0.99, n = 8; AgrpTrpv1: 8.38 ± 1.52, n = 13; U = 34, P = 0.20, Mann-Whitney Test; Figure 6R) or duration of attachments (control: 515.7 ± 78.68 s, n = 8; AgrpTrpv1: 502.6 ± 91.98 s, n = 13; U = 50, P = 0.91, Mann-Whitney Test; Figure 6T), but we found a decrease in the latency of attachment (control: 501.9 ± 68.91, n = 8; AgrpTrpv1: 335.2 ± 55.52, n = 13; U = 21, P = 0.02, Mann-Whitney Test; Figure 6S). Since dams were lactating, we also measured body weight changes in the pups as a measure of milk intake. Interestingly, upon activation of Agrp neurons, P10 mice ingested a lower amount of milk than controls during the test (control: 91.2 ± 19.9 mg; AgrpTrpv1: 43.7 ± 10.0 mg; pups that did not attach: −10.0 ± 2.9 mg, n = 12; F2,30 = 19.35, P < 10−5, one-way ANOVA; Figure 6U–V). These results suggest that activated Agrp neurons increase dam-seeking behavior, but not necessarily increase ingestion of milk.
Chemogenetic activation of Agrp neurons increases ingestive behaviors in P15 mice
Mice rapidly transition from breastfeeding to independent feeding during weaning period. At approximately P15, mice begin experimenting with food sources, but still rely on breastfeeding for nutrition (Hammond et al., 1996). We next examined the behavior of P15 mice towards the dam to investigate the ontogeny of Agrp neuron function. All tested P15 mice attached to the nipples of the anesthetized dam regardless of chemogenetic activation of Agrp neurons (n = 19 mice per group; Figure 7A). Activating Agrp neurons did not significantly change the distance travelled in the testing chamber (control: 2.03 ± 0.34 m; AgrpTrpv1: 2.20 ± 0.22 m; U = 145, P = 0.31, Mann-Whitney Test; Figure 7B). In contrast to P10 mice, chemogenetic activation of Agrp neurons in P15 mice showed a striking increase in the number of attachments to the dam’s nipples compared to control (control: 3.68 ± 1.02; AgrpTrpv1: 11.74 ± 1.80; U = 72.5, P = 0.001; Mann-Whitney Test; Figure 7C–D). We observed no statistical difference in the latency of the first attachment (control: 226.6 ± 50.8 s; AgrpTrpv1: 123.0 ± 16.92 s; U = 116, P = 0.06; Mann-Whitney Test; Figure 7E) or the total duration of nipple attachment (control: 711.6 ± 85.2 s; AgrpTrpv1: 798.9 ± 79.02 s; U = 143, P = 0.28; Mann-Whitney Test; Figure 7F).
Figure 7: Ontogeny of ingestive behaviors in mice.
(A-H) Suckling behavior in P15 mice using anesthetized non-lactating dams during a twenty-minute test. (A) All pups attached to the dam’s nipples during the test. (B) Total distance traveled. (C) Raster plot, ticks indicate nipple attachment. (D) Number of nipple attachments. (E) Latency to the first nipple attachment. (F) Total time attached to nipples. (G) Representative tracking data of nipple attachment. (H) Number of nipples explored.
(I-J) Nipple preference as measured by the number of nipple attachments per nipple row in P15 mice (n = 38; both control and AgrpTrpv1 mice were included).
(K-O) Suckling behavior in P15 mice using anesthetized lactating dams during a ten-minute test.
(K) Proportion of P15 mice that attached to the nipples of lactating dams. In (L), total distance traveled during the test. In (M-O), only showing mice that attached to nipples. In (M), the number of nipple attachments. In (N), latency to the first attachment. In (O), total time attached to nipples.
(P) Delta body weight after the suckling assay.
(Q) Chow intake during 30 min after activation of Agrp neurons in mice at 15, 18 and 21 days of age.
(R) Delta body weight in the same animals as in (Q).
In A and K, statistical analysis performed using the Chi-square test (2-tailed). In yellow, proportion of mice that attached to the dam’s nipples. In grey, mice that did not attach. In B, D, E, F, H, L, M, N, O, and P, violin plots represent the distribution of the data. Symbols represent individual values. Statistical analysis performed using Mann-Whitney Test. In (Q-R), differences tested using unpaired t test with Welch’s correction (unequal SDs). P values provided in the panels when statistically significant. Bars and symbols (in J, Q and R) represent mean ± SEM.
Since P15 mice displayed numerous nipple attachments (Figure 7D), we could track and quantify the number of nipples explored to measure nipple-shifting behavior (Figure 7G) (Cramer et al., 1980). Chemogenetic activation of Agrp neurons in P15 mice increased the total number of different nipples explored during the test (control: 2.68 ± 0.57 nipples; AgrpTrpv1: 5.21 ± 0.62 nipples; U = 92.5, P = 0.007; Mann-Whitney Test; Figure 7H). In these experiments, mice did not show a nipple preference (n = 38 mice; P = 0.42, one-way ANOVA; Figure 7I–J), which is a phenomenon observed in other species (Erwin et al., 1975; Hudson et al., 2009; Tomaszycki et al., 1998).
