Abstract
Aim:
Stress has been implicated in the pathogenesis of Hashimoto’s thyroiditis (HT), nevertheless evidence is scarce regarding the effect of stress management on individuals suffering from HT. The purpose of this study was to evaluate the impact of an 8-week stress management intervention on the anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-TG) antibodies as well as thyroid-stimulating hormone (TSH) levels of women with HT. Secondary endpoints included the effect on the patients’ lifestyle, body mass index (BMI), depression, anxiety and stress.
Methods:
This was a two-arm parallel group (stress management intervention vs. standard care groups) randomized controlled study. Adult women with Hashimoto’s thyroiditis, completed questionnaires on stress, anxiety, depression and lifestyle, at the beginning of the programme and 8 weeks later. Laboratory thyroid function tests (anti-TPO, anti-TG antibodies and TSH) were also measured at baseline and at the end of the study.
Results:
A total of 60 women with HT, aged 25–76 years, participated in the study (30 patients in each group). After eight weeks, patients in the intervention group demonstrated statistically significant beneficial decrements in the rate change of anti-TG titers and the levels of stress, depression and anxiety as well as better lifestyle scores, compared to the control group.
Introduction
Autoimmune thyroiditis affects a large percentage of the population worldwide and is attributed to a combination of genetic and environmental factors (Wasserman et al. 2009). Hashimoto’s thyroiditis (HT), the most common type of autoimmune thyroiditis, is characterized by the detection of antithyroid antibodies i.e. anti-thyroid peroxidase (anti -TPO) and anti-thyroglobulin (anti-TG) antibodies with or without symptoms of hypothyroidism (e.g. weight gain, fatigue, cold intolerance, hair loss, dry skin, constipation etc.) (Hadj-Kacem et al. 2009). Apart from physical health, patients may experience the ramifications of HT in their mental health and quality of life in general (Jonklaas & Burman, 2013) as they often report symptoms of chronic fatigue, irritability, memory or other cognitive problems (Ott et al. 2011). Several psychiatric co-morbidities have been reported (e.g. depression, anxiety, panic disorder etc.), although depression constitutes the capstone of these disorders especially in overt hypothyroidism (Kung 1995, Matsubayashi et al. 1996, Radosavljevic et al. 1996). The end result of these multiple health problems is that patients are subject to great stress.
Although stress has not been recognized as a risk factor for HT, it is well-established that it affects endocrine and immune mechanisms implicated also in HT (Chrousos & Elenkov 2006, Mikoś 2014, Tsatsoulis 2006). For instance, hyperactivity of the hypothalamic-pituitary- adrenal axis of stress results in increased levels of cortisol in the bloodstream causing downregulation of the thyroid hormones and hypothyroidism (Tsigos & Chrousos 2002). Also, acute stress has immuno-enhancing properties favoring inflammation (Dhabhar 2014, Tsigos & Chrousos 2002) which in the context of autoimmunity could result in clinical relapse. On the other hand, chronic stress is associated with many perturbations of the immune system, mainly with Th1 to Th2 shift, leading to enhanced humoral responses (Elenkov & Chrousos 1999, Wilder 2002).
So far, there is scarce evidence on the role of stress management in HT. Patients with HT might benefit from interventions focusing on ameliorating anxiety, stress, depression and improving healthy lifestyle. The biopsychosocial intervention NET (Neuro-Emotional Technique) showed no benefits for thyroid function tests and physical, mental and general health (Brown et al. 2015). On the other hand, the intervention held by Moncayo and Moncayo (2014), which included magnesium supplementation and relaxation treatment, was notably beneficial leading to improvement of the psychological stress and the thyroid-related symptoms in 90% of patients with thyroiditis.
The primary aim of this two-arm randomized controlled study was to examine the impact of a stress management program on the thyroid antibodies and thyroid-stimulating hormone (TSH) levels of women with HT. Secondary endpoints included the effect on body mass index (BMI), lifestyle, depression, anxiety and stress.
Materials and Methods
This two-arm, parallel group, non-blind, randomized controlled study was conducted at the Naval Hospital of Athens in Greece, from November 2015 to July 2016, after receiving approval by the Ethics and Education Committee of the hospital. Eligible participants were adult women (≥ 18 years of age) with Hashimoto’s thyroiditis, residents of Athens and literate in Greek. Patients were excluded if they suffered a mental illness, received any medication or participated in any other stress management program.
