Abstract
PURPOSE
Nivolumab, an anti–programmed death-1 monoclonal antibody, has demonstrated frequent and durable responses in relapsed/refractory classic Hodgkin lymphoma (cHL). We report results from Cohort D of the CheckMate 205 trial, which assessed nivolumab monotherapy followed by nivolumab plus doxorubicin, vinblastine, and dacarbazine (N-AVD) for newly diagnosed cHL.
METHODS
Patients 18 years of age or older with untreated, advanced-stage (defined as III to IV and IIB with unfavorable risk factors) cHL were eligible for Cohort D of this multicenter, noncomparative, phase II trial. Patients received nivolumab monotherapy for four doses, followed by 12 doses of N-AVD; all doses were every 2 weeks, and nivolumab was administered at 240 mg intravenously. The primary end point was safety. Efficacy end points included objective response rate and modified progression-free survival, defined as time to disease progression/relapse, death, or next therapy. Chromosome 9p24.1 alterations and programmed death-ligand 1 expression were assessed in Hodgkin Reed-Sternberg cells in evaluable patients.
RESULTS
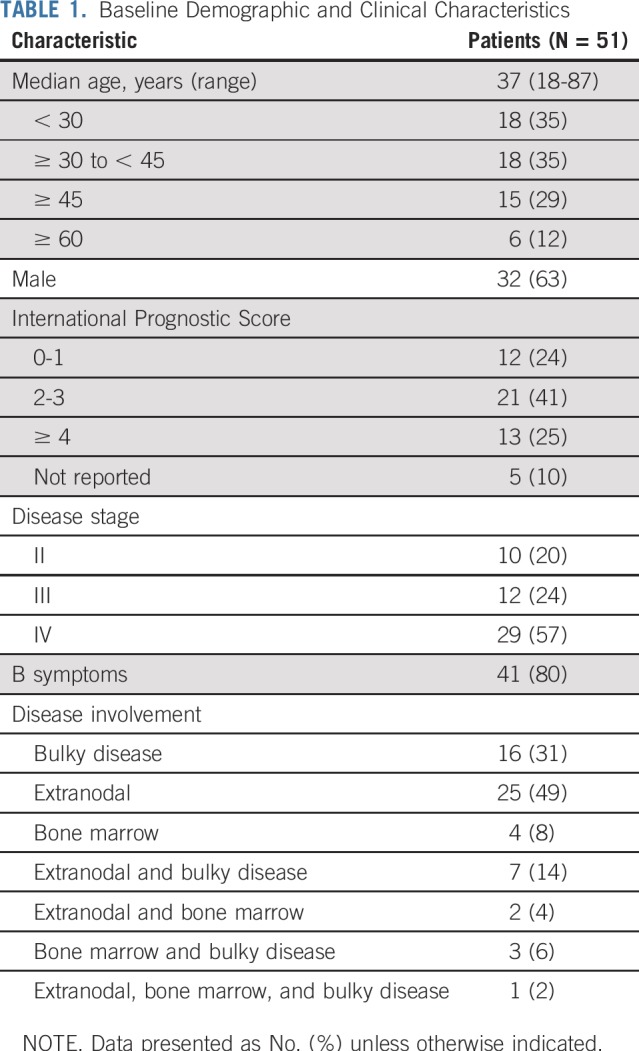
A total of 51 patients were enrolled and treated. At diagnosis, 49% of patients had an International Prognostic Score of 3 or greater. Overall, 59% experienced a grade 3 to 4 treatment-related adverse event. Treatment-related febrile neutropenia was reported in 10% of patients. Endocrine immune-mediated adverse events were all grade 1 to 2 and did not require high-dose corticosteroids; all nonendocrine immune-mediated adverse events resolved (most commonly, rash; 5.9%). At the end of therapy, the objective response rate (95% CI) per independent radiology review committee was 84% (71% to 93%), with 67% (52% to 79%), achieving complete remission (five patients [10%] were nonevaluable and counted as nonresponders). With a minimum follow-up of 9.4 months, 9-month modified progression-free survival was 92%. Patients with higher-level Hodgkin Reed-Sternberg programmed death-ligand 1 expression had more favorable responses to N-AVD (P = .041).
CONCLUSION
Nivolumab followed by N-AVD was associated with promising efficacy and safety profiles for newly diagnosed, advanced-stage cHL.
INTRODUCTION
Although treatment of newly diagnosed classic Hodgkin lymphoma (cHL) with multiagent chemotherapy results in high complete remission (CR) and cure rates, outcomes for patients with advanced-stage disease remain suboptimal.1-4 In contrast to earlier-stage disease, where front-line multiagent chemotherapy with or without radiotherapy may be associated with long-term remission in 85% to 95% of patients,4-6 disease progression or death within 5 years is seen in 20% to 30% of patients with advanced-stage cHL.1-3
Genetic alterations at 9p24.1, leading to overexpression of the programmed death-1 (PD-1) ligands 1 and 2 (PD-L1 and PD-L2), are a defining feature of cHL.7 High-magnitude 9p24.1 copy number alterations (CNAs) are more common in newly diagnosed stage III to IV cHL and have been associated with poorer progression-free survival (PFS) in patients receiving standard induction therapy.7 In patients with relapsed/refractory cHL receiving single-agent PD-1 blockade, high-magnitude 9p24.1 genetic alterations and PD-L1 expression in Hodgkin Reed-Sternberg (HRS) cells were associated with prolonged PFS.8 These observations provided the rationale for evaluating PD-1 blockade in the front-line setting in patients with advanced-stage cHL.
Nivolumab, a fully human immunoglobulin G4 anti–PD-1 immune checkpoint inhibitor monoclonal antibody, has demonstrated frequent and durable responses with a favorable safety profile as monotherapy in relapsed/refractory cHL.9 In heavily pretreated patients, nivolumab monotherapy was associated with an objective response rate (ORR) of 69% and a median PFS of 15 months.9 Nivolumab plus brentuximab vedotin (BV) demonstrated an ORR of 82%, with 61% CR in relapsed/refractory cHL, suggesting the potential benefit of combining PD-1 blockade with cytotoxic agents.10 Therefore, we hypothesized that combining nivolumab with chemotherapy would confer a therapeutic benefit in patients with advanced-stage, previously untreated cHL.
The high efficacy of anthracycline-containing chemotherapy regimens typically used in front-line treatment of cHL must also be balanced with their inherent toxicities, including late and persistent effects that may develop long after completing treatment.11,12 Outcomes are also particularly poor in elderly and frail patients, who may be unable to tolerate intensive chemotherapy.3,13-15 Response-adapted therapy, guided by 18F-labeled fluorodeoxyglucose–positron emission tomography (FDG-PET) scans after two treatment cycles, may reduce bleomycin-related pulmonary toxicity.16 However, many patients with FDG-PET–positive disease at the interim scan—who generally have a poor prognosis17—may still receive high-intensity chemotherapy.16,18,19 Furthermore, the risk of progression or death within 5 years in patients with an interim FDG-PET–negative scan after two cycles remains at approximately 20%.20 Replacing bleomycin with BV seems to improve modified PFS (mPFS) at 2 years, but the long-term efficacy and safety of BV plus doxorubicin, vinblastine, and dacarbazine (A-AVD) are yet to be established.21 Novel regimens with improved efficacy and manageable long-term safety profiles are therefore needed for newly diagnosed advanced-stage cHL.