We repeated the above experiments using anesthetized dams injected with oxytocin during a ten minutes test. In control mice, 50% of P15 mice attached to the dam’s nipples (4 out of 8 mice), while 91% of AgrpTrpv1 mice displayed the same behavior (11 out of 12 mice; P = 0.035, Chi-square Test; Figure 7K). Activating Agrp neurons did not significantly change the distance traveled in the testing chamber (control: 1.13 ± 0.26 m; AgrpTrpv1: 1.56 ± 0.21 m; U = 31, P = 0.20, Mann-Whitney Test; Figure 7L), but strongly increased the number of nipple attachments (control: 2.00 ± 0.70; AgrpTrpv1: 9.90 ± 1.82; U = 2.5, P = 0.007, Mann-Whitney Test; Figure 7M). The latency to the first nipple attachment (control: 162.0 ± 33.1 s; AgrpTrpv1: 119.9 ± 27.1; U = 14, P = 0.34, Mann-Whitney Test; Figure 7N) and the total duration of nipple attachment (control: 332.2 ± 11.9 s; AgrpTrpv1: 388.0 ± 34.7 s; U = 20, P = 0.85, Mann-Whitney Test; Figure 7O) were not changed upon chemogenetic activation of Agrp neurons. In contrast to P10 mice, chemogenetic activation of Agrp neurons in P15 mice did significantly increase milk intake in mice that attached to the nipples of lactating dams (control: 17.2 ± 26.1 mg; AgrpTrpv1: 109.5 ± 38.7 mg; U = 7, P = 0.02, Mann-Whitney Test; Figure 7P).
We also tested the extent to which activation of Agrp neurons induces ingestion of solid food during the weaning period. We did not observe changes in food intake in P15 mice, but we observed increased food intake in P18 and P21 mice upon chemogenetic activation of Agrp neurons (Figure 7Q). Similarly, we only found changes in body weight during the feeding test in P21 mice (Figure 7R). Together, this set of behavioral experiments suggest that Agrp neurons in P10 mice are not proximally involved in milk intake or signaling milk ingestion. We propose that these functional properties of Agrp neurons rapidly change (or appear) as mice approach weaning age.
Discussion
Overall, our results reveal functional properties of Agrp neurons in neonatal mice. These insights demonstrate developmental differences that emerge during ontogeny, so studying any complex system may remain incomplete without assessing its developmental properties (Tinbergen, 1963).
Our experiments unexpectedly revealed that Agrp neurons are functional during the first two postnatal weeks in mice despite their immature characteristics (Nilsson et al., 2005; Padilla et al., 2010). We showed that Agrp neurons in P10 mice did not respond to milk intake and their activation did not directly increase milk intake. Conversely, non-nutritive suckling and thermal insulation were key factors modulating the activity of Agrp neurons. These results are compatible with the physiology of breastfeeding in neonatal mammals. During breastfeeding, mice, like most other mammals, do not receive continuous milk ejection. Milk ejection remains under control of a neuroendocrine reflex (Cross and Harris, 1952) and occurs at random intervals, as demonstrated in rats (Lincoln et al., 1973). In spite of milk ejection patterns, neonatal rats stay attached to the dam’s nipple for at least 12 hours a day in the first days of life (Lincoln et al., 1973). In fact, homeostatic sensing of milk deprivation to modulate nutritive suckling behavior only develops later as shown in laboratory rodents (Ellis et al., 1984; Hall and Rosenblatt, 1978; Kenny et al., 1979). Thus, these studies strongly imply that nipple attachment serves as a stimulus for more than milk ejection. In fact, neonatal rodents develop filial huddling to dams triggered by thermo-tactile stimulation rather than provision of milk (Alberts, 2007; Alberts and May, 1984). Similar to rodents, neonatal monkeys prefer a cloth mother that provides thermal and tactile stimuli to a wired mother with a nursing bottle (Harlow, 1958), establishing that maternal comfort has a superior importance compared to milk intake in driving neonatal affectional responses. We posit that Agrp neurons of neonates drive this milk-independent encoding of the offspring-to-caregiver bond.
In our studies, thermal insulation was the primary factor modulating the activation of Agrp neurons in neonates following isolation from the nest. Notably, experiencing previous thermal challenges was not significant to activate neonatal Agrp neurons following isolation from the nest, which suggests an ‘innate’ property of Agrp neurons. Intriguingly, foster dams also provide thermal insulation for the neonates, but they do not suppress activation of Agrp neurons after isolation from the nest to the same degree as thermal support. This observation suggests that neonates integrate several sensory signatures coupled to the nest environment, such as sensory cues from the dam, siblings, home-nest odors or from all these sources, to create and build expectations of the external world. We unexpectedly propose that activity of Agrp neurons partially encodes this information. Future studies should address the exact nature of this information and the combination of sensory modalities needed to modulate the activity of neonatal Agrp neurons.
Our results provide further evidence to the proposal that Agrp neurons serve as motivational drivers in the mammalian brain. In adult mice, Agrp neurons may encode negative valence (Betley et al., 2015). Under this proposal, these neurons when active would generate an unpleasant state of hunger leading the adult mouse to engage in behaviors to eat and suppress this state (Betley et al., 2015). Our results support this functional property of Agrp neurons in encoding an overall negative state beginning in early development. Clearly, isolation from the nursing nest serves as a negative stimulus for the neonate, which triggers a rapid activation of Agrp neurons and emission of vocalizations. Conversely, reunion then serves as a positive stimulus for the isolated neonate, which immediately suppresses the activity of Agrp neurons. By studying the functional ontogeny of Agrp neurons, our results support a model by which Agrp neurons generate a motivational drive to suppress an overall negative state.
In our studies, we could not find increases in corticosterone levels upon isolation or an enhanced activation of Agrp neurons (as labeled by Fos) in the presence of predator odor. Despite these negative results, we cannot rule out the possibility that Agrp neurons early in life are responsive to general stressors.
