Abstract
Obesity and lack of physical exercise are associated with the increase of diabetes mellitus in women of reproductive age and during the gestational period. The objective of the present study was to evaluate physical activity levels during the pregnancy and postpartum periods and the influence of body mass index (BMI) in women with gestational diabetes mellitus (GDM) or low risk pregnancy. The Pregnancy Physical Activity Questionnaire (PPAQ), translated and validated for Portuguese, was used for the evaluation of physical activity (PA) level. The sample was stratified according to preconception BMI and the presence or absence of diagnosis of GDM, resulting in four groups with 66 participants each: low risk pregnancy (LRP) with normal weight (BMI ≥ 18.5 and ≤ 24 kg/m2), LRP and overweight/obese (BMI ≥ 25 kg/m2), GDM with normal weight and GDM with overweight/obese. The level of PA of each participant was measured as Metabolic Equivalent of Task (MET) during the preconceptional period (T0), in the third trimester of gestation (T1), and three months after delivery (T2). The comparison of the MET values showed that the values found in the evaluation three months after delivery (T2) were higher than 1.00 (1.10 MET for the LRP-normal weight, 1.06 MET for LRP-overweight/obese, 1.02 MET for the GDM- normal weight, 1.07 MET for the GDM-overweight/obese). On the pre-gestational (T0) and third trimester (T1) analyzes, the values were less than 1.00 MET. The analysis between groups in relation to BMI and diagnosis of GDM showed no difference.
Introduction
Lack of physical exercise associated with unhealthy diet contributes to obesity because they cause a change in body composition and alter the proportions of insulin receptors in muscles and adipose tissue. The prioritization of insulin action on adipocyte receptors leads to lower glucose uptake than to muscle receptors and consequently hyperglycemia, increased production of pancreatic insulin, and decreased body sensitivity to insulin [1]. Thus, the lack of regular exercise and obesity have been associated in the last decades to the increase in the prevalence of diabetes mellitus in the world population and, consequently, in women of reproductive age and during pregnancy. [2–4].
In 2015, the global prevalence of hyperglycemia first detected at any time during pregnancy was 16.9%, corresponding to 21.4 million exposed live-born infants [5]. In view of the severity of maternal-fetal and perinatal complications resulting from hyperglycemia [6,7], this metabolic disorder should be prevented or early controlled, with regular physical activity being one of the main strategies [8].
As a result of the several proven benefits, physical activity (PA) during pregnancy is usually recommended by different institutions, except in situations in which there are contraindication due to obstetric or clinical causes. The recommendation is a minimum of 30 minutes of moderate PA at least five times per week or totaling 150 minutes per week, avoiding intervals of more than 2 consecutive days without exercise [9–13]. However, despite the consolidated recommendations of exercise during pregnancy, a reduction in exercise levels are frequently observed during this period [8,12,14–20]. For example, in the United States a study showed that sixty percent of women reported not engaging in leisure time physical activity, and even for those who reported exercising regularly, physical activity progressively decreased by trimester [21].
Considering the evident need to elaborate and integrate exercise programs in prenatal care that combine effectiveness and greater adherence of the pregnant population, it is essential to estimate the level of PA in the population at low and intermediate obstetric risk, as well as to identify the variables that contribute to this scenario. The objective of the present study was to evaluate the influence of body mass index (BMI) in women with GDM on PA levels during gestational and postpartum periods.
Patients and methods
The study was approved by the Ethics Committee of the University Hospital of the Ribeirão Preto Medical School, University of São Paulo (HCFMRP-USP) (Approval No. 1.358.154) and did not interfere with the obstetric management adopted for the selected patients. We selected women ≥ 18 years, literate, with singleton pregnancies and gestational age > 32 weeks who had no contraindication to exercise and who were under low-risk prenatal follow-up, defined as women without any pathology except overweight or obesity, or were diagnosed with GDM at the Women’s Health Referral Center of Ribeirão Preto—MATER or at HCFMRP-USP between January 2016 and February 2017.
The pregnant women who accepted to participate in the study were divided into four groups according to gestational risk and BMI (normal weight BMI: ≥ 18.5 and ≤ 24.9 kg/m2; overweight/obese BMI: ≥ 25 kg/m2): low-risk pregnancy and normal weight BMI (LRP-BMI 0); low-risk pregnancy and overweight/obese BMI (LRP-BMI 1); GDM and normal weight BMI (GDM-BMI 0); GDM and overweight/obese BMI (GDM-BMI 1) (Fig 1). We selected the groups by convenience sampling methods. Each group should have at least 66 patients, considering a level of significance of 5% and power of the test of 80%.
Fig 1. Flowchart of the study casuistry.
