Abstract
STUDY QUESTION
Can time-lapse imaging systems make it possible to identify novel early non-invasive biomarkers to predict live birth?
SUMMARY ANSWER
From mostly high-grade embryos, out of 35 morphometric, morphologic and morphokinetic variables, only pronuclei (PN) position at time of PN juxtaposition and the absence of multinucleated blastomeres at the 2-cell stage (MNB2cell), were potentially associated with live birth.
WHAT IS KNOWN ALREADY
Previous studies indicate that some kinetic markers may be predictive of blastocyst development and embryonic implantation. Certain teams have suggested including some of them in decisional algorithms for embryo transfers.
STUDY DESIGN, SIZE, DURATION
Using a time-lapse incubator (EmbryoScope, Unisense FertiliTech), we retrospectively explored the associations between the morphometric, morphologic and morphokinetic parameters of oocytes, zygotes and embryos, and their associations with live birth. This study assessed 232 embryos from single embryo transfers after ICSI cycles performed between January 2014 and December 2017.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The morphometric, morphologic and morphokinetic parameters (18, 4 and 13, respectively) of oocytes, zygotes and early embryos were studied retrospectively. The associations between these parameters were examined using a Spearman’s correlation, Mann–Whitney or chi-squared test as appropriate. We examined whether these parameters were associated with outcomes in univariate and multivariate logistic regression analyses.
MAIN RESULTS AND THE ROLE OF CHANCE
Central PN juxtaposition was associated with a 2-fold increase in the odds of live birth (OR = 2.20; 95% CI, [1.26–3.89]; P = 0.006), while the presence of MNB2cell was associated with half the odds of live birth (OR = 0.51; 95% CI, [0.27–0.95]; P = 0.035). These two parameters were independent of embryo kinetics. The 33 remaining parameters had no significant association with the capacity of transferred embryos to develop to term.
LIMITATIONS, REASONS FOR CAUTION
Even though the population size was relatively small, our analyses were based on homogeneous cycles, i.e. young women whose transferred embryos were found to be high-grade according to conventional morphology evaluation. In addition, our conclusions were established from a specific, highly selected population, so other study populations, such as women in an older age bracket, may yield different results. Finally, because we assessed day 2/3 transfers, our findings cannot be generalized to embryos cultured up to the blastocyst stage.
WIDER IMPLICATIONS OF THE FINDINGS
It would be interesting to explore, prospectively, whether PN localisation is a relevant measure to predict embryo development when added into further algorithms and whether this parameter could be suitable for use in other IVF clinics. Further studies are needed, notably to explore the added value of timing evaluation in cohorts of embryos with low or intermediate morphology grade, as well as in other maternal populations (i.e. older women).
STUDY FUNDING/COMPETING INTEREST(S)
No external funding was used for this study. P. Sagot received funding from the following commercial companies: Merck Serono, Finox Biotech, Ferring, MSD France SAS, Teva Sante ´ SAS, Allergan France, Gedeon Richter France, Effik S.A., Karl Storz Endoscopie France, GE Medical Systems SCS, Laboratoires Genevrier, H.A.C. Pharma and Ipsen.
All the authors confirm that none of this funding was used to support the research in this study. There are no patents, products in development or marketed products to declare. This does not alter the authors’ adherence to all the journal policies on sharing data and materials.
Keywords: embryo morphology, embryo morphometric parameters, embryo kinetics, ICSI, live birth, time-lapse imaging system
Introduction
Single embryo transfers (SETs) reduce the incidence of multiple pregnancies after IVF (McLernon et al., 2010), but the selection of embryos with higher developmental potential is crucial to ensure high implantation and birth rates (Ziebe et al., 1997; Van Royen et al., 1999; De Neubourg et al., 2002). Traditionally, and according to the ESHRE/ALPHA consensus, embryo morphological grading, like the number of blastomeres and the degree of fragmentation, are useful criteria for embryo selection (ALPHA Scientists in Reproductive Medicine and ESHRE Special Interest Group Embryology, 2011). Over the last few years, time-lapse imaging (TLI) systems have been developed, giving embryologists the chance to use additional non-invasive criteria. Several markers of embryonic kinetics (Lemmen et al., 2008; Abeyta and Behr, 2014; Armstrong et al., 2015) have been studied from the first cleavage onwards, and some of them have been applied in decisional algorithms for embryo transfer (Meseguer et al., 2011; VerMilyea et al., 2014; Basile et al., 2015; Liu et al., 2016; Milewski et al., 2016; Petersen et al., 2016; Carrasco et al., 2017). However, there is still a need to determine whether kinetic parameters can be used as independent criteria to improve embryo selection (Goodman et al., 2016; Petersen et al., 2016; Armstrong et al., 2018).
A study published in 2018 employed TLI monitoring to investigate fertilisation events in detail (Coticchio et al., 2018). Even though the impact on clinical outcomes has not been assessed, the morphokinetic parameters linked to fertilisation were identified as potential novel embryonic developmental biomarkers (Coticchio et al., 2018). Furthermore, reports that look at oocyte size and morphology as indications of oocyte quality are scarce. The recently established relationship between the morphometric parameters of mature oocytes and the morphokinetic behaviour of subsequent embryos underline the need for further evaluation of oocyte morphology (Faramarzi et al., 2017).
The TLI system thus offers researchers the ability to explore the impact of other non-invasive parameters on outcomes, in the embryo as well as in the oocyte and zygote. However, the association between early oocyte- or fertilisation-related parameters and clinical outcomes has garnered little attention so far. This study provides a comprehensive morphometric, morphologic and morphokinetic description of embryos starting from the oocyte in a subset of SET. Our main objective was to expand the range of possible parameters that may be developed as non-invasive biomarkers of live birth.
Materials and Methods
Study design and population
This retrospective study from January 2014 to December 2017 at the University Hospital of Dijon included SET (elective or not, i.e. with remaining cryopreserved embryos or only one embryo available, respectively) at either day 2 or 3 from fertilized oocytes cultured in the time-lapse incubator (EmbryoScope, Unisense FertiliTech). Only ICSI cycles were included, allowing us to control the time of insemination and to report oocyte- and fertilisation-related measures. We only included one cycle per patient. Women over 42 years of age, egg donations, cases associated with disorders such as hydrosalpinx, obesity (BMI > 32) or uterine conditions and surgical spermatozoa or ICSI performed in a viral context were excluded. A total of 232 transferred embryos were studied.