The participants who met the inclusion criteria were randomized in two groups on the basis of random numbers generated by web-based random number generator to cosmic radiation (random.org). The participants of each group were informed about the purpose of the program. After obtaining written informed consent to participate in the study, they completed questionnaires for baseline measurements. The intervention group participated in an 8-week stress management program which included stress management and lifestyle counseling in 8 weekly sessions. Controls received standard care by their physicians. The timeline and content of the stress management program are presented in Table 1.
Table 1.
Timeline and content of the program sessions
| Sessions timeline |
Session content |
|---|---|
| 1st week | Lifestyle and routine changes to healthier direction |
| 2nd week | Diaphragmatic breathing |
| 3rd week | Progressive relaxation technique |
| 4th week | Cognitive reconstruction |
| 5th week | Diet adjustments |
| 6th week | Guided imagery |
| 7th, 8th week | Conversation and encouraging the adoption of the techniques |
Measurements
Socio-demographic and Anthropometric Characteristics
Participants’ socio-demographic characteristics i.e. age, education, marital and smoking status as well as BMI (i.e. their weight in kilograms divided by the square of their height in meters) were recorded upon entry and at the end of the study.
Levels of anti-TPO, anti-TG and TSH
Thyroid function tests were evaluated at baseline and after 8 weeks. Levels of thyroid autoantibodies (anti-TPO, anti-TG) were measured with the classic method of immunofluorescence and TSH was measured with the chemiluminescent third-generation method, as described elsewhere (Spencer 2013).
Health Lifestyle and Personal Control Questionnaire (HLPCQ)
The healthy lifestyle and personal control questionnaire (HLPCQ), is a psychometric tool consisting of 26-items, designed for and weighted in the Greek population (Darviri et al. 2014). Respondents are asked to indicate the frequency of some habits in their everyday life on a Likert-type scale (1 = never or rarely, 2 = sometimes, 3 = often 4 = always). Twelve questions refer to nutrition, 8 refer to daily time management, 2 refer to organized physical activity and 4 are about social support practices and positive thinking (eg, “cleaning” of the mind during sleep). Higher scores indicate healthier lifestyle. Separate scores can be obtained on the following subscales: healthy dietary choices, unhealthy dietary avoidance, daily routine, organized physical activity, social support and mental control.
Depression Anxiety Stress Scale-21 (DASS-21)
DASS-21 is a questionnaire that has three subscales: depression, anxiety and stress (Lovibond & Lovibond 1995). Each subscale includes 7 items to which the respondent is invited to answer via a 5-point Likerttype scale. Higher scores indicate higher stress, anxiety or depression. This scale has been validated in the Greek population (Lyrakos et al. 2009).
Statistical analysis
Baseline socio-demographic and outcome data are presented as means, standard deviations (SD), or frequencies within groups. Between group comparisons for baseline data were performed using Pearson’s chi-square and Student’s t tests for categorical and interval characteristics, respectively. There were no missing data in the dataset. Longitudinal changes in outcome measures from baseline to 8 weeks (or rate of outcome change) were analyzed using linear mixed-effects models with interaction terms for study group and time points. Random intercepts b0 were used for the random effect of each participant in the model using variance components structure. The models’ formula was the following:
where Yti is the outcome, b0 is the intercept, b1, b2, b3, are the fixed coefficients, b’0 is the random coefficient for intercept, TIMEti is the time point (t) for each individual (i), GROUPi is the intervention condition and eti is the time specific residual of the model. The H0 null hypothesis of interest was b3=0. By coding control group and baseline time as zeros (intervention and follow-up time as one) b3 represents the difference of the average rate of outcome of the intervention group relative to the control group.
The Reliable Change Index (RCI) was calculated for each outcome questionnaire (Jacobson & Truax, 1991). An absolute RCI above 1.96 denotes significant difference after taking into account scores, score variance and questionnaire’s reliability. RCI was calculated according to the formula: RCI = (X2-X1) ÷ √(SEM12 + SEM22 ), where X2 and X1 are the final and baseline outcome values and SEM is the standard error measurement calculated by the formula: standard deviation (in timel or 2) * √ (1-Cronbach’s alpha in time 1 or 2). The Number Needed to Treat (NNT) for each questionnaire was calculated (i.e. the number of individuals that we need to treat in order to attain significant improvement in 1 subject). All analyses were performed using SPSS 22.0v for Windows (Chicago IL).
Results
Figure 1 shows the study flowchart. Overall, 97 patients were approached and of these, 30 were excluded (19 did not meet the inclusion criteria, 11 refused to participate). Of the 67 participants, 34 were randomized to the intervention group, with four drop outs during the intervention (one patient left Athens and three failed monitoring due to increased family responsibilities). From the control group, three patients discontinued due to major life events. Finally, a total of 60 women were analyzed (30 patients in each group).