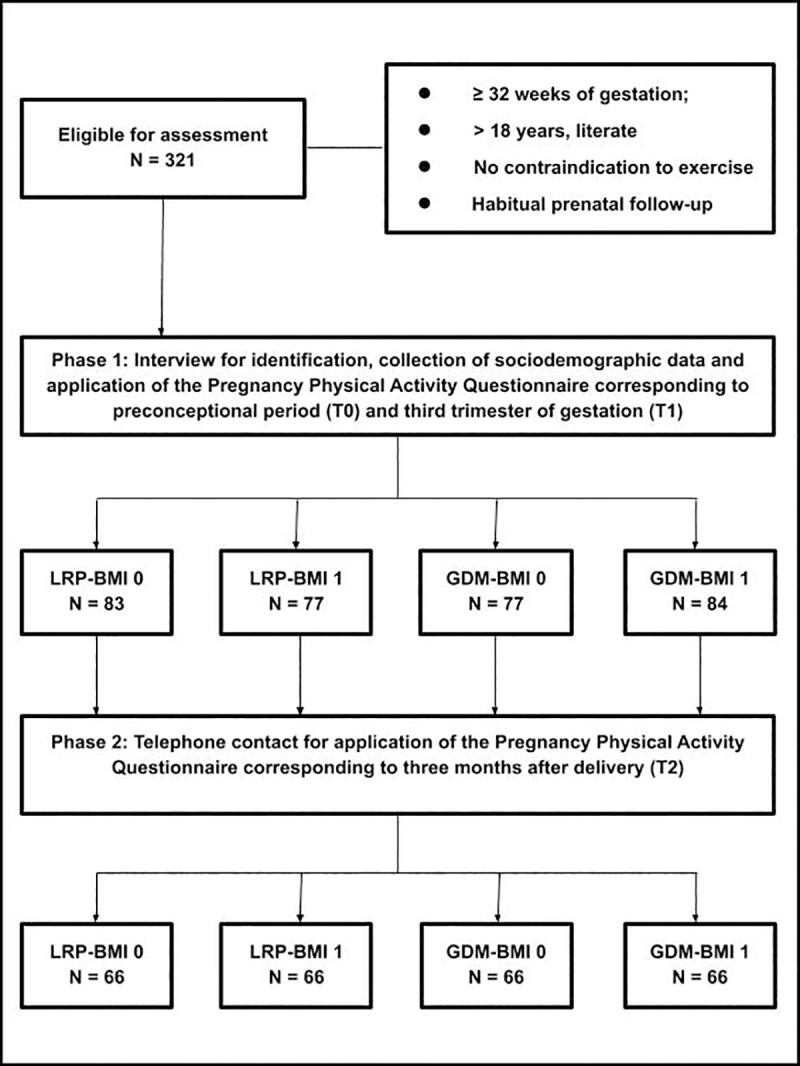
LRP = low-risk pregnancy; GDM = gestational diabetes mellitus; BMI 0 = normal weight body mass index; BMI 1 = overweight/obesity.
After inclusion and signing the free informed consent form, face-to-face interviews were held with the pregnant women to collect data such as maternal age, skin color, years of schooling, alcohol consumption, smoking, and self-reported preconception weight and height for characterization of the sample. The socioeconomic status of the patients was evaluated using the Brazilian Economic Classification Criteria [22] (Fig 1).
Physical activity was evaluated using the Pregnancy Physical Activity Questionnaire (PPAQ) [23], translated and validated for the Portuguese language by Silva et al [24]. The responses to the items of the questionnaire indicate the average time spent in each PA domain (in minutes or hours): leisure, household, caregiving, sports and exercise, transportation, and occupation. The women answered the PPAQ indicating the responses regarding the time spent that best fitted their activities during the preconception period (T0—referred about one month before pregnancy) and on the occasion of the interview (last trimester = T1). No instructions about additional PA were given during the interview to avoid interference with the prenatal follow-up of the patients. Three months after delivery (T2), the patients were contacted by telephone (data obtained from the hospital records) and responded again to the questionnaire for the period in question (Fig 1).
The level of PA was determined as metabolic equivalent (MET), which is defined as the energy expenditure at rest. MET is considered equivalent to oxygen consumption or VO2 (1 MET = 3.5 ml O2/kg/min) and to energy expenditure (1 MET = 1 kcal/kg/h) [25]. This resting value is used to quantify other activities, with each activity being expressed as a multiple of the resting metabolic rate [25]. Each activity is classified according to its intensity as sedentary (< 1.5 METs), light (1.5–2.9 METs), moderate (3.0–6.0 METs), or vigorous (> 6.0 METs) [23, 26, 27].
Differences in the qualitative variables between groups were determined by the chi-squared test and in the quantitative variables by analysis of variance. Tukey’s post-test was applied when necessary. A mixed effects linear regression model was fitted to compare the groups at each time point and time points in each group. The analysis was stratified by BMI and residual analysis using normality and dispersion graphs was performed for adjustment of the model. MET was the dependent variable and time and group were the independent variables. The model was implemented in the SAS 9.3 program using the PROC MIXED procedure.