We then completed a comprehensive morphometric, morphologic and morphokinetic description of the embryos. To improve the consistency and homogeneity of the results, the embryos were described systematically by the two same embryologists who were blinded to the outcome of the embryo transfer.
The information used in the study was collected for clinical care. According to French Public Health Law, non-interventional studies on humans do not require approval from Institutional Review Board or written consent from the participants. Nevertheless, we obtained formal confirmation that ethical approval was not required for this observational study.
ICSI protocol and embryo culture
The controlled ovarian stimulation (COS) protocols consisted of either long or short GnRH agonist downregulation followed by rFSH/hMG or antagonist protocols. The short agonist protocol was preferred for patients with an expected low ovarian response while the antagonist protocol was used to prevent ovarian hyperstimulation syndrome. A single injection of hCG (Ovitrelle; Merck Serono) was administered to induce final follicular maturation. The luteal phase was supported by 400 mg of vaginal progesterone started the day after oocyte retrieval. The dose was maintained until 6 weeks of gestation (WG) and then dropped to 200 mg per day until 8 WG. Oocyte retrieval was performed by transvaginal ultrasound-guided follicle aspiration 36 h after hCG injection. Sperm preparation for ICSI was performed as previously described (Barberet et al., 2018). The oocyte–cumulus complexes were denuded using hyaluronidase (Fertipro, Belgium). ICSI was performed within 2 h of oocyte retrieval on mature oocytes.
After micro-injection, inseminated oocytes were immediately transferred into pre-equilibrated Embryoslides (Unisense Fertilitech) with 25 μl of culture medium (Global, LifeGlobal) under oil (Nidoil, Nidacon). Then, the Embryoslides were incubated in the EmbryoScope at 37.0°C, 6%CO2, 5%O2. Embryo development was recorded every 20 min in seven different focal planes. The images and related data were stored in the EmbryoViewer (Unisense FertiliTech) and subsequently analysed.
Recording of morphologic-morphometric-morphokinetic parameters
The annotation options for all parameters described in Supplementary Table SI and Fig. 1 are not included in the Embryoscope software. Therefore, an independent, purpose-built database was created and used for data analysis.
Figure 1.
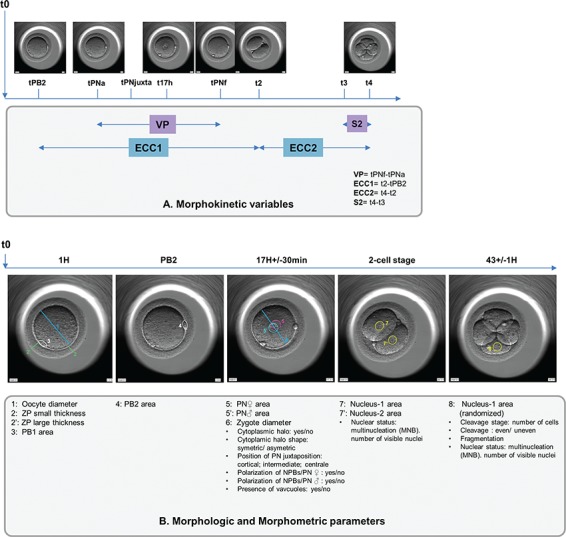
Description of recorded morphokinetic events (A) and monitored morphologic-morphometric parameters (B).
Oocyte
The oocyte diameter (corresponding more precisely to the ooplasm) and the first polar body (PB1) area were documented. The mean thickness of the thinnest and thickest zona pellucida (ZP) were used to estimate the thickness of the ZP.
Zygote
Similarly, we recorded the measurements of the second polar body (PB2). Nuclear events were annotated as suggested by Ciray et al. (2014): (i) the time of PB2 emission (tPB2), (ii) the time of appearance of the two pronuclei (tPNa) with the distinction of the female (identified as the one near the site of emission of the PBII) and male pronuclei (PN), (iii) the time of PN juxtaposition (tPNjuxta), (iv) the time of their fade out (tPNf) and (v) the time period in which the pronuclei were visible (VP).
At the tPNjuxta, we annotated the PN position (cortical, intermediate or central). Fertilisation status was checked 17 h ± 30 min after ICSI as recommended by the ESHRE/ALPHA consensus (2011). At this time, the areas of the PN (female and male) and the diameter of the zygote were also assessed. PB and PN surfaces were directly measured thanks to the EmbryoScope viewer’s elliptical measurement tool by circumscribing the outer periphery of PB or PN. The polarization of nucleolar precursor bodies (NPBs) was recorded distinctly within the female and male PN at 17 h +/− 30 min.
We indicated whether there was a cytoplasmic halo and its position relative to the centre of the oocyte (symmetrical/asymmetrical). The presence of one or more vacuoles was also recorded.
Embryos
The classic morphological appearance of the embryos was monitored: the number and the size of blastomeres (regular or irregular cleavage) and the percentage of anucleate fragments were evaluated once at 44 ± 1H on Day 2 and 68 ± 1H on Day 3 post-ICSI as recommended by the ESHRE/ALPHA consensus. The only criterion used to decide whether to transfer on Day 2 or 3 was the day of oocyte retrieval: Friday [embryo transfer at Day 3] or not [embryo transfer at Day 2]. The sequence of events was recorded according to previously established guidelines (Ciray et al., 2014). Therefore, cell stages were described as the time from ICSI to the first frame in which the membranes of the two, three or four blastomeres were completely separated (t2, t3, t4). Additionally, we calculated the duration of the first cell cycle (ECC1 = t2-tPB2), the second embryo cell cycle (ECC2 = t4-t2) and the time period to complete synchronous divisions (S2 = t4-t3). We checked for two possible anomalies in embryo cleavage: reverse cleavage, when a blastomere is re-absorbed after cleavage, and direct cleavage, when a single blastomere divides directly from one to three cells in less than 5 h at first cleavage.
