Abstract
Lung transplantation is a life-saving operation for patients with advanced lung disease. Pulmonary allografts eventually fail because of infection, thromboembolism, malignancy, airway complications, and chronic rejection, otherwise known as chronic lung allograft dysfunction (CLAD). Emerging evidence suggests that a highly-compromised airway circulation contributes to the evolution of airway complications and CLAD. There are two significant causes of poor perfusion and airway hypoxia in lung transplantation: an abnormal bronchial circulation which causes airway complications and microvascular rejection which induces CLAD. At the time of transplantation, the bronchial artery circulation, a natural component of the airway circulatory anatomy, is not surgically connected, and bronchi distal to the anastomosis become hypoxic. Subsequently, the bronchial anastomosis is left to heal under ischemic conditions. Still later, the extant microvessels in transplant bronchi are subjected to alloimmune insults that can further negatively impact pulmonary function. This review describes how airway tissue hypoxia evolves in lung transplantation, why depriving oxygenation in the bronchi and more distal bronchioles contributes to disease pathology and what therapeutic interventions are currently emerging to address these vascular injuries. Improving anastomotic vascular healing at the time of transplantation and preventing microvascular loss during acute rejection episodes are two steps that could limit airway hypoxia and improve patient outcomes.
Keywords: lung transplantation, hypoxia, hypoxia-inducible factors, anastomosis, microvasculature
1. Introduction
Lung transplantation is a viable option for many end-stage lung diseases but is limited by relatively poor five- and ten-year survival [1]. Biomedical scientists are seeking to address these poor outcomes by seeking the principal causes by increasing their focus on the airway microcirculation. Because the bronchial artery circulation is not restored at the time of transplantation, lung allograft airways are perfused by blood from the poorly-oxygenated pulmonary artery circulation and are relatively hypoxic relative to contralateral-native (non-transplanted) lung [2]. Immediately after surgery, the airway anastomosis is ischemic and susceptible to significant morbidities including infection, dehiscence, and stenosis [3]. Later, microvascular rejection occurs during acute rejection episodes, further compromising the vascular supply and contributing to the pathogenesis of CLAD [4,5]. Because of the etiologic relevance of microvascular loss in all forms of solid organ chronic rejection [4–10], there is interest in elucidating the pathways causing this microcirculatory attrition in allograft tissue. One such pathway, the hypoxia-inducible factors (HIFs), are central regulators of cellular responses to hypoxia and govern changes in cellular metabolism, proliferation, migration, and angiogenesis [11]. Hypoxia-inducible gene expression (i.e., VEGF-A, VEGF-C, HMOX1, and TIE2) is upregulated in transplanted allografts [12]. The degree of hypoxic gene expression in donor airways is directly linked to airway complications after lung transplantation. The abnormal vasculature is the proximate cause for this hypoxia. Specifically, in the early post-transplant period, the lungs receive perfusion from a single vascular bed, and in the late post-transplant period, the remnant vasculature suffers further damage through immune and non-immune mediated mechanisms [13].
Chronologically, the first reason for airway hypoxia in lung transplantation is due to organ procurement, which includes obligatory ischemia that can lead to ischemia-reperfusion injury (IRI). In the early post-transplant period, transplanted allografts are relatively hypo-perfused. Prior to transplantation, the lungs receive perfusion from the oxygen-rich bronchial arteries and the relatively hypoxic pulmonary arteries which form a complex vascular plexus in the airways [13]. The pulmonary arteries originate from the right ventricle, participate in gas exchange, are more muscular, and vasoconstrict in response to hypoxia. The bronchial arteries arise from the systemic vasculature, travel alongside the bronchi and form a vascular plexus with the pulmonary arteries near the respiratory bronchioles and alveoli. The pulmonary and systemic circulations each significantly contribute to bronchial blood flow [14]. The bronchial vasculature is responsible for supplying nutrients to the underlying bronchi and connective tissue. As a result, under native condition, the lung airways are relatively protected from hypoxemia. However, following lung transplantation, only the pulmonary circulation is preserved. While the bronchial vasculature may spontaneously re-connect, this often does not occur immediately; and may be absent up to one-year post-transplant [2]. Collateral systemic vessels that supply the donor bronchi may arise from the nearby coronary circulation in heart-lung transplants, giving further evidence to the inherent hypoxic quality of post-transplant airways [15,16]. This regional ischemia places the graft, especially the bronchial anastomosis, at risk for infectious, structural, and functional complications.
