Abstract
Background
Uncontrolled high blood pressure (UBP) can lead to various cardiovascular complications causing an estimated nine million deaths per year worldwide. In Meknes, epidemiologic data on UBP are scarce, depriving programs from evidence-based information that would allow a better management of hypertension. Hence, we aimed to assess UBP prevalence in hypertensive patients treated in Meknes and identify UBP-associated risk factors.
Methods
Between November and December 2017, we conducted a cross-sectional study enrolling 922 hypertensive patients managed at Meknes’s primary health care facilities using the multistage sampling method. We interviewed patients face to face to collect their socio-demographic-characteristics, lifestyle behaviours, clinical parameters and the triad care system-patient-physician. Another questionnaire was self-administered by physicians to characterize therapeutic inertia. A multivariate logistic regression analysis highlighted the risk factors associated with UBP.
Results
UBP prevalence was 73% with a mean age of 61±11 years (mean±standard deviation) and a male/female ratio of 1/3. Risk factors associated with UBP were: therapeutic inertia (adjusted odds ratio to other variables (AOR) = 18.2, 95% CI [8.35–39.84]), drug non-adherence (AOR = 1.8, 95% CI [1.07–3.04]), obesity/overweight (AOR = 1.6, 95% CI [1.03–2.58]), unemployment (AOR = 1.9, 95% CI [1.09–3.01]), low income (AOR = 2.6, 95% CI [1.01–6.86]), family history of hypertension (AOR = 1.5, 95% CI [1.07–2.08]) and male sex (AOR = 1.6, 95% CI [1.04–2.58]).
Conclusion
UBP prevalence is high in Meknes. Prevention should firstly focus on raised awareness of hypertensive patients’ self-care management. Secondly, health professionals should better comply to the guidelines of anti-hypertensive treatments. Lastly, health professionals should frequently be reminded to reach therapeutic goals to overcome therapeutic inertia.
Introduction
Abnormally high blood pressure values define hypertension. Hypertension is a major risk factor and the cause of an estimated nine million deaths per year worldwide [1–3]. In 2000, hypertension affected an estimated 972 million people, including 639 million in developing countries [4]. Projections based on these data suggest that the number will increase by 60% in 2025 [5]. Low- and middle-income countries account for more than three quarters of global deaths related to cardiovascular diseases according to the World Health Organization [1, 6]. Premature deaths, personal and family disruption, loss of income, and high health care costs associated with hypertension are a significant economic burden for families, communities, and public health system with 57 million disability-adjusted life year (DALY) at the global level and 658.301 DALY in Morocco [7]. Due to its chronicity and the need for lifespan treatment, hypertension is hence a factor of poverty [8].
The Moroccan budget allocated to tackle hypertension rose from 33 million dirhams in 2010 to 45 million dirhams in 2014 [7]. A first Moroccan national survey was conducted in 2000 on a sample of 1802 participants and revealed that hypertension had an estimated prevalence of 33% among people aged 20 and over [9]. According to regional records, the 46 primary health care facilities (PHCF) of the prefecture of Meknes followed approximately 15515 hypertensive patients in 2016, 1793 of whom had complications. Altogether, it appears that data on hypertension in Morocco are still very scarce. A better knowledge of the current characteristics of the hypertensive population would highly improve the clinical management of the disease and its cardiovascular complications. A multi-center epidemiological trial of hypertension in North Africa conducted between September 2008 and January 2009 involving 28.500 patients showed that 45% of the population were known as hypertensive and 64% of those individuals had uncontrolled blood pressure (UBP) [7, 10]. The benefit of antihypertensive treatment should correlate with the decline in blood pressure. UBP (systolic blood pressure greater than 140 mmHg and / or diastolic blood pressure greater than 90 mmHg after treatment) is often complicated by serious cardiovascular events for which it multiplies the risk of death [11, 12]. To date, there is no available data in Morocco regarding the prevalence of UBP and its associated determinants. The objective of this study is to assess the prevalence of UBP in hypertensive patients followed at PHCF in Meknes and to identify its associated risk factors.
Methods
Study design and population
We performed a cross-sectional study with descriptive and analytical purposes from November 1st to December 31st of 2017. We based sampling on a stratified two-stage survey. The primary unit consisted of the primary health care centers. The secondary unit consisted of hypertensive patients undergoing a medical treatment to correct high blood pressure for at least six months, residing in Meknes and attending one of the six health centers where the prevalence of hypertension is the highest and who have agreed to participate in the survey. We selected these six centers according to the reported prevalence of hypertension at the level of each PHCF and after weighting according to the urban (85%) or rural (15%) origin of the patients.
In each primary unit selected, we performed a random draw of the patient order numbers to determine the secondary unit. This technique facilitated the survey and reduced costs.
Sample size determination
Based on a 95% confidence interval, a prevalence of UBP estimate of 50%, setting the margin of error at 5%, achieving 20% inflation and a cluster effect of 2, we have set the minimum sample size to 922 hypertensive patients. We excluded pregnant women and individuals with mental disability from the study population. We also excluded seven patients presenting with secondary high blood pressure as documented in their medical records.
Data collection
We used two questionnaires: we applied the first standardized questionnaire to eligible patients who answered questions during a face-to-face individual interview. We collected the following data: socio-demographic background, perception of the disease by the patient, lifestyle and behavioural habits, clinical parameters and the triad care system-patient-physician. The second questionnaire was self-administered by physicians and focused on therapeutic inertia.