The nuclear area was assessed in embryos at the 2-cell stage (the area was measured in each visible nucleus and if two nuclei were observed, we calculated the mean and whether the difference between the nuclei was ≥25%) and the 4-cell stage (for one randomized nucleus). At the 2-cell stage, the nuclear and multinucleation status were also checked and recorded as MNB2cell if at least one blastomere was multinucleated. The presence of multinucleation at Day 2/3 was documented as was the number of visible mononucleated blastomeres at the 4-cell stage.
Embryos fertilized on Day 1 with regular 4- to 5-cell embryos at Day 2, <20% fragmentation and no MNB2cell were deemed ‘TOP’ grade (Van Royen et al., 1999; Fauque et al., 2013).
Outcomes
Live birth was the main endpoint of this study. We associated the features recorded in the early embryonic stages with the occurrence of live birth. We also investigated the potential associations between morphologic, morphometric and/or embryonic events.
Statistical analysis
Patient characteristics were described and compared depending on the outcome (live birth or no live birth) using a chi-squared test or Fisher’s exact test for categorical variables and a Student’s t-test or Mann–Whitney test for continuous variables.
The morphometric, morphologic and morphokinetic parameters of the embryos were first described and expressed as frequencies for categorical data, and as means with SD for time parameters and quantitative data. A box plot was used to summarize the distribution of time parameters. The associations between these parameters were then examined using Spearman’s rank correlation (ρ), a Mann–Whitney test or a chi-squared test as appropriate.
The relationship between morphometric, morphologic and morphokinetic parameters of embryos and the onset of live birth (independent variable) was first examined in univariate analysis using appropriate tests. Because there was no initial hypothesis regarding a threshold, morphometric parameters (oocyte or zygote diameters and the nuclear areas at the 2-cell and 4-cell stages) were converted from continuous variables into binary variables by dividing them into groups based on their 75th percentile. Then, the parameters that reached a significance of P < 0.15 in univariate analyses were introduced into a stepwise multivariate logistic regression model. A backward procedure was used, and variables were excluded if the corresponding P-value for the Wald test was higher than 0.05. Analyses were systematically adjusted for maternal age, which is known to impact the rate of live birth. The cause of female infertility and the COS protocol are linked, so we chose to include only the protocol in the model as an adjusted variable. Interactions were tested, and the adequacy of the model was assessed using the Hoswer–Lemershow test. Models were compared using the Akaike information criterion.
P < 0.05 was considered significant for all tests except the analysis of associations between morphologic, morphometric and/or embryonic events where a lower P-value (<0.01) was used to determine significance given the number of analyses. Odds ratios (OR) are provided with 95% confidence intervals (CI). All analyses were performed using SAS statistical software (version 9.4, SAS Institute, Cary, NC).
Results
Patient characteristics
From 232 SETs (85% and 15% at Days 2 and 3, respectively), 118 biochemical pregnancies and 99 live births of healthy children were achieved. The mean age of the women in our study was 30.8 ± 4.2 years. The mean value for anti-Müllerian hormone (AMH) was 4.4 ± 4.3 ng/ml, mean BMI was 23.8 ± 4.2 and the mean number of treatment cycles was 1.1 ± 0.3. Indication for treatment was male infertility alone in 38.4% of cycles and combined male and female infertility in 40.1% of cycles. Biochemical pregnancy, implantation and delivery rates were 50.9%, 45.3% and 42.7%, respectively.
First, we compared the characteristics of the two groups of SETs resulting in a live birth or not (Supplementary Table SII). The COS protocols were different while age, AMH values, BMI and cycle characteristics (such as number of retrieved oocytes) were similar (Supplementary Table SII).
Association between morphologic/morphokinetic/morphometric embryo parameters
The analyses of correlations revealed that oocyte diameter was not associated with any time parameters (Fig. 2).
Figure 2.
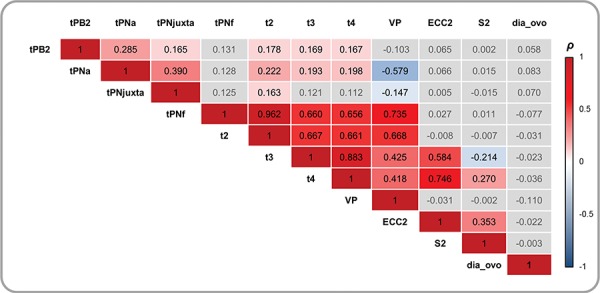
Correlations between quantitative variables (oocyte diameter and fertilisation/embryonic timings), Spearman’s correlation test (ρ). Grey boxes represent non-significant coefficients. tPB2, time of polar body (PB) 2 emission; tPNa, the time of appearance of the two pronuclei with the distinction between the female (identified as the one near the site of emission of the PBII) and male PN; tPNjuxta, the time of PN juxtaposition; tPNf, the time of pronuclear fade out; t2, t3, t4, the time to reach 2, 3, 4 cells; VP, the time period in which the pronuclei were visible; ECC2, the time period of the second cell cycle (t4-t2); S2, the time period to complete synchronous divisions (t4-t3).
Among kinetic embryo parameters, there was no correlation between tPB2, tPNa and tPNjuxta and any of the subsequent events. From the time of PN disappearance onwards, the timings of the various embryonic events were correlated together (Fig. 2). A significant correlation was found between ECC2 and t4 (ρ = 0.746; P < 0.001) as well as between VP and tPNf (ρ = 0.735; P < 0.001; Fig. 2).
The associations between the timings of embryonic events and morphological embryonic parameters were studied. The later first cleavage occurred, the later further embryo divisions occurred. The times at which PN faded were associated with the appearance of the first cleavage (Supplementary Table SIII). The central position of PN juxtaposition was not associated with any timing or morphological parameters except the time of PN juxtaposition (P < 0.001, Supplementary Table SIII).
Morphologic/morphokinetic/morphometric embryo parameters and outcomes
The next step was to compare the early embryo parameters recorded in the two outcome groups in order to detect potential new markers for embryos likely to result in live birth.