Later in the course of a lung transplant patient’s life, airway hypoxia may occur because microvessels are lost during episodes of alloimmune rejection, and this vascular injury, which is associated with significant regional tissue ischemia, may in turn give rise to CLAD, the main cause of long-term mortality [1]. The histopathologic hallmark of obstructive CLAD is the fibro-proliferative obliteration of the small airways, also known as obliterative bronchiolitis (OB). The Papworth investigators showed that pre-OB lesions have fewer microvessels, a finding that was interpreted to mean that the drop-out of the microvasculature was a direct cause of fibrotic airway remodeling [4,5]. This review reviews the mechanisms leading to airway hypoxia in the transplanted lung allograft, beginning with IRI, describes the biology governing subsequent tissue responses, and concludes with therapeutic concepts that emerge from these explanations.
2. Ischemia-reperfusion injury
Airway hypoxia in lung transplantation can occur as a result of IRI, more globally referred to as primary graft dysfunction (PGD). This form of whole graft hypoxia is not limited to the airways, per say, but involves the entire lung and is an unfortunate complication inherent to any solid organ that has an obligatory ex vivo period of low perfusion followed by sudden perfusion. PGD is the leading cause of early death following lung transplantation and is defined as the presence of hypoxemia, and diffuse radiographic infiltrates within 72 hours following transplantation, not attributable to any other identifiable cause [1]. Given that the exact mechanisms leading to the development of IRI have not been elucidated, treatment is generally supportive, rather than curative. IRI is generally due to the formation of reactive oxygen species, which results in activation of the innate immune system, increase in cytokines and damage associated molecular patterns (DAMPs) [17,18]. Ultimately, this immune activation causes endothelial barrier dysfunction and epithelial damage, resulting in diffuse alveolar damage, edema, and hypoxemia.
3. Vascular injury and airway hypoxia in lung transplantation.
The two main causes of airway hypoxia following lung transplantation occur because bronchial artery revascularization does not occur and because donor microvessels are susceptible to acute rejection. Because the current surgical grafting technique omits restoration of the bronchial artery circulation, the blood supply to the fresh bronchial anastomosis lacks the normal dual supply from bronchial and pulmonary arteries, and the connection site between recipient and donor is particularly ischemic (Figure 1). In this sense, the first vascular injury is attributable to the creation of an aberrant transplant circulation. Additionally, the more distal airway microvasculature is vulnerable to alloimmune-mediated rejection (Figure 2). Pre-clinical models suggest that when airway blood vessels are destroyed, regional ischemia and hypoxia last days occurs, followed by irreversible fibrotic remodeling. The loss of a functional microvasculature is closely linked to the inability to rescue airways from chronic rejection [19]. Consequently, intervening to both promote revascularization to protect the surgical anastomosis at the time of transplantation or, later, to protect blood vessels during acute rejection both have high potential to improve transplant outcomes.
Figure 1. Airway hypoxia in lung transplantation: the bronchial anastomosis at the time of transplantation.
Airways are supplied with blood by a vascular plexus which receives blood from both bronchial and pulmonary circulatory systems. In the early post-transplant period, the anastomosis site is ischemic and hypoxic and becomes, over time, susceptible to airway complications.
