Abstract
The process of neurogenesis in mammals, which is prolific and widespread at birth, gradually slows with aging and in humans becomes restricted to areas including the cerebellum and hippocampus. It has been reported that exposure to glucocorticoids can impair neurogenesis in both adults and children. Glucocorticoids are known to bind with high affinity to intracellular receptors. Glucocorticoid blood levels are normally regulated by environmental stresses, but because of their clinical utility, exogenous glucocorticoids are frequently administered in drug formulations. Consequently, concerns have arisen about the consequences of glucocorticoid use on neurogenesis and health, especially in the pediatric population. In this article we will review recent findings that a select number of related glucocorticoids also bind the Hedgehog pathway receptor Smoothened. We will discuss their pharmacology and also a most surprising result; that this select group of compounds, which includes FDA approved drugs, unlike typical glucocorticoids such as dexamethasone, stimulate stem cell growth and thus enhance neurogenesis.
Introduction:
The ability of stem cell neurons in the central nervous system of mammals to divide is not entirely lost with aging. It has recently been shown in humans that stem cell neuronal populations remain active in the hippocampus, suggesting that memory and behavior may remain plastic throughout adulthood and responsive to external stressors such as drugs (Schoenfeld and Gould, 2011). Mood disorders are prevalent in the general adult population and Lithium, a commonly used drug to treat depression, appears to enhance adult hippocampus neurogenesis by preventing GSK3β activity up-regulation mediated by nuclear glucocorticoid receptors (Boku et al., 2010). The ability of glucocorticoids to inhibit neurogenesis has become a clinical concern in children where negative effects on brain development are apparent in infants exposed to dexamethasone (Heine and Rowitch, 2009), and the chronic use of steroids has increased in the pediatric population for diseases like asthma. In general, children exposed to glucocorticoids over extended periods have an increased association with cognitive deficits and a reduction in brain size (Damsted et al., 2011).
Seven transmembrane receptors on stem cells regulate many of the growth and patterning pathways for organogenesis and tissue repair. For example, Frizzled receptors signal through GSK3β in the gastrointestinal tract to control levels of beta-catenin, whose elevation is associated with tumors (Doucas et al., 2005). Similarly, abnormally elevated signaling of the seven transmembrane receptor Smoothened (Smo), a Hedgehog pathway regulator normally suppressed by Patched, underlies development of medulloblastoma in the cerebellum (Corcoran and Scott, 2001). While endogenous Wnt agonists have been identified for Frizzled, no endogenous agonist has been discovered for Smo. A limited number of synthetic Smo agonists, however, have been discovered by screening compound libraries and testing their activity in the presence of the Smo antagonist cyclopamine (Chen et al., 2002). For our program in stem cell and regenerative medicine, we developed a similar strategy to identify Smoothened ligands using a novel β-arrestin-GFP translocation assay (Wang et al., 2010), but rather than screen combinatorial libraries we have concentrated our efforts on FDA approved drugs. In one such screen we surprisingly identified a restricted series of glucocorticoids with Smoothened activity (Wang et al., 2010). Moreover, these select compounds in contrast to glucocorticoids in general stimulate rather than inhibit the growth of cerebellar neuronal progenitor cells.
Select Glucocorticoids as Smo Agonists: Potential Effects for Neurogenesis:
The Hedgehog signaling pathway, mediated by Smo, is critical for embryonic patterning, stem cell growth, and central nervous system development (Ingham and McMahon, 2001; Marti and Bovolenta, 2002; Palma et al., 2005; Ruiz i Altaba et al., 2002). Therapeutic activation of Hedgehog signaling by Smo agonists has been proposed for restoring tissue function in the peripheral and central nervous systems for retinal injury, Parkinson’s disease, spinal cord damage, and neuronal degeneration (Bambakidis et al., 2009; Tsuboi and Shults, 2002; Wan et al., 2007; Li et al., 2008). The cerebellum contains one of the most abundant populations of neurons in the brain, derived from a stem cell population of cerebellar granular cell precursors (GCPs), and the in vivo expansion of these granule precursor cells is dependent on Hedgehog/Smo pathway signaling (Wechsler-Reya and Scott, 1999). For the mouse cerebellum in the first postnatal week, purkinje cell secreted sonic hedgehog (Shh) ligand stimulates GCP proliferation in the outer layer of the cerebellar surface (external granular layer). GCPs then exit the proliferative phase of the cell cycle, differentiate into granule neurons, and migrate inward into the internal granule layer (Figure 1).