Upon parental separation, some infant mammals and birds respond with protest, despair, and emit sounds to attract them (Hofer, 1994). Our results demonstrated that neonatal Agrp neurons are a critical component of the circuit modulating vocal behavioral response in mice. These findings provide further insight into the underlying neural pathways subserving neonatal vocal behavior (Curry et al., 2013; Mosienko et al., 2015; Winslow et al., 2000). Investigating downstream circuits linking Agrp neurons to brain regions involved in the emission of USVs (Arriaga and Jarvis, 2013; Arriaga et al., 2012) will reveal how the neonatal brain encodes sensory information and internal state-dependent variables to generate behavioral responses (Boulanger-Bertolus et al., 2017; Hofer, 1996).
Our results have a broad impact to understand the functional ontogeny of hypothalamic neurons and the importance of the neonatal period in brain and behavior development. Additionally, these studies establish a mechanistic substrate underlying infant-caregiver interaction, which suggests an initial population of neurons underlying the long-sought nature of this social bond in mammals (Harlow, 1958; Lewis et al., 2007).
STAR Methods
Contact for reagent and resource sharing
Further information and requests for reagents should be direct to and will be fulfilled by the Lead Contact, Marcelo Dietrich (marcelo.dietrich@yale.edu).
Experimental models and subject details
All preweaning mice used in the experiments were 10–21 days old from both genders. Dams used were 2 – 6 months old. In this study, we used the following mouse lines from The Jackson Laboratories: Agrptm1(cre)Lowl/J (AgrpCre) (JAX: 012899); B6;129P2- Gt(ROSA)26Sortm1(Trpv1,ECFP)Mde/J (R26LSL-Trpv1) (JAX: 008513); B6.129X1-Trpv1tm1Jul/J (Trpv1KO) (JAX: 003770); B6N.129-Rpl22tm11Psam/J (Rpl22LSL-HA) (JAX: 011029); 129S-Npytm1Rpa/J (NpyKO) (JAX: 004545); and Slc32a1tm1Lowl (or VgatFlox/Flox) (JAX: 012897).
AgrpTrpv1 mice were: AgrpCreTm/+::Trpv1−/−::R26-LSL-Trpv1Gt/+; control animals were Trpv1−/−:R26-LSL-Trpv1Gt/+ mice injected with capsaicin. AgrpCre and R26LSL-Trpv1 mice were backcrossed to Trpv1KO mice to avoid the peripheral actions of capsaicin when injected systemically to activate Agrp neurons (Arenkiel et al., 2008; Dietrich et al., 2015; Guler et al., 2012; Ruan et al., 2014). We have thoroughly characterized AgrpTrpv1 mice previously (Dietrich et al., 2015). AgrpHA mice were generated by crossing AgrpCre to Rpl22LSL-HA mice. Analysis of ectopic expression of Cre was performed by using a specific set of primers against the excised conditional allele, as characterized before (Dietrich et al., 2015); mice with ectopic expression of the excised allele were not used in the studies. AgrpVgat-KO mice were generated by crossing AgrpCre to VgatFlox/Flox mice to finally generate AgrpCre/+:: VgatFlox/Flox. Controls were Cre negative littermates. All mice were kept in temperature- and humidity-controlled rooms, in a 12/12 hr light/dark cycle, with lights on from 7:00 AM–7:00 PM. Food (Teklad 2018S, Envigo) and water were provided ad libitum unless otherwise stated. All procedures were approved by IACUC (Yale University).
Method details
Drugs:
capsaicin (10 mg/kg, s.c. or i.p.; 3.33 % Tween-80 in PBS; from Sigma) and oxytocin (5mg/kg, 15 IU/mg dissolved in PBS; from Sigma).
Immunohistochemistry:
Mice were deeply anesthetized and perfused with freshly prepared fixative (paraformaldehyde 4%, in PBS 1x [pH = 7.4]). Brains were post-fixed overnight in fixative and sectioned on a vibratome. Coronal brain sections (50 μm) were washed several times in PBS 1x (pH = 7.4) and pre-incubated with Triton X-100 (0.3% in PBS 1x) for 30 min. Sections were then incubated in a blocking solution (Triton 0.3%, Donkey Serum 10%, Glycine 0.3M in PBS 1x) for one hour. Sections were then incubated with rabbit polyclonal anti-Fos (1:200; sc-52, Santa Cruz Biotechnologies) or rabbit monoclonal anti-Fos (1:1000, #2250; Cell Signaling Technology) and mouse polyclonal anti-HA (1:1000; 901503, Biolegend) for 16 hrs. After, sections were extensively washed in 0.3% Triton in PBS and incubated with secondary fluorescent Alexa antibodies (1:500). Sections were mounted and visualized by a Leica TCS SP5 Spectral Confocal Microscope (Center for Cellular e Molecular Imaging, Yale University). During the entire procedure, investigators were blinded to the experimental groups. The ImageJ analysis program (version 1.51h, NIH, USA) (Girish and Vijayalakshmi, 2004; Schindelin et al., 2012) was utilized to count the number of –HA positive (AgrpHA neurons) and Fos-positive neurons manually.
Isolation from the nest (Figure 1A–D):
At postnatal day 10 (P10), neonates (AgrpHA) were divided into three conditions: (1) kept with the biological mother and littermates (nest); (2) isolated for 90 minutes and (3) isolated for 8 hours. Isolated animals were single-housed and placed in a clean chamber with fresh bedding. Pups were sacrificed and expression of Fos was evaluated. Samples were prepared for immunohistochemistry as described above. AgrpHA pups were used in these studies to allow identification of Agrp neurons. All samples were prepared and counted blinded for the experimental groups.