Results
The flowchart (Fig 1) shows the number of pregnant women in each phase of the study as well as the inclusion criteria. The initially calculated sample size was 264 patients, but the number of patients in each group was increased on average by 20% because of possible follow-up losses three months after delivery when the patients were contacted by telephone call. Thus, the initial sample consisted of 321 patients that answered the PPAQ regarding preconceptional and pregnancy periods, including 77 patients in LRP-BMI 1, 77 in GDM-BMI 0, 83 in LRP-BMI 0, and 84 in GDM-BMI 1. As a result of the loss to follow-up due to the impossibility of telephone contact after 10 attempts at different periods of the day during one week, the final sample for data collection by telephone contact 3 months after delivery consisted of 264 patients, with 66 patients per group.
The mean age (± standard deviation) of pregnant women with GDM was 26.55 (± 6.2) years in the BMI 0 group and 30.25 (± 6.03) years in the BMI 1 group, while the mean age of LRP women was 24.8 (± 5.16) and 27.52 (± 5.75) years in the BMI 0 and BMI 1 groups, respectively (Table 1). The mean maternal age was significantly higher in the GDM-BMI 1 group when compared to the GDM-BMI 0 and LRP-BMI 0 groups.
Table 1. Maternal clinical and sociodemographic characteristics (N = 321).
| Variable | Group | |||||
|---|---|---|---|---|---|---|
| LRP | GDM | |||||
| BMI 0 (N = 83) |
BMI 1 (N = 77) |
BMI 0 (N = 77) |
BMI 1 (N = 84) |
P | ||
| Age and gestational history | Maternal age | 24.80 (±5.16) | 27.52 (±5.75) | 26.55 (±6.20) | 30.25 (±6.03) | <0.0001⁑ |
| No. of pregnancies | 2.02 (±1.22) | 2.34 (±1.26) | 2.34 (±1.53) | 2.49 (±1.59) | 0.184⁑ | |
| Parity | 0.86 (±1.12) | 1.16 (±1.08) | 1.12 (±1.33) | 1.18 (±1.40) | 0.1823⁑ | |
| Abortions | 0.19 (±0.48) | 0.18 (±0.51) | 0.22 (±0.62) | 0.31 (±0.54) | 0.124⁑ | |
| Habits (N, %) |
Smoking | 6 (7.79%) | 4 (5.33%) | 5 (7.35%) | 5 (6.10%) | 0.926☼ |
| Alcohol consumption | 1 (1.30%) | 0 (0%) | 1 (1.47%) | 0 (0%) | 0.5327☼ | |
| Skin color (N, %) |
Non-white | 33 (39.76%) | 36 (46.75%) | 35 (45.45%) | 31 (36.90%) | 0.5406☼ |
| White | 50(60.24%) | 41 (53.25%) | 42 (54.55%) | 53 (63.10%) | ||
| Years of schooling (N, %) |
< 8 | 7 (8.43%) | 10 (12.99%) | 13 (16.88%) | 17 (20.24%) | |
| = 8 | 12 (14.46%) | 9 (11.69%) | 11 (14.29%) | 5 (5.95%) | ||
| > 8 and < 11 | 21 (25.30%) | 17 (22.08%) | 22 (28.57%) | 13 (15.48%) | 0.073☼ | |
| = 11 | 42 (50.60%) | 39 (50.65%) | 27 (35.06%) | 42 (50%) | ||
| > 12 | 1 (1.20%) | 2 (2.60%) | 4 (5.19%) | 7 (8.33%) | ||
| Household income, US$ (N, %) |
6,297.07 | 0 | 0 | 1 (1.30%) | 0 | |
| 2,758.58 | 0 | 0 | 0 | 3 (3.61%) | 0.1791☼ | |
| 1,374.96 | 10 (12.20%) | 13 (16.88%) | 11 (14.29%) | 15 (18.07%) | ||
| 748.14 | 25 (30.49%) | 24 (31.17%) | 16 (20.78%) | 25 (30.12%) | ||
| 449.15 | 36 (43.90%) | 30 (38.96%) | 31 (40.26%) | 26 (31.33%) | ||
| 198.70 | 11 (13.41%) | 10 (12.99%) | 18 (23.38%) | 14 (16.87%) | ||
| Route of delivery (N, %) |
Cesarean | 18 (23.38%) | 28 (37.33%) | 20 (29.41%) | 34 (41.46%) | 0.0751☼ |
| Vaginal | 59 (76.62%) | 47 (62.67%) | 48 (70.59%) | 48 (58.54%) | ||
LRP-BMI 0 = low-risk pregnancy and normal weight body mass index (≥ 18.5 and ≤ 24.9 kg/m2); LRP-BMI 1 = low-risk pregnancy and overweight/obese body mass index (≥ 25 kg/m2); GDM-BMI 0 = gestational diabetes mellitus and normal weight body mass index (≥ 18.5 and ≤ 24.9 kg/m2); 2; GDM-BMI 1 = gestational diabetes mellitus and overweight/obese body mass index (≥ 25 kg/m2); SD = standard deviation.