Figure 1.
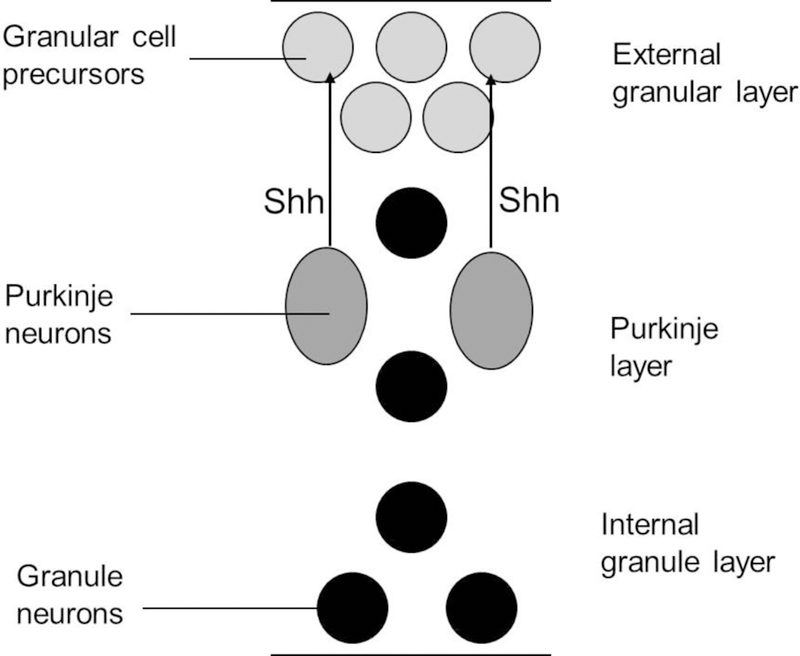
Primary neuronal granule cell precursor (GCP) proliferation in the cerebellum is Hedgehog signaling dependent.
Using a high-throughput screen of cells containing Smo and β-arrestin-GFP reporter that binds activated Smo, we identified four glucocorticoids - halcinonide, fluticasone propionate, clobetasol propionate, and fluocinonide as Smo agonists (Wang et al., 2010). Similar to some other known Smo agonists, SAG and purmorphamine, these glucocorticoids bind Smo, promote Smo internalization, and activate Gli. Consistent with Smo agonist activity, these select set of glucocorticoids stimulate the proliferation of GCPs alone and synergistically, and such stimulation was not affected by the glucocorticoid nuclear receptor antagonist Mifepristone (RU-486). In contrast, the glucocorticoid receptor (GR) agonist dexamethasone inhibited Shh-activated GCP proliferation. Additionally, GCP treatment with Shh, Purmorphamine, SAG, halcinonide, clobetasol propionate, and fluticasone propionate (but not the weak Smo agonist fluocinonide) inhibit caspase 3 degradation and block GCP apoptosis by increasing endogenous cyclin D2 protein expression. No such growth enabling responses were observed in GCPs treated with cortisone, dexamethasone, prednisolone, and corticosterone.
As expected the four glucocorticoid Smo agonists and dexamethasone had similar effects in activating GR in a GR-GFP nuclear translocation assay, yet dexamethasone produced an opposite response to the glucocorticoid Smo agonists on GCP proliferation and apoptosis. Taken together these data suggest that the GCP proliferative response is due directly to activation of Smo and is independent of glucocorticoid nuclear receptor signaling. The ability of some glucocorticoid drugs, particularly FDA approved compounds, to activate Hedgehog signaling has profound implications for pediatric therapeutics, stress related disorders, and regenerative medicine. These drugs have well-known safety profiles and are well characterized, so that their application in new therapeutic areas can be evaluated much more expeditiously that unapproved compounds isolated in screens of compound libraries.