In our sample, which was composed of more than 88% of TOP embryos, the four conventional morphologic parameters observed at Day 2 and 13 timings of embryonic events were not significantly different in the two groups (Table I, Supplementary Table SIV, Fig. 3).
Table I.
Morphologic parameters according to live birth outcome.
| Live birth | |||
|---|---|---|---|
| Parameter | No (n = 133) | Yes (n = 99) | P-value a |
| Cleavage stagec: | |||
| 2-cell | 1 (0.7) | 0 (0) | 1.000 |
| 4-cell | 125 (94.0) | 94 (95.0) | |
| 5-cell | 5 (3.8) | 3 (3.0) | |
| 6-cell | 2 (1.5) | 2 (2.0) | |
| 1st Cleavage | |||
| ≤26 h | 100 (75.2) | 81 (81.8) | 0.228 |
| >26 h | 33 (24.8) | 18 (18.2) | |
| Embryo fragmentationc: | |||
| [0–10]% | 119 (89.5) | 82 (82.8) | 0.318 |
| [10–20]% | 12 (9.0) | 14 (14.1) | |
| [20–50]% | 2 (1.5) | 3 (3.0) | |
| Cleavage typec: | |||
| regular | 124 (93.2) | 94 (94.9) | 0.587 |
| irregular | 9 (6.8) | 5 (5.1) | |
| ‘TOP’ embryob | 118 (88.7) | 88 (88.9) | 0.968 |
For categorical variables, n (%) is presented. For continuous variables, mean (± SD) and range are presented. IQ: interquartile.
aChi2 or Fisher’s exact tests were used for dichotomous variables.
b‘TOP’ embryo is fertilized at Day 1 without MNB2cell with regular 4- to 5-cell embryos at Day 2 with <20% fragmentation.
cAt 43H+/-1H.
Figure 3.
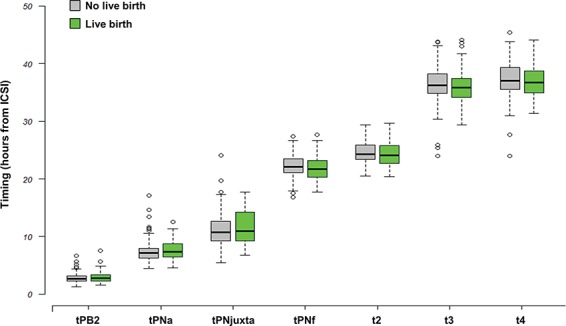
Timing of morphokinetics events occurring during early embryo development according to the live birth outcome. Boxes represent the interquartile range (IQR). Lines inside the boxes are the median. Whiskers represent the lowest datum still within 1.5 IQR of the lower quartile, and the highest datum still within 1.5 IQR of the upper quartile. tPB2, time of PB; 2 emission; tPNa, the time of appearance of the two pronuclei with the distinction between the female (identified as the one near the site of emission of the PBII) and male PN; tPNjuxta, the time of PN juxtaposition; tPNf, the time of pronuclear fade out; t2, t3, t4, the time to reach 2, 3, 4 cells.
Among the 18 morphometric parameters investigated (from oocyte to 4-cell stage, Fig. 1) with univariate analyses, no statistically significant differences were observed, except for PN position at the time of juxtaposition (Table II). The analysis of the live birth rates according to PN position and the features of the cytoplasmic halo showed that the live birth rate was the highest when the PN juxtaposition was central, whatever the type or presence of cytoplasmic halo (Supplementary Fig. S1).
Table II.
Morphometric parameters and abnormal cleavage according to live birth outcomes.
| Live birth | |||
|---|---|---|---|
| No (n = 133) | Yes (n = 99) | P-valuea | |
| Oocyte diameter (μm) | |||
| Mean | 115 ± 5 (106–131) | 115 ± 4 (102–125) | 0.258 |
| 75th percentile: 118 μm2 | |||
| <118 | 88 (66.2) | 76 (76.8) | 0.079 |
| ≥118 | 45 (33.8) | 23 (23.2) | |
| ZP mean thickness (μm) | 17.0 ± 2.3 (12.5–24.0) | 17.1 ± 2.5 (11.5–25) | 0.806 |
| PB area (μm2) | |||
| 1 | 290 ± 91 | 280 ± 89 | 0.376 |
| 2 | 243 ± 92 | 250 ± 116 | 0.633 |
| PN area (μm2) | |||
| ♀ | 523 ± 84 (333–787) | 529 ± 94 (315–773) | 0.606 |
| ♂ | 526 ± 92 (306–787) | 532 ± 83 (383–731) | 0.592 |
| Zygote diameter (μm) | |||
| Mean | 111 ± 5 (99–123) | 111 ± 5 (97–127) | 0.926 |
| 75th percentile: 113 μm2 | |||
| <113 | 94 (70.7) | 66 (66.7) | 0.514 |
| ≥113 | 39 (29.3) | 33 (33.3) | |
| Presence of vacuole(s) at zygote-stage (yes) | 18 (13.5) | 14 (14.1) | 0.8944 |
| Cytoplasmic halo: | |||
| No | 40 (30.1) | 25 (25.3) | 0.697* |
| Symmetrical | 21 (15.8) | 21 (21.2) | |
| Asymmetrical | 72 (54.1) | 53 (53.5) | |
| Position of PN juxtaposition: | |||
| Cortical | 6 (4.5) | 4 (4.1) | 0.056 |
| Intermediate | 78 (58.7) | 43 (44.3) | 0.017** |
| Central | 49 (36.8) | 50 (51.6) | |
| Polarization of NPBs/PN (♀/♂) | |||
| Yes/Yes | 43 (32.3) | 31 (31.3) | 0.690 |
| Yes/No | 36 (27.1) | 22 (22.2) | |
| No/Yes | 11 (8.3) | 12 (12.1) | |
| No/No | 43 (32.3) | 34 (34.3) | |
| At 2-cell stage, nuclear status: | |||
| 2 nuclei visible | 2 (1.5) | 4 (4.0) | 0.406 |
| 0 or 1 nucleus visible | 131 (98.5) | 95 (96.0) | |
| At 2-cell stage, nuclear area (μm2)b: | |||
| Mean | 425 ± 89 (213–649) | 431 ± 85 (255–663) | 0.634 |
| At 4-cell stage: | |||
| Random nucleus area (μm2) | |||
| Mean | 299 ± 75 (75–490) | 307 ± 70 (51–424) | 0.411 |
| Number of visible nuclei at 4-cell stage | |||
| <4 | 31 (23.3) | 26 (26.3) | 0.605 |
| 4 | 102 (76.7) | 73 (73.7) | |
| MNB2cell (Yes) | 45 (33.8) | 24 (24.2) | 0.114 |
| MNB4cell (Yes) | 4 (3.0) | 3 (3.0) | 1 |
| DC (Yes) | 3 (2.3) | 1 (1.0) | 0.638 |
| RC (Yes) | 1 (0.8) | 1 (1.0) | 1 |
For categorical variables, n (%) is presented. For continuous variables, mean (± SD) and range are presented. DC: direct cleavage, MNB: multinucleation blastomere, RC: reverse cleavage.