☼Chi-squared test
⁑Variance analysis
The number of pregnancies ranged from 2.02 to 2.49 (standard deviation of 1.22 to 1.59), with no difference between groups. The groups were homogenous in terms of the mean number of abortions, parity, alcohol consumption, and smoking (Table 1).
In all groups, most women belonged to socioeconomic class C2, which corresponds to a household income of US$ 449.15 (GDM-BMI 0 = 40.26%; GDM-BMI 1 = 31.33%; LRP-BMI 0 = 43.9%; LRP-BMI 1 = 38.96%). The second most prevalent socioeconomic class was C1, which corresponds to a household income of US$ 748.14 (20.78%, 30.12%, 30.49% and 31.17%, respectively) (Table 1).
The time factor was the most important parameter in the assessment of PA. Higher levels of PA were observed in the postpartum period when compared to the preconceptional and pregnancy periods, regardless of BMI stratification and identification of GDM (Table 2). Mean MET values > 1 were observed in all groups 3 months after delivery (T2) (1.10 MET for GDM-BMI 0 and 1.06 MET for GDM-BMI 1; 1.02 MET for LRP-BMI 0 and 1.07 MET for LRP-BMI 1). In contrast, MET values < 1 were calculated from the information reported by the patients in reference to the preconceptional period (T0) and in the third trimester (T1) (Table 2).
Table 2. Distribution of metabolic equivalent (MET) at each time point in the different groups according to body mass index category (N = 321).
| BMI | Group | Time | N | Mean ±SD | Median (Q1 –Q3) | Min–Max |
|---|---|---|---|---|---|---|
| Normal weight (18,5–24,9 Kg/m2) |
LRP | 0 | 83 | 0.82 ± 0.46 | 0.68 (0.49–1.15) | 0.10–2.27 |
| 1 | 83 | 0.65 ± 0.35 | 0.57 (0.38–0.82) | 0.11–1.55 | ||
| 2 | 66 | 1.02 ± 0.26 | 1.01 (0.91–1.15) | 0.17–1.86 | ||
| GDM | 0 | 77 | 0.81 ± 0.44 | 0.75 (0.49–1.06) | 0.06–2.05 | |
| 1 | 77 | 0.69 ± 0.42 | 0.56 (0.39–0.94) | 0.08–2.01 | ||
| 2 | 66 | 1.10 ± 0.35 | 1.06 (0.94–1.21) | 0.40–2.30 | ||
| Overweight/ obesity (≥ 25 Kg/m2) |
LRP | 0 | 77 | 0.99 ± 0.42 | 1.02 (0.70–1.25) | 0.14–1.95 |
| 1 | 77 | 0.76 ± 0.36 | 0.71 (0.46–1.03) | 0.15–1.66 | ||
| 2 | 66 | 1.07 ± 0.29 | 1.11 (0.87–1.27) | 0.49–1.93 | ||
| GDM | 0 | 84 | 0.80 ± 0.42 | 0.78 (0.46–1.02) | 0.15–2.09 | |
| 1 | 84 | 0.71 ± 0.37 | 0.61 (0.44–0.90) | 0.17–2.04 | ||
| 2 | 66 | 1.06 ± 0.36 | 1.05 (0.81–1.24) | 0.27–1.88 |
BMI = body mass index; LRP = low-risk pregnancy; GDM = gestational diabetes mellitus; time 0 = preconceptional; time 1 = third trimester of gestation; time 2 = 3 months after delivery; N = number of pregnant women; SD = standard deviation; Q1 = first quartile; Q3 = third quartile; Min = minimum; Max = maximum.
Distribution of quantitative variables in relation to the groups.
Comparison of time points in GDM-BMI 0 and GDM-BMI 1 showed a difference in mean METs at T2 when compared to T1 and the same difference was observed in mean METs at T2 when compared with to T0. Comparing the mean METs presented by the LRP-BMI group 0, a difference was observed between the three moments evaluated. In LRP-BMI 1, it was found difference between T2 and T1 and between T1 and T0 (Table 3). Finally, comparison of the GDM and LRP groups with overweight/obese BMI showed lower mean METs in the former at T0 (Table 3).