Operational definitions
We measured the blood pressure on a patient sitting his back against a chair at the end of the interview i.e. after 20 minutes of rest, his arm supported so that it was at the height of the heart, his feet on the ground while avoiding crossed legs and ensuring he was not speaking during the measurement. We used an electronic blood pressure monitor (MicroLife Pro M with an accuracy of ±3 mmHg) with a cuff sized for the age of the patient. We performed a preliminary blood pressure measurement on each arm to determine which had the highest blood pressure value. We then monitored two successive measurements of the blood pressure on the arm with the highest blood pressure with an interval of one minute between them. We defined the average of these last two measurements as the patient’s average arterial pressure. To ascertain UBP, we collected arterial pressure measurements from the patient's medical chart and we recorded the average of the last three arterial pressure measured over the preceding six months which include one done during this study. UBP was classified according to the criteria of the European Society of Hypertension and the European Society of Cardiology (ESH/ESC) guidelines [13]. According to these guidelines, UBP was identified in the treated general population when the systolic blood pressure (SBP) was greater than or equal to 140 mmHg whereas the diastolic blood pressure (DBP) was greater than or equal to 90 mmHg. High blood pressure was noted as controlled if the SBP was less than 140 mmHg and / or the DBP less than 90 mmHg. In diabetic patients, UBP was identified if, after treatment, the SBP was greater than or equal to 140 mmHg and / or the DBP greater than or equal to 85 mmHg. It was noted as controlled if the SBP was less than 140 mmHg and / or the DBP less than 85 mmHg. In patients with renal failure diagnosed at the end stage of kidney disease, UBP was acknowledged if, after treatment, the SBP was greater than or equal to 130 mmHg and / or the DBP greater than or equal to 90 mmHg. It was noted as controlled if the SBP was less than 130 mmHg and / or a DBP less than 90 mmHg. The level of hypertension was classified according to the criteria of ESH/ESC guidelines as [13]: grade I, SBP 140–159 mmHg and/or DBP 90-99mmHg; grade II, SBP 160–179 mmHg and/or DBP 100–109 mmHg; grade III, SBP 180 mmHg and/or DBP 110 mmHg; isolated systolic hypertension, SBP ≥140 mmHg and DBP<90mmHg. We defined obesity according to the WHO classification of the body mass index (BMI); abdominal obesity was defined by waist circumference measurement (males⩾ 102 cm; females⩾ 88 cm). Cardiovascular risk was split into two categories: high or low. Drug adherence was assessed by the Girerd Drug Adherence Evaluation Test [4, 14]. This test gathers six questions to which the patient must answer yes or no; the questions asked are included in the patient’s questionnaire in the section “compliance with treatment” “S1 Questionnaire”. Patients with a yes response number greater than 3 were rated poorly observing, patients with a yes response number between 1 and 2 were rated moderately observant and those with zero yes were rated good observant. The therapeutic inertia was evaluated by the Okonofua modified method as described previously [15]. Briefly, we considered that there was therapeutic inertia when there was no change in treatment despite an abnormal blood pressure value during two successive consultations and a minimum of one month between these two consultations and no change in treatment in the last 3 months. The 3 months with no change in treatment correspond to the minimum period of balanced patient monitoring, which is often the time for renewal visits for antihypertensive treatment.
Data management and statistical analysis
We maintained the consistency of the compiled data by editing and checking for duplicates. We removed duplicates before coding and analysis using Epi-Info-7.2.0.1. We calculated frequencies and percentages to identify the distribution of the socio-demographic characteristics. All tests were two-tailed, and we considered a p value < 0.05 to be statistically significant.
We expressed the results as mean ± standard deviation for quantitative variables or as percentages for qualitative variables. To address the objectives of the study, we performed a bivariate analysis to identify the factors associated with UBP in hypertensive patients and a multivariate analysis to neutralize the confounding factors and determine the simultaneous effect of independent variables on the poor control of HBP. We used logistic regression in the bivariate and multivariate analysis; we fitted into multivariable logistic regression any variable with a p-value up to 0.2 in the bivariate analysis. We used a backward stepwise logistic regression procedure to determine the final model. We estimated the association between each assumed risk factor and UBP using the adjusted odds ratio and its 95% confidence interval.
Ethical considerations
The ethical review board of the Faculty of Medicine of Rabat in Morocco reviewed and approved the study protocol. We obtained consent from all participants after detailed explanation of the research purpose and assurance of maintaining privacy and confidentiality. Thus, we rigorously implemented all precautions to respect anonymity and confidentiality of the patient’s information by using a coding system. Data were accessible only to the research team. A list containing the patient’s code, his surname and first name, his contact information was established; the coordinator of the study could, in case of needing further information, identify the patient from his code, by his name and surname. No one else, apart from the study coordinator, had access to this list.
Results
1. Results socio-demographic and economic characteristics
During the study period, 922 hypertensive patients were enrolled of whom 675 had uncontrolled hypertension corresponding to a prevalence of 73%. The male/female ratio was 1/3 and the average age was 61±11 years with a range from 30 to 100 years. Married individuals accounted for 63% of cases and illiterates accounted for 73% of cases. More than 88% of the cases were unemployed with 79% having a monthly income of less than or equal to 1500 MAD ($150) and 52% had a Moroccan medical assistance plan (RAMED) “Table 1”.
Table 1. Socio-demographic and economic characteristics of hypertensive patients followed up at primary health care facilities, Meknes, Morocco, 2017.
| Quantitative variables | n = 922 | Uncontrolled BP | Controlled BP | p-value | |
|---|---|---|---|---|---|
| Patients’ total no. (%) | 922 (100) | 675 (73.2) | 247 (26.8) | ||
| Age in years±sd | 61.4±10.8 | 61.4±10.5 | 61.6±11.9 | 0.96 | |
| Qualitative variables no. (%) | |||||
| Age group in years | 0.03 | ||||
| ≥80 | 68 (7.4) | 42 (61.8) | 26 (38.2) | ||
| [70–79] | 159 (17.2) | 118 (74.2) | 41 (25,8) | ||
| [60–69] | 333 (36.1) | 256 (76.9) | 77 (23.1) | ||
| [50–59] | 255 (27.6) | 190 (74.5) | 65 (25.5) | ||
| [40–49] | 94 (10.2) | 62 (66.0) | 32 (34.0) | ||
| [30–39] | 13 (1.4) | 7 (53.8) | 6 (46.2) | 1 | |
| Sex | Male | 205 (22.2) | 160 (78.0) | 45 (22.0) | 0.07 |
| Female | 717 (77.8) | 515 (71.8) | 202 (28.2) | 1 | |
| Area of residence | |||||
| Urban | 781 (84.7) | 574 (73.5) | 207 (26.5) | 0.64 | |
| Rural | 141 (15.3) | 101 (71.6) | 40 (28.4) | 1 | |
| Marital status | 0.71 | ||||
| Widower | 253 (27.4) | 192 (75.9) | 61 (24.1) | ||
| Divorced | 58 (6.3) | 41 (70.7) | 17 (29.3) | ||
| Single | 26 (2.8) | 19 (73.1) | 7 (26.9) | ||
| Married | 585 (63.4) | 423 (72.3) | 162 (27.7) | 1 | |
| Education | 0.45 | ||||
| Illiterate | 677 (73.4) | 490 (72.4) | 187 (27.6) | ||
| Elementary | 159 (17.2) | 125 (78.6) | 34 (21.4) | ||
| Middle school | 41 (4.4) | 28 (68.3) | 13 (31.7) | ||
| High school | 34 (3.7) | 25 (73.5) | 9 (26.5) | ||
| College | 11 (1.2) | 7 (63.6) | 4 (36.4) | 1 | |
| Occupation | |||||
| No | 814 (88.3) | 605 (74.3) | 209 (25.7) | 0.04 | |
| Yes | 108 (11.7) | 70 (64.8) | 38 (35.2) | ||
| Monthly income per household ($) | 0.13 | ||||
| <150 | 728 (79.0) | 539 (74.0) | 189 (26.0) | ||
| [150–200[ | 96 (10.4) | 69 (71.9) | 27 (28.1) | ||
| [200–300[ | 57 (6.2) | 42 (73.7) | 15 (26.3) | ||
| [300–500[ | 17 (1.8) | 13 (76.5) | 4 (23.5) | ||
| ≥500 | 24 (2.6) | 12 (50.0) | 12 (50.0) | 1 | |
| Health insurance plan | 0.68 | ||||
| Without | 189 (20.5) | 140 (74.1) | 49 (25.9) | ||
| RAMED | 480 (52.0) | 344 (71.7) | 136 (28.3) | ||
| Other | 28 (3.0) | 22 (78.6) | 6 (21.4) | ||
| Mutual | 225 (24.4) | 169 (75.1) | 56 (24.9) | 1 | |
RAMED: Moroccan medical assistance plan, sd: standard deviation. For qualitative variables, the Pearson chi-2 test estimated the association between the dependent variable and the independent variables when the conditions were valid. For the quantitative variables, we used a comparison test of two means; p-value was considered significant when it was less than 0.05.