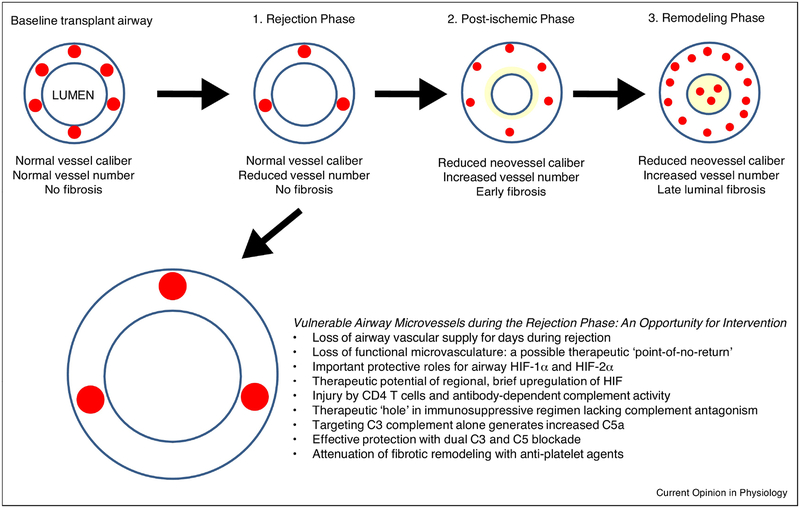
Figure 2. Airway hypoxia in lung transplantation: the airways during acute rejection episodes.
In the acute rejection phase, there is a loss of airway microvessels that may lead to the development of chronic rejection. The airway microvasculature is especially vulnerable during acute rejection and may be amenable to specific therapeutic interventions prior to airway remodeling in the post-ischemic and remodeling phases.
Given the importance of the microvasculature in maintaining lung allograft health, there has been significant interest in developing animal models to facilitate the study of vascular beds. The orthotopic tracheal transplant (OTT) model, in which a donor trachea is transplanted into a recipient mouse, is a validated and accepted tool to evaluate large airway vasculature [20]. Transplantation between different strains results in acute rejection pathology, similarly to lymphocytic bronchitis. Using the OTT model, our group has characterized the immune-mediated mechanisms that lead to vascular injury following tracheal transplantation. We found that CD4+ T cells and antibody-dependent complement activation are the main drivers of alloimmune-mediated vascular injury [21,22]. In addition to adaptive immunity, the innate immune system also propagates microvascular damage. Inhibition of adaptive and innate immunity, as accomplished by treatment with cyclosporine and elafin (neutrophil elastase inhibitor), provides greater protection of graft perfusion, as compared to cyclosporine treatment alone [23]. Elastase activity is a noteworthy contributor to microvascular injury and likely works in concert with the adaptive immune system to negatively impact the transplant.
As mentioned in the Introduction, the HIFs are central regulators of cellular responses to hypoxia. Under normoxic conditions, HIFs are degraded through a proteasome-mediated mechanism which includes hydroxylation of prolyl hydroxylase followed by von Hippel-Lindau protein (VHL)-mediated ubiquitination and degradation. HIF-1 is upregulated in airways undergoing allograft rejection, presumably as a stimulus to help remote microvascular repair and healing [24]. In this process, recipient-derived cells can contribute to vascular repair. Augmentation of HIF-1 signaling through gene therapy can bolster microvascular healing and limit fibrotic remodeling. Upregulation of HIF-1 and HIF-2 in VHL-haplodeficient airway donors is similarly protective [25]. Recently, our group found that endothelial HIF-2 (but not HIF-1) is an essential survival factor for airway microvascular cells [26]; HIF-2 promotes microvessel integrity through endothelial angiopoietin-1/TIE2 signaling and Notch activity. Genetically-upregulating HIF-2 in airway allografts provides robust protection from microvascular destruction and diminishes alloimmune inflammation. Transient upregulation of the HIFs in the peri-transplant period at the anastomosis site or during acute rejection episodes in the more distal airways have therapeutic potential for the two major vascular injuries described in Figures 1 and 2 [24,27,28]. To this end, iron-chelators are FDA-approved therapies for iron-overload-related disorders which stabilize the HIFs and their potential as a treatment is described in the Therapeutic Opportunities section below.