Table 3. Estimated difference between means for comparisons between time points and between groups in each body mass index category (N = 321).
| BMI | Comparison | Estimated difference between means | 95% CI | P | |
|---|---|---|---|---|---|
| Normal Weight (18.5 to 24.9 kg/m2) | (T0–T1) GDM | 0.1216 | 0.03371 | 0.2096 | 0.0069 |
| (T0–T2) GDM | -0.2962 | -0.3889 | -0.2036 | <0.0001 | |
| (T1–T2) GDM | -0.4179 | -0.5106 | -0.3252 | <0.0001 | |
| (T0–T1) LRP | 0.1778 | 0.09306 | 0.2624 | <0.0001 | |
| (T0–T2) LRP | -0.2136 | -0.3056 | -0.1216 | <0.0001 | |
| (T1–T2) LRP | -0.3914 | -0.4833 | -0.2994 | <0.0001 | |
| T0 (GDM—LRP) | -0.01339 | -0.1353 | 0.1085 | 0.829 | |
| T1 (GDM—LRP) | 0.04272 | -0.07917 | 0.1646 | 0.4909 | |
| T2 (GDM—LRP) | 0.06924 | -0.06092 | 0.1994 | 0.296 | |
| Overweight/ obesity (≥ 25 kg/m2) | (T0–T1) GDM | 0.08677 | 0.002436 | 0.1711 | 0.0438 |
| (T0–T2) GDM | -0.2729 | -0.3645 | -0.1814 | <0.0001 | |
| (T1–T2) GDM | -0.3597 | -0.4513 | -0.2681 | <0.0001 | |
| (T0–T1) LRP | 0.2305 | 0.1422 | 0.3188 | <0.0001 | |
| (T0–T2) LRP | -0.06306 | -0.1561 | 0.02996 | 0.1831 | |
| (T–T2) LRP | -0.2936 | -0.3863 | -0.2008 | <0.0001 | |
| T0 (GDM—LRP) | -0.1960 | -0.3127 | -0.07935 | 0.0011 | |
| T1 (GDM—LRP) | -0.05229 | -0.1690 | 0.06441 | 0.3786 | |
| T2 (GDM—LRP) | 0.01386 | -0.1116 | 0.1393 | 0.828 | |
GDM = gestational diabetes mellitus; LRP = low-risk pregnancy; BMI = body mass index; 95% CI = 95% confidence interval; T0 = preconceptional; T1 = third trimester of gestation; T2 = 3 months after delivery.
Mixed effects regression model.
Discussion
Comparison of time points showed a difference in METs postpartum compared to the third trimester of pregnancy and preconceptional periods in women with gestational diabetes mellitus and normal weight BMI and in women with gestational diabetes mellitus and overweight/obese BMI. On the other hand, in women with low-risk pregnancy and normal weight BMI, a difference in mean METs was observed between all time points. In women with low-risk pregnancy and overweight/obese BMI, a difference was found between pospartum and the third trimester of gestation periods and between this period and preconceptional period. This increase in mean METs over the period of 3 months after delivery might be due to the care demands of the newborn, which remain more intense up to 12 months after birth [28, 29]. It is emphasized that, regardless of the differences observed in intra and intergroup analysis, from a clinical point of view, no change in PA level was observed, with all participants continuing to be classified as sedentary (MET < 1.5) [27].
There is considerable heterogeneity in the classification and methods used to evaluate PA level. This controversy explains the differences found in the prevalence of sedentarism and impairs the comparison between studies involving different populations [30].
In contrast to the present study, other authors reported a reduction in PA level after birth due to the existence of personal and environmental barriers to exercise and sports. Personal factors include the influence of household income, care with other children, lack of a partner during exercise, and lack of exercise support from family [19, 31–33]. Another important personal factor are the high care needs of the newborn, highlighted in other studies as impairing the participation in sports and regular exercises and contributing to lower PA levels. However, it should be noted that the populations studied had higher initial PA levels than the sample of the present study [34]. Environmental factors that contribute to exercising less include those that cannot be controlled by the mother, such as access to public transportation, safe leisure-time facilities, and lack of a health information system [34].
The sedentary PA level is usually more common among individuals from lower socioeconomic classes [35]. The sample of the present study was predominantly composed of patients of socioeconomic class with a family income of approximately US $ 449.15, corresponding to 41.88% in the women with low-risk pregnancy group and to 35.63% in the women with gestational diabetes mellitus group. This finding might explain the predominance of sedentary level observed. A study conducted in Pelotas, Brazil, which involved more than 5,000 young adults who responded to the short version of the International Physical Activity Questionnaire, showed a lower than expected increase in PA rates from 41.1% in 2002 to 52.0% in 2007 in the low-income population. The responsible factors for decrease of physical activity would be industrial mechanization and a consequent decrease in human activity (considering that low-income workers tend to participate in activities related to manual labor), increased use of public transportation, as well as an increase in purchasing power which permits the acquisition of motorcycles and cars, thus reducing transport-related activity [36].
Alarming rates of sedentary PA level are observed in different countries. In the United States, children and adults spend approximately 55% of their waking hours (7.7 hours/day) in activities that result in very low levels of energy expenditure. This estimate reaches almost 60% (more than 8 hours/day) in adolescents and adults between 60 and 85 years of age. Regarding gender, women are more sedentary than men [37].