We present results from Cohort D of CheckMate 205, which assessed the safety and efficacy of nivolumab monotherapy followed by nivolumab plus AVD (N-AVD) for newly diagnosed, advanced-stage cHL. To evaluate the activity of immunotherapy alone for newly diagnosed cHL, a nivolumab monotherapy period before combination therapy was included, which also provided an opportunity to test the hypothesis of immune system priming before chemotherapy.
METHODS
Study Design and Patients
CheckMate 205 (ClinicalTrials.gov identifier: NCT02181738) is a multicenter, multicohort, noncomparative, phase II study of nivolumab for cHL. Patients 18 years of age and older with untreated, advanced-stage cHL, with an Eastern Cooperative Oncology Group performance status of 0 to 1 and hemoglobin-adjusted diffusing capacity of the lung for carbon monoxide more than 60%, were enrolled in Cohort D. Disease was staged per Cotswold-modified Ann Arbor staging22; advanced-stage disease was defined as stage III to IV, or stage IIB with bulky (node or nodal mass > 10 cm or a mediastinal mass with a maximum transverse to internal thoracic diameter ratio at T5/6 level of ≥ 1/3 by chest radiograph), or extranodal disease. Patients with planned post-treatment consolidative radiotherapy were not eligible.
This study was performed in accordance with the Declaration of Helsinki. Approval from the appropriate institutional review board and independent ethics committee was received for the protocol, amendments, and consent forms before initiating the study at each site. All patients provided written informed consent before enrollment.
Treatments
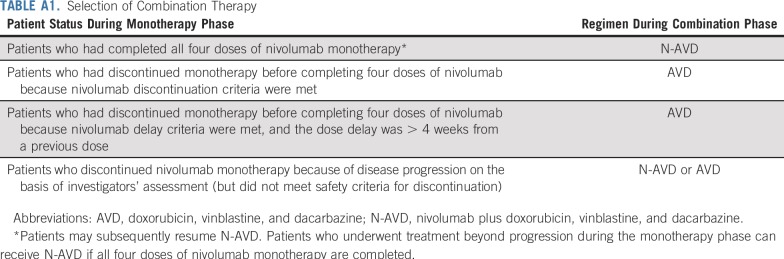
Patients were treated first with nivolumab monotherapy and then with N-AVD combination therapy. In the monotherapy phase, patients received nivolumab (240 mg intravenously over 30 minutes every 2 weeks) for four doses. Patients then entered the combination therapy phase, and received six combination cycles (12 doses, once every 2 weeks) of N-AVD (nivolumab 240 mg, doxorubicin 25 mg/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2, all intravenously). After combination therapy, patients entered follow-up, with radiographic assessments at 39, 65, and 104 weeks from the last dose. Patients who did not complete a phase were still observed and could enter subsequent phases. Patients who experienced study drug toxicity requiring discontinuation of nivolumab, or who experienced dose delays more than 4 weeks from the previous dose during the monotherapy phase, could receive AVD alone during the combination phase. Full combination therapy selection criteria are listed in Appendix Table A1 (online only).
End Points and Assessments
The primary end point was safety and tolerability, assessed by the incidence of grade 3 to 5 treatment-related adverse events (TRAEs) between the first dose and 30 days after the last dose. Immune-mediated adverse events (IMAEs) were assessed until 100 days after the last dose. Adverse events (AEs) were coded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Secondary end points included CR rate at the end of therapy (EOT), per independent radiology review committee (IRC) using 2007 International Working Group criteria23 and treatment discontinuation rate (in each phase and overall). Responses were assessed by FDG-PET plus computed tomography or magnetic resonance imaging at baseline, end of monotherapy, after two combination cycles, and at EOT (9 ± 2 weeks from last dose). FDG-PET was optional after two combination cycles if the previous scan was negative; computed tomography scans were required. Exploratory end points included CR (per investigator) and ORR (per IRC and investigator) at the end of monotherapy, after two combination cycles, and at EOT, as well as mPFS per IRC.
The definition of mPFS was time to relapse/progression, death, or subsequent therapy (the first nonpalliative radiotherapy, systemic cancer therapy, or transplantation), regardless of response (per IRC). Traditional PFS (if subsequent therapy was not considered an event and censored) was also evaluated.
Exploratory Biomarker Analysis
Genetic alterations at 9p24.1 in HRS cells from baseline tumor biopsies were evaluated by fluorescence in situ hybridization (FISH) as described previously7,8 and classified as unbalanced rearrangement, amplification, copy gain, polysomy, disomy (normal), or relative copy loss. CNAs were defined as previously described7,8 on the basis of the target:control signal ratio in 50 HRS cells per tumor. Nuclei with a target:control signal ratio of 3:1 or greater were defined as coamplified for PD-L1 and PD-L2, and those with a signal ratio of greater than 1:1 but less than 3:1 were classified as having relative copy gain of these loci. Nuclei with a signal ratio of 1:1, but more than two copies per probe were defined as polysomic for 9p24.1. Percentage and magnitude of 9p24.1 amplification, copy gain, polysomy, and disomy were defined for each patient, as previously described.7,8 The status of 9p24.1 for each patient was assigned using the highest observed level of 9p24.1 genetic alteration; those with 9p24.1 copy gain lacked amplification, and those with 9p polysomy lacked 9p24.1 copy gain or amplification.7,8
The expression of PD-L1 in HRS cells was measured by a modified H-score on the basis of double immunohistochemistry (IHC) staining of PD-L1 and PAX5, as described previously.7 Approximately 50 HRS cells per patient were assessed for PD-L1 H-score.
Statistical Analysis
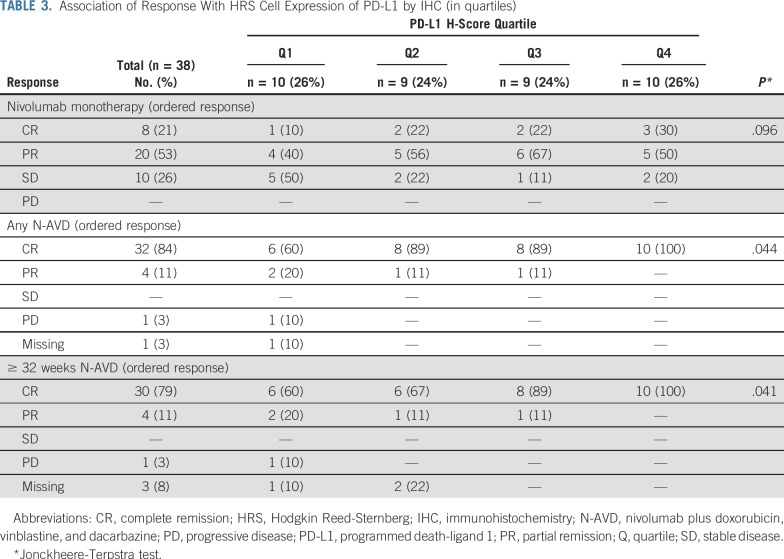
All patients who received one or more doses of nivolumab were followed for safety and efficacy. The sample size was determined to provide precision to estimate the proportion of patients who experienced one or more grade 3 to 5 TRAE (for a range of incidence rates from 16% to 46% on the basis of prior data24) and to understand the safety profile. Time-to-event data were estimated using the Kaplan-Meier method and, when appropriate, medians with 95% CIs were calculated using Brookmeyer and Crowley methodology or the Greenwood method. P values for PD-L1 H-scores and percentage disomic HRS cells were calculated using the Kruskal-Wallis rank-sum test. The categorical response and PD-L1 H-score (in quartiles) were treated as ordinal variables, and the Jonckheere-Terpstra trend test for double-ordered contingency tables was used to test for an association between response and H-score quartile.