2. Hypoxia and airway infection in lung transplantation.
Relative to other solid organ recipients, lung transplant recipients face an increased risk of infectious complications, due to higher levels of immunosuppression, constant and direct exposure to environmental pathogens, and impaired mucociliary clearance post-transplant. In the initial post-transplant period, the lung allograft is especially susceptible to Aspergillus fumigatus, a facultative anaerobe that thrives in hypoxic environments [29]. Infections can manifest as tracheobronchitis, invasive pulmonary aspergillosis, or bronchial anastomotic infections. Preventing these infections is of paramount importance as infection of the anastomotic sites is associated with stenosis, which can lead to significant pulmonary impairment, or more seriously, dehiscence, which is associated with significant mortality. A unique feature of Aspergillus is its ability to establish an ischemic microenvironment, via the production of anti-angiogenic factors such as gliotoxin, a metabolite that inhibits angiogenesis [30]. Therefore, to protect the lung allograft, many centers employ dual anti-fungal therapies in the initial post-transplant period. Improving the anastomotic circulation at the time of transplantation may lessen this airway complication, as described below.
3. Therapeutic Opportunities to Limit Airway Hypoxia
Given the importance of airway microvessels in maintaining allograft health, there is interest in developing directed interventions to preserve allograft vasculature and to promote its regrowth. As VEGF is a central regulator of vasculogenesis, modulators of VEGF expression are a possible option for intervention. However, aberrant vascular growth promoted by this growth factor can lead to edema and inflammation [31,32], limiting its benefit in to the transplant. Another approach is through the temporary stabilization of airway HIFs through topical application of iron chelators to the anastomosis site at the time of surgery. Iron chelators can promote neovascularization at the ischemic wound site whereas aerosolized iron-chelators can facilitate microvascular repair during acute rejection episodes [27]. Iron chelators stabilize HIFs by inhibiting the activity of prolyl hydroxylases through the depletion of Fe2+ [33,34]. With the topical application of an iron chelator at the time of transplantation, the drug can be applied directly to the anastomosis site to diminish ischemia as well as limit Aspergillus invasion [27,29]. To improve the bioavailability and distribution of iron chelators, these agents can be suspended in nanoparticle solutions which facilitate effective drug delivery [27]. With inhaled iron chelators during acute rejection episodes, formulations can be designed to penetrate to distal airways where fibrotic occlusions are prone to develop in CLAD. In this manner, blocking microvascular injury during periods of highest vulnerability (Figure 2), it may be possible to limit the development of CLAD. A self-limited period of anti-coagulation, such as with the anti-platelet agent, clopidogrel, could similarly be useful by preventing occlusive microvessel clots and thereby limit airway ischemia and hypoxia [35].
As mentioned above, it is possible that there is a therapeutic ‘hole’ existing in standard immunosuppressive regimens which could be addressed by adjunctive therapies. Pre-clinical modeling demonstrates that CD4 T cells and antibody-dependent complement activity are independently sufficient to destroy airway microvessels [22]. While transplant clinicians typically treat patients with steroids and calcineurin inhibitors which control CD4 T cell responses, the innate immune arm (including complement activation) is less-well targeted. To this end, add-on therapies (e.g., complement and neutrophil elastase inhibitors) could supplement typical methylprednisolone bursts to provide more-complete immune coverage to close the therapeutic hole [36,37]. Adjunctive approaches must also be considered for negative side-effects; broadening immunosuppression renders patients more susceptible to infections and malignancies. Similarly, upregulating HIF-1 (especially for prolonged periods) could stimulate Th1/Th17 immunity and be harmful to the transplant [38].