2. Perception of the disease by the patient
Among the 922 hypertensive subjects we enrolled, only 69 (7%) had a general knowledge of hypertension. The repartition by level of knowledge on this pathology showed that 57 (6%) knew the clinical signs of the disease, 423 (46%) knew its means of prevention and 264 (29%) knew its complications.
3. Lifestyle and behavioural characteristics
Analysis of the behavioural data showed that 716 hypertensive subjects (77%) had between one and two risk factors. Physical activity was unsatisfactory in 243 cases (26%). The sodium diet was reported in 162 hypertensive patients (18%) and the non- adherence with diet and lifestyle habits was recorded in 826 cases (90%) “Table 2”.
Table 2. Behavioural characteristics of hypertensive patients monitored at primary health care facilities, Meknes, Morocco, 2017.
| Qualitative variables no. (%) | n = 922 | Uncontrolled BP (n = 675) | Controlled BP (n = 247) | p-value | |
|---|---|---|---|---|---|
| Tobacco use | 0.88 | ||||
| Current smoker | 21 (2.3) | 15 (71.4) | 6 (28.6) | ||
| Former smoker | 58 (6.3) | 44 (75.9) | 14 (24.1) | ||
| Non-smoking | 843 (91.4) | 616 (73.1) | 227 (26.9) | 1 | |
| Alcohol consumption | 0.02 | ||||
| Frequent | 21 (2.3) | 20 (95.2) | 1 (4,8) | ||
| Occasional | 14 (1.5) | 8 (57.1) | 6 (42.9) | ||
| Never | 887 (96.2) | 647 (72.9) | 240 (27.1) | 1 | |
| Physical activity | |||||
| Unsatisfactory | 243 (26.4) | 176 (72.4) | 67 (27.6) | 0.74 | |
| Satisfactory | 679 (73.6) | 499 (73.5) | 180 (25.5) | 1 | |
| Stress | 0.57 | ||||
| Intense | 284 (30.8) | 204 (71.8) | 80 (28.2) | ||
| Intermediate | 489 (53.0) | 357 (73.0) | 132 (27.0) | ||
| None | 149 (16.2) | 114 (76.5) | 35 (23.5) | 1 | |
| Salty diet | 0.47 | ||||
| Salty | 162 (17.6) | 117 (72.2) | 45 (27.8) | ||
| Semi-salted | 713 (77.3) | 520 (72.9) | 193 (27.1) | ||
| Without | 47 (5.1) | 38 (80.9) | 9 (19.1) | 1 | |
| Duration of high blood pressure in years | |||||
| >5 | 464 (50.3) | 353 (76.1) | 111 (23.9) | 0.04 | |
| ≤5 | 458 (49.7) | 322 (70.3) | 136 (29.7) | 1 | |
| Family history of high BP | |||||
| Yes | 485 (52.6) | 371 (76.5) | 114 (23.5) | 0.01 | |
| No | 437 (47.4) | 304 (69.6) | 133 (30.4) | 1 | |
| Self-monitoring | |||||
| No | 810 (87.9) | 593 (73.2) | 217 (26.8) | 1.0 | |
| Yes | 112 (12.1) | 82 (73.2) | 30 (26.8) | 1 | |
| Lifestyle and dietary advice | |||||
| No | 826 (89.6) | 602 (72.9) | 224 (27.1) | 0.50 | |
| Yes | 96 (10.4) | 73 (76.0) | 23 (34.0) | 1 | |
| Annual biological check-up | |||||
| No | 727 (78.9) | 546 (75.1) | 181 (24.9) | 0.01 | |
| Yes | 195 (21.1) | 129 (66.1) | 66 (33.9) | 1 | |
| Number of risk factors | 0.22 | ||||
| ≥3 | 185 (20.1) | 134 (72.4) | 51 (27.6) | ||
| 1–2 | 716 (77.6) | 529 (73.9) | 187 (26.1) | ||
| None | 21 (2.3) | 12 (57.1) | 9 (42.9) | 1 | |
BP: blood pressure. For qualitative variables, the Pearson chi-2 test estimated the association between the dependent variable and the independent variables when the conditions were valid; p-value was considered significant when it was less than 0.05.
4. Clinical features
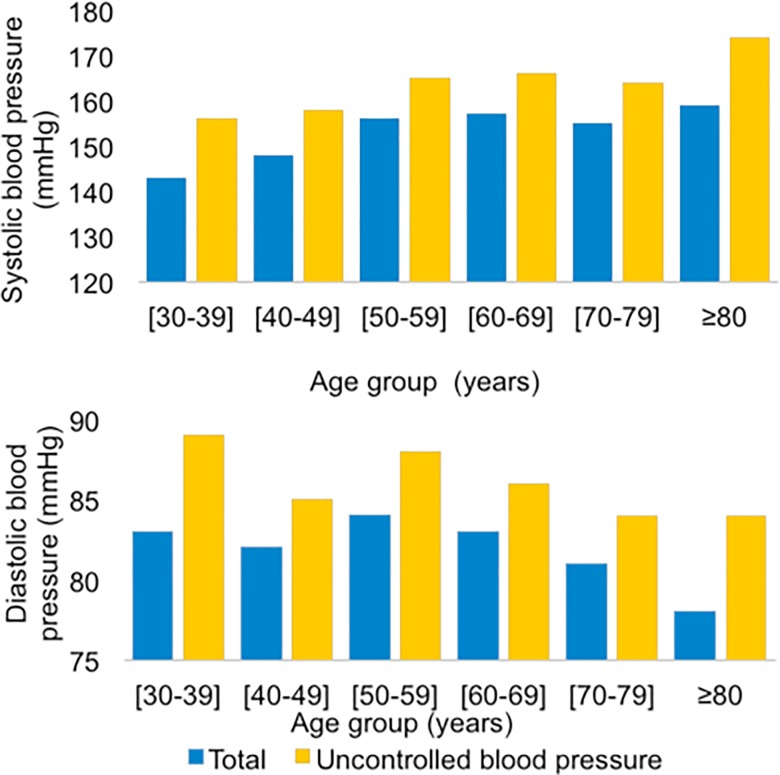
Mean SBP was 155.7±22.7 mmHg while DBP was 82.5±12.6 mmHg in the hypertensive patients recruited for the study. The data from the study showed that blood pressure increased gradually from the age of 40 years. As early as age 50, we found that SBP continues to increase as DBP gradually decreases. The presence of comorbidity associated with hypertension was found in 577 cases (63%) with a predominance of diabetes (56%) “Fig 1”. In terms of health characteristics, overweight and obesity were predominant in 782 (85%) hypertensive patients; the same is true for abdominal obesity in 717 hypertensive individuals (78%) “Table 3”. During the study period, 606 hypertensive patients had a high cardiovascular risk, 55% of whom did not have medical management of risk factors.
