Abstract
Addictive drugs affect sleep both in individuals currently using drugs and in individuals who have withdrawn from drugs. In fact, sleep disturbances are reported by individuals for some drugs long after they have quit taking them and after other withdrawal symptoms have subsided. This suggests that addictive drugs and sleep share some of the same neurobiological mechanisms. Sleep researchers may be studying the neurobiology of addictive drugs without knowing it. The purpose of this survey is to summarize the effects that addictive drugs have on sleep and stages of sleep. We demonstrate that different addictive drugs have differential effects on disturbance of sleep, in general, and on specific stages of sleep either while the drug is on board or after withdrawal. Accordingly, these results are intended to encourage sleep researchers to use their knowledge of sleep mechanisms to offer researchers of addictive drugs new insights of how addictive drugs might affect brain mechanisms. Also, these results should alert researchers of addiction that treatment for drug effects needs to consider treatment for sleep disturbances as well. Treatment for addiction is rarely accompanied by treatment for sleep disturbances even though this survey demonstrates they are clearly related.
Keywords: addictive drugs, sleep disturbances, circadian rhythm, cocaine, nicotine, cannabis, opioids, alcohol
Introduction
Sleep is a physiological necessity. Considerable research is continuing to uncover the complicated neurobiological interactions that govern the need for sleep, stages of sleep, causes and consequences of disturbed sleep and the interaction of disturbed sleep with many mental and physical diseases. While research on specific diseases is mostly funded by relevant institutes in the National Institutes of Health, research on disorders of sleep is not funded by one institute but rather by the institute whose disease focus is affected by sleep disturbance. Sleep disturbance associated with depression would be studied by the National Institute of Mental Health; sleep disturbances over the life span and particularly among the aging would be studied by the National Institute on Aging; sleep disturbance associated with breathing (e.g., apnea) would be studied by the National Heart, Lung, and Blood Institute, and so on.
Addictive drugs not only have effects on sleep patterns and circadian rhythm, but sleep disturbances act to exacerbate the adverse effects of psychoactive drugs. In fact, sleep disturbances are often the reason for taking drugs in the first place, staying on drugs, and relapsing by those wishing to quit. Studies of these interactions are part of the mandate of the National Institute on Drug Abuse. The neurobiological mechanisms underlying circadian rhythm are most commonly studied in the animal model, mostly rodents, where addictive drugs can be introduced to determine their effect on neural circuitry. These studies are designed to understand how drugs affect the brain with the goal of developing an appropriate intervention. Studies at the neurobiological level are more difficult in humans because of ethical concerns, on the one hand, and the limitation on invasive procedures. Neuroimaging is one method to study activation of brain function and integrity of brain structures in those who take drugs. Such studies are reviewed elsewhere [1,2].
Neuroimaging is technically difficult for studying the effects of drugs during sleep. Instead, research resorts to objective measures from polysomnography or devices that measure an individual’s activation throughout the day and night. Subjective measures are also utilized by means of self-report on validated questionnaires. While these various assessments cannot inform the neurobiology underlying sleep mechanisms, they can assess the architecture of the stages of sleep. Accordingly, they can be used to compare individuals with sleep disturbances to a comparison group, or they can determine changes in a sleep-disturbed group over time following disease progression or, contrastingly, during treatment. The purpose of this review is to chronicle the effect on sleep quality and efficiency, in general, and on stages of sleep, in particular, by each of the common addictive drugs. The neurochemistry of each drug is different, and the question is whether each would have a differential effect on sleep. Such differences may provide clues for prevention and treatment of both sleep disorders and drug addiction.
Method
Research articles addressing drug effects were obtained in two ways. First, several PubMed searches were made using key word combinations. Second, appropriate references were gleaned from the bibliographies in the PubMed list of articles. One series of PubMed searches paired each of the addictive drugs with “sleep quality” and with “sleep disturbance.” Another series paired each of the addictive drugs with “sleep” together with “withdrawal” or “abstinence.” The drugs were cocaine, nicotine, cannabis, opioids, and alcohol.
Articles of interest included those with objective measures of sleep stages affected by a specific drug. The most comprehensive objective measure is polysomnography where each of the Non-Rapid Eye Movement (Non-REM) Stages (1—4) of sleep plus REM sleep, as well as sleep onset and total sleep time, can be distinguished electrophysiologically. A second objective measure is actigraphy where the participant wears a device that can determine by body activity some of the stages of sleep and wakefulness. Also of interest are articles where subjective measures by the participants were determined by validated questionnaires. Summary of these articles is presented in Table 1. Statistical analyses of the data from these the studies are not possible due to small numbers of studies and the variety of research goals and methodologies.
Table 1.