Mechanism of Action
Glucocorticoid steroids are a standard of care in many clinical settings including rheumatic disorders, asthma, inflammation and cancer (Schimmer 2006). An excellent summary of the medicinal chemistry of steroid hormones and therapeutically related compounds and a review of the x-ray crystal structures of steroidal compounds provide keen insights into the medicinal chemistry of steroidal glucocorticoids (Proteau, 2004; Duax et al., 1988). Glucocorticoid steroids bind the glucocorticoid receptor, a member of the nuclear receptor superfamily (Figure 2). Upon binding to GR, the cytoplasmic form of the receptor moves to the nucleus and activates the transcription and repression of multiple gene products involved in metabolism and inflammation. In particular, glucocorticoids such as dexamethasone acting through the GR receptor inhibit cell growth in GCPs. Independent of GR signaling, select glucocorticoids such as halcinonide bind to the Smo receptor, activate Hedgehog signaling, and stimulate cell growth in GCPs (Figure 2). While these select glucocorticoids can be both GR agonists and Smo agonists, the Smo agonist effects appear to be the more dominant in GCPs.
Figure 2.
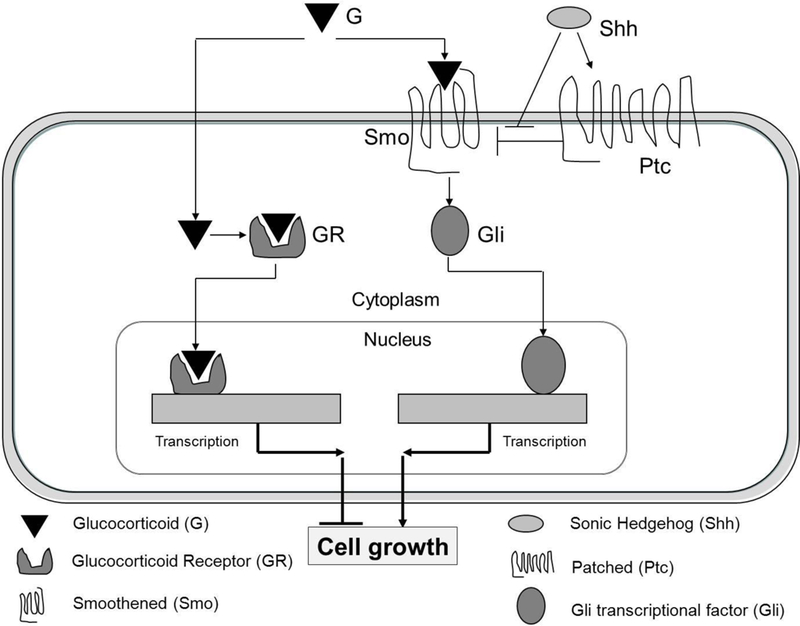
Select glucocorticoids affect cell growth through both the glucocorticoid receptor and the Smo receptor.
Structure-Activity Relationships of Smo Agonist Glucocorticoids
Subsequent screening of focused chemical libraries in the β-Arrestin2-GFP translocation assay has increased the number of glucocorticoid derivatives identified with Smo agonist activity (Table 1). These efforts have helped define key structure activity relationships (SAR) for Hedgehog agonist activity while also identifying a number of structurally-related glucocorticoid derivatives without Smo agonist activity (Table 2).
Table 1.
Summary of glucocorticoid derivatives with Smo agonist activity in the β-arrestin2-GFP translocation assay
Table 2.