Milk intake in the nursing nest (Figure 1E–F):
when ten days of age (P10), pups were divided into two groups: kept with the biological mother or isolated for 90 minutes. Isolated pups were reintroduced to the biological mother for another 90 minutes after isolation. Body weight was measured prior and after 90 minutes. To ensure that animals within each group had similar milk availability, they were tested using an equal number of animals. Upon reintroduction of the isolated pups to the home cage, pups that stayed with the dam were removed to avoid competition. These experiments were not blinded.
Measurement of corticosterone levels (Figure 1G):
At postnatal day 10, neonates were divided into two groups: kept with the biological mother or isolated for 90 minutes. After testing, neonates were deeply anesthetized, and blood samples were collected through cardiac puncture. A total of 150 μl of blood was collected. Blood samples were left at room temperate for one hour and centrifuged at 5000 rpm for 20 minutes. Plasma was collected and stored at −80°C. Corticosterone level was measured using an enzyme immunoassay (ELISA) kit (Enzo Life Sciences, Farmingdale, NY, USA) according to the manufacturer’s instructions.
Isolation from the nest in the presence of a predator odor (Figure 1H–J):
At postnatal day 10 (P10), pups (AgrpHA) were divided into three conditions: (1) kept with the biological mother and littermates (nest); (2) isolated for 90 minutes and (3) isolated for 90 minutes in the presence of a synthetic predator odor (2,4,5-Trimethylthiazole (mT), Sigma-Aldrich). Isolated animals were single-housed and placed in a clean chamber with fresh bedding. Ten microliters of mT odor were pipetted onto a small square nesting material (2 × 2 cm). To avoid contact with the odor, the chamber was divided by a wire mesh resulting in two small compartments, allowing the pups to smell the odor.
Isolation from the nest with milk infusion (Figure 1K–O):
The procedure of milk infusion consisted in the insertion of a polyurethane-based catheter tubing (Micro-Renathane® Tubing, MRE-033, Braintree Scientific, Inc.) attached to a pump. To avoid an invasive procedure, the inserted tube end was heated and bent to create a small U shape at its end tip. After insertion into the mouth, the tube was attached to the fur on the outside of the cheek using a small drop of crazy glue to hold it in place. The whole procedure did not require anesthesia and last less than 30 seconds. A total of 200 μl of milk was infused during the 90 minutes (15 μl ejections, every 5–15 minutes). The following types of milk were used: (1) commercial Half & Half cow’s milk (Organic Valley, Ultra Pasteurized Grade A); an (2) mouse milk collected from lactating dams. To confirm that milk was infused, a tasteless blue dye (Erioglaucine disodium salt, Sigma-Aldrich, Cat. 861146) was added (< 1 mg/mL) to the milk prior infusion. The stomach was excised to confirm the blue color indicating milk ingestion.
Mouse milk collection:
The milk collection was performed on lactating dams with litters between the ages of P8-P12. Dams were separated from their litter for 6 hours prior collection to ensure adequate milk production. Dams were lightly anesthetized with isoflurane and oxytocin (5 mg/kg, i.p) was administered to promote milk release. Milk was expressed from the nipples using pressure from the thumb and forefinger to gently massage and squeeze the mammary tissue in an upward motion until a visible bead of milk begins to form at the base of the teat. Then, milk was collected using a 20 μl calibrated pipette, pipetted into a 1.5 mL Eppendorf tube and storage at −20°C until the day of the test. The duration of the milk collection last less than 10 minutes.
Artificial feeding protocol (Figure 1P–R):
when seven days old, neonates were separated from the biological dam and kept with a non-lactating foster dam. Every 3–4 hours, the neonates were separated from the foster dam and milk was provided for ~30 minutes by the experimenter using a surrogate nipple attached to a tip in a 100 μL pipette. The volume of intake in each session varied between 80 μl to 150 μl of milk. Because of the limitation in getting mouse milk, we performed the artificial feeding using a more caloric formula of cow milk (Heavy Whipping Cream, Organic Valley) that resembles the nutrition facts of a mouse milk (Gors et al., 2009). At postnatal day 10, milk was provided for 30 minutes and immediately after neonates were separated into two groups: kept with the non-lactating foster dam and littermates (nest) or isolated for 90 minutes. All other procedures for Fos counting were as described above.
Assessment of maternal components with foster dams (Figure 2A–C):
At postnatal day 10, neonates were separated from the biological dam and placed in the cage of a foster dam. Foster dams in different lactation conditions were used: (1) non-lactating foster dam; (2) non-lactating foster dam with protruded nipples; (3) lactating foster dam. Dam rodents have the nipples still distended without milk release permitting suckling for two weeks after weaning if the female is not pregnant again. Lactating foster dams were chosen in a similar postnatal day of lactation, and their offspring was removed immediately before placing the alien/unfamiliar neonates. Neonates were divided into five groups: (1) kept with the biological dam and littermates (nest); (2) kept with a non-lactating foster dam; (3) kept with a non-lactating foster dam with protruded nipple;(4) kept with lactating foster dam with protruded nipple and (5) isolated for 90 minutes. All other procedures for Fos counting were as described above.
