Abstract
The Wnt signaling pathway is critical for normal tissue development and is an underlying mechanism of disease when dysregulated. Previously, we reported that the drug Niclosamide inhibits Wnt/β-catenin signaling by decreasing the cytosolic levels of Dishevelled and β-catenin, and inhibits the growth of colon cancers both in vitro and in vivo. Since the discovery of Niclosamide’s anthelmintic activity, a growing body of literature indicates that Niclosamide is a multifunctional drug. In an effort to identify derivatives of Niclosamide with improved pharmacokinetic properties that maintain the multifunctional drug activity of Niclosamide for clinical evaluation, we designed DK419, a derivative containing a 1H-benzo[d]imidazole-4-carboxamide substructure, using the structure-activity relationships (SAR) of the Niclosamide salicylanilide chemotype. Similar to Niclosamide, we found DK419 inhibited Wnt/β-catenin signaling, altered cellular oxygen consumption rate and induced production of pAMPK. Moreover, we found DK419 inhibited the growth of CRC tumor cells in vitro, had good plasma exposure when dosed orally, and inhibited the growth of patient derived CRC240 tumor explants in mice dosed orally. DK419, a derivative of Niclosamide with multifunctional activity and improved pharmacokinetic properties, is a promising agent to treat colorectal cancer, Wnt-related diseases and other diseases in which Niclosamide has demonstrated functional activity.
Keywords: Niclosamide, Wnt signaling inhibitor, Small molecule, Cancer, Drug design, Oxidative phosphorylation
Graphical Abstract:

1. Introduction
The Wnt signaling pathway is critical for stem cell development and the proper growth and maintenance of tissues1. Dysregulation of this pathway plays an important role in many diseases, particularly cancer1–3. In colorectal cancer (CRC), more than 93% of tumors had alteration in this pathway, ~80% of which were mutations in the Adenomatous polyposis coli (APC) or β-catenin genes that result in hyperactivation of Wnt signaling1, 4. Given the importance of the Wnt signaling pathway driving disease development, it is important to develop therapies that target the pathway1, 5.
Mechanistic studies have revealed key signaling molecules in the Wnt pathway1, 3, 6. In the canonical Wnt pathway, signaling activity is initiated by secreted Wnt protein ligands binding to the seven transmembrane receptor Frizzled and the single transmembrane receptors LRP5/6, resulting in activation of cytosolic Dishevelled (Dvl) proteins, internalization of the Frizzled receptor, and stabilization and translocation of cytosolic β-catenin proteins into the nucleus. Cytosolic β-catenin levels are controlled by a destruction complex consisting of Axin, APC, and two serine-threonine protein kinases, CK1α/δ and GSK3α/β. In CRC, most of the mutations observed in this pathway result in an increase in nuclear β-catenin protein and activation of its target genes. Overall, the Wnt signaling pathway consists of a series of intracellular protein-protein interactions, for which traditional drug discovery approaches have been difficult5, 7. To date, no drugs have been approved that specifically target the pathway8.
Recent efforts, however, have provided opportunities to inhibit the pathway1, 9–14. Our contribution to this field was the discovery that the anthelmintic drug Niclosamide (Fig. 1), inhibits Wnt/β-catenin signaling15, a discovery that has since been confirmed by others16–18.
Fig. 1. Inhibitor design.
The hydrogen-bond donating phenolic OH group of Niclosamide was replaced with a NH group in a heterocyclic imidazole ring and the pKa adjusted by inserting a CF3 substituent. The NO2 group of Niclosamide was replaced with a -CF3 group based on previous SAR studies. Properties calculated in Maestro (Schrodinger, LLC).
Niclosamide is a salicylic acid derivative that belongs to the salicylanilide class of anthelmintic agents. It was approved by the FDA in 1982 for use in humans to treat tapeworm infections19 and has been identified as an essential medicine by the World Health Organization20. Niclosamide has protonophore activity and has been shown to uncouple oxidative phosphorylation21–25. Structurally, Niclosamide contains an aryl β-hydroxy carbonyl pharmacophore element that is common in natural products and drugs that encompass a broad range of biological activity26. Since its discovery, Niclosamide has been shown to have a number of important biological activities that indicate it is a multi-functional drug26.
Niclosamide’s multi-functional activity has generated considerable interest in its use to treat cancer, rheumatoid arthritis, diabetes, and bacterial and viral infections25–32. In addition to our clinical trial in colorectal cancer (), there are now three additional clinical trials of Niclosamide listed in ClinicalTrials.gov in cancer (, , ), and one in rheumatoid arthritis (NCT03160001). As a potential anti-cancer agent, Niclosamide inhibits the growth of cancer cells from multiple tumor types and is active against cancers resistant to other drugs26, 33–36. In addition to inhibition of the Wnt signaling pathway, Niclosamide inhibits other key oncogenic signaling pathways such as mTOR , NF-κB, Notch and STAT-3, and has effects on metabolism, including activation of AMPK26.
Systemic exposure to Niclosamide from an oral dose is low. It is poorly absorbed and is cleared rapidly34, 37, 38. Although Niclosamide’s pharmacokinetic properties are appropriate for use as an anthelmintic agent, its pharmacokinetic and solubility properties may limit its utility in diseases where systemic exposure is required.
Given the need to identify drugs that inhibit Wnt/β-catenin signaling, Niclosamide’s spectrum of biological activity and the limitations of Niclosamide’s pharmacokinetic properties, we used the SAR of Niclosamide to design Wnt inhibitors with a similar spectrum of biological activity and improved pharmacokinetic exposure. This resulted in the identification of DK419 as one promising candidate.
2. Material and Methods
2.1. Chemical Property Calculations
cLogP and pKa values were calculated using Maestro (Schrodinger, LLC). cLogP values are QPLogP (octanol/water) calculated in Qikprops. The pKa values were calculated in water solvent in Epik (Version 10.3.015, Schrodinger, LLC).
2.2. Synthesis
DK419 [6-chloro-2-(trifluoromethyl)-N-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazole-4-carboxamide] was prepared in 5 steps from commercially available 2-methyl-6-nitroaniline
To a 2-necked round-bottom flask equipped with an addition funnel, a thermometer and a magnetic stir bar under an Argon atmosphere was added 12.97g (85.23 mmol) of 2-methyl-6-nitroaniline and 35 ml glacial acetic acid. The suspension was placed in a pre-heated oil bath at 50°C to produce a red solution. To the addition funnel was added a suspension of 11.95 g N-chlorosuccinimide (NCS) in ca. 45 mL of acetic acid. The NCS suspension was added dropwise over ca 15 minutes while maintaining the temperature around 55 °C. After the addition was complete, the addition funnel was rinsed with acetic acid (ca. 5 mL) and added. A brown homogeneous solution resulted during the course of the reaction. The progress of the reaction was followed by TLC using methylene chloride as the elutant. Upon completion of the reaction, the reaction mixture was cooled to room temperature, poured into 100 mL of water and cooled in an ice bath. The solids were filtered on paper under vacuum, and rinsed with a total of 130 mL of water. The solids were dried in air in the filter funnel under vacuum to yield 13.3 g of 4-chloro-2-methyl-6-nitroaniline as an orange-red solid. 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 2.2 Hz, 1H), 7.38 (br. s, 1H), 7.23 (br. s., 2H), 2.17 (s, 3H). MS (ESI+) m/z = 187 (M+1). This material was used in the subsequent reaction without further purification.