a Chi2 or Fisher’s exact tests were used for dichotomous variables and Student t-test or Mann–Whitney U-test for continuous variables.
*Statistical analyses comparing with and without cytoplasmic halo.
**Statistical analyses comparing central versus cortical/intermediate position of PN.
We then proceeded to a multivariate analysis that included the variables from the univariate analysis with a P-value below 0.15 in (i.e. the 75th oocyte diameter, the PN position at time of juxtaposition, the VP timing, the multinucleation at 2-cell stage). Adjustments were systematically made for maternal age and COS protocol. Finally, the presence of MNB2-cell and the central PN juxtaposition were significantly associated with live birth. The presence of MNB2-cell at the 2-cell stage was found to decrease the odds of live birth by almost half (OR = 0.51; 95% CI, [0.27–0.95]; P = 0.035, Table III). On the contrary, central PN juxtaposition increased the odds of live birth more than two folds (OR = 2.20; 95% CI, [1.26–3.89]; P = 0.006, Table III). This last result was consistent in the analysis of the subgroup of ‘TOP’ embryos (OR = 2.09; 95% CI, [1.17–3.79]; P = 0.014).
Table III.
Multivariate analysis for live birth outcome.
| OR* | 95% CI | P-value | |
|---|---|---|---|
| Maternal age (≥37 vs <37a) | 0.36 | [0.11–1.05] | 0.075 |
| COS protocols (others versus long agonista) | 0.40 | [0.21–0.74] | 0.004 |
| Position of juxtaposed PN (central versus intermediate/corticala) | 2.20 | [1.26–3.89] | 0.006 |
| MNB2-cell (Yes vs Noa) | 0.51 | [0.27–0.95] | 0.035 |
CI: confidence interval; COS: controlled ovarian stimulation; OR: odds ratio.
a Reference category.
*ORs computed from stepwise multivariate logistic regression.
Discussion
The selection of embryos with high developmental competence is an important issue in ART programs. Nowadays, thanks to TLI systems, we can simultaneously and thoroughly assess many potential non-invasive predictive markers without being detrimental at the early stages of preimplantation development. In this retrospective study, focused on SETs from ICSI attempts, we studied the potential impact of a total of 35 morphokinetic, morphometric and morphologic parameters on birth outcomes.
In the present study, 2 of the 18 morphometric characteristics were associated with transferred embryos developing to term: the position of the PN at the time of PN juxtaposition and the absence of MNB at the 2-cell stage. The likelihood of live birth was increased when the PN were located centrally instead of peripherally or intermediately at the time of juxtaposition.
To our knowledge, there are no previous studies assessing the impact of this parameter on live birth. PN location at fading time was not a determining factor for implantation as previously found with oocyte donor cycles (Aguilar et al., 2014). However, when Coticchio et al. (2018) investigated fertilisation events and the development of Day 3 embryos, they noticed that the more peripherally male PN appearance occurred, the later the time of PN breakdown and first cleavage. They hypothesized that a male PN in the peripheral position requires extra time to achieve central positioning, potentially influencing the timing of later events. Our findings confirmed that positional events during fertilisation may influence not only the timing of later events but also the birth potential of transferred embryos. Moreover, contrary to the cytoplasmic halo, this parameter could be a particularly discriminating biomarker for high-grade embryos. It would be interesting to prospectively explore whether this criterion is relevant for predicting embryo development (via decisional algorithms) and suitable for different clinical settings.
We also found that the presence of MNB at the 2-cell stage decreased the likelihood of live birth by almost half. The significant negative effect of multinucleation at the 2-cell stage observed in the present study is in line with previous reports (Ergin et al., 2014; Desch et al., 2017). This could be linked to the high rates of chromosomal aneuploidy in such embryos (Staessen and Van Steirteghem, 1998; Meriano et al., 2004).
For morphometric oocyte assessments, our mean oocyte diameter was similar to the measurements reported in two other studies (Cavilla et al., 2008; Romão et al., 2010). Faramarzi et al. (2017) recently reported shorter embryo cleavage times (tPB2, t5, t8) for oocytes with a larger diameter. Though oocyte diameter and embryo kinetics could be associated, we found no link between oocyte diameter and the timing of further embryonic events or the outcome of ICSI in multivariate analyses.
Overall, we found that kinetic parameters were not capable per se of predicting the live birth potential of early preimplantation embryos selected with conventional morphological assessments. We recognize that these results may seem a bit perplexing. However, while not serving as ultimate answer to the question of the interest of time-lapse records, this original comprehensive study using live birth as an endpoint reveals no marked timing differences between transferred embryos that result in a live birth and those that do not.