Isolation from the nest with thermal support (Figure 2D–G):
When ten-day-old, neonates were separated from the biological dam, and thermal support was provided using two different conditions. In the first condition, neonates were placed in a humidity and temperature-controlled climate chamber (70–80% of humidity, 35°Celsius, Sables Systems). In the second condition, a thermal support device set at 35° Celsius was placed underneath the chamber in which the neonates were separated. We confirmed appropriated thermal conditions by monitoring the temperature throughout testing using calibrated thermometers. Neonates were divided into four groups: (1) kept with the biological dam and littermates (nest); (2) isolated for 90 minutes at thermoneutrality; (3) isolated for 90 minutes in the thermal support device; and (4) isolated for 90 minutes without thermal support (room temperature). All other procedures for Fos counting were as described above.
Isolation from the nest in pups raised at thermoneutrality (Figure 2H–J):
The lactating dam was placed in a humidity and temperature-controlled climate chamber (70–80% of humidity, 35° Celsius, Sables Systems) two weeks before delivery to acclimate to the new environment. Temperature and humidity in the climate chamber were monitored twice a day until testing. At postnatal day 10, neonates were divided into three groups: (1) kept with the biological dam and littermates (nest); (2) isolated for 90 minutes at room temperature; and (3) isolated for 90 minutes at thermoneutrality. All other procedures for Fos counting were as described above.
Fiber photometry (Figure 3):
AgrpCre/Cre mouse neonates (P0-P1) were cryo-anesthetized. Neonates were placed on ice, using aluminum foil as a barrier to prevent direct contact with the ice. After 8 minutes, neonates were removed from the ice and placed onto a chilled rat/mouse neonatal frame (Stoelting Co., Wood Dale, IL). A Cre-dependent adeno-associated virus (AAV) encoding the calcium sensor jGCaMP7s (AAV8-CAG-Flex-jGCaMP7s-SV40, Penn Vector Core) was injected unilaterally at a volume of 300 nl using following coordinates from lambda: AP = +.98 ML, lateral = −0.3mm, DV = −4.1mm. On postnatal day 12, a fiber optic cannula (NA = 0.48, core diameter = 400 μm from Doric Lenses) was placed over the arcuate nucleus using following coordinates from bregma: AP = −1.38 mm, lateral = −0.3mm, DV= −5.8 mm. One to two days after placing the fiber optic cannula, experimental mice were placed in a Plexiglas cage (10 cm × 8 cm × 6 cm) with 4 siblings and bedding from home cage. After 5 minutes of baseline fiber photometry recordings (see below), mice connected to the fiber photometry system were moved to an identical adjacent Plexiglas cage for a period of ten minutes of isolation. Subsequently, experimental mice were return to the cage with the siblings for 5 minutes. The fiber photometry system consisted of two different sets of LEDs: 405 nm LED sinusoidally modulated at 211 Hz and a 460 nm LED sinusoidally modulated at 333 Hz. Both light streams were merged into an optical fiber patch using a minicube (Doric Systems). The fiber optic patch was connected to the cannula on the mouse pup. Fluorescence emitted by jGCaMP7s in response to light excitation was collected with same fiber patch cord and focused into a photodetector (Newport). The signal collected at the photodetector was collected in a digital fiber photometry processor (RZ5P, Tucker-Davis Technologies). Signal was processed and pre-analyzed using the Synapse Software Suite (Tucker-Davis Technologies). The data was exported to MATLAB for post-processing. First, the isosbestic channel (405 nm excitation) was fitted to the calcium-dependent channel (460 nm excitation, denoted as F) using first order polynomial fitting (F0 denotes the fitted isosbestic). The calcium fluorescence activity was calculated as: (F − F0)/F0. The high frequency components of the fluorescence activity were then filtered out by a low pass filter at 0.5 Hz. We then down sampled the signal by averaging it in non-overlapping windows of 0.1s. The Z-score was calculated considering the minute before isolation as the baseline.
Recording of ultrasonic vocalizations (Figures 4–5):
P10 mice were separated from the dam and placed in a soundproof chamber. In the thermoneutrality experiment, pups were divided into two groups: (1) isolated for 90 minutes at room temperature or (2) isolated for 90 minutes at thermoneutrality (70–80% of humidity, 35° Celsius, Sables Systems). USVs were recorded for 90 minutes. In experiments with the NpyKO and AgrpVgat-KO mice, USVs were recorded for ten minutes immediately following separation from the dam in the soundproof chamber. In the experiment with AgrpTrpv1 mice, this initial ten minutes was considered as a baseline before activation of Agrp neurons. Then, P10 mice were injected with capsaicin (10 mg/kg, s.c) and USVs were recorded for an additional twenty minutes. USVs were recorded using an UltraSoundGate Condenser Microphone CM 16 (Avisoft Bioacoustics, Berlin, Germany) placed 10 cm above the animals. The microphone was connected via an UltraSoundGate 416 USGH audio device and recorded with a sampling rate of 250,000 Hz by the software Avisoft RECORDER (version 4.2.16; Avisoft Bioacoustics).
Maternal preference test (Figure 5N–P):
The maternal preference test was performed in a three-chamber apparatus (65 × 42 × 23 cm) and comprised of three stages: Stage 1 – acclimation: the dam was allowed to explore the apparatus without the presence of pups for ten minutes. Stage 2 – exploration: two P10 mice (control and AgrpTrpv1, n = 25 pairs) were placed on each side of the apparatus inside of an inverted metal wire cup and the dam was allowed to explore the pups for ten minutes. Stage 3 - preference: dam was restricted to the center compartment, pups were injected with capsaicin (10 mg.kg, s.c) and placed in the cups and then the dam was allowed to explore all compartments for 20 minutes. Groups were randomly alternated between both sides to avoid preference for one side of the chamber. Time spent interacting with the pups was measured using Any-maze (Stoelting Co., Wood Dale, IL).