To a round-bottom flask equipped with a Claisen adapter and a magnetic stir bar under an argon atmosphere was added 2.24 g (11.95 mmol) of 4-chloro-2-methyl-6-nitroaniline and 95% ethanol (56 mL). To this mixture was added an aqueous (pH ca 7–8) suspension of RaNi (ca. 2 g). The reaction flask was evacuated and charged 3 times with hydrogen, and then placed in an oil bath pre-heated to 60–70 °C. After 4 hours, the reaction was judged to be complete by TLC (3% methanol/methylene chloride). The reaction mixture was then evacuated and charged 3–4 times with Argon to remove hydrogen gas, and celite was added. The mixture was filtered through celite, rinsed sequentially with ethanol and then water, and the filtrate concentrated in vacuo to a brownish oil. The oil was suspended in water (40 mL) and 2.7 mL of trifluoroacetic acid was added, and the resultant mixture refluxed for 2.5 hr. The reaction mixture was then cooled to room temperature. Sodium bicarbonate was added carefully to adjust the pH to ca. 7–8, and the aqueous reaction mixture extracted 2 times with ethyl acetate. The ethyl acetate layers were combined and washed with brine, dried over sodium sulfate, filtered, and concentrated in vacuo to give 6-chloro-4-methyl-2-(trifluoromethyl)-1H-benzo[d]imidazole as a solid. MS (ESI+) m/z = 235, 233 (M+1). This material was used in subsequent reaction without further purification.
To a round-bottom flask equipped with a magnetic stir bar, and a thermometer and reflux condenser was added 2.11 g ( 9 mmol) of 6-chloro-4-methyl-2-(trifluoromethyl)-1H-benzo[d]imidazole and 126 ml of 0.5M NaOH. The reaction mixture was placed at reflux and 7.56g KMnO4 (44.97 mmol) was added in 5 portions over 4 hours. The reaction mixture was then refluxed for 3 additional hours and removed from the oil bath and cooled to ca 50°C. Celite and sodium metabisulfite was added to the reaction flask, and the mixture filtered hot through celite. The filtrate was cooled in an ice-bath, and HCl added to adjust the pH to 3. The resultant aqueous mixture was extracted 3 times with ethyl acetate, and the ethyl acetate layers were combined, dried over sodium sulfate, filtered, and concentrated onto a plug of silica gel. The silica gel plug was loaded onto a column of silica gel and eluted with 10–80% methanol in methylene chloride. The desired fractions were combined, heptane was added, and the fractions were concentrated in vacuo to give 0.9 g of 6-chloro-2-(trifluoromethyl)-1H-benzo[d]imidazole-4-carboxylic acid as a white-tan solid. 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 7.89 (s, 1H). MS (ESI-) m/z = 263,265 (M-1)
To a dry round-bottomed flask was added 2.16 g (8.17 mmol) of 6-chloro-2-(trifluoromethyl)-1H-benzo[d]imidazole-4-carboxylic acid and 45 mL of dry DMF. To the resultant solution was added a solution of 3.42g (8.99 mmol) HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate) in a total of 15mL of dry DMF. The mixture was stirred at room temperature for 40 min, at which time 1.1 mL (1.45 g, 8.99 mmol) of 4-(trifluoromethyl)aniline and 4.3 mL (3.16g, 24.55 mmol) of Hunig’s base was added. The reaction mixture was stirred at room temperature for 24 hr and the progress of the reaction monitored by TLC using 3% EtOAc/CH2Cl2-Hexane (1:1). The amber solution was poured into 500 mL of water and the pH adjusted to 3 by the addition of 2N HCl. The resultant white precipitate was stirred for 10 min, filtered, washed with water, and air-dried in the filter funnel. The solids were dissolved in EtOAc, and the solution dried with Na2SO4, and filtered. To the filtrate was added heptane and the solution was concentrated under vacuum on a rotary evaporator to give white solid (3.1 g). The solids were loaded onto ca. 40 mL of silica gel by dissolving the solids in EtOAc, adding heptane, and concentrating under vacuum, and these solids were eluted on a column of ca. 500 mL of silica gel with a gradient of 1 – 7 % EtOAc/CH2Cl2. Pure fractions were set aside, and mixed fractions were re-chromatographed. Pure fractions were combined and concentrated. The solids were dissolved in EtOAc, heptane added to the solution, and concentrated to give 1.7 g (51%) of 6-chloro-2-(trifluoromethyl)-N(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazole-4-carboxamide as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 11.3 (br s, 1 H), 8.05 – 8.07 (br s, 1H), 7.96 (br d, J = 8.00 Hz, 3H), 7.76 (d, J = 8.69 Hz, 2H). MS (ESI+) m/z = 408, 410 (3:1) (M+1); MS (ESI-) m/z = 406, 408 (3:1) (M-1).
2.3. Frizzled internalization assay
The Frizzled1-GFP (Fzd1-GFP) assay was performed following a procedure similar to that previously published15. Briefly, U2OS cells stably expressing Fzd1-GFP were plated in confocal dishes and incubated at 37C with 5% CO2. After 24 hours the cells were treated with 12.5μM of test compound (dissolved in DMSO) or DMSO alone in media as a control for 6 hours at 37C and then fixed with 4% paraformaldehyde. The cells were then examined by microscopy using an LSM 510-Meta confocal microscope (Carl Zeiss, Thornwood, NY, USA) equipped with 40× and 100× apo chromat objectives. GFP was excited using a 488-nm argon laser line. Images were processed using the LSM software Image Browser.