4. Conclusions
Lung transplants are unique for being the only solid organ allograft in which the native vascular supply (in the case of lung transplants, a dual supply) is not restored at the time of transplantation. The immediate effect of not restoring the bronchial artery circulation is that the anastomotic site where the recipient airway is joined to the donor airway is highly ischemic and hypoxic post-operatively. This physiologic derangement renders the airway anastomosis susceptible to stenosis, dehiscence, and infection [3]. This problem could be addressed by including a bronchial artery revascularization step, as advocated by Gosta Pettersson (Cleveland Clinics) [39], but the collective surgical community has not yet embraced this approach because of the technical challenges and its currently-unconfirmed benefits. One solution to this issue is to promote angiogenesis with locally-applied medications (such as an iron chelator solution) at the time of transplantation. The second major microvascular issue that emerges is attributable to local ischemia and hypoxia that likely occurs in the distal airways during the time of allograft rejection [23]. There may be a direct causal link between this microvascular injury and the subsequent development of CLAD. Intervening by directly upregulating HIFs during acute rejection episodes or, other strategies, such as limited anti-coagulation, could benefit patients by directly working to preserve a functional airway circulation. Alternatively, targeting the therapeutic ‘hole’ in immunosuppression to limit innate immune injury during acute rejection episodes could similarly promote microvascular health. In closing, there is now a strong rationale to optimize microvascular health in solid organ transplants through the testing of novel therapies which limit tissue hypoxia.
Highlights.
Highlights are not required for this journal.
Acknowledgments:
This work was supported by the National Institutes of Health [HL095686, HL138473, HL122887, HL014985, HL129970].
Abbreviations:
- BOS
bronchiolitis obliterans syndrome
- CLAD
chronic lung allograft dysfunction
- IR
ischemia-reperfusion
- OB
obliterative bronchiolitis
- PGD
primary graft dysfunction
- VHL
von Hippel-Lindau protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
Mark Nicolls, is an inventor on a patent that is directly germane to the ideas that are proposed in this review. U.S. Application Serial No. 14/653,245 Entitled: Iron Chelators and Use Thereof for Reducing Transplant Failure During Rejection Episodes First Named Inventor: Nicolls, Mark R. Your Ref.: S11–300; C11657_P11657–03 Our Ref.: STAN-891 Patent No. 9763899. Although, only in its incipient stages, a company, which Mark Nicolls is involved with, is being formed around the concept of using iron chelators in lung transplant recipients, which is a concept referred to multiple times as a promising approach within the manuscript.
References
- 1.Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J: The registry of the international society for heart and lung transplantation: Thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: Allograft ischemic time. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation (2017) 36(10):1047–1059. [DOI] [PubMed] [Google Scholar]
- 2.Dhillon GS, Zamora MR, Roos JE, Sheahan D, Sista RR, Van der Starre P, Weill D, Nicolls MR: Lung transplant airway hypoxia: A diathesis to fibrosis? Am J Respir Crit Care Med (2010) 182(2):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespo MM, McCarthy DP, Hopkins PM, Clark SC, Budev M, Bermudez CA, Benden C, Eghtesady P, Lease ED, Leard L, D’Cunha J et al. : Ishlt consensus statement on adult and pediatric airway complications after lung transplantation: Definitions, grading system, and therapeutics. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation (2018) 37(5):548–563.* The problem of airway complications following transplantation is described and a pathological grading scale is proposed which may help guide future clinical trials which seek to prevent and treat this important lung transplant morbidity.