Studies included in the Results
| Author(s), Year, Ref No. |
Number of participants |
Assessment measure |
Addictive drugs considered |
Sleep target |
|---|---|---|---|---|
| Chronotype | ||||
| Adan A, 1994 [3] | 537 healthy undergraduate students, or workers | questionnaires: circadian typology, alcohol & tobacco use | alcohol, tobacco, (caffeine) | chronotype |
| Prat G, Adan A, 2011 [4] | 517 healthy undergraduate students | questionnaires: circadian typology, alcohol & drug use | nicotine, cannabis, cocaine, heroin, others | chronotype |
| Tavernier R et al, 2015 [5] | 942 healthy undergraduate students | questionnaires: circadian typology, social jetlag, academic achievement; Insomnia Severity Index | alcohol, marijuana | “sleep problems” |
| Subjectivity | ||||
| Babson KA, 2013 [11] | 115 cannabis dependent | clinical interview for substance use; Pittsburgh Sleep Quality Index | cannabis | sleep quality, sleep disturbances |
| Brantstetter SA et al, 2016 [6] | 10,508 (using national surveys) | questionnaires: alcohol & cigarette use, sleep disturbances | nicotine, alcohol | sleep efficiency |
| Hartwell EE et al, 2014 [8] | 68: 33 non-treatment-seeking, opioid dependents; 35 healthy, non-users | Clinical interview for substance use; Pittsburgh Sleep Quality Index, Insomnia Severity Index | opioids, other | sleep efficiency |
| Morasco BJ et al, 2014 [7] | 284: 72 chronic pain + opioid prescription; 104 pain, no prescription; 91 no pain, no prescription | Clinical interview for substance use; Pittsburgh Sleep Quality Index, Multidimensional Pain Inventory | opioids | sleep quality, sleep efficiency |
| Thomas MB et al, 2016 [9] | 33 healthy children8 | Childhood Sleep Questionnaire, Lifetime Drug & Alcohol Use History | alcohol, cannabis | sleep duration, sleep quality |
| Wong MM et al, 2010 [10] | 386 healthy children | questionnaires: drinking & drug use | alcohol | sleep disturbances |
| Objective drug/sleep interaction | ||||
| Angarita et al, 2014 [28] | 20 cocaine dependent | polysomnogram | cocaine | sleep stages |
| Angarita et al, 2016 [23] | not available (review) | “objective” (polysomnogram?) | alcohol, cannabis, cocaine, opioids | sleep stages, sleep efficiency |
| Asaad TA et al, 2011 [45] | 33 opioid dependent | polysomnogram | opioids | sleep architecture |
| Bolla KI et al, 2008 [36] | 17 cannabis users | polysomnogram | cannabis | sleep architecture |
| Bolla KI et al, 2010 [38] | 18 cannabis users | polysomnogram | cannabis | sleep architecture |
| Conroy DA, Arnedt JT, 2014 [25] | not available (review) | “objective” (polysomnogram?) | alcohol, nicotine, cannabis, cocaine, heroin, others | sleep architecture |
| Drummond SPA et al, 1998 [48] | 29 alcohol dependent | polysomnogram | alcohol | sleep architecture |
| Feinberg I et al, 1975 [35] | 7 cannabis users | electro-encephalogram (2-channel) | cannabis | sleep architecture |
| Feinberg I et al, 1976 [34] | 4 cannabis users | electro-encephalogram (2-channel) | cannabis | sleep architecture |
| Gann H et al, 2004 [49] | 40 alcohol dependent | polysomnogram | alcohol | sleep architecture |
| Garcia AN, Salloum IH 1986 [21] | not available (review) | polysomnogram | nicotine, opioids, cocaine, cannabis | sleep architecture |
| Howe RC et al, 1980 [44] | 20 heroin dependent | electro-encephalogram (2-channel) | opioids | REM |
| Howe RC et al, 1980 [46] | 20 heroin dependent | electro-encephalogram (2-channel) | opioids | sleep architecture |
| Imatoh N et al, 1986 [47] | 6 alcohol dependent | polysomnogram | alcohol | non-REM, REM |
| Irwin MR et al, 2016 [29] | 213: 32 abstinent cocaine; 73 abstinent alcohol; 108 non-users | polysomnogram | alcohol, cocaine | sleep architecture |
| Jaehne A et al, 2015 [32] | 33 smokers | polysomnogram | nicotine | sleep architecture |
| Matuskey D et al, 2011 [27] | 28 cocaine dependent | polysomnogram, sleep quality questionnaire | sleep architecture | |
| Mehtry V et al, 2014 [43] | 15 opioid dependent | polysomnogram | opioids | sleep architecture |
| Moreno-Coutiño A et al, 2006 [33] | 15 chronic smokers | polysomnogram | nicotine | sleep architecture |
| Morgan PT et al, 2006 [24] | 12 chronic cocaine users | polysomnogram, sleep quality questionnaire | cocaine | sleep architecture |
| Schierenback T et al, 2008 [22] | review: 8 studies; 59 subjects | polysomnogram | cocaine, stimulants | sleep architecture |
| Snyder S, Karacan I, 1985 [50] | 52: 26 abstinent alcohol dependent; 26 non-users | polysomnogram | alcohol | sleep architecture |
| Soldatos CR et al, 1980 [30] | 100: 50 chronic smokers; 50 non-smokers | electro-encephalogram | nicotine | sleep architecture |
| Thompson PM et al, 1995 [26] | 50: 16 alcohol dependent; 14 stimulant dependent; 20 non-users | electro-encephalogram | alcohol, stimulants | sleep architecture |
| Vandrey R et al, 2011 [37] | 20 cannabis users | polysomnogram, Pittsburgh Sleep Quality Index | cannabis | sleep architecture |
| Zhang L et al, 2006 [31] | 6400 (from Sleep Heart Health Study) | polysomnogram | nicotine | sleep architecture |
NOTE: The make-up of “sleep architecture” varies among studies but always includes the non-REM and REM sleep stages, and variously includes sleep onset, efficiency, total sleep.
The goal in this survey is to organize the research findings to determine patterns of how sleep is affected in current users, compared to abstinent users, for each drug, and to determine if there are differential effects in cross-comparing different drugs. Ultimately, these patterns are intended to provide insight into the neurobiological effect of the drugs. Sleep researchers who know or who are studying the neurobiology underlying the stages of sleep can infer how the drugs may impart their effects. It is hoped this insight will point to research directions that will lead to prevention and treatment not only for drug use but also for the associated sleep disturbances. This is important because it is not clear whether diseases of addiction for a specific drug, or sleep disturbances, should be addressed separately or together.
Results
Chronotype
The tendency for individuals to go to bed at night and wake up in the morning at particular times is called chronotype. Those who have early bedtimes and who rise early are “morning” types (“larks”); those who stay up late and sleep late are “evening” types (“owls”). Determination of chronotype is usually established by self-report questionnaires. The proportions in each category depend on the population being assessed, and their ages. Adolescents and early adults tend to be evening types; older people tend to be morning types. Overall, about 40% would fall in the “neither” category; approximately equal numbers are in the evening and morning category. The question is whether a particular chronotype has advantages or adverse consequences related to health, performance, cognitive ability or personality factors. For the purposes of this review, the question is whether chronotype is related to drug use or diseases of addiction.
One the earliest studies of 537 students and workers reported significantly more alcohol and tobacco use for evening types [3]. Morning types used these substances the least; “neither” types were in between. While these two substances (plus caffeine drinks) were all that were assessed, a later study on a similar sample size produced the same results on nicotine plus a wide range of illegal drugs [4]. Significantly more (72%) evening type subjects used nicotine compared to “neither” types (46%) or morning types (37%). For cannabis, the proportions were similar: evening type, 67%, neither type, 41%, and morning type, 26%. For ecstasy, it was 17%, 6%, and 4% for evening, neither, morning types respectively. Similar proportions were seen for cocaine, heroin, amphetamines, and hallucinogens but the numbers were too small to reach significance.