Analysis of ultrasonic vocalizations:
Ultrasonic vocalizations (USV) were automatically extracted from the audio recordings by using spectral analysis through image processing. Each audio file was analyzed in segments of 1 minute long and then Short Fourier transformed with a Hamming windowing function (window = 256), NFFT = 1024 sampling points and an overlap between successive windows equal to half of the window size. These parameters generate a spectrogram with resolution of 0.5 ms and 244 Hz. The spectrograms were converted to grayscale images and the USVs were segmented on the spectrogram through a sequence of image processing techniques, which included the contrast enhancement of the image (γ=1), the application of an adaptive threshold (sensitivity of 20%) followed by a series of morphological operations and identification of connected components. The segmented USV candidates were then analyzed by a local median filtering (LMF) to eliminate segmentation noise based on the contrast between an USV candidate and its background. The minimum contrast acceptable between an USV candidate and its background was automatically estimated based on a differential geometry analysis of the contrast of all the USV candidates detected in an audio recording. USVs less than 10 ms apart were considered as part of the same syllable. Next, all the USVs were classified in 11 distinct call types (Grimsley et al., 2011) by a Convolutional Neural Network, which had the AlexNet architecture as starting point. The network was trained for USV classification with over 14,000 samples of real USVs, which were then augmented in order to increase the variability of the samples, resulting in >57,000 samples. The output consisted of a table summarizing the main features of the USVs detected. This table contains the start and end time of the USVs, as well as its mean, maximum and minimum frequency, mean intensity and other relevant spectral features such as the existence of harmonic components. Each vocalization received a label based on the most likely call type label attributed by the Convolutional Neural Network. The label of each USV is also available as a probability distribution function over all the call types. The software was custom developed in our laboratory and is available upon request. The details of the software will be published elsewhere.
Mouse pup behavior towards the anesthetized dam (Figure 6A–G, 7A–J):
Animals were recorded under infrared illumination and assessed for 20 minutes at postnatal day 10 (P10) and postnatal day 15 (P15). Each animal was tested at one age only. Before the experiment, the dam was anesthetized (100 mg/kg Ketamine + 10 mg/kg Xylazine). The maximum number of pups tested per dam was eight. Animals received an injection of capsaicin (10 mg/kg, s.c.) before the experiment. We used a custom built-chamber (20 × 15 cm built in opaque Plexiglas). The dam was placed at an angle of 45° on her back along the edge. Pups were placed on the other edge of the chamber, ~20 cm away from their dam. Parameters such as latency to attach to the dam’s nipple, distance traveled, and the number of nipple attachments were assessed using Any-Maze (Stoelting Co., Wood Dale, IL). Experiments were performed blinded for the genotype.
Mouse pup behavior towards the anesthetized dams injected with oxytocin (Figure 6P–V, 7K–P):
In anesthetized dams, milk ejection is largely decreased, and dams are considered non-lactating (Lincoln et al., 1973). To circumvent this issue, we performed a similar experiment as described above but injected the anesthetized dams with oxytocin (5 mg/kg, i.p) immediately before each test. Nipples were manually expressed to confirm that there was milk ejection before the experiment. P10 mice received an injection of capsaicin (10 mg/kg, s.c) and were subsequently assessed for 20 minutes. In this assay, a second injection of oxytocin (5 mg/kg, i.p) was given at 10 minutes of test. P15 mice received an injection of capsaicin (10 mg/kg, s.c) and were subsequently assessed for 10 minutes. The duration of the testing period was shorter because P15 mice quickly attached to the dam’s nipple in preliminary experiments. Parameters such as latency to attach to the dam’s nipple, distance traveled, and the number of nipple attachments were analyzed using Any-Maze (Stoelting Co., Wood Dale, IL). Body weight was measured prior and after testing. Experiments were performed blinded for the genotype.
Independent feeding (Figure 7Q–R):
Mice were tested at postnatal days P15, P18, and P21. Naive animals were used for each postnatal age and mice were acclimated to the behavior room for one-hour before the experiment. Food was left inside the cage to prevent a state of deprivation. Animals were tested in a mouse cage filled with home bedding and two petri dishes placed in opposite corners. After the acclimation period (1 hour), the experiment was performed. Animals were removed from the cage, received an injection of capsaicin (10 mg/kg, i.p.) and were returned to the cage. One Petri dish was empty; the other had a pellet of chow diet. Body weight and food intake were evaluated after 30 minutes. Experiments were performed blinded for the genotype.
Quantification and statistical analysis
MATLAB (2016a or above) and Prism 8.0 were used to analyze data and plot figures. All figures were edited in Adobe Illustrator CS6/CC. Illustrations were designed by Mind the Graph (MindtheGraph.com). Data were first subjected to a normality test using the D’Agostino & Pearson normality test or the Shapiro-Wilk normality test. When homogeneity was assumed, a parametric analysis of variance test was used. The student’s t test was used to compare two groups. Welch’s correction was used when standard deviations were unequal between groups. ANOVA was used to compare multiple groups. Tukey-Kramer’s multiple comparisons test was used to find post-hoc differences among groups and calculate 95% confidence intervals to report effect size. When 95% confidence intervals were not calculated, then the Holm-Sidak’s multiple comparisons test was used. When homogeneity was not assumed, the Kruskal-Wallis nonparametric ANOVA was selected for multiple statistical comparisons. The Mann-Whitney U test was used to determine significance between groups. Two sample Kolmogorov–Smirnov test was used to calculate the statistical differences between features of ultrasonic vocalizations. Chi-squared test was used to find differences in the number of pups that attached to nipples in the behavior tests performed in neonates. One- or two-tail tests were used based on prior experimental hypothesis. Statistical data are provided in text and in the figures. In the text, values are provided as mean ± SEM. P < 0.05 was considered statistically significant.