RESULTS
Baseline Patient Characteristics and Disposition
Fifty-one patients were enrolled and treated in Cohort D of CheckMate 205; baseline characteristics are listed in Table 1. At clinical cutoff (determined by the last patient, last visit), median follow-up was 11.1 months (range, 1.2 to 16.4 months).
TABLE 1.
Baseline Demographic and Clinical Characteristics
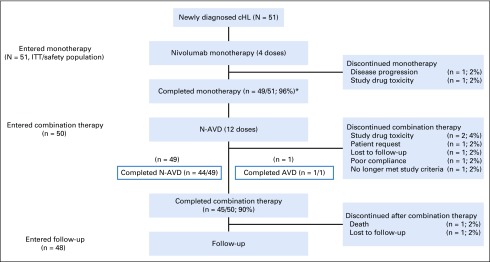
Of the 51 patients, 49 (96%) completed monotherapy (Appendix Fig A1, online only). Fifty patients entered the combination therapy phase (one discontinued the study after one dose of nivolumab because of disease progression); 49 received N-AVD, and one received AVD only because of study drug toxicity in the monotherapy phase. Ninety percent of patients completed combination therapy (44 of 49 completed N-AVD and one of one completed AVD); 48 entered follow-up. Of the six patients who did not complete combination therapy, two discontinued because of study drug toxicity, one no longer met the study criteria, one requested to discontinue, one was lost to follow-up, and one had poor treatment compliance.
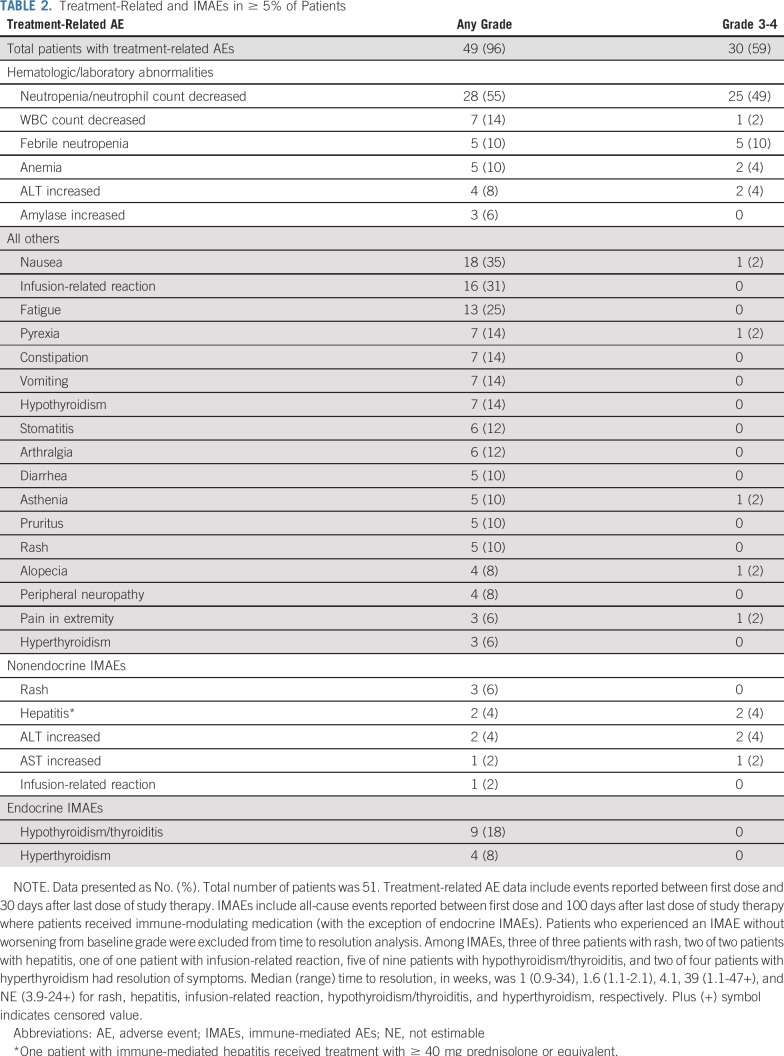
Safety
In total, 59% of patients experienced a grade 3 to 4 TRAE (Table 2), most commonly neutropenia (49%). Febrile neutropenia was reported in five patients (10%), and 30 (59%) received growth factors, all after starting combination therapy and mainly as secondary prophylaxis (27 patients; 90%). Overall, eight patients (16%) experienced a treatment-related infection; two (4%) were grade 3 to 4 (one each of gastroenteritis and respiratory tract infection). Infusion-related reactions were reported in 15 patients (29%) during monotherapy and in three patients (6%) during combination therapy; all were grade 1 to 2.
TABLE 2.
Treatment-Related and IMAEs in ≥ 5% of Patients
The median reduction from baseline in pulmonary function (hemoglobin-adjusted diffusing capacity of the lung for carbon monoxide) was 1.5 mL/min/mm Hg (3% of predicted reduction); no pneumonitis was reported. Treatment-related nervous system disorders were reported in 12 patients (24%) and were all grade 1 to 2; the most commonly reported were peripheral neuropathy in four patients (8%), peripheral sensory neuropathy in two patients (4%), and polyneuropathy in two patients (4%). Treatment-related serious AEs were reported in 14% of patients overall, most commonly febrile neutropenia in two patients (4%) and infection in two patients (4%). The most common nonendocrine IMAE was rash in three patients (6%); grade 3+ nonendocrine IMAEs were reported in two patients (4%) who experienced grade 3 increased ALT (both patients) and grade 3 increased AST (one patient). The most common endocrine IMAE was hypothyroidism in eight patients (16%, all grade 1 to 2; Table 2). All nonendocrine IMAEs resolved, and seven of 13 endocrine IMAEs resolved. Overall, four patients (8%) experienced an AE leading to discontinuation: one patient with febrile neutropenia (grade 3 to 4) and one each with hyperthyroidism, abnormal hepatic function, and interstitial lung disease (each grade 1 to 2). Overall, 31 patients (61%) had one or more doses of nivolumab delayed; of 703 nivolumab doses (across monotherapy and combination therapy) in patients who received N-AVD, 55 (8%) were delayed, with 39 (71%) of these delays resulting from an AE.