Several authors have indicated that morphokinetic parameters are characteristic of blastocyst formation (Wong et al., 2010; Conaghan et al., 2013; Milewski et al., 2015; Motato et al., 2016) and pregnancy or implantation (Meseguer et al., 2011; VerMilyea et al., 2014; Basile et al., 2015; Liu et al., 2016; Milewski et al., 2016; Motato et al., 2016; Petersen et al., 2016; Carrasco et al., 2017) but the general applicability of former morphokinetic algorithms for pregnancy, implantation or birth prediction is currently subject to controversy. Some authors who were not involved in developing the algorithms have maintained that time-lapse algorithms have a significantly higher predictive power than conventional scoring (Adamson, 2016; Kieslinger et al., 2016; Adolfsson et al., 2018; Liu et al., 2018), while other authors were unable to show a significant predictive capability for pregnancy, implantation or live birth (Kirkegaard et al., 2014; Yalçınkaya et al., 2014; Fréour et al., 2015; Ahlstrom et al., 2016; Goodman et al., 2016; Barrie et al., 2017; Adolfsson et al., 2018). These discrepancies are probably due to the fact that timing is markedly influenced by the fertilisation method (Lemmen et al., 2008), culture medium (Ciray et al., 2012), oxygen level (Kirkegaard et al., 2013a), population features (Fréour et al., 2013; Wissing et al., 2014) and controlled stimulation protocols. Moreover, the addition of time-lapse kinetics was not found to improve embryo selection in a recent randomized controlled trial (Goodman et al., 2016). This is in line with Ahlstrom’s conclusions specifying that for transfers of fresh Day 2 embryos, conventional morphology can be used to predict live birth more accurately than kinetics (Ahlstrom et al., 2016). In addition, most algorithms used early embryonic cleavage parameters (mainly based on t2, t3 and t4) (Meseguer et al., 2011; VerMilyea et al., 2014; Basile et al., 2015; Milewski et al., 2015; Liu et al., 2016; Petersen et al., 2016) which we found to be interdependent. Finally, Azzarello et al. (2012) and Aguilar et al. (2014) who investigated the possible effects of the timing of PN fading on implantation and live birth rates, respectively, reported discordant results. While Azzarello et al. found a longer mean PN fading time for zygotes resulting in live birth, Aguilar reported earlier timing in embryos that implanted successfully. In the current study, PN fading did not contribute to the live birth outcome. Similarly, the VP was not significant in our multivariate analysis, contrary to Ahlstrom et al. (2016). But, almost all of the transferred embryos in our population had a high-grade morphology (>88% of TOP embryo), potentially reducing the chance of finding a discriminative VP range. We can therefore hypothesize that timing may be more relevant for cohorts of embryos with ‘intermediate’ grade morphology. Further studies are needed, notably to explore the value of timing in embryonic cohorts that are not predominantly ‘TOP’ embryos. Even though we found no relevant time-related measurements, the time-lapse system remains the only tool to detect abnormal embryo cleavage (i.e. reverse or direct cleavage), which is detrimental for early and late embryo development (Rubio et al., 2012; Liu et al., 2014; Barrie et al., 2017). The present study was not tailored to evaluate the effect of abnormal cleavage and MNB at Day 2, especially since embryos with such irregularities were not selected for transfer when possible.
For the purposes of our study, we included SETs only (elective or not) in order to exclude a potential bias linked to multiple-embryo transfers (patient-related factors such as the receptivity of the endometrium). Of course estimations can be made to statistically account for dependencies within individuals and to exclude potential bias in the known implantation data-KID studies, but this risk cannot be fully mastered, especially with relatively small cohorts. In our multivariate analyses, contrary to the type of COS protocol, maternal age was not found to be a major criterion for ICSI outcomes. This can be explained by our young maternal population (averaging 30 years old).
Moreover, because previous studies have demonstrated relatively broad morphokinetic time ranges for optimum blastocyst formation or implantation (Meseguer et al., 2011; Azzarello et al., 2012; Dal Canto et al., 2012; Hlinka et al., 2012; Chamayou et al., 2013; Kirkegaard et al., 2013b), the sample size of our study may be not sufficient to establish clear differences in timing for embryos with a high potential for implantation and development to birth and those with a low potential. Our conclusions, established from a selected population, may yield different results in other study populations, for instance women in an older age bracket. Finally, because we assessed Day 2/3 transfers, our findings may potentially not be extrapolated to embryos cultured up to the blastocyst stage.
Conclusions
To our knowledge, this is the first analysis of its kind to use birth as an endpoint and to investigate as many morphometric, morphokinetic and morphologic parameters. From this analysis, we can underline one novel non-time-related parameter—PN position at the time of PN juxtaposition—which was found to be associated with live birth. The use of this new potential biomarker as well as the screening of multinucleation at the 2-cell stage, is all the more interesting as the embryos are of high-grade morphology. In addition, these qualitative parameters are clinically easier to apply than quantitative parameters, which tend to be difficult to reproduce and more sensitive to variations in clinic-specific characteristics.
While our study was not designed to assess time-lapse algorithms, our data underline that most morphokinetic parameters up to Day 2 cannot distinguish the ‘TOP’ embryos that develop to term from those that end in a failure (around half). We believe that it is not worth taking the time to scrupulously annotate and analyse the timing of events throughout preimplantation development for a good quality embryo that has already undergone conventional grading. The use of Artificial Neural Network, a promising tool for automated annotation of morphokinetic data generated with a TL device, could overcome this issue (Wolczyński et al., 2009; Mölder et al., 2015). We expect that the growing use of genetic screening and the resulting use of these measurements may help us to better select embryos with high developmental competence and to decipher whether some of these non-invasive parameters could be considered as additional independent predictors.
Supplementary Material
Acknowledgments
We thank all the technicians of the Reproductive Laboratory of Dijon University Hospital and Nicolas Braquehais for their valuable help. We thank Suzanne Rankin for proofreading the manuscript.
Funding
None.
Authors’ roles
P.F., J.B. and C.B. were the principal investigators and take primary responsibility for the paper. C.B., E.V., J.C. and C.A.N. collected the data. C.C. and P.S. were involved in the clinical management of patients. L.J. and A.S. did the statistical analyses. P.F., J.B. and C.B. coordinated the research and drafted the manuscript.
Conflict of interest
P. Sagot received funding from the following commercial companies: Merck Serono, Finox Biotech, Ferring, MSD France SAS, Teva Sante ´ SAS, Allergan France, Gedeon Richter France, Effik S.A., Karl Storz Endoscopie France, GE Medical Systems SCS, Laboratoires Genevrier, H.A.C. Pharma and Ipsen.