Supplementary Material
Supplementary Figure 1: PCR-based analysis for genotyping AgrpTrpv1 and control mice, Related to Figure 5.(A) Illustrative diagram of the tissue collection and PCR-based analysis genotyping.
(B) Genomic DNA samples from P15 mice were extracted and amplified using primers for Trpv1 knockout allele. The lower band is the knockout allele for the Trpv1 gene.
(C) Genomic DNA samples from P15 mice were extracted and amplified using primers for ectopic Trpv1 allele. The upper band (667 bp) shows that the excised allele for Trpv1 is specifically expressed in the arcuate nucleus of the hypothalamus.
Highlights.
Isolation from the nest activates Agrp neurons in neonatal mice.
Care and warmth, but not by milk, blunts activation of Agrp neurons.
Neonatal Agrp neurons modulate isolation-induced ultrasonic vocalizations.
Agrp neurons increase milk ingestion in fifteen-, but not in ten-day-old mice.
Acknowledgements
We thank Jeremy Bober for technical support. We thank lab members as well as Dr. Ruslan Medzhitov, Dr. Ivan de Araujo, and Dr. Esther Florsheim for critical insights in the manuscript. M.O.D. was supported by a NARSAD Young Investigator Grant ID 22709 from the Brain & Behavior Research Foundation, by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health (R01DK107916), by a pilot grant from the Yale Diabetes Research Center (P30 DK045735), by the Yale Center for Clinical Investigation Scholar Award, by the Whitehall Foundation, by the Charles H. Hood Foundation, Inc. (Boston, MA) and by a pilot grant from the Modern Diet and Physiology Research Center (The John B. Pierce Laboratory). M.O.D. also received support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. M.R.Z., A.F., and R.D.P. were partially supported by scholarships from CNPq and CAPES. We thank Life Science Editors for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information
Supplemental information includes one figure and can be found with this article online.
Declaration of interests
The authors declare no conflict of interest.
References
- Alberts JR (2007). Huddling by rat pups: ontogeny of individual and group behavior. Developmental psychobiology 49, 22–32. [DOI] [PubMed] [Google Scholar]
- Alberts JR, and May B (1984). Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental psychobiology 17, 161–181. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Klein ME, Davison IG, Katz LC, and Ehlers MD (2008). Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nature methods 5, 299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, and Jarvis ED (2013). Mouse vocal communication system: are ultrasounds learned or innate? Brain and language 124, 96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, Zhou EP, and Jarvis ED (2012). Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PloS one 7, e46610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros VN, Mundim M, Galindo LT, Bittencourt S, Porcionatto M, and Mello LE (2015). The pattern of c-Fos expression and its refractory period in the brain of rats and monkeys. Front Cell Neurosci 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, and Sternson SM (2015). Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger-Bertolus J, Rincon-Cortes M, Sullivan RM, and Mouly AM (2017). Understanding pup affective state through ethologically significant ultrasonic vocalization frequency. Scientific reports 7, 13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer CP, Blass EM, and Hall WG (1980). The ontogeny of nipple-shifting behavior in albino rats: mechanisms of control and possible significance. Developmental psychobiology 13, 165–180. [DOI] [PubMed] [Google Scholar]
- Cross BA, and Harris GW (1952). The role of the neurohypophysis in the milk-ejection reflex. J Endocrinol 8, 148–161. [DOI] [PubMed] [Google Scholar]
- Curry T, Egeto P, Wang H, Podnos A, Wasserman D, and Yeomans J (2013). Dopamine receptor D2 deficiency reduces mouse pup ultrasonic vocalizations and maternal responsiveness. Genes, brain, and behavior 12, 397–404. [DOI] [PubMed] [Google Scholar]
- Dana H, Sun Y, Mohar B, Hulse B, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, Macklin JJ, et al. (2018). High-performance GFP-based calcium indicators for imaging activity in neuronal populations and microcompartments. bioRxiv. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, et al. (2012). AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nature neuroscience 15, 1108–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, and Horvath TL (2015). Hypothalamic Agrp Neurons Drive Stereotypic Behaviors beyond Feeding. Cell 160, 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Axt K, and Epstein AN (1984). The arousal of ingestive behaviors by chemical injection into the brain of the suckling rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 4, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin J, Anderson B, and Bunger D (1975). Nursing behavior of infant pigtail monkeys (Macaca nemestrina): preferences for nipples. Percept Mot Skills 40, 592–594. [DOI] [PubMed] [Google Scholar]
- Girish V, and Vijayalakshmi A (2004). Affordable image analysis using NIH Image/ImageJ. Indian J Cancer 41, 47. [PubMed] [Google Scholar]
- Gors S, Kucia M, Langhammer M, Junghans P, and Metges CC (2009). Technical note: Milk composition in mice--methodological aspects and effects of mouse strain and lactation day. J Dairy Sci 92, 632–637. [DOI] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, and Wenstrup JJ (2011). Development of social vocalizations in mice. PloS one 6, e17460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. (2005). Agouti-related peptide-expressing neurons are mandatory for feeding. Nature neuroscience 8, 1289–1291. [DOI] [PubMed] [Google Scholar]