Fig 1. Distribution of means of systolic and diastolic blood pressure values by age groups, Meknes, Morocco, 2017.
Table 3. Clinical features of hypertensive patients at primary health care facilities, Meknes, Morocco, 2017.
| Qualitative variables no. (%) | n = 922 | Uncontrolled BP (n = 675) | Controlled BP (n = 247) | p-value | |
|---|---|---|---|---|---|
| Comorbidity | |||||
| Yes | 577 (62.6) | 421 (73.0) | 156 (27.0) | 0.82 | |
| No | 345 (37.4) | 254 (73.6) | 91 (26.4) | 1 | |
| Diabetes | |||||
| Yes | 515 (55.8) | 378 (73.4) | 137 (26.6) | 0.88 | |
| No | 407 (44.1) | 297 (73.0) | 110 (27.0) | 1 | |
| Cardiovascular disease | |||||
| Yes | 87 (9.4) | 60 (69.0) | 27 (31.0) | 0.37 | |
| No | 835 (90.6) | 615 (73.7) | 220 (26.3) | 1 | |
| Dyslipidemia | |||||
| Yes | 146 (15.8) | 105 (72.0) | 41 (28.1) | 0.70 | |
| No | 776 (84.2) | 570 (73.5) | 206 (26.5) | 1 | |
| Kidney disease | |||||
| Yes | 24 (2.6) | 13 (54.2) | 11 (45.8) | 0.03 | |
| No | 898 (97.5) | 662 (73.7) | 236 (26.3) | 1 | |
| Overweight/obesity | |||||
| Yes | 782 (84.8) | 580 (74.2) | 202 (25.8) | 0.12 | |
| No | 140 (15.2) | 95 (67.9) | 45 (32.1) | 1 | |
| Abdominal obesity | |||||
| Yes | 717 (77.7) | 519 (72.4) | 198 (27.6) | 0.28 | |
| No | 205 (22.2) | 156 (76.1) | 49 (23.9) | 1 | |
| Waist-hip ratio | |||||
| Android obesity | 716 (77.6) | 519 (72.5) | 197 (27.5) | 0.32 | |
| Gynoid obesity | 206 (22.3) | 156 (75.7) | 50 (24.3) | 1 | |
BP: blood pressure. For qualitative variables, the Pearson chi-2 test estimated the association between the dependent variable and the independent variables when the conditions were valid; p-value was considered significant when it was less than 0.05.
5. Triad care system-patient-physician
Among the 922 hypertensive patients surveyed, 665 (72%) had an average satisfaction with the health care system. Poor adherence with the drug was reported in 244 cases (27%). The patient-physician relationship was considered to be poor in 367 (40%) hypertensive subjects “Table 4”.
Table 4. Characteristics of the triad care system-patient-physician amongst hypertensive patients followed at the level of primary health care facilities, Meknes, Morocco 2017.
| Qualitative variables no. (%) | n = 922 | Uncontrolled BP (n = 675) | Controlled BP (n = 247) | p-value | |
|---|---|---|---|---|---|
| Relationship with the health care system | 0.92 | ||||
| Bad | 36 (3.9) | 26 (72.2) | 10 (27.8) | ||
| Intermediate | 665 (72.1) | 485 (72.9) | 180 (27.1) | ||
| Good | 221 (23.9) | 164 (74.2) | 57 (25.8) | 1 | |
| Drug adherence | <0.01 | ||||
| Bad | 244 (26.5) | 172 (70.5) | 72 (29.5) | ||
| Intermediate | 593 (64.3) | 453 (76.4) | 140 (23.6) | ||
| Good | 85 (9.2) | 50 (58.8) | 35 (41.2) | 1 | |
| Physician-patient relationship | 0.40 | ||||
| Bad | 367 (39.8) | 274 (74.7) | 93 (25.3) | ||
| Intermediate | 537 (58.2) | 386 (71.9) | 151 (28.1) | ||
| Good | 18 (2.0) | 15 (83.3) | 3 (16.7) | 1 | |
| Therapeutic inertia | |||||
| Yes | 238 (25.8) | 231 (97.0) | 7 (3.0) | <0.01 | |
| No | 684 (74.2) | 444 (64.9) | 240 (35.1) | 1 | |
| Medical management of risk factors | |||||
| No | 547 (59.3) | 396 (72.4) | 151 (27.6) | 0.49 | |
| Yes | 375 (40.7) | 279 (74.4) | 96 (25.6) | 1 | |
| Duration of treatment in months | |||||
| >6 | 836 (90.7) | 622 (74.4) | 214 (25.6) | 0.01 | |
| ≤ 6 | 86 (9.3) | 53 (61.6) | 33 (38.4) | 1 | |
| Number of molecules | 0.49 | ||||
| Monotherapy | 829 (89.9) | 604 (72.9) | 225 (27.1) | ||
| Bitherapy | 90 (9.8) | 68 (75.6) | 22 (24.4) | ||
| Tritherapy | 3 (0.3) | 3 (100.0) | 0 (0.0) | 1 | |
| Type of treatment | |||||
| Generic | 879 (95.3) | 646 (73.5) | 233 (26.5) | 0.38 | |
| Brand/trademark | 43 (4.7) | 29 (67.4) | 14 (32.6) | 1 | |
For qualitative variables, the Pearson chi-2 test estimated the association between the dependent variable and the independent variables when the conditions were valid; p-value was considered significant when it was less than 0.05.