There was one treatment-related death. The patient (68 years of age) died 38 days after the last dose of N-AVD (combination cycle 5), having experienced four serious AEs: acute respiratory infection, febrile neutropenia, congestive heart failure (each grade 4), and acute respiratory failure (grade 5), all considered related to N-AVD. The patient was in CR after two combination cycles. Of the other five patients older than 60 years of age (ages 61 to 87 years), three experienced grade 3 to 4 TRAEs (most commonly neutropenia), all during combination therapy, none of which led to discontinuation.
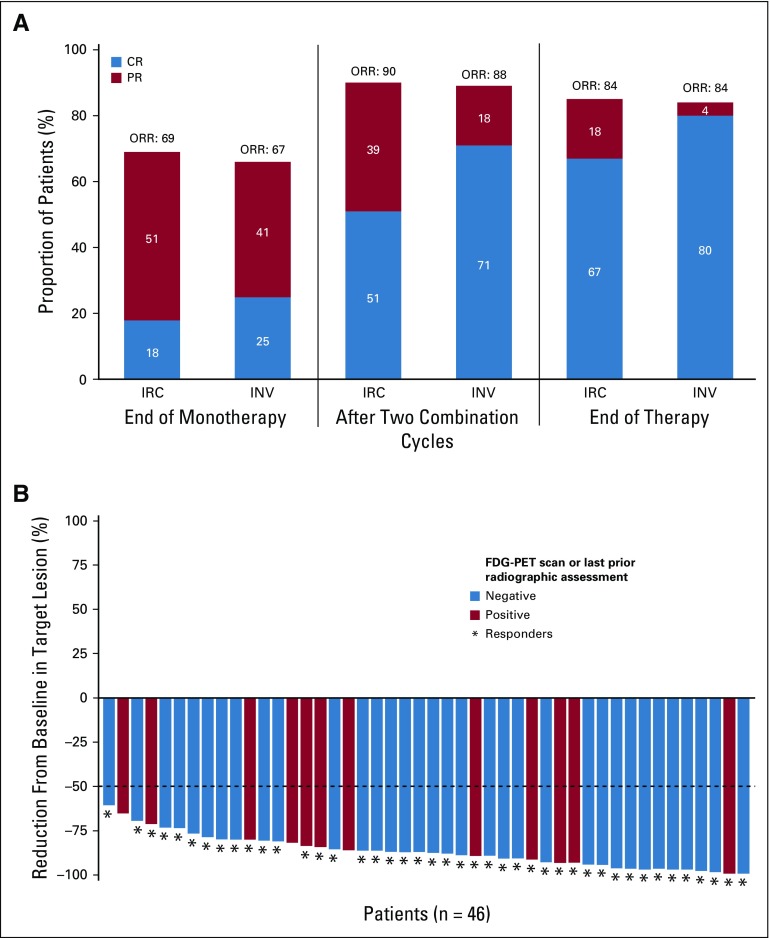
Efficacy
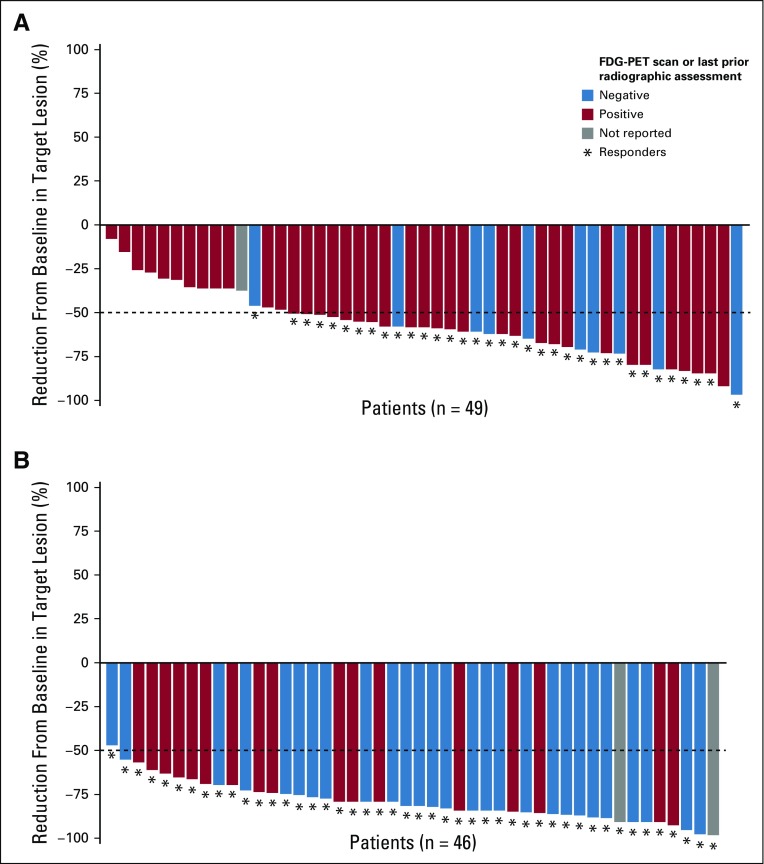
At the end of monotherapy, ORR (95% CI) per IRC (n = 51) was 69% (54% to 81%), with 18% (8% to 31%) achieving CR; after two combination cycles, ORR was 90% (79% to 97%), with 51% (37% to 65%) achieving CR; and at EOT, ORR was 84% (71% to 93%), with 67% (52% to 79%) achieving CR (Fig 1A). At the end of monotherapy, after two combination cycles, and at EOT, 35 of 49 (71%), 45 of 46 (98%), and 46 of 46 (100%) response-evaluable patients had a target lesion tumor burden reduction of more than 50%, respectively (Fig 1B; Appendix Fig A2, online only).
FIG 1.
(A) Objective response rate (ORR) per independent radiology review committee (IRC) and investigator at end of monotherapy, after two combination cycles, and at end of therapy. Total number of patients was 51. (B) Change in target lesion per IRC in response-evaluable patients at end of treatment. Horizontal line indicates 50% reduction consistent with 2007 International Working Group response criteria. Three patients had greater than 50% reduction in target lesion burden but were considered to have new or 18F-labeled fluorodeoxyglucose–positron emission tomography (FDG-PET) positive lesions by the IRC. CR, complete remission; INV, investigator assessment; PR, partial remission.
Per investigator, the ORR (95% CI) at EOT was 84% (71% to 93%), with an 80% (67% to 90%) CR rate. Five patients (10%) were not response evaluable and were counted as nonresponders: one withdrew consent, one died, one was lost to follow-up, one started nivolumab monotherapy at the end of combination therapy (a protocol violation; the patient was in CR after two combination cycles and at the last available assessment at EOT), and one did not have an EOT assessment (and was in CR after two combination cycles). Among the 46 response-evaluable patients at EOT, ORR per IRC was 93%, with 74% achieving CR.