The education level is directly related to a reduction in PA level. Pregnant women with higher education spend fewer hours in sedentary activities than those who only have a high school diploma [38]. Data from the Brazilian Institute of Geography and Statistics have shown that the larger the number of years of schooling, the higher the percentage of individuals practicing some sport [38]. In addition to education level, the socioeconomic class seems to influence the participation in PA and sports, which is more widespread in classes of higher monthly household income per capita [39, 40]. Taken together, our results of low PA levels are compatible with the low education level of the patients.
Specifically for leisure-time PA, studies also suggest an association between the high prevalence of inactivity and sociodemographic indicators [41]. The lower the education level and household income, the lower the chance of engagement in leisure-time PA because of the lack of social and environmental resources and information [42, 43].
With respect to the impact on physical health, exercise is related to the prevention and treatment of diseases such as GDM, gestational hypertension and obesity [44–48], while sedentarism results in a significant increase in health expenditures [49,50]. Specifically for diabetes, an important aspect is the economic impact caused by the disease and its complications, with substantial economic loss to patients and their families, as well as to health systems and national economies [4]. This economic burden is expressed directly as medical costs and is indirectly associated with the loss of productivity, premature mortality and a negative impact on the nation’s gross domestic product [4]. In 2011, the Harvard School of Public Health reported global gross domestic product losses due to direct and indirect costs from diabetes totaling US$ 1.7 trillion [51].
The homogenous finding of sedentary PA level in the sample studied contrasts with the consolidated concepts of the relevant benefits of PA during pregnancy and postpartum for the mother-fetus and newborn [44, 52–55], but is compatible with the global epidemiological scenario. A recent review demonstrated that pregnant women spend more than 50% of their time (57.1 to 78%) in sedentary activities, i.e., those using less than 1.5 MET [30].
Despite these data, programs or booklets with detailed information and instructions that incorporate exercises in the routine of the pregnant population are sparse in Brazil. It is therefore necessary that health managers and healthcare workers from the private and public sector elaborate exercise programs for all pregnant women [46].
This study presented some limitations to be considered. This was an observational study with a casuistry composed of women belonging to socioeconomic stratification predominantly with shorter schooling time and low family income, with no representation of all socioeconomic classes. It should be emphasized that the instrument used is characterized by a subjective measure in the form of a questionnaire, which was not compared with a direct physical activity measures, which are considered to present better precision.
Conclusion
In the present study, neither BMI nor a diagnosis of GDM interfered with the PA level of pregnant women. On the other hand, the time factor, specifically the postpartum period, determined higher PA levels when compared to preconception and pregnancy in both women with low-risk pregnancy and women with a diagnosis of GDM. Despite the statistical difference, there was no clinical relevance in the MET's difference because the patients still remained on the sedentary physical activity level according to PPAQ.
In view of the growing sedentary PA level in the population studied, the importance of exercise for the prevention and treatment of GDM and the severe complications of hyperglycemia, the development of public health policies providing safer instructions and exercise programs for pregnant women should be reinforced.
Supporting information
Reproduced from Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004; 36(10): 1750–60.
(PDF)
(PDF)
Brazilian economic Classification Criteria from Associação Brasileira de Empresas e Pesquisas (Brazilian Association of Companies and Research).
(PDF)
L Time 0: preconceptional period; Time 1: third trimester of gestation; Time 2: three months after delivery; GDM: gestational diabetes mellitus; LRP: low-risk pregnancy; BMI: body mass index; Normal weight (≥ 18,5 and ≤ 24,9 Kg/m2); Overweight/Obese (≥ 25 Kg/m2); MET: metabolic equivalent of task.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), wich provided a master scholarship to CSO (1538886 / 2015-2017). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eaton SB, Eaton SB. Physical Inactivity, Obesity, and Type 2 Diabetes: An Evolutionary Perspective, Res Q Exerc Sport. 2017; 88(1):1–8. 10.1080/02701367.2016.1268519 [DOI] [PubMed] [Google Scholar]
- 2.Chasan-Taber L, Schmidt MD, Pekow P, Sternfeld B, Manson JE, Solomon CG et al. Physical Activity and Gestational Diabetes Mellitus among Hispanic Women. J Womens Health. 2008; 17(6):999–1008. [DOI] [PubMed] [Google Scholar]
- 3.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: Implications for the application of screening guidelines. Aust NZ J Obstet Gynaecol. 2011; 51:26–30 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Report on Diabetes 2016. Publications of the World Health Organization are available on the WHO website (http://www.who.int).