2.4. TOPFlash reporter assay
The procedure used followed that adapted from Chen et al15. Wnt-3A conditioned medium was prepared using L WNT-3A cells (ATCC® CRL-2647™) purchased from ATCC, and was obtained using published protocols (http://www.atcc.org/Products/All/CRL-2647.aspx#culturemethod)15. HEK293 cells were stably transfected with p8xTOPFlash, Renilla luciferase plasmid pRL-TK, and pLKO.1 as previously published15. Briefly, stably transfected cells were seeded in 100 μl of cell growth medium (MEM, Sigma, catalog number M4655) supplemented with 10% FBS (Atlanta Biologicals, catalog number S11050), and 1ug/ml puromycin (Sigma, catalog number P8833), 100U/ml penicillin-streptomycin (Invitrogen, catalog number 15140122)/well in 96-well plates and incubated in 5% CO2 at 37°C overnight to 100% confluency. Fifty microliters of Wnt-3A conditioned medium containing the chemical compounds to be tested or DMSO was added to each well. After 6 h treatment, the cells were washed once with PBS and lysed with 55 μl of Passive Lysis Buffer supplied in the Dual-Luciferase Reporter Assay kit (Promega, Catalog number E1960). Twenty-five microliters of cell lysate was used for measuring luciferase activity in a 96-well plate reader (FluoStar Optima, BMG Labtech, Chicago, IL).
2.5. Evaluation of Wnt signaling inhibition by Western blot
All cells were plated in 6 well-plates and allowed to attach overnight in a 5% CO2 incubator at 37°C to reach 50–70 percent confluency. The cells were then treated with the indicated compound for 18 hours. Whole cell lysates were prepared by adding 200μL 1.5x Laemmli sample buffer. Cytosolic lysates were prepared by treating cells with hypotonic buffer and dispersing cell aggregates by drawing the lysate buffer in and out a syringe equipped with 25Gx1 needles. The cytosolic lysate was centrifuged at 15,000rpm for 120 minutes, 200μL of cytosol lysate was collected and 67μL of 4x Laemmli sample buffer was added. Whole cell lysates were subjected to Western blot analysis using antibodies to c-Myc (Santa Cruz Biotechnology, catalog number SC-40), Cyclin D1 (Cell Signaling Technology, catalog number 2978), and Survivin (Cell Signaling Technology, catalog number 2808). Cytosol lysate were subjected to Western blot analysis using antibodies to β-catenin (Santa Cruz Biotechnology SC-7963) and Axin2 (Cell Signaling Technology, catalog number 2151). An antibody to β-actin (Santa Cruz Biotechnology, catalog number SC-47798) was used as a loading control.
2.6. Evaluation of phospho-AMPK levels by Western blot
12-well plates were incubated with 250μL poly-D-lysine solution (Sigma, catalog number P6407) at 10 μg/ml for 30 minutes at room temperature, the poly-D-lysine solution was removed, and the wells washed twice with 500μL distilled water. Colonic epithelial CCD841Co cells were cultured in MEM medium plus 1mM sodium pyruvate (Invitrogen, catalog number 11360070), 1x MEM Non-Essential Amino Acids (Gibco catalog number 11140), 10% FBS, and 100 U/ml penicillin-streptomycin. 0.27 million CCD841Co cells per well were plated in culture medium in the poly-D-lysine coated 12-well plates and allowed to attach overnight at 37C in a 5% CO2 incubator. The next morning, the media was replaced with DMSO vehicle in cell media, DK419 or Niclosamide in DMSO diluted to the indicated concentration with DMEM medium (Sigma, catalog number D5030) supplemented with 2mM sodium pyruvate and 2mM L-glutamine (Invitrogen, catalog number 25030081). The medium does not contain glucose. After 30 minutes treatment, the media was removed and the cells were lysed with 120μL 1X Laemmli sample buffer. Immunoblots of the cell lysates using antibodies to phospho-AMPK (pAMPK) (Cell Signaling Technology, catalog number 2535) and AMPK (Cell Signaling Technology, catalog number 2532) was used to detect phosphorylated AMPK (p-Threonine 172) levels and total AMPK levels, respectively. Antibodies to β-actin were used as loading controls.
2.7. Assessment of Oxygen consumption rate
Colonic epithelial CCD841Co cells were plated in Seahorse XFp 8-well miniplates at 8000 cells/well and allowed to attach overnight at 37C in a 5% CO2 incubator. Polarographic analysis of oxygen consumption rate (OCR) was performed using Seahorse XF analyzer (Agilent Technologies, Santa Clara, CA) and a Seahorse XFp Cell Mito Stress Test Kit (Agilent Technologies, catalog number 103010–100). OCR was measured under basal conditions after the additions of 1μM Niclosamide, 1μM DK419, or DMSO in media, following the additions of 1μM Oligomycin, 1μM FCCP, and 0.5μM Rotenone & Antimycin A at the times indicated. Data were analyzed by the software Wave (Version 2.3.0.20, Agilent Technologies, Santa Clara, CA).
2.8. Inhibition of cell proliferation by MTS assay
Colon cancer cell lines SW948, HT-29, HCT116, CRC240, SW480, and DLD-1 were used in the cell proliferation assay. All cell lines, except CRC240, were from ATCC. CRC240, a colorectal cancer patient derived xenograft (PDX) cell line, was obtained from the Hsu lab (Duke University). Generation of CRC240 cell line was previously reported39. SW948 cells were cultured in Leibovitz’s medium (Gibco, catalog number 21083–027). HT-29 and HCT116 cells were cultured in McCoy’s 5A medium (Gibco, catalog number 16600–082). SW480 cells were cultured in MEM medium. CRC240 and DLD-1 cells were cultured in RPMI medium (Gibco, catalog number 11875–093). All media were supplemented with 10% FBS and 100 U/ml penicillin-streptomycin. The cells were plated at 5000 cells per well in 100 μl of culture medium in 96-well plates, and treated with Niclosamide from concentrations of 0.04 to 10 μM or DK419 from concentrations of 0.001 to 10 μM for 72 h, after which time the cells were analyzed by colorimetric MTS assay (Promega, catalog number G3581). IC50 values calculated using Graphpad Prism.
2.10. Pharmacokinetic analysis
DK419 was dissolved in 10% N-Methyl pyrrolidinone (Sigma, catalog number 328634) and 90% PEG300 (Sigma, catalog number 20237) at 0.2 mg/mL, and dosed orally to NOD/SCID mice (n=4) at 1mg/kg. Blood samples (~20 μL) were collected serially from tail vein into vials containing 1 μL of 1000 U/mL heparin at the following time-points: pre-dose, 0.25, 0.5, 1, 2, 4, 8, 24, 48, and 72h after drug administration. After centrifugation at 1300 g for 5 min at room temperature, plasma was separated and stored at −80 °C until the analysis.