A study using path analysis [5] showed chronotype predicted both substance use and academic adjustment—defined as self-perceived ability to manage academic assignments—and social jetlag—defined as the difference in bedtime for weekends compared to weekdays. The subjects were college students assessed over three years and analyzed as to whether self-perception of chronotype in the 1st year predicted substance use in the 2nd and 3rd years. Those who considered themselves to be evening persons tended to have greater substance use in year 2 continuing into year 3. These individuals also tended to have lower achievement, but they were not necessarily those who had social jetlag. By contrast, those who reported social jetlag in year 1 tended to have increased substance use in year 2.
Subjectivity
Studies that probe addicted or abstinent individuals’ impressions of their quality of sleep quality and whether they achieve a full night’s sleep can offer insights to etiologies, treatment and prevention strategies. Such impressions include soundness, continuity, or restfulness of sleep determined by self-assessments of whether or not they fall asleep readily, stay asleep all night, wake up often, or tend to wake early. For example, in a secondary analysis of a large dataset where smokers reported their sleep habits, it was found, using “time to first cigarette” as a measure, that smoking severity was related only to “waking too early” and not to time to fall asleep or waking too often [6]. The authors hypothesized that nicotine addiction adversely affected brain circuitry involved with arousal. Patients taking prescription opioids to manage pain reported more disturbed sleep and taking longer to fall asleep than people with pain who were not taking prescription opioids [7]. The same was true for those with prescription opioid dependence who not only took longer to fall asleep but had less sleep and less efficient sleep even after three days abstinence [8].
An interesting correlative observation was reported for subjective assessment of sleep difficulties in children followed by self-report of alcohol or cannabis use by these subjects when they became adolescents or young adults [9]. Mothers of 11-year-old boys in a low SES neighborhood estimated “sleep duration” and “sleep quality” of their sons. At age 20–22 these children were assessed for “first use,” “intoxication,” and “repeated use” of either alcohol or cannabis. Both, reports of reduced sleep duration and sleep quality in childhood, significantly predicted adverse outcomes of all three of these drug use variables with the only exception that sleep quality did not predict first use of cannabis. A more extensive study [10] with parental report of sleeping in even younger children (ages 3–5 in boys, 6–11 in girls) supported and extended these results. Specifically, “overtiredness” reported in these children by their mothers predicted binge drinking, experiencing blackouts, driving under the influence of alcohol as determined by self-report by these children when they reached late adolescence. For illicit drug use, sleep difficulties reported for the children had only an indirect influence. Sleep difficulties in childhood predicted sleep difficulties in adolescence which, in turn, predicted illicit drug use. Another indirect pathway provided a potential insight as to the neurobiological basis for this observation. Overtiredness in the children predicted poorer performance on a response inhibition (stop signal) task. Similar to adolescent sleep difficulties, poor performance on this task predicted the number of illicit drugs used by late adolescents. Poor performance on response inhibition—an executive function task—is well known to be related to increased risk for addictive behavior.
Perception of adverse sleep was also shown to affect cannabis use in individuals attempting to quit [11]. The participants were 102 cannabis dependent military veterans wishing to quit. They were assessed at baseline for sleep quality and sleep efficiency. Quality measurement is a subjective (questionnaire-generated) assessment of onset latency, nighttime disturbances and tiredness during the day. Efficiency refers to the amount of time asleep relative to the amount of time in bed and duration (total hours asleep). These assessments were then repeated several times during the months after the initial attempts to quit. It was found that sleep quality was not related to cannabis use at baseline but was significantly and negatively related to amount of cannabis use during the quit period. However, the results for sleep efficiency/duration were the opposite; efficiency and duration were related to use at baseline but not during the quit period.
Circadian rhythm
The mammal’s share of research on the effect of addictive drugs on the mechanics of circadian rhythm is, and has been, done in rodents. Circadian rhythms are driven primarily by light from the retina through a pathway to the suprachiasmatic nucleus (SCN) which, in turn, drives cyclic behavior of transcription factors and associated proteins throughout the body. Consideration of the drug effects on the circadian system in the animal model will not be considered here, though thoroughly reviewed elsewhere [12]. In the rodent model, it is possible to employ polymorphisms in critical circadian genes and observe their interaction with addictive drugs, especially cocaine and alcohol [13]. Most often targeted is the clock gene because of its regulation in the dopaminergic system [14,15]. Clock genes regulate the expression of period genes which also are affected by cocaine [16]. It is hoped that continued study at the molecular level will provide targets for pharmacological therapy in humans whose rhythms—sleep as detailed in this report—are affected by addictive drugs. To date, the largest body of research in humans is the effect of alcohol on circadian rhythm [17].
As indicated in the previous paragraph, circadian rhythms are driven by light. The major receptors in the retina are intrinsically photosensitive retinal ganglion cells (ipRGC’s) which are maximally sensitive to blue light. These cells express melanopsin and are ultimately responsible for stimulating the SCN [18]. Regarding blue light, it is interesting to relate an observation in abstinent cocaine patients conducted in years just prior to the discovery and description of this ipRGC—SCN pathway [19,20]. These patients were found to have significantly reduced electroretinogram responses to flashes to blue light. And furthermore, the reduction was correlated with increased craving. Explanations at the time involved possible reduced levels of dopamine in the retina; dopamine is inextricably related to cocaine addiction.
Objective drug/sleep interactions
To get an accurate assessment of what effect drug use or drug withdrawal has on sleep stages, polysomnography is the method to use. Electrodes are attached to specified places on the skull or, in some studies, a “nightcap” with attached electrodes is worn. Although definitions have varied slightly over the years, sleep is characterized as either Rapid Eye Movement (REM) or as Non-REM (Non-REM). Non-REM is often divided into four stages (1—4) determined by the slowness and amplitude of the EEG recording; Stages 3 and 4 are the slowest with largest amplitudes and are called Slow Wave Sleep (SWS). REM sleep is characterized by fast EEG activity and, characteristically, accompanied by eye movements. The key for providing insight to drug effects is that REM and Non-REM stages are driven by differences in neurobiological systems and thus could be differentially affected by the various addictive drugs. The following is a survey of those changes in sleep stages depicted for current users and users abstinent for 1 week or more in Figure 1. Effects for shorter term abstinence are not shown in the figure but are detailed in the following narrative.