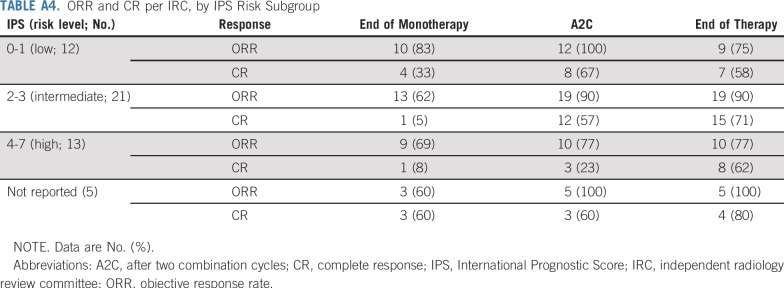
Of the 12 patients not in CR per IRC at EOT, seven were deemed in CR per investigator and did not receive subsequent therapy (Appendix Table A2, online only). Overall, three patients (6%) had progressive disease per IRC at EOT; per investigator, two (4%) had progressive disease. Of the six patients older than 60 years of age, five achieved CR per IRC at any time during treatment, with all six achieving CR per investigator (Appendix Table A3, online only). Response per IRC by International Prognostic Score risk subgroup is listed in Appendix Table A4 (online only).
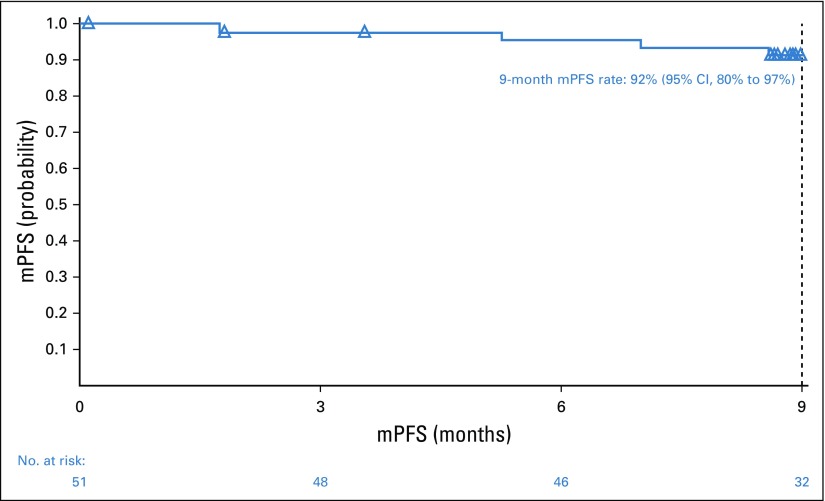
With a minimum follow-up of 9.4 months, the 9-month mPFS rate was 92% (95% CI, 80% to 97%; Fig 2). The traditional PFS Kaplan-Meier curve would be similar to the mPFS curve; median PFS was not reached. The 9-month OS rate was 98% (95% CI, 86% to 100%).
FIG 2.
Modified progression-free survival (mPFS) per independent radiology review committee. mPFS was defined as time to progression, death, or next subsequent therapy, regardless of response.
Baseline 9p24.1 Alterations and PD-L1 Expression
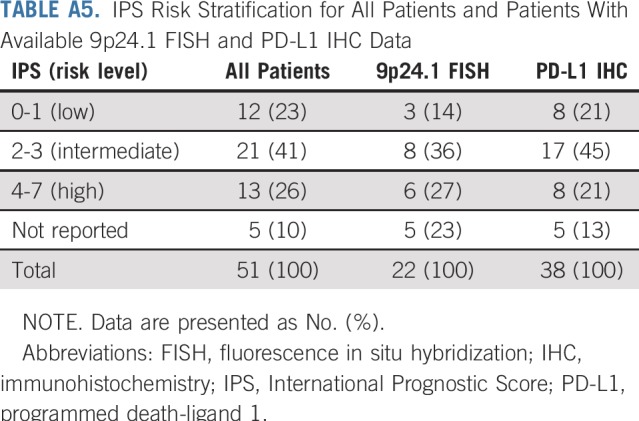
Twenty-two patients were evaluable for HRS cell 9p24.1 status by FISH; 38 were evaluable for HRS cell PD-L1 expression by IHC. Patients with evaluable 9p24.1 status and PD-L1 expression were comparable to the full cohort in International Prognostic Score risk (Appendix Table A5, online only).
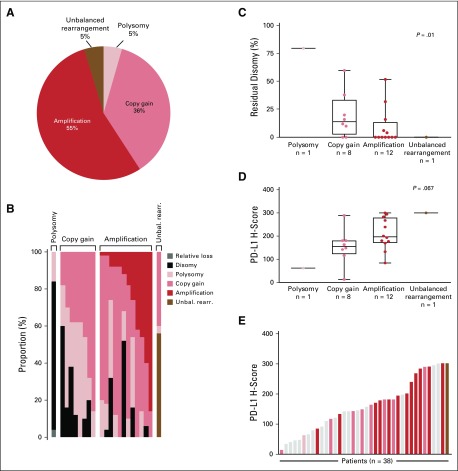
All 22 patients with evaluable 9p24.1 FISH in baseline tumor specimens had detectable CNAs (unbalanced rearrangement, amplification, copy gain, or polysomy) in HRS cells; 12 of 22 patients (55%) had amplification as the highest level CNA (Fig 3A and 3B; Appendix Fig A3, online only). Additional disomic HRS cells were detected in 12 patients (Fig 3B), and the proportion of disomic cells was inversely correlated with the magnitude of 9p24.1 alterations (P = .01; Fig 3C). A positive trend between the magnitude of 9p24.1 CNA and PD-L1 expression (P = .067; Fig 3D) was observed. One patient had an unbalanced rearrangement at 9p24.1 and the highest level of PD-L1 expression among evaluable samples (Fig 3E).
FIG 3.
Chromosome 9p24.1 alterations and programmed death-ligand 1 (PD-L1) expression (H-scores) in evaluated patients with classic Hodgkin lymphoma (cHL) from Cohort D. (A) Prevalence of 9p24.1 genetic alterations in 22 evaluable patients with cHL. (B) The spectrum of 9p24.1 alterations in evaluated cHLs. Each patient is classified by the highest observed level of 9p24.1 alteration in Hodgkin Reed-Sternberg (HRS) cells: polysomy, copy gain, amplification, or unbalanced rearrangement. Individual patients are represented by columns on the x axis, and the percentage of HRS cells with relative loss (dark gray), disomy (black), polysomy (light pink), copy gain (medium pink), amplification (red), or unbalanced rearrangement (brown) is depicted on the y axis. In patients classified by the highest observed level of 9p24.1 alteration, additional HRS cells had lower-level 9p24.1 copy number alterations, as previously described.7,8 For example, patients classified as having 9p24.1 amplification had additional HRS cells with 9p24.1copy gain, 9p24.1 polysomy, and/or 9p24.1 disomy. cHLs identified as having 9p24.1 copy gain included additional HRS cells with 9p24.1 polysomy and/or 9p24.1 disomy; patients classified as polysomic for chromosome 9p24.1 had additional HRS cells that were disomic for 9p24.1, as previously described.7,8 (C) Percentage of disomic HRS cells in cHLs classified by 9p24.1 alterations. The percentage of 9p24.1 disomic HRS cells was highest in tumors classified as polysomic for 9p24.1, intermediate in tumors with 9p24.1 copy gain, and lowest in tumors with 9p24.1 amplification, as previously described.7,8 P value calculated using the Kruskal-Wallis rank-sum test. (D) PD-L1 H-scores in cHLs classified by 9p24.1 alterations (22 evaluable patients). PD-L1 H-scores are calculated by multiplying the percentage of PD-L1-positive HRS cells (Pax5dim+; 0% to 100%) and the average intensity of PD-L1 staining (0 to 3+) in evaluated HRS cells. P value calculated using the Kruskal-Wallis rank-sum test. (E) PD-L1 H-scores in all evaluable patients with cHL (n = 38; 18 patients were evaluable for PD-L1 immunohistochemistry but not fluorescence in situ hybridization). Individual samples are visualized as columns on the x axis. Columns in light gray were not evaluable for 9p24.1 genetic alterations. Additional columns are colored by 9p24.1 alterations (see key). Unbal. rearr., unbalanced rearrangement.