Quantification of DK419 in mouse plasma was started by vigorous agitation of 4 μL sample, 10 μL of 20 ng/mL DK4–106-3 (in-house made internal standard analog), 20 μL of water, and 100 μL of ethyl-acetate. After centrifugation at 16,000 g for 5 min at room temperature, 80 μL of organic (upper) layer was dried under nitrogen stream, residue reconstituted with a mixture of 50% mobile phase A (10 mM ammonium acetate, 0.1% formic acid) and 50% mobile phase B (methanol), and 5 μL injected into LC/MS/MS system. Analysis was performed on Shimadzu series 20A LC / Applied Biosystems/SCIEX API 4000 QTrap MS/MS instrument. Column: Agilent Eclipse 4.6×50 mm, 1.8 μm at 50 °C. Elution gradient: 0–2 min 50–90%B, 2–2.5 min 90%B, 2.5–2.7 min, 90–50%B; flow 1 min/mL. MS/MS transitions followed: 405.8/218.9 (DK419) and 331.8/311.8 (DK4–106-3; int. std.). Run time: 5 min. Calibration samples were prepared by adding pure DK419 to pooled mouse plasma in 2.4 ng/mL (LLOQ)-1000 ng/mL range and analyzed alongside the PK study samples. The calibration curve was linear (r = 0.999).
2.11. Xenograft tumor studies
The development of CRC240 PDX was previously reported39. Briefly, CRC240 PDX tissue were washed in PBS and then minced in PBS at 150mg/ml. 200μl of the PDX tissue suspension were implanted into the flanks of NOD/SCID mice. When PDX tumor volume reached about 100 mm3, mice were randomized and were orally dosed for 11 days daily by Niclosamide, DK419, or the vehicle control (10% 1-methyl-2-pyrrolidinone and 90% PEG300). Niclosamide was suspended in the vehicle at 144mg/ml and dosed at 72 mg/kg. DK419 was dissolved in the vehicle at 0.2mg/ml and dosed at 1mg/kg. Tumor size and body weight were measured at day 0, 4, 8, and 11. The day after the last dose, animals were euthanized and tumor samples were collected and processed for Western blot analysis. Briefly, tumor samples were lysed in RIPA Buffer (Thermofisher Scientific, catalog number 89900). Sample lysates were subjected to Western blot using antibodies to Survivin, c-Myc, and Axin2 as described above. β-actin was used as a loading control.
3. Results
3.1. Design and synthesis of DK419
Central in our design was the removal of substituents in Niclosamide’s structure associated with its poor PK properties while keeping SAR features associated with inhibition of Wnt signaling and uncoupling of oxidative phosphorylation. In the design, we sought to remove the 2-hydroxy and 4’-nitro substituents that were known to be sites of metabolism while at the same time providing a hydrogen bond donating group at the 2-position with a pKa of ~6–7, the ability to hydrogen bond to and delocalize its corresponding conjugate base, and a clogP ≥ 3. Toward this end, we replaced the 2-hydroxy group of the salicylic motif with a heterocyclic NH group and modulated its pKa by the appropriate placement of electron-withdrawing substituents (Fig. 1). In the case of DK419, we used an imidazole ring substituted with a trifluoromethyl group. In the anilide ring, we replaced the 4’- nitro group with a trifluoromethyl group based on previous SAR studies that indicated it was suitable replacement for the nitro group40. Upon calculation of the pKa and LogP of DK419, we found that its calculated pKa = 7.5 and its calculated LogP (cLogP) = 5.5, and that it met the other design criteria. DK419 was then synthesized in 5 steps starting from commercially available 2-methyl-6-nitroaniline (Fig. 2).
Fig. 2. Synthesis of DK419.
i. NCS, AcOH ii. RaNi, H2 iii. TFA iv. KMnO4 v. HATU, 4-trifluoroaniline
3.2. Inhibition of Wnt/β-catenin signaling
Niclosamide’s activity against the Wnt/β-catenin pathway was first discovered by its ability to internalize the Fzd1 receptor using a novel Fzd1-GFP internalization assay15. Similar to previous work that characterized its inhibition of Wnt/β-catenin signaling15, 34, DK419 was evaluated in cellular assays to assess internalization of Fzd1-GFP receptor, inhibition of β-catenin gene transcription in the Wnt3A-stimulated TOPFlash reporter assay, and the reduction of Wnt/β-catenin signaling proteins and target genes by Western blot (Fig. 3). In the Fzd1-GFP internalization assay, U2OS cells stably expressing Fzd1-GFP protein were treated with Niclosamide or DK419 at 12.5 μM for 6 hours and compared to control media containing equivalent amounts of DMSO. Frizzled receptor internalization was assessed by the production of a green fluorescent internal puncta using confocal microscopy (Fig. 3A). Both DK419 and Niclosamide produced a robust punctate pattern compared to control, indicating that both molecules induce internalization to the receptor. We next assessed by dose response the ability of DK419 to inhibit Wnt/β-catenin gene transcription using the Wnt3A-stimulated β-catenin TOPFlash reporter assay (Fig. 3B) and found that DK419 inhibited Wnt/β-catenin signaling with an IC50 of 0.19 ± 0.08 μM, comparable to Niclosamide (IC50 of 0.45 ± 0.14 μM). To confirm these results, we next compared DK419 and Niclosamide in two colorectal cancer cell lines that harbor mutations in the Wnt/β-catenin signaling pathway (HCT-116, β-catenin mutation; SW-480, APC mutation) and in patient-derived colorectal tumor CRC-240 cells by Western blot (Fig. 3C). Consistent with the results of the Wnt3A-stimulated TOPFlash assay, both DK419 and Niclosamide demonstrated reduced levels of Axin2, β-catenin, c-Myc, Cyclin D1 and Survivin in all three CRC tumor cell models.
Fig. 3. Inhibition of Wnt/β-catenin signaling by DK419 and Niclosamide.
(A) DK419 and Niclosamide induce Fzd1-GFP internalization. Fzd1-GFP stable U2OS cells were treated for 6 h at 37C with DMSO (i), 12.5μM niclosamide (ii), or 12.5μM DK419 (iii). Internalized vesicles are noted with arrowheads. (B) DK419 and Niclosamide inhibit Wnt/β-catenin signaling in TOPFlash assay. HEK293 cells stably expressing TOPFlash luciferase and Renilla luciferase reporters were treated with Wnt3A-conditioned medium in the presence of DMSO or compounds from 0.04 to 10 μM. The TOPFlash reporter activity of Wnt3A with DMSO treatment was set as 100%. DK419 IC50 = 0.19 ± 0.08μM; Niclosamide IC50 = 0.45 ± 0.14 μM. Data were fit using GraphPad Prism (mean ± SEM, n = 3). (C) Reduction of Wnt/β-catenin target gene protein levels in HCT116, SW-480 and CRC240 cells by DK419 or Niclosamide. Cells were treated with DMSO or compounds in DMSO at 5μM for 18h. Cytosolic fractions and whole cell lysate were analyzed by western blot. β-actin was used as a loading control.