6. Characteristics of antihypertensive treatment
Among the 922 hypertensive patients enrolled in the study, 836 (91%) had been on the same treatment for more than six months. 829 (90%) were on monotherapy and 90 (10%) on dual therapy. Generic treatment was predominant in 879 (95%) hypertensive patients “Table 4”.
7. Uncontrolled blood pressure
Among the 922 hypertensive patients included in the study, 675 had an UBP, which means a prevalence of 73%. Their mean age was 61±10 years with extremes ranging from 30 to 98 years and a sex ratio male/female of 1/3. Analysis of the socio-economic data showed that out of 675 hypertensive subjects with UBP, 89% were unemployed and 80% had a monthly income per household of less than 150$ “Table 1”.
Behavioural data analysis revealed that of the 675 UBP, 89% did not comply with lifestyle and dietary measures. The lack of annual physical exam and check-up amongst patients included in the study, an essential element in the management of hypertension, was noted in 81% of patients with UBP, i.e. 546 hypertensive patients. Similarly, 73% of these hypertensive patients did not perform blood pressure self-measurement, corresponding to 593 hypertensive patients.
The distribution of hypertensive subjects with UBP by level of blood pressure revealed that 48% had isolated systolic blood pressure, 16% had blood pressure grade I, 7% had blood pressure grade II and 2% had blood pressure grade III.
Analysis of the clinical data of 675 hypertensive subjects with UBP showed that 2% had chronic kidney disease “Table 3” and 67% followed a monodrug therapy “Table 4”. Among 589 hypertensive patients with high cardiovascular risk and for whom hypertension was uncontrolled, 45% did not have a good management of risk factors.
Therapeutic inertia was prevalent in 34% of the study population, which means 231 UBP for whom the antihypertensive treatment was not intensified or modified during the last two consultations “Table 4”.
8. Bivariate analysis
We enrolled 922 hypertensive patients included in the study; 675 (73%) of them had UBP. The cut-off p-value after the bivariate analysis was set at p<0.2. Accordingly, we identified thirteen factors associated with UBP: 1. therapeutic inertia (p = 0.01), 2. treatment longer than six months (p = 0.01), 3. absence of an annual physical exam (p = 0.01), 4. family history of hypertension (p = 0.01), 5. drug non-adherence (p = 0.01), 6. alcohol consumption (p = 0.02), 7. age (p = 0.04), 8. non-employment (p = 0.04), 9. a duration of high blood pressure of more than 5 years (p = 0.04), 10. renal disease (p = 0.04), 11. male sex (p = 0.07),12. low monthly income per household (p = 0.13), and 13. overweight/obesity (p = 0.12) “Table 5”.
Table 5. Multivariate analysis (odds ratio, p-value) of uncontrolled blood pressure in hypertensive patients followed at primary health care facilities, Meknes, 2017.
| Qualitative variables | Bivariate analysis | Multivariate analysis complete model | Multivariate analysis final model | |||||
|---|---|---|---|---|---|---|---|---|
| COR 95%CI | p-value | AOR 95%CI | p-value | AOR 95%CI | p-value | |||
| Male/female ratio | 1.4 [0.96–2.01] | 0.07 | 1.6 [1.00–2.52] | 0.05 | 1.6 [1.04–2.58] | 0.03 | ||
| Age in years | 0.04 | |||||||
| ≥80 | 1.4 [0.41–4.57] | 0.59 | 1.4 [0.30–6.30] | 0.66 | 1.4 [0.31–6.46] | 0.65 | ||
| [70–79] | 2.5 [0.78–7.76] | 0.12 | 2.2 [0.51–9.38] | 0.28 | 2.2 [0.51–9.41] | 0.29 | ||
| [60–69] | 2.8 [0.93–8.73] | 0.06 | 2.8 [0.70–11.84] | 0.14 | 2.9 [0.69–11.93] | 0.14 | ||
| [50–59] | 2.5 [0,81–7.72] | 0.10 | 2.7 [0.66–11.20] | 0.16 | 2.6 [0.63–10.88] | 0.18 | ||
| [40–49] | 1.7 [0.51–5.35] | 0.39 | 1.6 [0.38–7.03] | 0.50 | 1.5 [0.35–6.66] | 0.56 | ||
| [30–39] | 1 | 1 | 1 | |||||
| Unemployment | 1.6 [1.03–2.40] | 0.04 | 1.8 [1.06–2.95] | 0.02 | 1.8 [1.09–3.01] | 0.02 | ||
| Monthly income per household in $ | ||||||||
| <150 | 2.8 [1.25–6.45] | 0.01 | 2.8 [1.07–7.34] | 0.03 | 2.6 [1.01–6.86] | 0.04 | ||
| [150–200[ | 2.5 [1.02–6.37] | 0.04 | 2.3 [0.80–6.68] | 0.11 | 2.1 [0.74–6.22] | 0.15 | ||
| [200–300[ | 2.8 [1.03–7.55] | 0,04 | 2.3 [0.71–7.27] | 0.16 | 2.1 [0.66–6.73] | 0.20 | ||
| [300–500[ | 3.2 [0.81–12.86] | 0.09 | 2.9 [0.63–13.45] | 0.17 | 2.8 [0.61–13.04] | 0.18 | ||
| ≥500 | 1 | 1 | 1 | |||||
| Alcohol consumption | ||||||||
| Frequent | 7.4 [0.99–55.55] | 0.05 | 7.9 [0.97–69.47] | 0.05 | 7.7 [0.95–62.06] | 0.06 | ||
| Occasional | 0.4 [0.17–1.44] | 0.19 | 0.2 [0.06–0.93] | 0.04 | 0.2 [0.06–0.93] | 0.04 | ||
| Never | 1 | 1 | 1 | |||||
| Obesity/overweight | 1.4 [0.92–2.00] | 0.12 | 1.5 [0.96–2.45] | 0.07 | 1.6 [1.03–2.58] | 0.04 | ||
| Annual biological check-up | 1.5 [1.09–2.17] | 0.01 | 1.4 [0.93–2.03] | 0.10 | 1.4 [0.97–2.08] | 0.07 | ||
| Familial history | 1.4 [1.06–1.90] | 0.01 | 1.4 [1.04–2.02] | 0.03 | 1.5 [1.07–2.08] | 0.02 | ||
| Duration of high blood pressure for more than 5 years | 1.3 [1.00–1.79] | 0.04 | 1.3 [0.93–1.82] | 0.12 | ***** | ***** | ||
| Duration of treatment less than 6 months | 1.8 [1.14–2.87] | 0.01 | 1.4 [0.84–2.34] | 0.20 | ***** | ***** | ||
| Kidney disease | 0.4 [0.18–0.95] | 0.04 | 0.5 [0.19–1.29] | 0.15 | ***** | ***** | ||
| Drug adherence | ||||||||
| Bad | 1.7 [1.00–2.79] | 0.04 | 1.4 [0.78–2.46] | 0.26 | 1.4 [0.80–2.52] | 0.21 | ||
| Intermediate | 2.3 [1.41–3.63] | <0.01 | 1.7 [1.01–2.89] | 0.04 | 1.8 [1.07–3.04] | 0.02 | ||
| Good | 1 | 1 | 1 | |||||
| Therapeutic inertia | 17.8 [8.27–38.45] | <0.01 | 18.1 [8.30–39.6] | <0.01 | 18.2 [8.35–39.84] | <0.01 | ||
COR: crude odds ratio, AOR: adjusted odds ratio, CI: confidence interval
9. Multivariate analysis
After adjusting for the other variables, the following factors were identified as predictor factors of UBP in hypertensive patients treated at Meknes’ PHCF: 1. therapeutic inertia (adjusted odd ratio (AOR) = 18.2, 95% CI [8.35–39.84]), 2. the low income per household (AOR = 2.6, 95% CI [1.01–6.86]), 3. non-employment (AOR = 1.8, 95% CI) [1.09–3.01]), 4. drug non-adherence (AOR = 1.8, 95% CI [1.07–3.04]), 5. the male sex (AOR = 1.6, 95% CI [1.04–24.88]), 6. overweight/obesity (AOR = 1.6, 95% CI [1.03–2.58]), 7. and the family history of hypertension (AOR = 1.5, 95% CI [1.07–2.08]) “Table 5".