- 5.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017; 128:40–50. 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 6.Negrato CA, Mattar R e, Gomes MB. Adverse pregnancy outcomes in women with diabetes. Diabetol Metab Syndr. 2012; 4(1): 41 10.1186/1758-5996-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feghali MN, Abebe KZ, Comer Dm, Caritis S, Catov JM, Scifres CM. Pregnancy outcomes in women with an early diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2018; 8 (138): 177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Obstetrician and Ginecologists Committee opinion Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002; 99(1): 171–3. [DOI] [PubMed] [Google Scholar]
- 10.Colbert SR, Castorino K e, Javanovič L. Prescribing physical activity to prevent and manage gestational diabetes. World J Diabetes. 2013; 4 (6): 256–62. 10.4239/wjd.v4.i6.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2015; 38(1): S1–S2. [DOI] [PubMed] [Google Scholar]
- 12.American College Obstetrics and Gynecology. ACOG Committee opinion. Number 650, December 2015: Exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015; 126 (6): 135–42. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016; 39: 2065–79. 10.2337/dc16-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magro-Malosso ER, Saccone G, Di Tommaso M, Roman A, Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(8): 921–31. 10.1111/aogs.13151 [DOI] [PubMed] [Google Scholar]
- 15.Perales M, Artal R, Lucia A. Exercise during pregnancy. JAMA. 2017; 317(11):1113–14. 10.1001/jama.2017.0593 [DOI] [PubMed] [Google Scholar]
- 16.Sklempe Kokic I, Ivanisevic M, Kokic T, Simunic B, Pisot R. Acute responses to structured aerobic and resistance exercise in women with gestational diabetes mellitus. Scand J Med Sci Sports. 2018. [DOI] [PubMed] [Google Scholar]
- 17.Borodulin K, Evenson KR, Wen F, Herring AH, Benson AM. Physical activity patterns during pregnancy. Med Sci Sports Exerc.2008; 40(11), 1901–08. 10.1249/MSS.0b013e31817f1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaston A e, Cramp A. Exercise during pregnancy: a review of patterns and determinants. J Sci Med Sport. 2011; 14(4): 299–305. 10.1016/j.jsams.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 19.Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM et al. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore Matern Child Health J. 2015; 19(11):2523–35. [DOI] [PubMed] [Google Scholar]
- 20.Hayman M, Short C, Reaburn P. An investigation into the exercise behaviours of regionally based Australian pregnant women. J Sci Med Sport. 2016; 19 (8): 664–8. 10.1016/j.jsams.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 21.Di Mascio D, Magro-Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2016; 215(5):561–571 10.1016/j.ajog.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 22.ABEP—Associação Brasileira de Empresas e Pesquisas: Critério de Classificação Econômica Brasil 2015 –CCEB 2015. http://www.abep.org/criterio-brasil. Accessed October 4, 2015. Portuguese.
- 23.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004; 36(10): 1750–60. [DOI] [PubMed] [Google Scholar]
- 24.Silva FT, Araujo Júnior E, Santana EF, Lima JW, Cecchino GN, Silva Costa FD. Translation and cross-cultural adaptation of the Pregnancy Physical Activity Questionnaire (PPAQ) to the Brazilian population. Ceska Gynekol. 2015; 80(4): 290–98. [PubMed] [Google Scholar]
- 25.Byrne NM, Hills AP, Hunter GR, Weinsier RL, Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol. 2005; 99(3): 1112–9. 10.1152/japplphysiol.00023.2004 [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993; 25(1): 71–80. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000; 32(9): S498–504. [DOI] [PubMed] [Google Scholar]
- 28.Evenson KR1, Aytur SA, Borodulin K. Physical activity beliefs, barriers, and enablers among postpartum women. J Womens Health (Larchmt). 2009. December;18(12):1925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borodulin K, Evenson KR e Herring HA. Physical activity patterns during pregnancy through postpartum. BMC Women's Health 2009; 9:32 10.1186/1472-6874-9-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King AC, Castro C, Wilcox S, Eyler AA, Sallis JF, Brownson RC. Personal and environmental factors associated with physical inactivity among different racial-ethnic groups of U.S. middle-aged and older-aged women. Health Psychol. 2000; 19(4):354–64. [DOI] [PubMed] [Google Scholar]
- 31.Coll CVN, Domingues MR, Hallal PC, da Silva ICM, Bassani DG, Matijasevich A et al. Changes in leisure-time physical activity among Brazilian pregnant women: comparison between two birth cohort studies (2004–2015). BMC Public Health. 2017; 17:119 10.1186/s12889-017-4036-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symons-Downs DS and Hausenblas HA. Women's exercise beliefs and behaviors during their pregnancy and postpartum. J Midwifery Womens Health. 2004; 49:138–144. 10.1016/j.jmwh.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 33.Evenson KR, Moos MK, Carrier K, Siega-Riz AM. Perceived Barriers to Physical Activity among Pregnant Women. Matern Child Health J. 2009; 13(3): 364–375. 10.1007/s10995-008-0359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. 2017; 14(1):32 10.1186/s12966-017-0485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallal PC, Matsudo SM, Matsudo VKR, Araújo TL, Andrade DR, Bertoldi AD. Physical activity in adults from two Brazilian areas: similarities and differences. Cad. Saúde Pública.2005; 21(2):573–80. [DOI] [PubMed] [Google Scholar]
- 36.Knuth AG, Bacchieri G, Victoria CG, Hallal PC. Changes in physical activity among Brazilian adults over a 5-year period. J Epidemiol Community Health 2010;64:591–95. 10.1136/jech.2009.088526 [DOI] [PubMed] [Google Scholar]
- 37.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR et al. Amount of Time Spent in Sedentary Behaviors in the United States, 2003–2004. Am J Epidemiol. 2008; 167(7): 875–81. 10.1093/aje/kwm390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch KE, Landsbaugh JR, Whitcomb BW, Pekow P, Markenson G, Chasan-Taber L. Physical Activity of Pregnant Hispanic Women. Am J Prev Med. 2012; 43(4): 434–39. 10.1016/j.amepre.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Instituto Brasileiro de Geografia e Estatística 2014—Síntese de indicadores da população brasileira. Rio de Janeiro, Brasil. Portuguese.