3.3. Increase in oxygen consumption rate and induction of pAMPK
Niclosamide induces activating phosphorylation of AMPK and increases cellular oxygen consumption rate, both likely a result of its uncoupling of mitochondria oxidative phosphorylation21–25. To determine if the molecular principles used in the design of DK419 were able to reproduce Niclosamide’s ability to uncouple oxidative phosphorylation and induce AMPK phosphorylation, we compared the ability of DK419 and Niclosamide to increase the oxygen consumption rate and induce phosphorylation of AMPK in CCD841Co cells, a normal epithelial colonic cell line (Fig. 4). To assess the change in oxygen consumption rate, cells were treated with DK419 or Niclosamide at 1μM and oxygen consumption was compared versus DMSO control media using a Seahorse XFp analyzer (Fig. 4A). At the end of the experiment, Oligomycin, FCCP, Rotenone and Antimycin A were added sequentially to assess mitochondria status. FCCP, in particular, uncouples oxidative phosphorylation and increases oxygen consumption rate, as witnessed by the increased rate of oxygen consumption in the presence of the ATP synthase inhibitor Antimycin A in the cells treated with DMSO control media. Niclosamide also increases the oxygen consumption rate, indicating its ability to uncouple of oxidative phosphorylation in these cells. As anticipated by its molecular design41, DK419 increases the rate of oxygen consumption, consistent with the expectation of a molecule that uncouples oxidative phosphorylation. We found that the kinetic profile and the magnitude of the uncoupling effect is different at the single doses of Niclosamide and DK419 used. The reason for this difference is not known but may reflect a difference in potency between the two compounds or differences in cell or mitochondrial membrane permeability. Overall, both Niclosamide and DK419 at 1μM caused oxygen consumption to increase and then decrease over time. The data from this experiment is consistent with the ability of these compounds to inhibit the spare respiratory capacity of mitochondria as demonstrated by the finding that treated cells did not respond to the subsequent addition of FCCP. The effects observed were consistent with a report that Niclosamide at similar concentrations (0.5 and 1.5 μM) caused oxygen consumption rate to increase in HCT116 cells for which later addition of FCCP caused no further increase in oxygen consumption rate42. As expected, addition of Rotenone and Antimycin A at the end of the experiment decreased oxygen consumption, indicating that the mitochondria of cells treated with the uncoupling agent were still actively respiring.
Fig. 4. DK419 increases oxygen consumption rate and induces pAMPK in cell culture.
(A) Oxygen consumption rate in CCD841Co colonic cells in the presence of 1μM Niclosamide, 1μM DK419, or DMSO was measured using a Seahorse XFp analyzer. Compound addition noted by arrows. Control compounds 1μM Oligomycin, 1μM FCCP, and 0.5μM Rotenone & Antimycin added at the end of the experiment (arrows). Data are mean ± SD, n = 2. OCR was measured under basal conditions. (B) CCD841Co colonic cells were treated for 2 hours with DK419 or Niclosamide and the whole cell lysate analyzed by Western blot. β-actin was used as a loading control.
To assess the ability of DK419 to induce phosphorylation of AMPK, CCD841Co cells were treated with DK419, Niclosamide or control media containing DMSO, and compared by Western blot (Fig. 4B). Niclosamide increased pAMPK levels versus control as expected. Again, as anticipated by the design, DK419 also increased the levels of pAMPK. Cellular levels of AMPK did not appear to change (data not shown). Taken together, these data indicate that DK419, like Niclosamide, has effects on oxidative phosphorylation and impacts the levels of pAMPK.
3.4. Inhibition of colorectal cancer cell proliferation
To assess the ability of DK419 to inhibit the proliferation of CRC tumor cell lines, we evaluated the effect of DK419 and Niclosamide on cell proliferation using a MTS assay in six CRC tumor cell lines (Table 1). DK419 inhibited the proliferation of each of the six CRC cell lines with IC50 values ranging from 0.07 to 0.36 μM. Niclosamide also inhibited the proliferation of these cell lines, with IC50 values that ranged from 0.11 to 2.39 μM. Overall, DK419 demonstrated the ability to inhibit the proliferation of CRC cells and showed a trend toward greater potency in these cell lines compared to Niclosamide.
Table 1.
Inhibition of cell proliferation by DK419 and Niclosamide a
| Cell Line | DK419 | Niclosamide |
|---|---|---|
| SW948 | 0.07 ± 0.02 | 0.11 ± 0.02 |
| HT-29 | 0.12 ± 0.03 | 0.13 ± 0.04 |
| HCT116 | 0.28 ± 0.04 | 0.41 ± 0.06 |
| CRC240 | 0.32 ± 0.12 | 0.89 ± 0.16 |
| SW480 | 0.33 ± 0.05 | 0.98 ± 0.21 |
| DLD-1 | 0.36 ± 0.07 | 2.39 ± 0.76 |
IC50 values in μM from MTS assay at 72 hr using Graphpad Prism, n = 3.
3.5. Systemic exposure from oral dose – plasma pharmacokinetics
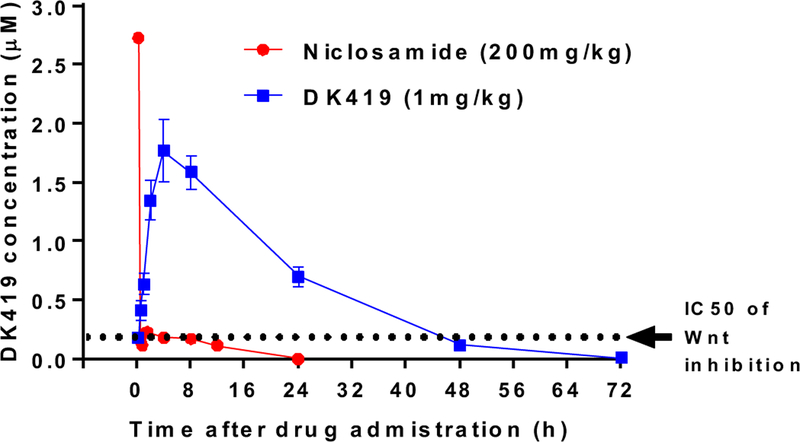
A central element in the design of DK419 was to identify Wnt/β-catenin inhibitors with improved oral exposure by the removal of substituents in the structure of Niclosamide that undergo metabolism and impact its absorption. In the case of DK419, the nitro group of Niclosamide was replaced with a trifluoromethyl group and the phenol replaced with an amine group within the benzimidazole ring system. To assess whether these design elements in DK419 offered improvements in systemic exposure from oral dosing, mice were dosed orally with DK419 at 1mg/kg and the plasma concentration of DK419 evaluated over 72 hours (Fig. 5). Here, we were delighted to find that DK419 produced significantly higher levels of the inhibitor in plasma that were sustained for more than 24 hours. Namely, at this dose, the plasma concentration of DK419 at 24 hours was 3-fold higher than its IC50 for inhibition of Wnt/β-catenin signaling and CRC proliferation in the TOPFlash and MTS assays. Overall, a single 1mg/kg oral dose of DK419 produced much higher and more sustained systemic exposure than in case of a single 200 mg/kg oral dose of Niclosamide34 (AUC/DOSE and t1/2 values, Table 2.), supporting the notion that our design strategy resulted in much-improved pharmacokinetic properties of the new compound.