Discussion
In the current study, the prevalence of UBP was 73%. To our knowledge, there is no previous national study estimating nor analysing of UBP prevalence amongst hypertensive patients followed at PHCF in Morocco. In countries where the socio-economic and demographic statuses of the population are close to those existing in Morocco, the prevalence of UBP was similar. Indeed, in 2012, in Oran, Algeria, the prevalence of UBP was 69% when analysing a sample of 253 hypertensive patients [11]. In Blidia, Algeria, between 2014 and 2016, the analysis of a sample of 3622 hypertensive patients revealed that the prevalence of UBP was 70% [16].
In a multi-center survey of 12933 known hypertensive patients residing in Tunisia, Algeria or Morocco, the prevalence of UBP was 64% [7, 10]. However, the results did not report data specific to Morocco. In developed countries, the prevalence of UBP was lower. In France, between 2014 and 2016, the prevalence of UBP in a sample of 2169 hypertensive patients was 45% [17].
Moreover, in our work, age increased the risk for the frequency of UBP. Our study is consistent with results reported and published from studies conducted in Algeria and Burkina Faso [11, 18] as well as results reported from a study conducted in the USA [19]. The 1948 Framingham survey, conducted on a sample of 5000 people, showed that SBP increased linearly with age until age 80–90, while DBP increased until age 80–90. It has been reported that starting at age 50, there is a loss of elasticity of the arterial walls [19, 20] that would account for the continuous increase of SBP observed. We report the same observation in our study. Anti-hypertensive treatment administered to hypertensive patients had a preferential action on DBP and was less effective on SBP. This is why in a large proportion of elderly people undergoing treatment, systolic-diastolic hypertension progresses to isolated systolic hypertension [19]. This could explain the predominance of isolated SBP in our survey.
In our findings, males are more likely to be associated with UBP. This finding is similar to the results of the cross-sectional study conducted in Congo on a sample of 620 hypertensive patients [21]. However, different results were obtained in a study conducted in Zimbabwe and Malaysia that have been in contrast with our current findings [22, 23].
Lack of employment and low monthly income per household are more likely associated with UBP. This result has been reported in other studies, particularly in Burkina-Faso, Zimbabwe, China and other countries [18, 23–29].
Illiteracy rates at a high-level among hypertensive patients; it was predominant in our study and was significantly associated with UBP. This is similar to the results reported by the multi-center study conducted in Tunisia, Algeria and Morocco [10].
With regard to the medical plan, among the 922 hypertensive patients, 480 (52%) have a RAMED card and 225 (24%) a health care plan. However, more than 727 (79%) did not get their annual physical exam and check-up necessary for systematic medical monitoring including 423 (46%) patients with UBP. This study was an opportunity to raise the awareness of the benefits offered by the RAMED program to cover the most fragile population as well as providing information about the complications of hypertension. Another reason could be adding appointments that are widely spaced in time, which can range from 3 to 6 months in order to benefit from a specialized consultation.
In our study, occasional consumption of alcohol seemed to be a protective factor against uncontrolled hypertension. Indeed, multiple reports have demonstrated that regular and moderate consumption of alcohol is associated with a decrease in the overall risk of cardiovascular disease. This decrease is due to the beneficial effects of wine on lipoproteins and coagulation factors. However, it is important to remember that frequent alcohol consumption has no positive effect on blood pressure figures, rather, it is associated with increased hypertension [30].
Although a sedentary lifestyle plays an important role in UBP, this has not emerged in our study. It is the same for the excessive consumption of salt. Indeed, many studies have criminalized the sodium diet as a risk factor associated with UBP [11, 31] or even the appearance of cardiovascular events [32, 33]. A meta-analysis showed that in a sample of 175.000 people, an average salt intake greater than 5 g per day increased the risk of stroke by 1.2 in exposed versus unexposed patients. In other words, a reduction, even a small one, of the risk on the whole population could lead to a considerable number of cardiovascular events avoided [34].
The family history or genetic factors of hypertension are associated with UBP in our study. This result is similar to those reported by the sub-Saharian countries [18, 21, 35–37]; this could be explained by a demotivation of the patients to fight the disease.
Stress, a mental health problem, can lead to certain unhealthy mechanisms such as excessive consumption of alcohol, smoking or binge eating or even forgetting to take medication, which is a risk of increasing blood pressure and allows compromising efforts to control blood pressure [38]. Thus, the literature reports stress as a risk factor associated with UBP [39], however this did not emerge in our survey.
Numerous studies have reported overweight and obesity as risk factors associated with UBP [23, 39]. Each ten kilograms excess in comparison to the ideal weight results in a rise of 3 mmHg of the SBP and 2 mmHg for the DBP [40]. This association is more marked in case of so-called visceral android obesity. We found the same results in our study.
Non-adherence to the drug, marked by the lack of the drug prescription monitoring would be associated with UBP. This same finding has been reported in Sudan, Ethiopia, Cameroon and Tanzania [41–44]. This could be due to 1. insufficient stocks of anti-hypertensive drugs at PHCF that can last several months, 2. expensive anti-hypertensive drugs in pharmacies, 3. low income per household, 4. non-prioritization of RAMED beneficiaries during the distribution of drugs at the PHCF, 5. lack of family support, 6. unsatisfactory relationship between the patient and the care team, and 7. irregular medical follow-up of hypertensive patients.