Figure 1.
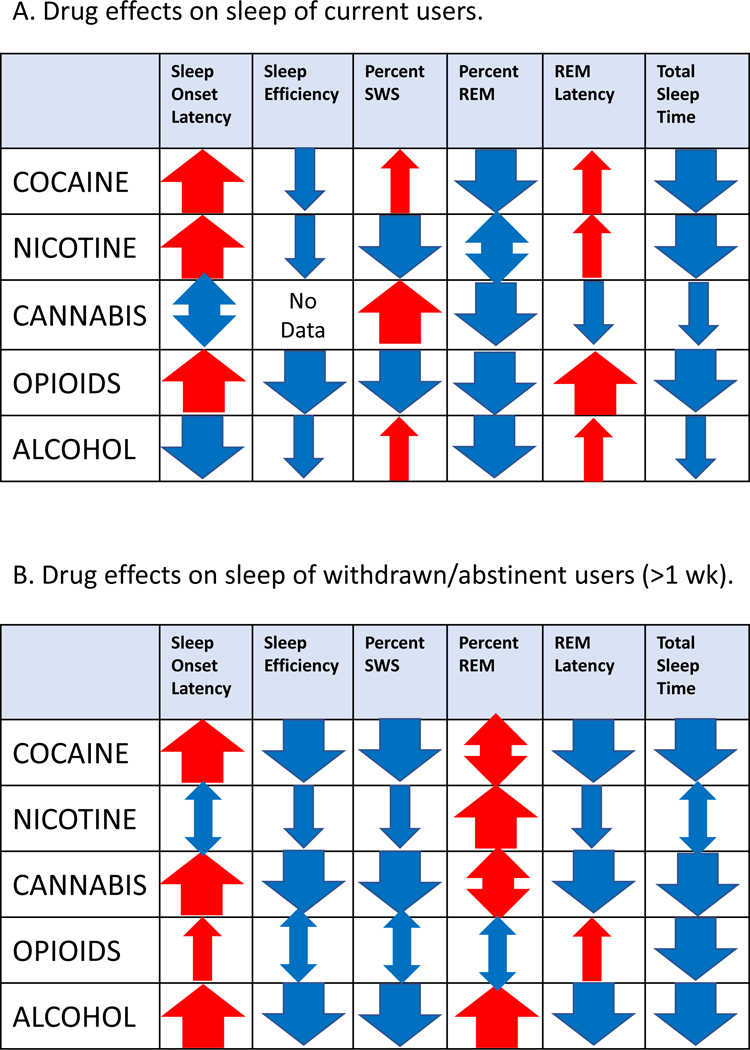
Graphical representation of the number of studies reporting objective effects of addictive drugs on aspects of sleep. A. Individuals currently using drugs. B. Individuals having withdrawn from drugs for a week or more. Red arrows pointing upward indicate increase; blue arrows pointing downward indicate decrease (e.g., increase/decrease in Sleep Onset Latency, etc.). Thick arrows indicate 3 or more studies report the same effect either unanimously or with one exception. Thinarrowsindicateonly1or 2 studies. Double-headed arrows indicate an inconsistent effect (some studies report anincrease; others a decrease) where color indicates predominance of an increase (red) or decrease (blue) of the effect. NOTE: These results are for long term withdrawal (> 1 wk.) but they are similar for withdrawal/abstinence for short term (less than a week) for almost all drugs and aspects of sleep. Exceptions are for cocaine where Sleep Onset Latency and Total Sleep Time are reportedly decreased in some studies. Total Sleep Time is increased in short term withdrawal from nicotine. Percent REM tends to be more often increased in short term withdrawal from cannabis.
Cocaine.
Intoxicated users have an increased onset latency [21–25]; reduced sleep efficiency [22,25], reduced total sleep time [21,24,25] and, in one study [24] increased wakings after sleep onset. Also, there is an increase in REM latency [21,25] with reduced percentage of REM sleep [21–23,25]. In patients during short term abstinence there are mixed reports on sleep onset latency where two studies say it is increased [26,22] while four say it is reduced [21,23–25]. However, by contrast for longer term abstinence, increased sleep onset latency is consistently reported [21–24,26,27]. Sleep efficiency is decreased in the short term [22,26] although one analysis reported an increase [25]. In the long term, efficiency is consistently reported to be decreased [21–23,26,27]. Slow wave sleep is reduced in the short term [23,26,28,29]) which remains reduced in the long term according to most studies [22,23,25,26,28], but increased for one other study [27]. REM latency is reduced [21,23,26,28] and REM percentage increased [21,22,26,28,29] in the short term; however, there are mixed reports for REM latency in the long term where four studies report continuing reduction [22,25,26,28] for REM latency but one study [27] reports increased latency. Reports are also mixed for percentage of REM in the long term where two studies report an increase (similar to the short term) [22,25] and several studies report a decrease [21,23,27,28]. These discrepancies may be attributed to assessments that are made at variable times after quitting. Total sleep time is reportedly reduced in the short term for some studies [23,24,28] but reportedly increased for other studies [21,25]. In the long term all studies report reduced total sleep time [21–24,26–28].
Nicotine.