Figure. 5. Plasma concentration of DK419 vs. time in mice.
Adult mice were dosed orally with DK419 at 1mg/kg of body weight. Blood samples were drawn serially at 0.25, 0.5, 1, 2, 4, 8, 24 h, 48, and 72 h after drug administration. N = 4 per time point. Quantification of DK419 in mouse plasma was done by LC/MS–MS and reported as ng/ml. The data presented are mean ± SEM. The dotted line is the IC50 of DK419 inhibition of Wnt/β-catenin signaling in the TOPFlash assay. For comparison, the plasma concentration of Niclosamide orally dosed at 200mg/kg is also presented34. Both DK419 and Niclosamide were administered in the vehicle of 10% 1-methyl-2-pyrrolidinone and 90% PEG300 to NOD/SCID mice.
Table 2. Pharmacokinetic parameters.
Both compartmental and non-compartmental modeling (WinNonlin) of DK419 plasma concentration-time data (Fig. 5) was performed.
| PK Parameter | DK419 compartmental 1st order extrav. input, 1st order elimination |
SEM | DK419 non-compartmental extravascular input |
SEM | Niclosamide* non-compartmental extravascular input |
|---|---|---|---|---|---|
| DOSE, mg (per kg BW) | 1 | 1 | 200 | ||
| Tmax, h | 5.85 | 0.52 | 4.5 | 0.51 | 0.25 |
| Cmax, μg/mL | 0.73 | 0.04 | 0.75 | 0.09 | 0.89 |
| Cmax, μM | 1.79 | 0.09 | 1.84 | 0.23 | 2.73 |
| AUCinf, h μg/mL (per kg BW) | 16.3 | 1.8 | 16.9 | 1.4 | 1.02 |
| AUCinf/DOSE, h μg/mL (per mg) | 16.3 | 1.8 | 16.9 | 1.4 | 0.005 |
| AUC0-LAST h μg/mL (per kg BW) | - | - | 16.8 ( 0–72 h) | 1.4 | 1.01 ( 0–24 h) |
| t1/2 - elimination, h | 10.8 | 2.1 | 9.2 | 0.2 | 3.1 |
| t1/2 - absorption, h | 2.0 | 0.4 | - | - | - |
| Vd/F, L (per kg BW) | 0.98 | 0.12 | 0.80 (Vz) | 0.07 | 0.90 (Vz) |
| CL/F, L/h (per kg BW) | 0.06 | 0.007 | 0.06 | 0.004 | 0.20 |
| MRT, h | - | - | 15.8 | 0.9 | 6.8 |
For comparison, included are Niclosamide PK parameters from reference 34 Animal experiment and dosing conditions explained in Fig. 5.
3.6. Inhibition of CRC tumor growth in vivo
To assess the ability of DK419 to inhibit the growth of CRC tumors in vivo, DK419 was evaluated in a patient-derived tumor mouse xenograft model. In this model, NOD/SCID mice bearing CRC240 PDX tumors were dosed orally once a day with DK419 at 1 mg/kg (2.4 μmoles/kg), Niclosamide at 72 mg/kg (220 μmole/kg), or vehicle. Tumor volume and body weight were measured over the study. At the conclusion of the study, tumors were collected and analyzed for Wnt target gene protein levels (Axin2, Survivin and c-Myc). As shown in Fig. 6A, DK419 inhibited the growth of CRC240 PDX tumors compared to vehicle control. When compared against mice treated with Niclosamide, DK419 at 1/90th the molar dose inhibited tumor growth to a similar level as Niclosamide. Neither DK419 nor Niclosamide produced a change in body weight (Fig. 6B). Moreover, in DK419 treated animals, the levels of each of the Wnt/β-catenin target genes in tumors were reduced significantly (Fig. 6 C, D). Overall, these studies demonstrate that DK419 can inhibit Wnt/β-catenin signaling and inhibit the growth of CRC tumors in vivo in a patient-derived CRC tumor model. Consistent with the pharmacokinetic studies indicating improved systemic exposure, DK419 demonstrated similar antitumor activity as Niclosamide but at a significantly lower dose.
Fig. 6. In vivo inhibition of tumor growth by DK419 in mouse CRC240 PDX tumor model.
NOD/SCID mice bearing CRC240 PDX tumors were randomly allocated to three groups and dosed orally daily for 11 days with vehicle control, Niclosamide at 72mg/kg, or DK419 at 1mg/kg. Tumor size and body weight were measured at day 0, 4, 8, and 11. (A) Tumor volume. (B) Body weight. Data represent means ± SEM, n = 3. *p<0.05 (two-tailed Student’s t test between vehicle vs. Niclosamide , or vehicle vs. DK419 treatment). (C) Tumors collected from mice were evaluated by Western blot for levels of Wnt pathway target gene proteins Axin2, Survivin and c-Myc. β-actin used as a loading control. (D) The levels of Wnt target gene proteins from Western blots in panel C were quantified by normalizing to β-actin and found to decrease vs. vehicle control. Data represent mean ± SEM, n = 3. *P <0.05 by a two-tailed Student’s t test of DK419 treated group compared to the control group.
4. Discussion
In the studies herein, we describe the design of a novel inhibitor of Wnt/β-catenin signaling based on the structure and SAR of Niclosamide. In our design, we sought to remove substituents in Niclosamide’s structure thought to contribute to its poor systemic exposure upon oral dosing. Key in the design was the replacement of the nitro and phenol substituents in Niclosamide. To our knowledge, this is the first example of a Niclosamide derivative in which the phenol can be replaced without a significant loss of Wnt/β-catenin inhibitory activity. In vitro, DK419 has similar potency to Niclosamide in the Fzd1-GFP internalization assay and in the Wnt3A-stimulated TOPFlash reporter assay. In multiple CRC cell lines that harbor mutations in the Wnt signaling pathway, DK419 reduced the levels of Wnt/β-catenin target gene proteins consistent with the results from the internalization and reporter assays. DK419 also inhibited the growth of multiple CRC cell lines in culture. In vivo, DK419 has good oral exposure in mice, producing plasma concentrations of DK419 24 hours after a single 1mg/kg oral dose that are above its in vitro IC50 values in the Wnt3A TOPFlash and the CRC cell proliferation assays. The exposure of DK419 at 1mg/kg significantly exceeds the exposure of Niclosamide at a dose of 200mg/kg. Consistent with pharmacokinetic studies that show improved exposure, DK419 at a significantly lower dose inhibited the growth of tumors comparable to Niclosamide in a human patient-derived colorectal PDX xenograft model and without effects on mouse body weight.