All the authors confirm that none of this funding was used to support the research in this study. There are no patents, products in development or marketed products to declare. This does not alter the authors’ adherence to all the journal policies on sharing data and materials.
References
- Abeyta M, Behr B. Morphological assessment of embryo viability. Semin Reprod Med 2014;32:114–126. [DOI] [PubMed] [Google Scholar]
- Adamson GD, Abusief ME, Palao L, Witmer J, Palao LM, Gvakharia M. Improved implantation rates of day 3 embryo transfers with the use of an automated time-lapse–enabled test to aid in embryo selection. Fertil Steril 2016;105:369–375. [DOI] [PubMed] [Google Scholar]
- Adolfsson E, Porath S, Andershed AN. External validation of a time-lapse model; a retrospective study comparing embryo evaluation using a morphokinetic model to standard morphology with live birth as endpoint. JBRA Assist Reprod 2018;22:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar J, Motato Y, Escribá MJ, Ojeda M, Muñoz E, Meseguer M. The human first cell cycle: impact on implantation. Reprod Biomed Online 2014;28:475–484. [DOI] [PubMed] [Google Scholar]
- Ahlstrom A, Park H, Bergh C, Selleskog U, Lundin K. Conventional morphology performs better than morphokinetics for prediction of live birth after day 2 transfer. Reprod Biomed Online 2016;33:61–70. [DOI] [PubMed] [Google Scholar]
- ALPHA Scientists in Reproductive Medicine, ESHRE Special Interest Group Embryology Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online 2011;22:632–646. [DOI] [PubMed] [Google Scholar]
- Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev 2015;2:CD011320. [DOI] [PubMed] [Google Scholar]
- Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev 2018;5:CD011320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzarello A, Hoest T, Mikkelsen AL. The impact of pronuclei morphology and dynamicity on live birth outcome after time-lapse culture. Hum Reprod 2012;27:2649–2657. [DOI] [PubMed] [Google Scholar]
- Barberet J, Chammas J, Bruno C, Valot E, Vuillemin C, Jonval L, Choux C, Sagot P, Soudry A, Fauque P. Randomized controlled trial comparing embryo culture in two incubator systems: G185 K-System versus EmbryoScope. Fertil Steril 2018;109:302–309. [DOI] [PubMed] [Google Scholar]
- Barrie A, Homburg R, McDowell G, Brown J, Kingsland C, Troup S. Preliminary investigation of the prevalence and implantation potential of abnormal embryonic phenotypes assessed using time-lapse imaging. Reprod Biomed Online 2017;34:455–462. [DOI] [PubMed] [Google Scholar]
- Basile N, Vime P, Florensa M, Aparicio Ruiz B, García Velasco JA, Remohí J, Meseguer M. The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithm for embryo selection. Hum Reprod 2015;30:276–283. [DOI] [PubMed] [Google Scholar]
- Carrasco B, Arroyo G, Gil Y, Gómez MJ, Rodríguez I, Barri PN, Veiga A, Boada M. Selecting embryos with the highest implantation potential using data mining and decision tree based on classical embryo morphology and morphokinetics. J Assist Reprod Genet 2017;34:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavilla JL, Kennedy CR, Byskov AG, Hartshorne GM. Human immature oocytes grow during culture for IVM. Hum Reprod 2008;23:37–45. [DOI] [PubMed] [Google Scholar]
- Chamayou S, Patrizio P, Storaci G, Tomaselli V, Alecci C, Ragolia C, Crescenzo C, Guglielmino A. The use of morphokinetic parameters to select all embryos with full capacity to implant. J Assist Reprod Genet 2013;30:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciray HN, Aksoy T, Goktas C, Ozturk B, Bahceci M. Time-lapse evaluation of human embryo development in single versus sequential culture media—a sibling oocyte study. J Assist Reprod Genet 2012;29:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, Sayed S, Time-Lapse User Group . Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod 2014;29:2650–2660. [DOI] [PubMed] [Google Scholar]
- Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, Baker VL, Adamson GD, Abusief ME, Gvakharia M et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril 2013;100:412–419. [DOI] [PubMed] [Google Scholar]
- Coticchio G, Mignini Renzini M, Novara PV, Lain M, De Ponti E, Turchi D, Fadini R, Dal Canto M. Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability. Hum Reprod 2018;33:23–31. [DOI] [PubMed] [Google Scholar]
- Dal Canto M, Coticchio G, Mignini Renzini M, De Ponti E, Novara PV, Brambillasca F, Comi R, Fadini R. Cleavage kinetics analysis of human embryos predicts development to blastocyst and implantation. Reprod Biomed Online 2012;25:474–480. [DOI] [PubMed] [Google Scholar]
- Desch L, Bruno C, Luu M, Barberet J, Choux C, Lamotte M, Schmutz E, Sagot P, Fauque P. Embryo multinucleation at the two-cell stage is an independent predictor of intracytoplasmic sperm injection outcomes. Fertil Steril 2017;107:97–103. [DOI] [PubMed] [Google Scholar]
- Ergin EG, Çalişkan E, Yalçinkaya E, Öztel Z, Çökelez K, Özay A, Özörnek HM. Frequency of embryo multinucleation detected by time-lapse system and its impact on pregnancy outcome. Fertil Steril 2014;102:1029–1033. [DOI] [PubMed] [Google Scholar]
- Faramarzi A, Khalili MA, Omidi M. Morphometric analysis of human oocytes using time lapse: does it predict embryo developmental outcomes? Hum Fertil 2017;20:1–6. [DOI] [PubMed] [Google Scholar]
- Fauque P, Audureau E, Leandri R, Delaroche L, Assouline S, Epelboin S, Jouannet P, Patrat C. Is the nuclear status of an embryo an independent factor to predict its ability to develop to term? Fertil Steril 2013;99:1299–1304. [DOI] [PubMed] [Google Scholar]
- Fréour T, Dessolle L, Lammers J, Lattes S, Barrière P. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril 2013;99:1944–1950. [DOI] [PubMed] [Google Scholar]
- Fréour T, Le Fleuter N, Lammers J, Splingart C, Reignier A, Barrière P. External validation of a time-lapse prediction model. Fertil Steril 2015;103:917–922. [DOI] [PubMed] [Google Scholar]