Similar to cocaine intoxication, current smokers experience increased sleep onset latency [21,25,30,31], reduced sleep efficiency [25,31], and reduced total sleep time [21,25,31]. Also, similar to cocaine, there is an increase in REM latency [21,25] with a concomitant decrease in percent REM [21,25], although one study [31] reports an increase of REM as well as increases in sleep Stages 1 and 2 and a decrease in slow wave sleep. Other studies [21,25,30] also report reduction in slow wave sleep. Onset latency for short term withdrawal is generally reported to be reduced [21,30,32] with one exception [25]—a more consistent picture compared to cocaine short term abstinence. However, the long-term abstinence from smoking differed from cocaine in that sleep onset latency is generally reduced [25,30,32] whereas it is increased for cocaine. Similar to cocaine abstinence, slow wave sleep is decreased both in the short term [30] and in the long term [25,33] with one exception [32]. Also, similar to short term cocaine abstinence, REM latency is decreased [21,25] and REM percentage increased [21,25,30]. In the long term, REM latency is also decreased [25,32,33] and percent REM increased [30,33]. This differed from cocaine where reports of REM latency and REM percentage were mixed. Two studies [32,33] addressed Stage 1 or Stage 2 sleep. One of the studies [32] reports a decrease in Stage 2 in both the short and long term; the other [33] reports a decrease in Stage 1 and an increase in Stage 2. Total sleep time after short-term withdrawal is increased for two studies [21,25] but reduced in the long term [25,33]. This observation is similar to abstinence from cocaine with mixed results of total sleep time in the short term and more consistent reduction of total sleep time in the long term.
Cannabis.
A few studies have focused on the effects on sleep during marijuana use; more studies focused on sleep effects during withdrawal. In cannabis users, most studies report increased slow wave sleep and decreased REM sleep [21–23,25,34,35]. Two studies report longer sleep onset latencies [21,25]; one study reports a shorter latency [23]. During withdrawal or abstinence from cannabis, sleep onset is increased in both the short [22,23,25,34,36,37] and long-term abstinence [21,25]. By contrast, latency to REM is shortened [23,25,34–37]. Concomitant with the shortened REM latency, the percent of REM sleep is increased in the short term of less than a week [23,25,34,37]. There are mixed reports of percent REM for longer term abstinence where both increased [21,25] and decreased [23,38] REM are reported. Most studies report reduced slow wave sleep [22,23,25,35,36] with one exception reporting increased slow wave sleep[34]. Total sleep time is consistently reportedly reduced [23,25,34,36–38] owing, perhaps, to increased waking after sleep onset [21,23,34,38]. Accordingly, sleep efficiency is reduced [25,36–38].
Unique to many subjects withdrawing from cannabis, strange dreams or nightmares are reported. An early study [39] systematically assessed withdrawal symptoms in 18 adult (average age = 31) current marijuana users in a 31-day follow-up period of verified abstinence. Of all the symptoms, only reports of “strange dreams” persisted unabated for the entire follow-up period. Indeed, for those subjects who were followed for up to 45 days, strange dreams continued to be reported. Since these are averaged data, the number of subjects who report strange dreams is not mentioned. In contrast, while other sleep difficulties (i.e., less total sleep time, reduced sleep efficiency, more awakenings) increase in the immediate abstinent period, they subside over time. Of the other withdrawal symptoms, anger and aggression last almost three weeks; some somatic complaints (i.e., sweating, stomach pains, nervousness) last for about two weeks. These results are corroborated in a study [40] where nightmares or strange dreams were judged to be the most intense out of 26 symptoms reported during a two-week abstinence period. However, in a judgement of distress (to the subject), nightmares ranked only 10th whereas “trouble getting to sleep” ranked 1st. Other items rated in that study were behavioral (angry outbursts, uphill struggle), somatic (nauseous), or mood (depressed, nervous) symptoms. Finally, in a 30-day inpatient study [41] strange or vivid dreams increased over the abstinence period as did difficulty of getting off to sleep. However, sleep depth and frequency of waking improved.
A similar result of sleep difficulties is seen in a study of 71 adolescents (average age = 16) seeking treatment for cannabis use [42]. Data were collected via self-report of withdrawal symptoms from the most recent abstinence period (number of days is not reported). Forty-three percent of the participants reported sleep difficulties ranging from mild to severe; 26 percent reported strange dreams of which eight percent were reportedly severe. However, a greater number of participants reported mood and behavioral disorders than sleep and dreaming difficulties. But since these were one-time assessments with unreported days of abstinence, persistence in these symptoms is not known.
Opioids.
The effect on sleep of persons taking opioids is consistent. Sleep onset latency and REM latency are increased [23,25,43]. There are greater awakenings after sleep onset which negatively affect sleep efficiency. Total sleep time is also reduced. There is an increase in Stages 1 and 2 with a concomitant decrease in both slow wave sleep and REM sleep, all of which suggest a poorer quality of sleep [23]. Abstinence from opioids does not seem to improve the increased sleep onset latency [8,23] or REM onset latencies [23,44] except perhaps in the longer term for REM percentage [45]. In short term abstinence, reports are mixed as to the effect on sleep stages. While early reports indicate reduction in all phases of sleep [44,46] or just for sleep efficiency and total sleep time in patients withdrawing from non-prescription opioids [8], a later report indicates an increase in slow wave and REM sleep [25]. In long-term abstinence, one study reports the same effect on persons who are not abstinent—increased Stages 1 and 2, reduced slow wave and REM sleep [45]. This result is contrasted with a case control study [43] of opioid-dependent patients after one week of abstinence where Stage 1 is reduced, as is total sleep time and sleep efficiency while sleep onset latency and waking after sleep onset are increased.
Alcohol.
Alcohol use seems to reduce sleep onset latency [21,25] with concomitant increase in slow wave sleep. This effect on sleep is essentially the opposite from nicotine. However, similar to nicotine (and cocaine), REM latency is increased [21,25] with percent REM decreased [21,23,25]. Sleep onset latency is increased for both short [26] and long-term abstinence [23,25,26,48,50] which, again, is opposite to the case of withdrawal from nicotine. However, increased sleep onset latency for long term abstinence is similar to long term abstinence from cocaine. Slow wave sleep is reduced for both short [26,47] and long term abstinence [25,26,29,43,47–49] with one study [47] reporting a concomitant increase in Stages 1 and 2. In general, REM percentage is increased for both short and long-term abstinence [25,26,29,48]; REM latency is decreased, especially in the long term [25,26,48–50]. And, as might be expected, both sleep efficiency[25,26,48–50] and total sleep time[23,25,26,48–50] are reduced in long and short-term abstinence.