Therapeutic inertia could be explained by: 1. an overestimation of the effect of the prescribed treatments, 2. the physician’s doubts on the adherence to the treatment, 3. the assumption by the physician that the patient does not lead a healthy lifestyle prevents the decision to intensify the treatment, 4. a lack of physician’s training to achieve therapeutic goals [45], 5. the influence of the socio-economic background of the hypertensive patient on the medical decision 6. the lack of the appropriate antihypertensive treatment at PHCF; and 7. non-adherence to the reference frames for the management of hypertensive patients.
Limitations of the study
Our study had a few limitations, including its cross-sectional design. Investigators use this design for its speed, easiness, and cost-efficiency. Cross-sectional designs are suited to investigate chronic diseases such as hypertension; however, they have a limited ability to document the causal relationship between exposure and uncontrolled blood pressure measured at the same time. In this study, the information collected about the sodium diet was qualitative, based on the declaration of the patient, which did not quantify the salt consumed. We also acknowledged this prevarication bias when collecting data in relation to alcohol consumption, tobacco or salt, physical activity and income per household.
Conclusion
Our study shows a high prevalence of UBP amongst subjects followed at PHCF in Meknes. Therapeutic-inertia, non-adherence to drug, non-employment, low monthly income per household, overweight/obesity, family history of hypertension and male sex were associated with UBP. Based on our findings we recommend: i) improving adherence of health professionals to the guidelines and standards of management of hypertensive patients; ii) improving adherence to the drug; and iii) promoting a healthy lifestyle.
Supporting information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Panorama mondial de l'hypertension. Un "tueur silencieux" responsable d'une crise de santé publique mondiale 2013. WHO:[Available from: http://apps.who.int/iris/bitstream/handle/10665/85334/WHO_DCO_WHD_2013.2_fre.pdf?sequence=1
- 2.Campos CL, Pierin AMG, Pinho NA. Hypertension in patients admitted to clinical units at university hospital: post-discharge evaluation rated by telephone. Einstein. 2017;15(1):45–9. 10.1590/S1679-45082017AO3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortez-Dias N, Martins SR, Belo A, Fiuza M, investigators VS. Association of metabolic risk factors with uncontrolled hypertension: comparison of the several definitions of metabolic syndrome. Journal of hypertension. 2013;31(10):1991–7. 10.1097/HJH.0b013e32836342f7 . [DOI] [PubMed] [Google Scholar]
- 4.Girerd X, Hanon O, Pannier B, Mourad JJ, Vaisse B. [Determinants of controlled hypertension in patients treated with antihypertensive drugs in France: The French League Against Hypertension Survey (FLAHS 2015)]. Annales de cardiologie et d'angeiologie. 2016;65(3):219–22. 10.1016/j.ancard.2016.04.019 . [DOI] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. 10.1016/S0140-6736(05)17741-1 . [DOI] [PubMed] [Google Scholar]
- 6.Organisation Mondiale de la Santé. Maladies cardiovasculaires. 2017. [Google Scholar]
- 7.Service des maladies cardiovasculaires, Direction des maladies nin transmissibles. Programme de prévention et de contrôle de l’hypertension artéruelle. In: Ministère de la Santé Maroc, editor. 2017. [Google Scholar]
- 8.Doulougou B. Hypertension artérielle dans la population adulte du Burkina faso: Prévalence, détection, traitement et contrôle [Public Health]: Université de Montréal; 2014. [Google Scholar]
- 9.Tazi MA, Abir-Khalil S, Chaouki N, Cherqaoui S, Lahmouz F, Srairi JE, et al. Prevalence of the main cardiovascular risk factors in Morocco: results of a National Survey, 2000. Journal of hypertension. 2003;21(5):897–903. 10.1097/01.hjh.0000059034.65882.83 . [DOI] [PubMed] [Google Scholar]
- 10.Nejjari C, Arharbi M, Chentir MT, Boujnah R, Kemmou O, Megdiche H, et al. Epidemiological Trial of Hypertension in North Africa (ETHNA): an international multicentre study in Algeria, Morocco and Tunisia. Journal of hypertension. 2013;31(1):49–62. 10.1097/HJH.0b013e32835a6611 . [DOI] [PubMed] [Google Scholar]
- 11.Mesli MF, Raiah M, Mohammedi B, Dida A. [Factors associated with poor blood pressure control in 253 treated hypertensive patients]. Annales de cardiologie et d'angeiologie. 2015;64(1):32–8. 10.1016/j.ancard.2014.04.009 . [DOI] [PubMed] [Google Scholar]
- 12.Plouin P.F. CG, Pagny J.Y., Lang T. Hypertension artérielle: épidémiologie, hémodynamique et physiopathologie. Stratégie de l’exploration et de la prise en charge. Encycl Méd Chirurg, Paris, Coeur-Vaisseaux. 1986:11302-A10:3. [Google Scholar]
- 13.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European heart journal. 2013;34(28):2159–219. 10.1093/eurheartj/eht151 . [DOI] [PubMed] [Google Scholar]
- 14.Hypertension artérielle et facteurs de risque associés. Une nouvelle étude de l’Assurance Maladie sur les évolutions entre 2000 et 2006. Caisse Nationale de l'Assurance Maladie.