Of interest, there was a trend toward more favorable responses to nivolumab monotherapy in patients with higher HRS cell PD-L1 expression (P = .096; Table 3). In addition, a greater proportion of patients with PD-L1 expression in quartile 3 or 4 had deeper and more durable responses to N-AVD (P = .041; Table 3).
TABLE 3.
Association of Response With HRS Cell Expression of PD-L1 by IHC (in quartiles)
DISCUSSION
In this first phase II study of a checkpoint inhibitor in previously untreated cHL, nivolumab monotherapy followed by N-AVD had a safety profile consistent with historical analyses for nivolumab9 and AVD,16 with no new safety signals observed. Notably, nearly all patients entered (98%) and completed (90%) the combination therapy phase. These data are consistent with previously reported discontinuation rates of 9% to 16% in studies with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD).2,24,25
Bleomycin was excluded in this study because of potential pulmonary toxicity and data suggesting bleomycin omission from ABVD may have the least impact on outcomes.26 Bleomycin-associated pulmonary toxicity results in approximately 4% mortality, can happen late after therapy, and has a negative impact on survival outcomes in cHL.27,28 N-AVD seems to have a favorable pulmonary safety profile, with a low reduction in pulmonary function and no pneumonitis reported. Alternative strategies to limit bleomycin exposure include omission of bleomycin after two cycles of ABVD in patients with PET-negative disease, which has demonstrated similar efficacy to ABVD with decreased pulmonary toxicity.16,20
Elderly patients, who have a greater risk of AEs and typically have poorer outcomes, make up a notable proportion of patients with cHL; in 2015, an estimated 27% of patients were 60 years of age or older,29 and 5-year OS was reported at 58% in these patients, compared with 90% in patients younger than 60 years of age.13 Treatment guidelines recommend that escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone be used with caution in elderly patients because of excess toxicity.30 Data from this study suggest that deep responses are possible with nivolumab followed by N-AVD treatment in elderly patients, with five of six patients (ages 61 to 87 years) achieving CR per IRC and all achieving CR per investigator. The most frequent grade 3+ TRAE in these patients was neutropenia, similar to the overall population. Data from a single-arm study including patients 60 years of age and older with untreated cHL showed a 2-year PFS rate of 84% with sequential BV and AVD treatment; however, the completion rate was relatively low (48% did not complete all planned treatment).31
This study was designed with a nivolumab monotherapy period before N-AVD combination therapy. Nivolumab monotherapy has demonstrated frequent and durable responses in patients with relapsed/refractory cHL (Cohorts A, B, and C of CheckMate 205).9 It is possible that immune checkpoint blockade before the immunosuppressive and myelosuppressive effects of chemotherapy may allow greater depth of response because of the relative preservation of effector immune cells in newly diagnosed patients and the potential for priming the immune system before chemotherapy. In Cohort D, after only 2 months of monotherapy, response rates were similar to results in relapsed/refractory cHL.9 Although the efficacy of this regimen is promising, larger comparative studies are required to assess whether this approach results in successful priming.
Combining a targeted therapy with a chemotherapeutic regimen may offer efficacy benefits and reduce the nonspecific cytotoxicity and intensity of untargeted chemotherapy. The ECHELON-1 study, which evaluated A-AVD for newly diagnosed, stage III to IV cHL, showed a 4.9% improvement in 2-year mPFS versus ABVD.21 However, there were higher rates of peripheral neuropathy and febrile neutropenia in the A-AVD arm, and patients 60 years of age and older did not seem to derive an mPFS benefit with A-AVD (hazard ratio, 1.00; 95% CI, 0.53 to 1.94).21 Extended follow-up is required to fully assess the long-term efficacy and safety of A-AVD. The combinations of N-AVD and A-AVD in stage III to IV cHL will be evaluated in the phase III randomized SWOG S1826 trial.32
Limitations of this study include its small sample size. This may preclude detection of rare AEs and limit precision on estimates of CR rates. However, the sample size was determined to evaluate the primary end point and is comparable to many phase II trials. There was some discordance between investigator- and IRC-assessed CR rates in this study. Although ORRs per IRC and investigator were both 84% at EOT, the investigator-assessed CR rate was 13% greater than the IRC assessment (80% v 67%). Furthermore, seven of 12 patients who were not in CR per IRC were deemed in CR by investigator and received no subsequent therapy. Quantitative PET scoring could have improved concordance between IRC and investigator-assessed responses; however, this study used the 2007 International Working Group response criteria,23 because it was designed prior to the publication of the 2014 Lugano criteria.33 In addition, atypical response patterns with checkpoint inhibitors make PET interpretation more challenging using conventional response criteria. Updated criteria that account for these phenomena may allow more accurate evaluation of checkpoint inhibitor efficacy in future studies.34
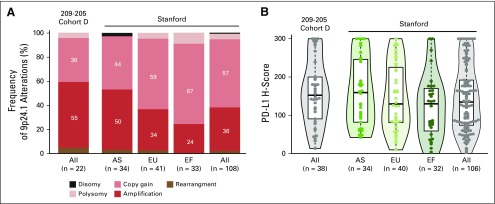
In a recently described cohort of patients with newly diagnosed cHL (the Stanford cohort), 9p24.1 amplification was significantly more common in patients with stage III to IV disease.7 Data from the currently reported multicenter phase II study confirmed this association; a similar proportion of patients in Cohort D had 9p24.1 amplification (55%) as among stage III to IV patients in the Stanford cohort (50%; Appendix Fig. A3).7 In the Stanford cohort, patients with 9p24.1 amplification had significantly shorter PFS after induction therapy.7 The current study addresses the possibility that the addition of PD-1 blockade may be particularly beneficial to patients with adverse clinical features, high-level 9p24.1 alterations, and increased PD-L1 expression. In this regard, it is notable that patients with higher PD-L1 expression had significantly higher response rates to N-AVD.
Initial data from Cohort D of CheckMate 205 suggest that combining the checkpoint inhibitor nivolumab with multiagent AVD chemotherapy is a promising and well-tolerated alternative treatment option for newly diagnosed, advanced-stage cHL. Longer follow-up and larger studies may confirm whether substitution of nivolumab for bleomycin in ABVD has long-term safety and OS benefits.
ACKNOWLEDGMENTS
The authors thank the patients, their families, and all co-investigators who participated in the trial. Donna Neuberg provided helpful input to the exploratory biomarker analysis. Medical writing and editorial support, under the direction of the authors, were provided by Adam Gill at Caudex and were funded by Bristol-Myers Squibb. The views expressed in this article are the authors’ own and not an official position of Bristol-Myers Squibb or the authors’ respective institutions. Bristol-Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
APPENDIX
FIG A1.