- Goodman LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril 2016;105:275–285. [DOI] [PubMed] [Google Scholar]
- Hlinka D, Kal’atova B, Uhrinova I, Dolinska S, Rutarova J, Rezacova J, Lazarovska S, Dudas M. Time-lapse cleavage rating predicts human embryo viability. Physiol Res 2012;61:513. [DOI] [PubMed] [Google Scholar]
- Kieslinger DC, De Gheselle S, Lambalk CB, De Sutter P, Kostelijk EH, Twisk JWR, Rijswijk, van J, Van den Abbeel E, Vergouw CG. Embryo selection using time-lapse analysis (early embryo viability assessment) in conjunction with standard morphology: a prospective two-center pilot study. Hum Reprod 2016;31:2450–2457. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Campbell A, Agerholm I, Bentin-Ley U, Gabrielsen A, Kirk J, Sayed S, Ingerslev HJ. Limitations of a time-lapse blastocyst prediction model: a large multicentre outcome analysis. Reprod Biomed Online 2014;29:156–158. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril 2013a;99:738–744. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Kesmodel US, Hindkjær JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod 2013b;28:2643–2651. [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online 2008;17:385–391. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chapple V, Feenan K, Roberts P, Matson P. Time-lapse deselection model for human day 3 in vitro fertilization embryos: the combination of qualitative and quantitative measures of embryo growth. Fertil Steril 2016;105:656–662. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chapple V, Roberts P, Matson P. Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the Embryoscope time-lapse video system. Fertil Steril 2014;102:1295–1300. [DOI] [PubMed] [Google Scholar]
- Liu Y, Feenan K, Chapple V, Matson P. Assessing efficacy of day 3 embryo time-lapse algorithms retrospectively: impacts of dataset type and confounding factors. Hum Fertil 2018;21:1–9. [DOI] [PubMed] [Google Scholar]
- McLernon DJ, Harrild K, Bergh C, Davies MJ, Neubourg D de, JCM D, Gerris J, Kremer JA, Martikainen H, Mol BW et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ 2010;341:c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriano J, Clark C, Cadesky K, Laskin CA. Binucleated and micronucleated blastomeres in embryos derived from human assisted reproduction cycles. Reprod Biomed Online 2004;9:511–520. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod 2011;26:2658–2671. [DOI] [PubMed] [Google Scholar]
- Milewski R, Kuć P, Kuczyńska A, Stankiewicz B, Łukaszuk K, Kuczyński W. A predictive model for blastocyst formation based on morphokinetic parameters in time-lapse monitoring of embryo development. J Assist Reprod Genet 2015;32:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewski R, Milewska AJ, Kuczyńska A, Stankiewicz B, Kuczyński W. Do morphokinetic data sets inform pregnancy potential? J Assist Reprod Genet 2016;33:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölder A, Drury S, Costen N, Hartshorne GM, Czanner S. Semiautomated analysis of embryoscope images: using localized variance of image intensity to detect embryo developmental stages. Cytometry A 2015;87:119–128. [DOI] [PubMed] [Google Scholar]
- Motato Y, los SMJ, Escriba MJ, Ruiz BA, Remohí J, Meseguer M. Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertil Steril 2016;105:376–384. [DOI] [PubMed] [Google Scholar]
- Neubourg DD, Gerris J, Royen EV, Mangelschots K, Valkenburg M. Prevention of twin pregnancy after IVF/ICSI using embryo transfer. Verh K Acad Geneeskd Belg 2002;64:361–370. [PubMed] [Google Scholar]
- Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Hum Reprod 2016;31:2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romão GS, Araújo MCPM, de MAS, Navarro PA de AS, Ferriani RA, dos RRM. Oocyte diameter as a predictor of fertilization and embryo quality in assisted reproduction cycles. Fertil Steril 2010;93:621–625. [DOI] [PubMed] [Google Scholar]
- Royen EV, Mangelschots K, Neubourg DD, Valkenburg M, Meerssche MV d, Ryckaert G, Eestermans W, Gerris J. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod 1999;14:2345–2349. [DOI] [PubMed] [Google Scholar]
- Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá M-J, Bellver J, Meseguer M. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril 2012;98:1458–1463. [DOI] [PubMed] [Google Scholar]
- Staessen C, Van Steirteghem A. The genetic constitution of multinuclear blastomeres and their derivative daughter blastomeres. Hum Reprod 1998;13:1625–1631. [DOI] [PubMed] [Google Scholar]
- VerMilyea MD, Tan L, Anthony JT, Conaghan J, Ivani K, Gvakharia M, Boostanfar R, Baker VL, Suraj V, Chen AA et al. Computer-automated time-lapse analysis results correlate with embryo implantation and clinical pregnancy: a blinded, multi-centre study. Reprod Biomed Online 2014;29:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissing ML, Bjerge MR, Olesen AIG, Hoest T, Mikkelsen AL. Impact of PCOS on early embryo cleavage kinetics. Reprod Biomed Online 2014;28:508–514. [DOI] [PubMed] [Google Scholar]
- Wolczyński S, Szamatowicz J, Milewska AJ, Domitrz J, Jamiołkowski J, Milewski R. Prognosis of the IVF ICSI/ET procedure efficiency with the use of artificial neural networks among patients of the Department of Reproduction and Gynecological Endocrinology. Ginekol Pol 2009;80:900–906. [PubMed] [Google Scholar]
- Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, Pera RAR. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol 2010;28:1115–1121. [DOI] [PubMed] [Google Scholar]
- Yalçınkaya E, Ergin EG, Çalışkan E, Öztel Z, Özay A, Özörnek H. Reproducibility of a time-lapse embryo selection model based on morphokinetic data in a sequential culture media setting. J Turk Ger Gynecol Assoc 2014;15:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod 1997;12:1545–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