- 40.Eime RM, Charity MJ, Harvey JT, Payne WR. Participation in sport and physical activity: associations with socio-economic status and geographical remoteness. BMC Public Health. 2015; 15:434 10.1186/s12889-015-1796-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dias-da-costa JS, Hallal PC, Wells JCK, Daltoé T, Fuchs SC, Menezes AMB et al. Epidemiology of leisure-time physical activity: a population-based study in southern Brazil. Cad. Saúde Pública. 2005; 21(1):275–82. [DOI] [PubMed] [Google Scholar]
- 42.Sales-da-costa R, Werneck GL, Lopes CS, Faerstein E. Associação entre fatores sócio-demográficos e prática de atividade física de lazer no Estudo Pró-Saúde. Cad Saúde Pública. 2003; 19(4):1095–1105. [DOI] [PubMed] [Google Scholar]
- 43.Cerin E and Leslie E. How socio-economic status contributes to participation in leisure-time physical activity. Soc Sci Med. 2008; 66(12):2596–609. 10.1016/j.socscimed.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 44.Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care. 2011; 34(1): 223–29. 10.2337/dc10-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrlich SF, Hedderson MM, Brown SD, Sternfeld B, Chasan-Taber L, Feng J et al. Moderate intensity sports and exercise is associated with glycaemic control in women with gestational diabetes. Diabetes Metab. 2017; 43(5):416–23. 10.1016/j.diabet.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.do Nascimento SL, Godoy AC, Surita FG, Pinto e Silva JL. Recommendations for physical exercise practice during pregnancy: a critical review. Rev. Bras. Ginecol. Obstet. 2012; 36 (9) 423–31. [DOI] [PubMed] [Google Scholar]
- 47.Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014; 25(3):331–43. 10.1097/EDE.0000000000000036 [DOI] [PubMed] [Google Scholar]
- 48.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015; 125(3):576–82. 10.1097/AOG.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 49.Bueno DR, Marucci MFN, Codogno JS, Roediger MA. Os custos da inatividade física no mundo: estudo de revisão. Ciênc. saúde coletiva [online]. 2016; 21 (4), 1001–10. [DOI] [PubMed] [Google Scholar]
- 50.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016; 388(10051):1311–24. 10.1016/S0140-6736(16)30383-X [DOI] [PubMed] [Google Scholar]
- 51.Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S et al. The global economic burden of noncommunicable diseases (Working Paper Series). Geneva: Harvard School of Public Health and World Economic Forum; 2011.
- 52.Brown W. The benefits of physical activity during pregnancy. J Sci Med Sport. 2002; 5(1): 37–45. [DOI] [PubMed] [Google Scholar]
- 53.Gavard JA e Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008; 51(2): 467–80. 10.1097/GRF.0b013e31816feb1d [DOI] [PubMed] [Google Scholar]
- 54.Prather H, Spitznagle T e Hunt D. Benefits of exercise during pregnancy. PM R. 2012; 4(11): 845–50. 10.1016/j.pmrj.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 55.Harrison CL, Brown WJ, Hayman M, Moran LJ, Redman LM. The Role of Physical Activity in Preconception, Pregnancy and Postpartum Health. Semin Reprod Med. 2016; 34(2):e28–37. 10.1055/s-0036-1583530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reproduced from Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004; 36(10): 1750–60.
(PDF)
(PDF)
Brazilian economic Classification Criteria from Associação Brasileira de Empresas e Pesquisas (Brazilian Association of Companies and Research).
(PDF)
L Time 0: preconceptional period; Time 1: third trimester of gestation; Time 2: three months after delivery; GDM: gestational diabetes mellitus; LRP: low-risk pregnancy; BMI: body mass index; Normal weight (≥ 18,5 and ≤ 24,9 Kg/m2); Overweight/Obese (≥ 25 Kg/m2); MET: metabolic equivalent of task.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