Figure 1.
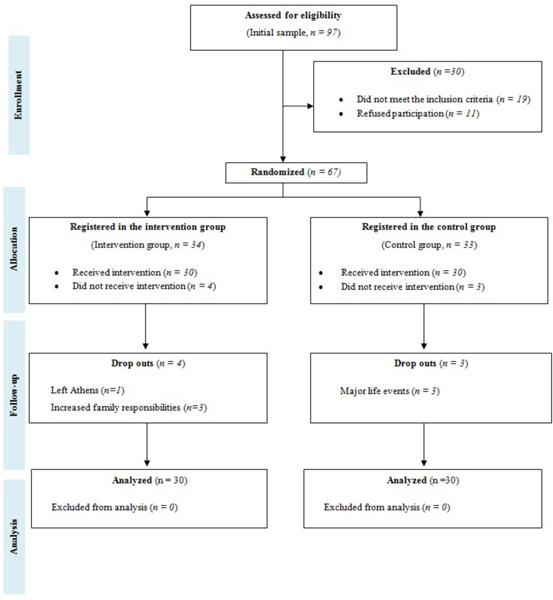
Diagram depicting the study flow chart.
Table 2 presents the socio-demographic characteristics of the study sample. Most women were of middle age, married, of tertiary education and nonsmokers. No statistically significant group comparisons were noted. Table 3 presents baseline measurements for all the outcomes’ scores. There were no statistically significant differences between the study groups at baseline.
Table 2.
Baseline socio-demographic characteristics of the study sample (N=60).
| Characteristic | Intervention (N=30) |
Control (N=30) |
P value |
|---|---|---|---|
| Age, mean ± SD1 | 45.7 ± 11.6 | 46.8 ± 11.7 | 0.72 |
| Married, N (%)2 | 16 (53.3) | 22 (73.3) | 0.18 |
| Education2 | 0.79 | ||
| Primary, N (%) | 2 (6.7) | 3 (10) | |
|
Secondary, N (%) |
9 (30) | 7 (23.3) | |
| Tertiary, N (%) | 19 (63.3) | 20 (66.7) | |
| Smoking, N (%)2 | 6 (20) | 8 (26.7) | 0.76 |
Table 3.
Baseline outcome characteristics of the study groups (N=60)1.
| Characteristic | Intervention (N=30) Mean ± SD |
Control (N=30) Mean ± SD |
P value |
|---|---|---|---|
| anti-TPO (IU/ml) | 506.2 ± 1248.2 | 352.0 ± 525.6 | 0.54 |
| anti-TG (IU/ml) | 451.1 ± 808.4 | 385.1 ± 759.5 | 0.75 |
| TSH (IU/ml) | 2.2 ± 1.5 | 1.9 ± 1.4 | 0.58 |
| BMI (Kg/m2) | 24.4 ± 4.7 | 25.8 ± 5.4 | 0.30 |
| HLPCQ | 66.2 ± 13.1 | 64.6 ± 15.2 | 0.65 |
| Stress | 7.5 ± 4.2 | 6.9 ± 4.7 | 0.65 |
| Depression | 4.4 ± 4.2 | 4.8 ± 5.2 | 0.74 |
| Anxiety | 4.5 ± 4.2 | 3.0 ± 2.9 | 0.13 |
BMI: Body Mass Index, anti-TPO: Thyroid Peroxidase Antibody, anti-TG: Thyroglobulin Antibody, TSH: Thyroid Stimulating Hormone, HLPCQ: Healthy Lifestyle and Personal Control Questionnaire
Student’s t-test
Table 4 presents the results of the mixed effects models for the rates of outcomes’ changes across time. Significant interaction between group and time (b3 in the model equation) was recorded for anti-TG, indicating a favorable effect of the intervention on these antibody titers. Patients in the intervention group demonstrated statistically significant beneficial decrements in the rate change of stress, depression and anxiety. The HLPCQ score was also significantly increased in the intervention group compared to the control group. Although, there was a decreasing trend of anti-TPO and TSH in the intervention group compared to controls, the differences were not statistically significant.
Table 4.