The biological target that binds to DK419 and Niclosamide that results in inhibition of Wnt/β-catenin signaling is not known. This fact is due to the reverse chemical genetic screening method used in which the screening assay measures a functional output and not activity against a specific target. As a result, this approach requires subsequent deconvolution to identify the binding target. Niclosamide was initially identified from a high-throughput assay that screened for agents that induce internalization of the Frizzled receptor. Subsequent in vitro studies found that Niclosamide decreased cellular levels of Dvl and cytosolic β-catenin, decreased levels of Wnt/β-catenin target gene proteins, and inhibited the proliferation of CRC cells in culture15. In vivo, Niclosamide inhibited the growth of CRC tumors in mouse models and reduced the levels of Wnt/β-catenin target proteins in the CRC tumors even though its systemic exposure was generally low34. Being designed from Niclosamide and incorporating key SAR features, DK419 has a similar Wnt/β-catenin and CRC cell proliferation inhibitory profile, yet represents a significant advance due to its improved systemic exposure. The low exposure of Niclosamide in humans is considered a risk to its success in multiple ongoing clinical trials. DK419 provides a framework to optimize its activity within a chemotype that demonstrates better exposure.
Initial SAR studies suggested that inhibition of Wnt signaling may be separate from the mechanism associated with its anthelmintic activity, namely uncoupling of oxidative phosphorylation43. Additional SAR studies demonstrated the ability to separate the two effects. Although the SAR suggests differences, there are also close similarities such that there may in fact be a relationship at some level between Wnt signaling and mitochondrial status44. It is well known that Wnt and mitochondrial function are both critical for cancer growth and metastasis. Mitochondria in cancer cells are often compromised, and a growing body of literature demonstrates uncoupling mitochondrial oxidative phosphorylation can overcome resistance to anticancer drugs45–50. Thus, the multifunctional activity of DK419 may have additional benefits in the treatment of cancer. Further studies are needed.
5. Conclusion
Using the SAR of Niclosamide, we designed DK419, a novel inhibitor of Wnt/β-catenin signaling that mimics Niclosamide’s inhibition of Wnt/β-catenin signaling and uncoupling of oxidative phosphorylation yet overcomes Niclosamide’s poor exposure when dosed orally. DK419 represents a new chemotype of Wnt/β-catenin inhibitors. Additional studies to optimize the activity of this chemotype and to assess its ability to inhibit other key oncogenic signaling pathways such as mTORC, STAT3, NF-κB, and Notch, and to inhibit tumor growth in additional tumor models are needed. Overall, DK419 holds promise as an agent to treat cancers and other Wnt-related diseases, as well as diseases in which Niclosamide has demonstrated important medicinal activity.
Acknowledgements
This work was funded in part by 5 R01 CA172570 (WC) and Clinical Oncology Research Center Development Grant 5K12-CA100639–08 (RAM). Wei Chen is a V Foundation Scholar and an American Cancer Society Research Scholar. NMR instrumentation in the Duke NMR Spectroscopy Center was funded by the NIH, NSF, NC Biotechnology Center and Duke University. The authors gratefully acknowledge this support and the support of Dr. David Gooden and Professor Eric Toone of the Duke Small Molecule Synthesis Facility. The assistances of Dr. Hailan Paio in conducting the Western blot studies in CRC cell lines is also gratefully acknowledged. The authors thank Dr. Richard Premont for reviewing the manuscript.
Abbreviations:
- AMPK
AMP-activated protein kinase
- APC
Adenomatous Polyposis Coli
- CRC
colorectal cancer
- Dvl
Dishevelled
- FCCP
trifluorocarbonylcyanide phenylhydrazone
- Fzd1
Frizzled1
- GFP
green fluorescent protein
- OCR
oxygen consumption rate
- SAR
structure-activity relationships
References
- 1.Nusse R; Clevers H Cell 2017, 169, 985. [DOI] [PubMed] [Google Scholar]
- 2.Zhan T; Rindtorff N; Boutros M Oncogene 2017, 36, 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clevers H; Nusse R Cell 2012, 149, 1192. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network. Nature 2012, 487, 330.22810696 [Google Scholar]
- 5.Barker N; Clevers H Nature Reviews Drug Discovery 2006, 5, 997. [DOI] [PubMed] [Google Scholar]
- 6.Chen W; ten Berge D; Brown J; Ahn S; Hu LA; Miller WE; Caron MG; Barak LS; Nusse R; Lefkowitz RJ Science 2003, 301, 1391. [DOI] [PubMed] [Google Scholar]
- 7.Meireles LMC; Mustata G Curr. Top. Med. Chem 2011, 11, 248. [DOI] [PubMed] [Google Scholar]
- 8.Sebio A; Kahn M; Lenz H-J Expert Opinion on Therapeutic Targets 2014, 18, 611. [DOI] [PubMed] [Google Scholar]
- 9.Mook RA Jr.; Ren XR; Wang J; Piao H; Barak LS; Kim Lyerly H; Chen W Bioorg Med Chem 2017, 25, 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabatabai R; Linhares Y; Bolos D; Mita M; Mita A Target Oncol 2017, 12, 623. [DOI] [PubMed] [Google Scholar]
- 11.Yang K; Wang X; Zhang H; Wang Z; Nan G; Li Y; Zhang F; Mohammed MK; Haydon RC; Luu HH; Bi Y; He TC Lab Invest 2016, 96, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran FH; Zheng JJ Protein Sci 2017, 26, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y; Oliver PG; Lu W; Pathak V; Sridharan S; Augelli-Szafran CE; Buchsbaum DJ; Suto MJ Cancer Lett 2017, 389, 41. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Li B; Orton D; Neitzel LR; Astudillo L; Shen C; Long J; Chen X; Kirkbride KC; Doundoulakis T; Guerra ML; Zaias J; Fei DL; Rodriguez-Blanco J; Thorne C; Wang Z; Jin K; Nguyen DM; Sands LR; Marchetti F; Abreu MT; Cobb MH; Capobianco AJ; Lee E; Robbins DJ Science Signaling 2017, 10, eaak9916.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MY; Wang JB; Lu JY; Bond MC; Ren XR; Lyerly HK; Barak LS; Chen W Biochemistry 2009, 48, 10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sack U; Walther W; Scudiero D; Selby M; Kobelt D; Lemm M; Fichtner I; Schlag Peter M; Shoemaker Robert H; Stein U Journal of the National Cancer Institute 2011, 103, 1018. [DOI] [PubMed] [Google Scholar]