- Grove KL, and Smith MS (2003). Ontogeny of the hypothalamic neuropeptide Y system. Physiology & behavior 79, 47–63. [DOI] [PubMed] [Google Scholar]
- Guler AD, Rainwater A, Parker JG, Jones GL, Argilli E, Arenkiel BR, Ehlers MD, Bonci A, Zweifel LS, and Palmiter RD (2012). Transient activation of specific neurons in mice by selective expression of the capsaicin receptor. Nature communications 3, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, and Schwartz MW (1998). Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature neuroscience 1, 271–272. [DOI] [PubMed] [Google Scholar]
- Hall WG, and Rosenblatt JS (1978). Development of nutritional control of food intake in suckling rat pups. Behav Biol 24, 413–427. [DOI] [PubMed] [Google Scholar]
- Hammond KA, Lloyd KC, and Diamond J (1996). Is mammary output capacity limiting to lactational performance in mice? The Journal of experimental biology 199, 337–349. [DOI] [PubMed] [Google Scholar]
- Harlow HF (1958). The nature of love. American Psychologist 13, 673–685. [DOI] [PubMed] [Google Scholar]
- Hofer MA (1994). Hidden regulators in attachment, separation, and loss. Monographs of the Society for Research in Child Development 59, 192–207. [PubMed] [Google Scholar]
- Hofer MA (1996). Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology 21, 203–217. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, and Leranth C (1997). Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain research 756, 283–286. [DOI] [PubMed] [Google Scholar]
- Hudson R, Raihani G, Gonzalez D, Bautista A, and Distel H (2009). Nipple preference and contests in suckling kittens of the domestic cat are unrelated to presumed nipple quality. Dev Psychobiol 51, 322–332. [DOI] [PubMed] [Google Scholar]
- Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, et al. (2012). Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. The EMBO journal 31, 4276–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny JT, Stoloff ML, Bruno JP, and Blass EM (1979). Ontogeny of preference for nutritive over nonnutritive suckling in albino rats. Journal of comparative and physiological psychology 93, 752–759. [Google Scholar]
- Lewis T, Amini F, and Lannon R (2007). A General Theory of Love (Knopf Doubleday Publishing Group; ). [Google Scholar]
- Lincoln DW, Hill A, and Wakerley JB (1973). The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol 57, 459–476. [DOI] [PubMed] [Google Scholar]
- Lincoln DW, and Paisley AC (1982). Neuroendocrine control of milk ejection. J Reprod Fertil 65, 571–586. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez F, Hnasko T, and Palmiter R (2005). NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Beis D, Alenina N, and Wohr M (2015). Reduced isolation-induced pup ultrasonic communication in mouse pups lacking brain serotonin. Mol Autism 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I, Johansen JE, Schalling M, Hokfelt T, and Fetissov SO (2005). Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Brain Res Dev Brain Res 155, 147–154. [DOI] [PubMed] [Google Scholar]
- Noirot E (1966). Ultra-sounds in young rodents. I. Changes with age in albino mice. Animal behaviour 14, 459–462. [DOI] [PubMed] [Google Scholar]
- Noirot E (1968). Ultrasounds in young rodents. II. Changes with age in albino rats. Animal behaviour 16, 129–134. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, and Zeltser LM (2010). Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature medicine 16, 403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, et al. (2014). O-GlcNAc Transferase Enables AgRP Neurons to Suppress Browning of White Fat. Cell 159, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PJ, and Hofer MA (1978). Oxytocin reinstates maternal olfactory cues for nipple orientation and attachment in rat pups. Physiology & behavior 20, 385–389. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, and Cone RD (2005). Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146, 1043–1047. [DOI] [PubMed] [Google Scholar]
- Tinbergen N (1963). On aims and methods of Ethology. Zeitschrift für Tierpsychologie 20, 410–433. [Google Scholar]
- Tomaszycki M, Cline C, Griffin B, Maestripieri D, and Hopkins WD (1998). Maternal cradling and infant nipple preferences in rhesus monkeys (Macaca mulatta). Dev Psychobiol 32, 305–312. [DOI] [PubMed] [Google Scholar]
- Van Segbroeck M, Knoll AT, Levitt P, and Narayanan S (2017). MUPET-Mouse Ultrasonic Profile ExTraction: A Signal Processing Tool for Rapid and Unsupervised Analysis of Ultrasonic Vocalizations. Neuron 94, 465–485 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorherr H, Kleeman CR, and Lehman E (1967). Oxytocin-induced stretch reaction in suckling mice and rats: a semiquantitative bio-assay for oxytocin. Endocrinology 81, 711–715. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, and Insel TR (2000). Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Hormones and behavior 37, 145–155. [DOI] [PubMed] [Google Scholar]
- Zippelius H-M, and Schleidt WM (1956). Ultraschall-Laute bei jungen Mäusen. Die Naturwissenschaften 43, 502–502. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: PCR-based analysis for genotyping AgrpTrpv1 and control mice, Related to Figure 5.(A) Illustrative diagram of the tissue collection and PCR-based analysis genotyping.
(B) Genomic DNA samples from P15 mice were extracted and amplified using primers for Trpv1 knockout allele. The lower band is the knockout allele for the Trpv1 gene.
(C) Genomic DNA samples from P15 mice were extracted and amplified using primers for ectopic Trpv1 allele. The upper band (667 bp) shows that the excised allele for Trpv1 is specifically expressed in the arcuate nucleus of the hypothalamus.