- 4.Luckraz H, Goddard M, McNeil K, Atkinson C, Charman SC, Stewart S, Wallwork J: Microvascular changes in small airways predispose to obliterative bronchiolitis after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation (2004) 23(5):527–531. [DOI] [PubMed] [Google Scholar]
- 5.Luckraz H, Goddard M, McNeil K, Atkinson C, Sharples LD, Wallwork J: Is obliterative bronchiolitis in lung transplantation associated with microvascular damage to small airways? Ann Thorac Surg (2006) 82(4):1212–1218. [DOI] [PubMed] [Google Scholar]
- 6.Bishop GA, Waugh JA, Landers DV, Krensky AM, Hall BM: Microvascular destruction in renal transplant rejection. Transplantation (1989) 48(3):408–414. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto Y, McCaughan GW, Painter DM, Bishop GA: Evidence that portal tract microvascular destruction precedes bile duct loss in human liver allograft rejection. Transplantation (1993) 56(1):69–75. [DOI] [PubMed] [Google Scholar]
- 8.Revelo MP, Miller DV, Stehlik J, Brunisholz K, Drakos S, Gilbert EM, Everitt M, Budge D, Alharethi R, Snow G, Hammond EH et al. : Longitudinal evaluation of microvessel density in survivors vs. Nonsurvivors of cardiac pathologic antibody-mediated rejection. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology (2012) 21(6):445–454. [DOI] [PubMed] [Google Scholar]
- 9.Bijkerk R, Florijn BW, Khairoun M, Duijs J, Ocak G, de Vries APJ, Schaapherder AF, Mallat MJK, de Fijter JW, Rabelink TJ, van Zonneveld AJ et al. : Acute rejection after kidney transplantation associates with circulating micrornas and vascular injury. Transplant Direct (2017) 3(7):e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loupy A, Cazes A, Guillemain R, Amrein C, Hedjoudje A, Tible M, Pezzella V, Fabiani JN, Suberbielle C, Nochy D, Hill GS et al. : Very late heart transplant rejection is associated with microvascular injury, complement deposition and progression to cardiac allograft vasculopathy. Am J Transplant (2011) 11(7):1478–1487. [DOI] [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular o2 tension. Proc Natl Acad Sci U S A (1995) 92(12):5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraft BD, Suliman HB, Colman EC, Mahmood K, Hartwig MG, Piantadosi CA, Shofer SL: Hypoxic gene expression of donor bronchi linked to airway complications after lung transplantation. Am J Respir Crit Care Med (2016) 193(5):552–560.** An important clinical study suggesting a strong relationship between airway hypoxia and airway complications
- 13.Nicolls M, Zamora MR: Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr Opin Organ Transplant (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barman SA, Ardell JL, Parker JC, Perry ML, Taylor AE: Pulmonary and systemic blood flow contributions to upper airways in canine lung. Am J Physiol (1988) 255(5 Pt 2):H1130–1135. [DOI] [PubMed] [Google Scholar]
- 15.Guthaner DF, Wexler L, Sadeghi AM, Blank NE, Reitz BA: Revascularization of tracheal anastomosis following heart-lung transplantation. Invest Radiol (1983) 18(6):500–503. [DOI] [PubMed] [Google Scholar]
- 16.Singh SP, Nath H, McGiffin D, Kirklin J: Coronary tracheal collaterals after heart-lung transplant. Am J Cardiol (2003) 92(12):1490–1492. [DOI] [PubMed] [Google Scholar]
- 17.Laubach VE, Sharma AK: Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant (2016) 21(3):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL: Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol (2010) 299(5):H1283–1299. [DOI] [PubMed] [Google Scholar]
- 19.Babu AN, Murakawa T, Thurman JM, Miller EJ, Henson PM, Zamora MR, Voelkel NF, Nicolls MR: Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest (2007) 117(12):3774–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lama VN, Belperio JA, Christie JD, El-Chemaly S, Fishbein MC, Gelman AE, Hancock WW, Keshavjee S, Kreisel D, Laubach VE, Looney MR et al. : Models of lung transplant research: A consensus statement from the national heart, lung, and blood institute workshop. JCI insight (2017) 2(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan MA, Nicolls MR: Complement-mediated microvascular injury leads to chronic rejection. Advances in experimental medicine and biology (2013) 735(233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan MA, Jiang X, Dhillon G, Beilke J, Holers VM, Atkinson C, Tomlinson S, Nicolls MR: Cd4+ t cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants. Circulation research (2011) 109(11):1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolls MR, Hsu JL, Jiang X: Microvascular injury after lung transplantation. Curr Opin Organ Transplant (2016) 21(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, Yoder MC, Semenza GL, Nicolls MR: Adenovirus-mediated hif-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest (2011) 121(6):2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Hsu JL, Tian W, Yuan K, Olcholski M, Perez Vde J, Semenza GL, Nicolls MR: Tie2-dependent vhl knockdown promotes airway microvascular regeneration and attenuates invasive growth of aspergillus fumigatus. J Mol Med (Berl) (2013) 91(9):1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X, Tian W, Tu A, Pasupneti S, Shuffle E, Dahms P, Zhang P, Haoliang C, Dinh T, Liu B, Giaccia A et al. : Endothelial hif-2 is required for the maintenance of airway microvasculature. Circulation (2018. (in press)).** In this paper, endothelial HIF-2 is found to be a key survival factor for airway vasculature in transplants. Upregulating HIF-2 not only promotes microvascular survival in transplants but is also noted to be anti-inflammatory.