- 15.Bencherif S., Khau S., Bloede F., Lamy J.B., H. F. Adaptation du traitement chez les hypertendus: inertie thérapeutique ou pratique centrée sur le patient La revue française de médecine générale: Paris 5; 2010. [Google Scholar]
- 16.Bachir Cherif A, Bouamra A, Taleb A, Nedjar R, Bouraghda A, Hamida F, et al. Differences in prevalence, treatment and control rates of hypertension between male and female in the area of Blida (Algeria). Annales de cardiologie et d'angeiologie. 2017;66(3):123–9. 10.1016/j.ancard.2017.04.009 . [DOI] [PubMed] [Google Scholar]
- 17.Perrine A.L., Lecoffre C., Blacher J., L’hypertension V. O. artérielle en France: prévalence, traitement et contrôle en 2015 et évolutions depuis 2006. Bull Epidémiol Hebd. 2018;10:170–9. [Google Scholar]
- 18.Yameogo NV, Kagambega LJ, Millogo RC, Kologo KJ, Yameogo AA, Mandi GD, et al. [Factors associated with poor blood pressure control in hypertensive black Africans: cross-sectional study of 456 hypertensive patients from Burkina Faso]. Annales de cardiologie et d'angeiologie. 2013;62(1):38–42. 10.1016/j.ancard.2012.05.001 . [DOI] [PubMed] [Google Scholar]
- 19.Giroux E. Origines de l'étude prospective de cohorte: Épidémiologie cardio-vasculaire américaine et étude de Framingham. Revue d'histoire des sciences. 2011;2:p. 297–318. 10.3917/rhs.642.0297 [DOI] [Google Scholar]
- 20.Sabbahi A, Arena R, Kaminsky LA, Myers J, Phillips SA. Peak Blood Pressure Responses During Maximum Cardiopulmonary Exercise Testing: Reference Standards From FRIEND (Fitness Registry and the Importance of Exercise: A National Database). Hypertension. 2018;71(2):229–36. 10.1161/HYPERTENSIONAHA.117.10116 . [DOI] [PubMed] [Google Scholar]
- 21.Ikama MS, Nsitou BM, Makani J, Nkalla-Lambi M, Passi-Louamba C. [Arterial hypertension and control in Brazzaville (Congo): role of ambulatory blood pressure monitoring (ABPM)]. Annales de cardiologie et d'angeiologie. 2015;64(2):76–80. 10.1016/j.ancard.2015.01.007 . [DOI] [PubMed] [Google Scholar]
- 22.Cheong AT, Sazlina SG, Tong SF, Azah AS, Salmiah S. Poor blood pressure control and its associated factors among older people with hypertension: A cross-sectional study in six public primary care clinics in Malaysia. Malaysian family physician: the official journal of the Academy of Family Physicians of Malaysia. 2015;10(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 23.Goverwa TP, Masuka N, Tshimanga M, Gombe NT, Takundwa L, Bangure D, et al. Uncontrolled hypertension among hypertensive patients on treatment in Lupane District, Zimbabwe, 2012. BMC research notes. 2014;7:703 10.1186/1756-0500-7-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong GH, Sun ZQ, Zhang XZ, Li JJ, Zheng LQ, Li J, et al. Prevalence, awareness, treatment & control of hypertension in rural Liaoning province, China. The Indian journal of medical research. 2008;128(2):122–7. . [PubMed] [Google Scholar]
- 25.Mainous AG, 3rd, King DE, Garr DR, Pearson WS. Race, rural residence, and control of diabetes and hypertension. Annals of family medicine. 2004;2(6):563–8. 10.1370/afm.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majernick TG, Zacker C, Madden NA, Belletti DA, Arcona S. Correlates of hypertension control in a primary care setting. American journal of hypertension. 2004;17(10):915–20. 10.1016/j.amjhyper.2004.05.016 . [DOI] [PubMed] [Google Scholar]
- 27.Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obesity research. 2000;8(3):270–8. 10.1038/oby.2000.32 . [DOI] [PubMed] [Google Scholar]
- 28.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine. 2001;344(1):3–10. 10.1056/NEJM200101043440101 . [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Meng Y, Yang Y, Liu Y, Dong C, Xiao J, et al. Major inducing factors of hypertensive complications and the interventions required to reduce their prevalence: an epidemiological study of hypertension in a rural population in China. BMC public health. 2011;11:301 10.1186/1471-2458-11-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcohol: Health effects. INSERM Collective Expert Reports. Paris 2000. 21348151 [Google Scholar]
- 31.Hamida F, Atif ML, Temmar M, Chibane A, Bezzaoucha A, Bouafia MT. [Prevalence of hypertension in El-Menia oasis, Algeria, and metabolic characteristics in population]. Annales de cardiologie et d'angeiologie. 2013;62(3):172–8. 10.1016/j.ancard.2013.04.008 . [DOI] [PubMed] [Google Scholar]
- 32.Steichen O, Plouin PF. [Current management of arterial hypertension]. La Revue de medecine interne. 2014;35(4):235–42. 10.1016/j.revmed.2013.06.013 . [DOI] [PubMed] [Google Scholar]
- 33.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. Bmj. 2009;339:b4567 10.1136/bmj.b4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. The New England journal of medicine. 2010;362(7):590–9. 10.1056/NEJMoa0907355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dzudie A, Kengne AP, Muna WF, Ba H, Menanga A, Kouam Kouam C, et al. Prevalence, awareness, treatment and control of hypertension in a self-selected sub-Saharan African urban population: a cross-sectional study. BMJ open. 2012;2(4). 10.1136/bmjopen-2012-001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudina EK, Michael Y, Assegid S. Prevalence of hypertension and its risk factors in southwest Ethiopia: a hospital-based cross-sectional survey. Integrated blood pressure control. 2013;6:111–7. 10.2147/IBPC.S47298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams EA, Keenan KE, Ansong D, Simpson LM, Boakye I, Boaheng JM, et al. The burden and correlates of hypertension in rural Ghana: a cross-sectional study. Diabetes & metabolic syndrome. 2013;7(3):123–8. 10.1016/j.dsx.2013.06.015 . [DOI] [PubMed] [Google Scholar]
- 38.Le directeur et comite de rédaction. Cadre Pancanadien pour la prévention et le contrôle de la pression artérielle, Canada: 2011–2012. 2012. [Google Scholar]
- 39.Jarab A., Al Zabadi H., Hallak H., Khdour M., Al-Shahed Q., T M. Determinants of Poor Blood Pressure Control in Hypertensive Patients: Findings from the Baseline Survey in West Bank, Palestine. Palestinian Medical and Pharmaceutical Journal (PMPJ). 2017. [Google Scholar]
- 40.Geronooz I J.M. K. Obésité et hypertension artérielle: de la physiopathologie au traitement. Revue Médicale de Liège. 2000;55(10):921–28. [PubMed] [Google Scholar]
- 41.Asgedom SW, Gudina EK, Desse TA. Assessment of Blood Pressure Control among Hypertensive Patients in Southwest Ethiopia. PloS one. 2016;11(11):e0166432 10.1371/journal.pone.0166432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maginga J, Guerrero M, Koh E, Holm Hansen C, Shedafa R, Kalokola F, et al. Hypertension Control and Its Correlates Among Adults Attending a Hypertension Clinic in Tanzania. Journal of clinical hypertension. 2016;18(3):207–16. 10.1111/jch.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menanga A, Edie S, Nkoke C, Boombhi J, Musa AJ, Mfeukeu LK, et al. Factors associated with blood pressure control amongst adults with hypertension in Yaounde, Cameroon: a cross-sectional study. Cardiovascular diagnosis and therapy. 2016;6(5):439–45. 10.21037/cdt.2016.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omar SM, Elnour O, Adam GK, Osman OE, Adam I. Assessment of blood pressure control in adult hypertensive patients in eastern Sudan. BMC cardiovascular disorders. 2018;18(1):26 10.1186/s12872-018-0769-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, et al. Clinical inertia. Annals of internal medicine. 2001;135(9):825–34. 10.7326/0003-4819-135-9-200111060-00012 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.