Results of the linear mixed-effects models for the rates of outcome change.
| Outcomes | b for group × time interaction1 (SE) |
P value |
|---|---|---|
| anti-TPO (IU/ml) |
−307.4 (211.6) | 0.15 |
| anti-TG (IU/ml) | −112.4 (43.6) | 0.01* |
| TSH (IU/ml) | −0.6 (0.3) | 0.07 |
| BMI (Kg/m2) | −0.48 (0.2) | 0.06 |
| HLPCQ | 2O.2 (2.8) | <0.001* |
| Stress | −4.0 (0.8) | <0.001* |
| Depression | −1.9 (0.7) | 0.01* |
| Anxiety | −1.9 (0.7) | 0.01* |
BMI: Body Mass Index, anti-TPO: Thyroid Peroxidase Antibody, anti-TG: Thyroglobulin Antibody, TSH: Thyroid Stimulating Hormone, HLPCQ: Healthy Lifestyle and Personal Control Questionnaire, SE: Standard Error, b: b coefficient of the linear mixed-effect model for the Rates of Outcome Change.
Reference categories: control group and baseline (both coded as zeros)
P<0.05
According to Table 5, 23.3–50.0% of patients had significant score changes according to the RCI index used for the questionnaires. Based on NNT the strongest effect was noted for lifestyle as assessed by the HLPCQ.
Table 5.
Number of individuals with beneficial significant change scores according to the Reliable Change Index and the corresponding Number Needed to Treat values (NNT).
| Characteristic (Cronbachs’ alpha before, after) |
Intervention N, (%) | Control N, (%) | NNT |
|---|---|---|---|
| HLPCQ (0.90, 0.94) | 15 (50) | 0 (0) | 2 |
| Stress (0.84, 0.83) | 8 (26.7) | 0 (0) | 12.5 |
| Depression (0.88, 0.89) | 7 (23.3) | 3 (10) | 25 |
| Anxiety (0.81, 0.79) | 7 (23.3) | 0 (0) | 14.3 |
HLPCQ: Healthy Lifestyle and Personal Control Questionnaire
Discussion
In this randomized controlled study, an 8-week stress management program was implemented in women with HT, to assess its impact on their thyroid function tests, mental health and lifestyle. Overall, the study showed that patients in the intervention group decreased the anti-TG titers, their stress, anxiety and depression levels and adopted a healthier lifestyle. The latter effect was found to be the strongest among the study measurements based on questionnaires. So far, there are no similar studies which corroborate or contradict these results. The NET study (which resulted in no clinical benefit for the patients) used a different approach to stress management in relation to our techniques (Brown et al. 2015). The WOMED approach for Hashimoto disease by Moncayo and Moncayo (2014), showed treatment success in 90% of the cases, using techniques that involved acupuncture for stress relief only to patients with high stress levels and magnesium supplementation to patients with low serum magnesium and residual symptoms of hypothyroidism. Therefore, the results of our study are not comparable to any of the studies’ findings mentioned above.
A limitation of the study was the lack of objective ascertainment of compliance to the stress management program. Patients were asked to keep a diary of the techniques they performed at home. Although their diary records showed in general perfect compliance, this is still a self-report assessment. Secondly, HT-related symptoms, which are more indicative of the disease severity, were not evaluated, although most patients were in clinical remission. Finally, the study did not seek for long-term data, after the end of the 8-week period.
To our knowledge, this is the first study showing a reduction of anti-TG titers in women with HT undergoing a stress management intervention. Although, the observed benefits in lifestyle and psychological health (i.e. stress, depression, anxiety) could account for this finding, more targeted research on the subject is needed to draw such a conclusion. So far, there is no definite epidemiological evidence linking stress to HT. Presumably, stress reduction could lead to decreased humoral immune responses which are responsible for antibody production (Dhabhar 2014). With regards to pathophysiology, although the connection of stress with Grave’s disease has been adequately evidenced, this is not the case with HT (Damian et al. 2016). The pathogenesis of HT includes both cell- (Th1 and Th17) and humoralmediated (anti-TPO, anti-TG) immune mechanisms, thus the role of stress should be complex. However, there is still enough room for speculation; acute stress might exacerbate HT, while chronic stress favoring a Th1 to Th2 shift might be related to increased blood levels of the anti-TPO and anti-TG antibodies (Dhabhar 2014). As such, it is reasonable to expect from effective stress management intervention to lower the levels of these antibodies in the bloodstream.
In conclusion, short-term stress management had beneficial effects on the anti-TG titers, the lifestyle and the psychological status of women with HT. Given the absence of similar studies, further research is warranted. The role of stress management in the everyday clinical practice of physicians treating patients with HT is still questionable. However, the remote possibility of causing any harm, renders stress management a safe personalized choice for physicians and patients.
Acknowledgments
We would like to thank the Endocrinologists Nikolaos Mazarakis, Ioannis G. Komninos and the Nephrologist Ioannis Tsouras, for their assistance, well as the patients for their commitment to the study.
Footnotes
Conflict of Interest
Authors declare no conflicts of interest.