Discussion
Chronotype
Evening chronotype is consistently linked to increased drug and alcohol use, especially among adolescents. The question is “why?” A simple answer may be that adolescents in college stay up late and are socially more active which would include drinking and using drugs. However, examination of the data argues against this simple explanation. For one thing, several mental disorders especially depression and anxiety disorders are consistently found among evening types and these may be associated with underlying sleep-affected mechanisms such as altered reward processing, sensation-seeking and impulsivity [51]. Secondly, cognitive performance differs between morning and evening types suggesting a neurobiological link. For example, in a specific test of reward response, eveningness assessed at age 20 was significantly correlated with neural response in two brain reward areas—medial prefrontal cortex and ventral striatum—two years later [52]. In addition, eveningness in these subjects was also correlated with alcohol use and dependence as well as cannabis use two years later. Response inhibition—a test of impulsivity—is well-known to be associated with addictive behavior, especially in structures in the right hemisphere [1]. A study of morning types and evening types used a stop signal task—a common task for assessing brain activation during response inhibition—in a morning session and in an evening session [53]. Performance across subjects was better in the evening but there were no group differences nor interactions. However, there were significant interactions in activation of the usual brain areas performing the task where morning types had greater activation in the morning session which diminished in the evening session; the reverse was the case for the evening types. While the authors did not speculate as what this might mean in terms of drug use, the results nevertheless demonstrate neural response differences to cognitive performance depending on chronotype.
Subjective measures
The neural correlates of poor performance on response inhibition tests may also be explained by poor sleep quality observed during childhood. This was assessed in self-ratings of sleep quality by early adolescents (ages 14–16) that correlated with decreased activation of the right dorsolateral prefrontal cortex during a go/no-go test and increased insula activation in a monetary rewards task [54]. The likelihood that sleep disturbances observed in childhood follow a developmental trajectory that persists into adolescence may be one explanation of why childhood assessment of sleep difficulties predicts substance use. However, another explanation may be that the observation of childhood sleep disturbances is a precursor to ADHD which also is associated with sleep difficulties as well as drug use in adolescents [55].
It is worth pointing out that self-perception of sleep quality is not always reliable in the face of objective measures. During abstinence in a study with inpatient chronic cocaine patients, sleep quality was rated by the participants as improving while objective measures demonstrated sleep deterioration. This phenomenon was termed “occult insomnia” [24]. A follow-up study [56] showed that patients underestimated sleep onset latency, amount of time awake after sleep onset and overestimated total sleep time. Understanding of this phenomenon may provide insight to the underlying effects of cocaine (in this case) on sleep circuitry.
Objective measures
The take home lesson from the objective measures of sleep stage disturbances in persons taking or withdrawing from addictive drugs is that the effects of no two drugs are alike. That is, each drug has its unique effect on sleep stages and sleep quality. In addition, the drugs differ in their effect on sleep between current use and long-term abstinence. Also, in some instances, the drugs even differ in their effects between short-term and long-term abstinence. For example, current users of cocaine have delayed onset of sleep whereas alcohol users have reduced onset. However, both have increases in slow wave sleep and decreases in REM sleep. Withdrawal from either of these drugs seems to have similar effect on sleep stages. By contrast, smokers are similar to opioid users in their delayed onset of sleep, decreased slow wave sleep and decreased REM sleep. However, they appear to differ on the sleep stages during withdrawal. These observations should encourage sleep researchers who know the neural circuitry of these sleep stages, to characterize the effect of drugs on those circuitries. That is, leveraging the knowledge of the neurobiology underlying the different stage of sleep should provide insight as to how addictive drugs are affecting the brain. Further, since each drug affects sleep differently, unique effects can be deduced and studied. The two-fold goals would be to determine medical interventions that serve to reduce the adverse drug effects on sleep and on the addiction process itself.
Neurobiological aspects
Sleep is initiated by several initiating factors that build up during wakefulness (sleep pressure) eventually promoting sleep. One of these factors is adenosine on which the effect of drugs has been reviewed [57]. It was pointed out that 1) there is an interaction between opioid- and adenosine-mediated signaling pathways which are affected by opioid administration, 2) a close relationship between adenosine and dopamine signaling systems facilitates the modulation of cocaine-mediated actions by adenosine receptor ligands, 3) alcohol directly interacts with adenosine system by blocking a nucleoside transporter leading to elevated endogenous adenosine levels, and 4) that the cannabinoid (CB1) receptor is modulated by the adenosinergic system. These are several examples of how to focus knowledge from sleep research to understand the biological effect of addictive drugs.
Understanding the neurocircuitry affected by the circadian circuitry and accompanying genetic influences is no small matter. Several neurotransmitters are involved. In a thorough review of sleep cycles [58] it is noted that REM sleep is triggered by cholinergic “REM-on” cells of the medial pontine reticular formation. “REM-off” cells are aminergic—noradrenergic in locus coeruleus and serotonergic in the dorsal raphe. Activation of these cells (and mutual inhibition) is supported by the mechanisms of circadian rhythm—a molecular clock. The clock consists of various genes and proteins in a series of transcriptional and translation feedback loops in and out of the cell nucleus, present in all cell types of the body. There are considerable research activities at these molecular levels conducted in animal (usually rodent) models. Summary of these is beyond the scope of this human-focused survey. But it is clear these mechanisms are affected by addictive drugs [13]. So, again, the task for continuing research is to determine which aspects of this circadian circuitry are affected by which drugs with the aim of uniquely characterizing drug affects in the brain in the hope of developing treatment for addiction.
Conclusion
The most important message to be learned by the various studies reviewed here is that sleep and sleep stages are differentially affected in users according to the drug to which they are addicted. Furthermore, abstinent or withdrawn users are also differentially affected according to their drug of addiction and the effect also differs from the effect while addicted. For example, users of either cocaine or nicotine similarly have increased latency to sleep onset and to latency to REM sleep but differ in the percentage of slow wave sleep. By contrast, those who withdraw from these two substances differ from each other for latency to sleep onset, but are similar to each other for REM onset latency and for slow wave sleep. Another example is that sleep onset latency is different for opioid users versus alcoholics while REM onset latency is similar between the groups. By contrast, when withdrawn from drugs, these two groups are similar in sleep onset latency but differ in REM latency. It can be concluded from these differences that the different neurobiological effects of the drugs affect different aspects of the sleep/wake cycle on the one hand, as well as ultradian sleep stages themselves. In other words, these drugs differ in their effect on the neurosystems of the brain and, in turn, affect different parts of the sleep mechanism.