- 17.Lu W; Lin C; Roberts MJ; Waud WR; Piazza GA; Li Y Plos One 2011, 6, e29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arend RC; Londono-Joshi AI; Samant RS; Li Y; Conner M; Hidalgo B; Alvarez RD; Landen CN; Straughn JM; Buchsbaum DJ Gynecologic Oncology 2014, 134, 112. [DOI] [PubMed] [Google Scholar]
- 19.Pearson RD; Hewlett EL Annals of Internal Medicine 1985, 102, 550. [DOI] [PubMed] [Google Scholar]
- 20.WHO, Ed. The Selection and Use of Essential Medicines World Health Organization; Geneva, 2007. [Google Scholar]
- 21.Weinbach EC; Garbus J Nature 1969, 221, 1016. [DOI] [PubMed] [Google Scholar]
- 22.Williamson RL; Metcalf RL Science 1967, 158, 1694. [DOI] [PubMed] [Google Scholar]
- 23.Frayha GJ; Smyth JD; Gobert JG; Savel J General Pharmacology 1997, 28, 273. [DOI] [PubMed] [Google Scholar]
- 24.Swan GE J. S. Afr. Vet. Assoc 1999, 70, 61. [DOI] [PubMed] [Google Scholar]
- 25.Tao H; Zhang Y; Zeng X; Shulman GI; Jin S Nature Medicine 2014, 20, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W; Mook RA Jr.; Premont RT; Wang J Cellular Signalling 2018, 41, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajamuthiah R; Fuchs BB; Conery AL; Kim W; Jayamani E; Kwon B; Ausubel FM; Mylonakis E Plos One 2015, 10, e0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gooyit M; Janda KD Sci Rep 2016, 6, 33642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M; Lee EM; Wen Z; Cheng Y; Huang WK; Qian X; Tcw J; Kouznetsova J; Ogden SC; Hammack C; Jacob F; Nguyen HN; Itkin M; Hanna C; Shinn P; Allen C; Michael SG; Simeonov A; Huang W; Christian KM; Goate A; Brennand KJ; Huang R; Xia M; Ming GL; Zheng W; Song H; Tang H Nature Medicine 2016, 22, 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YM; Lu JW; Lin CC; Chin YF; Wu TY; Lin LI; Lai ZZ; Kuo SC; Ho YJ Antiviral Research 2016, 135, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang M; Zeng S; Qiu Q; Xiao Y; Shi M; Zou Y; Yang X; Xu H; Liang L Int. Immunopharmacol 2016, 31, 45. [DOI] [PubMed] [Google Scholar]
- 32.Liang L; Huang M; Xiao Y; Zen S; Lao M; Zou Y; Shi M; Yang X; Xu H Inflammation Res. 2015, 64, 225. [DOI] [PubMed] [Google Scholar]
- 33.Li Y; Li PK; Roberts MJ; Arend RC; Samant RS; Buchsbaum DJ Cancer Lett 2014, 349, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osada T; Chen MY; Yang XY; Spasojevic I; Vandeusen JB; Hsu D; Clary BM; Clay TM; Chen W; Morse MA; Lyerly HK Cancer Research 2011, 71, 4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C; Lou W; Zhu Y; Nadiminty N; Schwartz CT; Evans CP; Gao AC Clinical Cancer Research 2014, 20, 3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R; Hu Z; Sun SY; Chen ZG; Owonikoko TK; Sica GL; Ramalingam SS; Curran WJ; Khuri FR; Deng X Mol Cancer Ther 2013, 12, 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews P, Thyssen J, Lorke D Pharmac. Ther 1983, 19, 245. [DOI] [PubMed] [Google Scholar]
- 38.Chang Y-W; Yeh T-K; Lin K-T; Chen W-C; Yao H-T; Lan S-J; Wu Y-S; Hsieh H-P; Chen C-M; Chen C-T Yaowu Shipin Fenxi 2006, 14, 329. [Google Scholar]
- 39.Lu M; Zessin AS; Glover W; Hsu DS PLoS One 2017, 12, e0169439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mook RA Jr.; Wang J; Ren XR; Chen M; Spasojevic I; Barak LS; Lyerly HK; Chen W Bioorg Med Chem 2015, 23, 5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terada H Environmental Health Perspectives 1990, 87, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senkowski W; Zhang X; Olofsson MH; Isacson R; Hoeglund U; Gustafsson M; Nygren P; Linder S; Larsson R; Fryknaes M Mol. Cancer Ther 2015, 14, 1504. [DOI] [PubMed] [Google Scholar]
- 43.Mook RA Jr.; Chen M; Lu J; Barak LS; Lyerly HK; Chen W Bioorg Med Chem Lett 2013, 23, 2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamanaka RB; Glasauer A; Hoover P; Yang S; Blatt H; Mullen AR; Getsios S; Gottardi CJ; DeBerardinis RJ; Lavker RM; Chandel NS Sci Signal 2013, 6, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg SE; Chandel NS Nat Chem Biol 2015, 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf DA Cancer Cell 2014, 26, 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G; Frederick DT; Wu L; Wei Z; Krepler C; Srinivasan S; Chae YC; Xu X; Choi H; Dimwamwa E; Ope O; Shannan B; Basu D; Zhang D; Guha M; Xiao M; Randell S; Sproesser K; Xu W; Liu J; Karakousis GC; Schuchter LM; Gangadhar TC; Amaravadi RK; Gu M; Xu C; Ghosh A; Xu W; Tian T; Zhang J; Zha S; Liu Q; Brafford P; Weeraratna A; Davies MA; Wargo JA; Avadhani NG; Lu Y; Mills GB; Altieri DC; Flaherty KT; Herlyn M The Journal of Clinical Investigation 2016, 126, 1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roesch A; Vultur A; Bogeski I; Wang H; Zimmermann KM; Speicher D; Korbel C; Laschke MW; Gimotty PA; Philipp SE; Krause E; Patzold S; Villanueva J; Krepler C; Fukunaga-Kalabis M; Hoth M; Bastian BC; Vogt T; Herlyn M Cancer Cell 2013, 23, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X; Fryknas M; Hernlund E; Fayad W; De Milito A; Olofsson MH; Gogvadze V; Dang L; Pahlman S; Schughart LA; Rickardson L; D’Arcy P; Gullbo J; Nygren P; Larsson R; Linder S Nat Commun 2014, 5, 3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shikata Y; Kiga M; Futamura Y; Aono H; Inoue H; Kawada M; Osada H; Imoto M Cancer Sci 2017, 108, 772. [DOI] [PMC free article] [PubMed] [Google Scholar]