Patient disposition. (*) One patient experienced study drug toxicity during the monotherapy phase and received doxorubicin, vinblastine, and dacarbazine (AVD) only during combination therapy. cHL, classic Hodgkin lymphoma; ITT, intent-to-treat; N-AVD, nivolumab plus AVD.
FIG A2.
Change in target lesion per independent radiology review committee (IRC) at (A) end of monotherapy and (B) after two combination cycles. FDG-PET, 18F-labeled fluorodeoxyglucose–positron emission tomography.
FIG A3.
Chromosome 9p24.1 alterations and programmed death-ligand 1 (PD-L1) expression in diagnostic biopsies from patients enrolled in CheckMate 205 Cohort D and the Stanford cohort.7 (A) Frequency of 9p24.1 alterations in newly diagnosed patients from CheckMate 205 Cohort D (all) and the Stanford cohort (shown by clinical stage: advanced stage [AS], early stage-unfavorable [EU], early stage-favorable [EF], and all). The color key for 9p24.1 copy number alteration is the same as that used in Fig 3. (B) Distribution of PD-L1 H-scores in newly diagnosed patients from the same cohorts. Violin plots show the minimum, median, quartiles, and maximum.
TABLE A1.
Selection of Combination Therapy
TABLE A2.
Status of IRC Non-CR Patients at End of Therapy
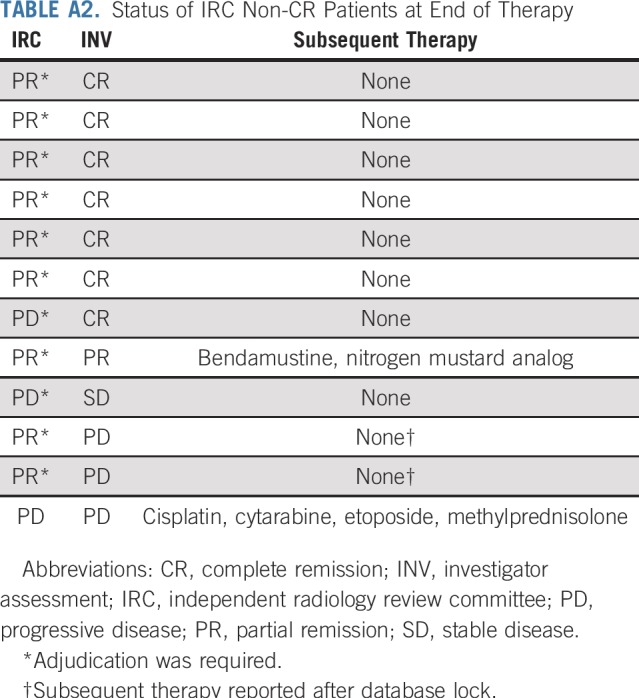
TABLE A3.
Best Overall Response per IRC in Patients Older Than 60 Years of Age (n = 6)
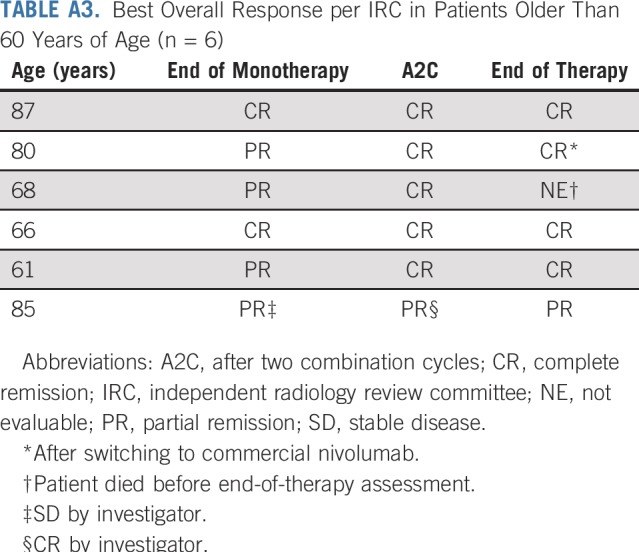
TABLE A4.
ORR and CR per IRC, by IPS Risk Subgroup
TABLE A5.
IPS Risk Stratification for All Patients and Patients With Available 9p24.1 FISH and PD-L1 IHC Data
Footnotes
Top-line results from CheckMate 205 Cohort D were presented at the American Society of Hematology Annual Meeting, Atlanta, GA, December 9 to 12, 2017; and the European Hematology Association Congress, Stockholm, Sweden, June 14 to 17, 2018.
Supported by Bristol-Myers Squibb, which also funded medical writing support. Also supported by the Center for Immuno-Oncology of the Dana-Farber Cancer Institute (S.J.R.), National Cancer Institute Grant No. R01 CA161026 (M.A.S.), and the Miller Family Fund (M.A.S.).
Clinical trial information: NCT02181738.
AUTHOR CONTRIBUTIONS
Conception and design: Radhakrishnan Ramchandren, Mariano Provencio, Margaret A. Shipp, Anne Sumbul, Philippe Armand, Stephen M. Ansell
Administrative support: Hun Ju Lee
Provision of study materials or patients: Antonio Rueda, Marek Trněný, Tatyana A. Feldman, Mariano Provencio, Christian Sillaber, Wolfgang Willenbacher, Scott J. Rodig
Collection and assembly of data: Radhakrishnan Ramchandren, Eva Domingo-Domènech, Antonio Rueda, Marek Trněný, Tatyana A. Feldman, Hun Ju Lee, Mariano Provencio, Christian Sillaber, Kerry J. Savage, Wolfgang Willenbacher, Azra H. Ligon, Jin Ouyang, Scott J. Rodig, Anne Sumbul, Philippe Armand
Data analysis and interpretation: Radhakrishnan Ramchandren, Eva Domingo-Domènech, Antonio Rueda, Marek Trněný, Hun Ju Lee, Mariano Provencio, Jonathon B. Cohen, Kerry J. Savage, Wolfgang Willenbacher, Azra H. Ligon, Robert Redd, Scott J. Rodig, Margaret A. Shipp, Mariana Sacchi, Anne Sumbul, Philippe Armand, Stephen M. Ansell
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Radhakrishnan Ramchandren
Consulting or Advisory Role: Seattle Genetics, Sandoz-Novartis, Pharmacyclics/Janssen, Bristol-Myers Squibb
Research Funding: Merck (Inst), Seattle Genetics (Inst), Janssen (Inst), Genentech (Inst)
Eva Domingo-Domènech
Consulting or Advisory Role: Takeda
Research Funding: Bristol-Myers Squibb, Takeda
Travel, Accommodations, Expenses: Takeda
Antonio Rueda
Honoraria: Roche, Bristol-Myers Squibb, Merck Serono
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, Merck Serono, MSD, Takeda, Novartis
Marek Trněný
Honoraria: Janssen, Gilead Sciences, Takeda, Bristol-Myers Squibb, Amgen, AbbVie, Roche, MorphoSys, Incyte
Consulting or Advisory Role: Takeda, Bristol-Myers Squibb, Incyte, AbbVie, Amgen, Roche, Gilead Sciences, Janssen, Celgene, MorphoSys
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Bristol-Myers Squibb, Roche, Janssen, AbbVie
Tatyana A. Feldman
Honoraria: Seattle Genetics, Pharmacyclics/Janssen, AbbVie, Bristol-Myers Squibb, Kite Pharma, Bayer, Takeda
Consulting or Advisory Role: Seattle Genetics, Bayer, Bristol-Myers Squibb
Speakers' Bureau: Seattle Genetics, Kite Pharma, Pharmacyclics, AbbVie, Janssen, Celgene
Research Funding: Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Portola Pharmaceuticals (Inst), Eisai (Inst), Kyowa Hakko Kirin (Inst), Amgen (Inst), Viracta Therapeutics (Inst), Cell Medica (Inst), Kyowa Hakko Kirin (Inst), Roche (Inst), Trillium Therapeutics (Inst), Pfizer (Inst), Viracta Therapeutics (Inst)
Travel, Accommodations, Expenses: Seattle Genetics, Kite Pharma, Pharmacyclics, AbbVie, Takeda
Mariano Provencio
Consulting or Advisory Role: Bristol-Myers Squibb, Boehringer Ingelheim, Roche, MSD, Pfizer, Novartis
Research Funding: Pierre Fabre (Inst), Roche (Inst), Boehringer Ingelheim (Inst), Bristol-Myers Squibb (Inst)
Christian Sillaber
Consulting or Advisory Role: Celgene, Bluebird Bio
Speakers' Bureau: Celgene, Bluebird Bio
Jonathon B. Cohen
Consulting or Advisory Role: Celgene, Seattle Genetics, Novartis, Infinity Pharmaceuticals, AbbVie, Janssen
Research Funding: Bristol-Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Takeda (Inst), AI Therapeutics (Inst), Genentech (Inst), ASH (Inst), Lymphoma Research Foundation (Inst)
Kerry J. Savage
Honoraria: Seattle Genetics, Bristol-Myers Squibb, Merck, Takeda
Consulting or Advisory Role: Seattle Genetics, Bristol-Myers Squibb, Merck, Servier, Takeda, Verastem