A second message is that those with “morning” or “evening” chronotypes are differentially at risk to develop drug addiction. Specifically, evening types are more likely to be using drugs than morning types. Importantly, this observation is not only due to the social habits of late-night individuals but also related to a neurobiological difference. Research is needed to determine what this difference might be. The relationship between sleep quality and risk for addiction was also demonstrated in longitudinal studies with children. Young children described by their mothers as having sleep difficulties were those more likely to engage in risky behavior and drug use when they became adolescents. The underling mechanism by which these observations are connected would provide insight to understanding the neurobiological risk factors for addiction. This understanding would go a long way to provide clues for prevention.
The most important conclusion derived from these studies for research on the one hand, and for prevention and treatment of addiction, on the other, is that there is an understudied neurobiological connection between sleep characteristics and the neurobiological effects of different drugs of abuse. Evaluation of clients seeking treatment for addiction should include an inventory of sleep habits and, perhaps, a laboratory investigation of disturbances of sleep stages as well. There is no protocol or agreement of when or how to treat sleep disturbances as part of the treatment for addiction. Should sleep disturbances and addiction be treated in parallel? Would prior treatment for sleep disturbances modify subsequent treatment for addiction? Since studies demonstrate residual sleep disturbance following drug withdrawal, it is imperative that treatment for sleep cannot be ignored. Indeed, relapse may well be due to untreated sleep disturbance following (presumably) successful treatment for addiction. The conclusions from these data should be red flags to include a focus on sleep as part of assessment and treatment of drug addiction.
Acknowledgements:
I thank Khushi Soni for help in compiling references.
Abbreviations:
- ADHD
Alzheimer’s disease hyperactive disorder
- EEG
electroencephalogram
- ipRGC
intrinsically photosensitive retinal ganglion cells
- REM
rapid eye movement
- SCN
suprachiasmatic nucleus
- SES
socioeconomic status
Footnotes
Disclaimer: The views and opinions expressed in this manuscript are those of the author only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Conflict of Interest: The author has no conflict of interest.
References
- [1].Gordon HW. Laterality of brain activation for risk factors of addiction. Curr Drug Abuse Rev 2016;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gordon HW. Hemispheric asymmetry of development due to drug exposure. J Sys Integrative Neurosci 2017;3(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction 1994;89:455–62. [DOI] [PubMed] [Google Scholar]
- [4].Prat G, Adan A. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int 2011;28(3):248–57. [DOI] [PubMed] [Google Scholar]
- [5].Tavernier R, Munroe M, Willoughby T. Perceived morningness-eveningness predicts academic adjustment and substance use across university, but social jetlag is not to blame. Chronobiol Int 2015;32(9):1233–45. [DOI] [PubMed] [Google Scholar]
- [6].Branstetter SA, Horton WJ, Mercincavage M, Buxton OM. Severity of nicotine addiction and disruptions in sleep mediated by early awakenings. Nicotine Tob Res 2016;18(12): 2252–8. [DOI] [PubMed] [Google Scholar]
- [7].Morasco BJ, O’Hearn D, Turk DC, Dobscha SK. Associations between prescription opioid use and sleep impairment among veterans with chronic pain. Pain Medicine 2014;15:1902–10. [DOI] [PubMed] [Google Scholar]
- [8].Hartwell EE, Pfeifer JG, McCauley JL, Moran-Santa Maria M, Back SE. Sleep disturbance and pain among individuals with prescription and opioid dependence. Addictive Behav 2014;39: 1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thomas MB, Shaw DS, Forbes EE, Sitnick SL, Hasler BP. The hazards of bad sleep—sleep duration and quality as predictors of adolescent alcohol and cannabis use. Drug Alcohol Depen 2016;168:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcoholism: Clin Exp Res 2010;34(6):1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Babson KA, Boden MT, Bonn-Miller MO. The impact of perceived sleep quality and sleep efficiency/duration on cannabis use during a self-guided quit attempt. Addictive Behav 2013;38:2707–13. [DOI] [PubMed] [Google Scholar]
- *[12].Gulick D, Gamsby JJ. Racing the clock: the role of circadian rhythmicity in addiction across the lifespan. Pharmacol Ther 2018;128:124–39. [DOI] [PubMed] [Google Scholar]
- [13].Logan RW, Williams W, McClung CA. Circadian rhythms and addiction: Mechanistic insights and future directions. Behav Neurosci 2014;128(3):387–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. PNAS 2005;102(26):9377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Parekh PK, Ozburn aR, McClung CA. Circadian clock genes: Effects on dopamine, reward and addiction. Alcohol 2015;49:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Falcon E, Ozburn A, Mukherjee S, Roybal K, McClung CA. Differential regulation of the Period Genes in striatal regions following cocaine exposure. PLOS ONE 2013;8(6):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Med Rev 2012;16:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schmoll C, Lascaratos G, Dhillon B, Skene D, Riha RL. The role of retinal regulation of sleep in health and disease. Sleep Med Rev 2011;15:107–13. [DOI] [PubMed] [Google Scholar]
- [19].Roy M, Roy A, Smelson D, Brown S. Reduced blue cone electroretinogram in withdrawn cocaine dependent patients: a replication. Biol Psychiat 1997;42:631–3. [DOI] [PubMed] [Google Scholar]
- [20].Smelson Da, Roy A, Roy M, Tershakovec D, Englehart C, Losonczy MF. Electroretinogram and cure-elicited craving in withdrawn cocaine-dependent patients: a replication. Am J Drug Alcohol Abuse 2001;27(2):391–7. [DOI] [PubMed] [Google Scholar]
- *[21].Garcia AN, Salloum IH. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: a focused review. Am J Addictions 2015;24:590–8. [DOI] [PubMed] [Google Scholar]
- [22].Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine ecstasy, and marijuana. Sleep Med Rev 2008;12:381–9. [DOI] [PubMed] [Google Scholar]