References
- Brown BT Graham PL Bonello R& Pollard H 2015. Biopsychosocial approach to primary hypothyroidism: treatment and harms data from a randomized controlled trial. ChiroprMan Therap 23 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP & Elenkov IJ 2006. Interactions of the endocrine and immune systems. In: DeGroot LJ, Jameson JL (eds.), Endocrinology, Saunders Elsevier, Philadelphia, PA, Vol. 1 pp. 799–818 [Google Scholar]
- Damian L Ghiciuc CM Dima-Cozma LC Ungureanu MC Cozma S Patacchioli FR & Lupusoru CE 2016. No definitive evidence for a connection between autoimmune thyroid diseases and stress in women. Neuro Endocrinol Lett 37 155–162 [PubMed] [Google Scholar]
- Darviri C Alexopoulos EC Artemiadis AK Tigani X Kraniotou C Darvyri P & Chrousos GP 2014. The Healthy Lifestyle and Personal Control Questionnaire (HLPCQ): a novel tool for assessing self empowerment through a constellation of daily activities. BMC Public Health 14 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS 2014. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 58 193–210 [DOI] [PubMed] [Google Scholar]
- Elenkov IJ & Chrousos GP 1999. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab 10 359–368 [DOI] [PubMed] [Google Scholar]
- Hadj-Kacem H Rebuffat S Mnif-Féki M Belguith-Maalej S Ayadi H & Péraldi-Roux S 2009. Autoimmune thyroid diseases: genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. Int JImmunogenet 36 85–96 [DOI] [PubMed] [Google Scholar]
- Jacobson NS & Truax P 1991. Clinical significance: a statistical approach to defining meaningful change in psychotherapy-research. J Consult Clin Psychol 59 12–19 [DOI] [PubMed] [Google Scholar]
- Jonklaas J Burman KD Bianco AC & American Thyroid Association Spring 2013. Program Committee 20l3 Treatment of hypothyroidism: possibilities on the horizon. Thyroid 23 ix–xi. [DOI] [PubMed] [Google Scholar]
- Kung AW 1995. Life events, daily stresses and coping in patients with Graves’ disease. Clin Endocrinol 42 303–308 [DOI] [PubMed] [Google Scholar]
- Lovibond SH & Lovibond PF 1995. Manual for the Depression Anxiety Stress Scales, 2nd Ed, Sydney: Psychology Foundation, ISBN 7334–1423–0 [Google Scholar]
- Lyrakos G & Arvaniti C 2009. Greek translation of the DASS. Retrieved from http://www2.psy.unsw.edu.au/dass/Greek/Greek.htm on June 30 2019
- Matsubayashi S Tamai H Matsumoto Y Tamagawa K Mukuta T,Morita T & Kubo C 1996. Graves’ disease after the onset of panic disorder. Psychother Psychosom 65 277–280 [DOI] [PubMed] [Google Scholar]
- Mikoś H Mikos M Obara-Moszyńska M & Niedziela M 2014. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (AITD). EndokrynolPol 65 150–155 [DOI] [PubMed] [Google Scholar]
- Moncayo R & Moncayo H 2014. The WOMED model of benign thyroid disease: acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin 3 44–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J Promberger R Kober F Neuhold N Tea M Huber JC & Hermann M 2011. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid 21 161–167 [DOI] [PubMed] [Google Scholar]
- Radosavljevic VR Jankovic SM & Marinkovic JM 1996. Stressful life events in the pathogenesis of Graves’ disease. Eur J Endocrinol 134 699–701 [DOI] [PubMed] [Google Scholar]
- Spencer CA 2013. Assay of Thyroid Hormones and Related Substances. www.endotext.org. Retrieved 5 November 2014
- Tsatsoulis A 2006. The role of stress in the clinical expression of thyroid autoimmunity. Ann NY Acad Sci 1088 382–395 [DOI] [PubMed] [Google Scholar]
- Tsigos C & Chrousos GP 2002. Hypothalamicpituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53 865–871 [DOI] [PubMed] [Google Scholar]
- Wasserman EE Nelson K Rose NR Rhode C Pillion JP Seaberg E Talor MV Burek L Eaton W Duggan A & Yolken RH 2009. Infection and thyroid autoimmunity: A seroepidemiologic study of TPOaAb. Autoimmunity 42 439–446 [DOI] [PubMed] [Google Scholar]
- Wilder RL 2002. Neuroimmunoendocrinology of the rheumatic diseases: past, present, and future. Ann N Y Acad Sci 966 13–19 [DOI] [PubMed] [Google Scholar]