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Seattle Genetics
Wolfgang Willenbacher
Honoraria: Celgene, Roche, Amgen, Gilead Sciences, Janssen, Novartis, Takeda
Consulting or Advisory Role: Celgene, Roche, Amgen, Gilead Sciences, Bristol-Myers Squibb, Merck, Takeda, Novartis, Janssen
Speakers' Bureau: Gilead Sciences
Research Funding: Takeda, Amgen, Celgene, Janssen
Azra H. Ligon
Leadership: Travera (I)
Stock and Other Ownership Interests: Travera (I)
Consulting or Advisory Role: Travera (I)
Scott J. Rodig
Honoraria: Perkin Elmer, Bristol-Myers Squibb
Consulting or Advisory Role: AstraZeneca, Perkin Elmer
Research Funding: Bristol-Myers Squibb, Merck, Affimed Therapeutics, Kite Pharma
Patents, Royalties, Other Intellectual Property: Patent pending for use of anti-galectin1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb
Margaret A. Shipp
Honoraria: Bristol-Myers Squibb, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst), Bayer (Inst), Merck (Inst)
Anne Sumbul
Employment: Bristol-Myers Squibb
Philippe Armand
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Pfizer, Affimed Therapeutics, Adaptive Biotechnologies
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Affimed Therapeutics (Inst), Adaptive Biotechnologies (Inst), Genentech (Inst), Tensha Therapeutics (Inst), Otsuka (Inst), Sigma-Tau (Inst)
Travel, Accommodations, Expenses: Genmab
Stephen M. Ansell
Honoraria: WebMD, Research to Practice, Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Affimed Therapeutics (Inst), Regeneron (Inst), Pfizer (Inst), LAM Therapeutics (Inst), Trillium Therapeutics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: Report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 2.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 4.Allen PB, Gordon LI. Frontline therapy for classical Hodgkin lymphoma by stage and prognostic factors. Clin Med Insights Oncol. 2017;11:1179554917731072. doi: 10.1177/1179554917731072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 7.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roemer MGM, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol. 2018;36:942–950. doi: 10.1200/JCO.2017.77.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36:1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131:1183–1194. doi: 10.1182/blood-2017-10-811224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 12.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 13.Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of Adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: A comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161:76–86. doi: 10.1111/bjh.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böll B, Görgen H, Fuchs M, et al. ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol. 2013;31:1522–1529. doi: 10.1200/JCO.2012.45.4181. [DOI] [PubMed] [Google Scholar]
- 15.Wongso D, Fuchs M, Plütschow A, et al. Treatment-related mortality in patients with advanced-stage Hodgkin lymphoma: An analysis of the German Hodgkin study group. J Clin Oncol. 2013;31:2819–2824. doi: 10.1200/JCO.2012.47.9774. [DOI] [PubMed] [Google Scholar]
- 16.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to International Prognostic Score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 18.Press OW, Li H, Schöder H, et al. US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34:2020–2027. doi: 10.1200/JCO.2015.63.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152:551–560. doi: 10.1111/j.1365-2141.2010.08485.x. [DOI] [PubMed] [Google Scholar]
- 20.Trotman J, Fosså A, Federico M, et al. Response-adjusted therapy for advanced Hodgkin lymphoma (RATHL) trial: Longer follow up confirms efficacy of de-escalation after a negative interim PET scan (CRUK/07/033) Hematol Oncol. 2017;35:65–67. [Google Scholar]
- 21.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 24.Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 25.Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: Results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi trial. J Clin Oncol. 2009;27:805–811. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- 26.Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–1427. doi: 10.1016/S0140-6736(14)61469-0. [DOI] [PubMed] [Google Scholar]
- 27.Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23:7614–7620. doi: 10.1200/JCO.2005.02.7243. [DOI] [PubMed] [Google Scholar]
- 28.Willenbacher W, Mumm A, Bartsch HH. Late pulmonary toxicity of bleomycin. J Clin Oncol. 1998;16:3205. [PubMed] [Google Scholar]
- 29.Noone AM, Howlader N, Krapcho M, et al. (eds): SEER Cancer Statistics Review, 1975-2015. Bethesda, MD, National Cancer Institute, 2018. https://seer.cancer.gov/archive/csr/1975_2015/
- 30. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Hodgkin lymphoma. V3.2018. Fort Washington, PA, National Comprehensive Cancer Network, 2018. https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. [Google Scholar]
- 31. doi: 10.1200/JCO.2018.79.0139. Evens AM, Advani RH, Helenowski IB, et al: Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol 10.1200/JCO.2018.79.0139 [epub ahead of print on September 4, 2018] [DOI] [PubMed] [Google Scholar]
- 32. Stephens DM, Li H, Schoder H, et al: Long-term follow-up of SWOG S0816: Response-adapted therapy for stage III/IV Hodgkin Lymphoma demonstrates limitations of PET-adapted approach. Presented at the American Society of Hematology Annual Meeting, San Diego, December 1-4, 2018. [Google Scholar]
- 33.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–2496. doi: 10.1182/blood-2016-05-718528. [DOI] [PubMed] [Google Scholar]