- *[23].Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract 2016;11:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[24].Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia, Drug Alcohol Depen 2006;82:238–49. [DOI] [PubMed] [Google Scholar]
- *[25].Conroy DA, Arnedt JT. Sleep and substance use disorders: an update. Curr Psychiat Rep 2014;16(10):487–93. [DOI] [PubMed] [Google Scholar]
- [26].Thompson PM, Gillin JC, Golshan S, Irwin M., Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiat 1995;38:831–6. [DOI] [PubMed] [Google Scholar]
- [27].Matuskey D, Pittman B, Forselius E, Malison RT, Morgan PT. A multistudy analysis of the effects of early cocaine abstinence on sleep. Drug Alcohol Depen 2011;115:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Angarita GA, Canavan SV, Forelius E, Bessette A, Pittman B, Morgan PT. Abstinence-related changes in sleep during treatment for cocaine dependence. Drug Alcohol Depen 2014;134:343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Irwin MR, Bjurstrom MF, Olmstead R. Polysomnographic measures of sleep in cocaine dependence and alcohol dependence: Implications for age-related loss of slow wave, stage 3 sleep. Addiction 2016;111:1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Soldatos CR, Kales JD, Scharf MB, Bixler EO, Kales A. Cigarette smoking associated with sleep difficulty. Science 1980;207(4430):551–3. [DOI] [PubMed] [Google Scholar]
- [31].Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol 2006;164(6):529–37. [DOI] [PubMed] [Google Scholar]
- [32].Jaehne A, Unbehaun T, Feige B, Cohrs S, Rodenbeck A, Schutz A-L, et al. Sleep changes in smokers before, during and 3 months after nicotine withdrawal. Addict Biol 2015;20:747–65. [DOI] [PubMed] [Google Scholar]
- [33].Moreno-Coutiño A, Calderón-Ezqquerro C, Drucker-Colín R. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine Tob Res 2006;9(3):389–96. [DOI] [PubMed] [Google Scholar]
- [34].Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther 1976;19(6):782–94. [DOI] [PubMed] [Google Scholar]
- [35].Feinberg I, Jones R, Walker JM, Cleve MS, Cavness BA, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther 1975;17(4):458–66. [DOI] [PubMed] [Google Scholar]
- [36].Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, et al. Sleep disturbance in heavy marijuana users. Sleep 2008;31(6):901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depen 2011;117:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang N-Y, Funderburk FR, et al. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med 2010;11:882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Budney AJ, Moore BA, Vandrey RG, Huges JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol 2003;112(3): 393–402. [DOI] [PubMed] [Google Scholar]
- [40].Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depen 2011;119:123–9. [DOI] [PubMed] [Google Scholar]
- [41].Lee D, Schroeder JR, Karschner EL, Goodwin RS, Hirvonen J, Gorelick DA, et al. Cannabis withdrawal in chronic, frequent cannabis smokers during sustained abstinence with a closed residential environment. Am J Addictions 2014;23:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depen 2005;78:205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mehtry V, Nizamie SH, Parvez N, Pradhan N. Sleep profile in opioid dependence: a polysomnographic case-control study. J Clin Neurophys 2014;31(6):517–22. [DOI] [PubMed] [Google Scholar]
- [44].Howe RC, Hegge FW, Phillips JL. Acute heroin abstinence in man: II. Alterations in rapid eye movement (REM) sleep. Drug Alcohol Depen 1980;6:149–61. [DOI] [PubMed] [Google Scholar]
- [45].Asaad TA, Ghanem MH, Samee AMA, El-Habiby MM. Sleep profile in patients with chronic opioid abuse. Addict Dis Treatment 2011;10(1):21–8. [Google Scholar]
- [46].Howe RC, Hegge FW, Phillips JL. Acute heroin abstinence in man: I. Changes in behavior and sleep. Drug Alcohol Depen 1980;5:341–56. [DOI] [PubMed] [Google Scholar]
- [47].Imatoh N, Nakazawa Y, Ohshima H, Ishibashi M, Yokoyama T. Circadian rhythm of REM sleep of chronic alcoholics during alcohol withdrawal. Drug Alcohol Depen 1986;18:77–85. [DOI] [PubMed] [Google Scholar]
- [48].Drummond SPA, Gillin JC, Smit TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: Natural course and relationship to relapse. Alcoholism: Clin Exp Res 1998;22(8):1796–802. [PubMed] [Google Scholar]
- [49].Gann H, van Calker D, Feige B, Cloot O, Brück R, Berger M, et al. Polysomnographic comparison between patients with primary alcohol dependency during subacute withdrawal and patients with a major depression. Eur Arch Psychiat Clin Neurosci 2004;254:263–71. [DOI] [PubMed] [Google Scholar]
- [50].Snyder S, Karacan I, Sleep patterns of sober chronic alcoholics. Neuropsychobiology 1985;13:97–100. [DOI] [PubMed] [Google Scholar]
- [51].Taylor BJ, Hasler BP. Chronotype and mental health: Recent advances. Curr Psychiat Rep 2018;20:59–69. [DOI] [PubMed] [Google Scholar]
- [52].Hasler BP, Casement MD, Sitnick SL, Shaw DS, Forbes EE. Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence two years later. Behav Brain Res 2017;327:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Song J, Feng P, Zhao X, Xu W, Xiao L, Zhou J, Zheng Y. Chronotype regulates the neural basis of response inhibition during the daytime. Chronobiol Int 2018;35(2):208–18. [DOI] [PubMed] [Google Scholar]
- [54].Telzer EH, Fuligni AJ, Lieberman MD, Galván A. the effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage 2013;71:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Berro LF, Tufik S, Andersen ML. Sleep impairment: the possible link between childhood ADHD, sensation seeking, and cocaine dependence. J Atten Disord 2015;19(4):351. [DOI] [PubMed] [Google Scholar]
- [56].Hodges SE, Pittman B, Morgan PT. Sleep perception and misperception in chronic cocaine users during abstinence. Sleep 2017;40(3):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hack SP, Christie MJ. Adaptations in adenosine signaling in drug dependence: Therapeutic implications. Crit Rev Neurobiol 2003;15(3&4):235–74. [DOI] [PubMed] [Google Scholar]
- *[58].Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: Reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev 2007;31:775–824. [DOI] [PMC free article] [PubMed] [Google Scholar]