- 27.Jiang X, Malkovskiy AV, Tian W, Sung YK, Sun W, Hsu JL, Manickam S, Wagh D, Joubert LM, Semenza GL, Rajadas J et al. : Promotion of airway anastomotic microvascular regeneration and alleviation of airway ischemia by deferoxamine nanoparticles. Biomaterials (2014) 35(2):803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heim C, Motsch B, Jalilova S, Bernhardt WM, Ramsperger-Gleixner M, Burzlaff N, Weyand M, Eckardt KU, Ensminger SM: Reduction of obliterative bronchiolitis (ob) by prolyl-hydroxylase-inhibitors activating hypoxia-inducible transcription factors in an experimental mouse model. Transpl Immunol (2016) 39(66–73. [DOI] [PubMed] [Google Scholar]
- 29.Hsu JL, Manouvakhova OV, Clemons KV, Inayathullah M, Tu AB, Sobel RA, Tian A, Nazik H, Pothineni VR, Pasupneti S, Jiang X et al. : Microhemorrhage-associated tissue iron enhances the risk for aspergillus fumigatus invasion in a mouse model of airway transplantation. Science translational medicine (2018) 10(429).* In this study, iron deposited in tissues because of microvascular injury is found to be an independent risk factor for fungal invasion in airway transplant recipients.
- 30.Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP: Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood (2009) 114(26):5393–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA: Vascular endothelial growth factor (vegf) induces remodeling and enhances th2-mediated sensitization and inflammation in the lung. Nat Med (2004) 10(10):1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weis SM, Cheresh DA: Pathophysiological consequences of vegf-induced vascular permeability. Nature (2005) 437(7058):497–504. [DOI] [PubMed] [Google Scholar]
- 33.Wang GL, Semenza GL: Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood (1993) 82(12):3610–3615. [PubMed] [Google Scholar]
- 34.Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S: Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol (2008) 172(3):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preidl RH, Eckl S, Ramsperger-Gleixner M, Koch N, Spriewald BM, Weyand M, Ensminger SM: Clopidogrel reduces post-transplant obliterative bronchiolitis. Transpl Int (2013) 26(10):1038–1048. [DOI] [PubMed] [Google Scholar]
- 36.Khan MA, Maasch C, Vater A, Klussmann S, Morser J, Leung LL, Atkinson C, Tomlinson S, Heeger PS, Nicolls MR: Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc Natl Acad Sci U S A (2013) 110(15):6061–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X, Nguyen TT, Tian W, Sung YK, Yuan K, Qian J, Rajadas J, Sallenave JM, Nickel NP, de Jesus Perez V, Rabinovitch M et al. : Cyclosporine does not prevent microvascular loss in transplantation but can synergize with a neutrophil elastase inhibitor, elafin, to maintain graft perfusion during acute rejection. Am J Transplant (2015) 15(7):1768–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkes DS: Chronic lung allograft rejection and airway microvasculature: Is hif-1 the missing link? J Clin Invest (2011) 121(6):2155–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong MZ, Johnston DR, Pettersson GB: The role of bronchial artery revascularization in lung transplantation. Thorac Surg Clin (2015) 25(1):77–85. [DOI] [PubMed] [Google Scholar]