Smo agonist activity in β-arrestin2-GFP translocation assay of representative glucocorticoid derivatives
| Non-Cyclic Series (NC) |  |
Cyclic Series(C) |  |
Activity in β-Arrestin translocation assay at 12.5uM Active (A), Inactive (IA) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Series | Name | D1,2 | R6 | R9 | R11 | R16 | R17α | R17 β | |
| NC | Fluticasone propionate | y | F | F | HO-b | CH3-a | O-CO-CH2-CH3 | CO-S-CH2-F | A |
| C | Halcinonide | n | H | F | HO-b | -O-C(CH3)2-O- | Cyclized with R16 | CO-CH2-Cl | A |
| NC | Clobetasol propionate | y | H | F | HO-b | CH3-b | O-CO-CH2-CH3 | CO-CH2-Cl | A |
| C | Fluocinonide | y | F | F | HO-b | -O-C(CH3)2-O- | Cyclized with R16 | CO-CH2-OCO-CH3 | A |
| NC | Fluticasone | y | F | F | HO-b | CH3-a | OH | CO-S-CH2-F | A |
| NC | Halobetasol propionate | y | F | F | HO-b | CH3-b | O-CO-CH2-CH3 | CO-CH2-Cl | A |
| C | Amcinonide | y | H | F | HO-b | -O-C(C5H11)-O- | Cyclized with R16 | CO-CH2-O-CO-CH3 | A |
| C | Fluocinolone acetonide | y | F | F | HO-b | -O-C(CH3)2-O- | Cyclized with R16 | CO-CH2-OH | A |
| C | Triamcinolone acetonide | y | H | F | HO-b | -O-C(CH3)2-O- | Cyclized with R16 | CO-CH2-OH | A |
| NC | Dexamethasone | y | H | F | HO-b | CH3-a | OH | CO-CH2-OH | IA |
| NC | Hydrocortisone-acetate | n | H | H | HO-b | H | OH | CO-CH2-O-CO-CH3 | IA |
| NC | Dexamethasone 21 acetate | y | H | F | HO-b | CH3-a | OH | CO-CH2-O-CO-CH3 | IA |
| NC | Prednisolone | y | H | H | HO-b | H | OH | CO-CH2-OH | IA |
| NC | Cortisone | n | H | H | O= | H | OH | CO-CH2-OH | IA |
| NC | Fluticasone furoate | y | F | F | HO-b | CH3-a | O-CO-(2-Furyl) | CO-S-CH2-F | IA |
| NC | Flumethasone | y | F | F | HO-b | CH3-a | OH | CO-CH2-OH | IA |
| NC | Betamethasone 21 acetate | y | H | F | HO-b | CH3-b | OH | CO-CH2-O-CO-CH3 | IA |
| NC | Betamethasone | y | H | F | HO-b | CH3-b | OH | CO-CH2-OH | IA |
D1,2 (double bond between C1 and C2) y = yes, n = no
Stereochemistry: a = alpha face, b = beta face of steroid
The most dominant trends noted for Smo agonist activity to date are the nature of the substituents at C-17, a hydroxyl group in the beta configuration at C-11, and a fluorine atom on the alpha face at C-9. Of these trends, a review of the inactive compounds suggests the most dominate feature for Smo agonist activity may be the nature of the substitution at the C17 position in conjunction with a hydroxyl group in the beta position at C-11. Though each of the active Smo agonists contains a hydroxyl group in the beta configuration at C-11, there are a number of glucocorticoid derivatives with an 11-beta hydroxyl group that are inactive Smo agonists. These inactive Smo agonists often possess a C-17 substituent that is more hydrophilic, less branched and generally smaller. Some of these compounds also have a fluorine atom in the C-9 alpha position. Based on these observations, a working model was created to guide SAR exploration and to aid our understanding of the structural features of glucocorticoid derivatives that drive potency and activation of the hedgehog pathway (Figure 3).
Figure 3.
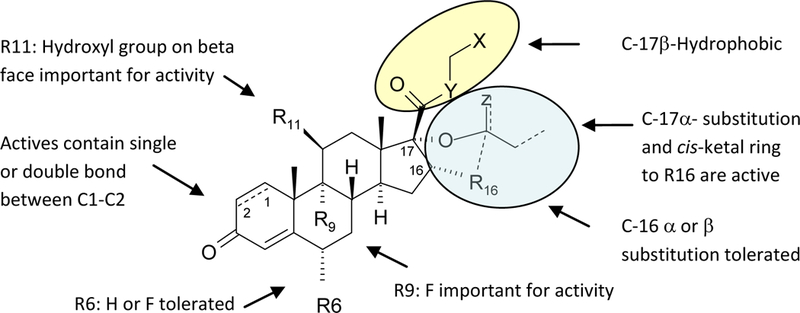
Working model of glucocorticoid Smo agonist activity.
Conclusion
Although glucocorticoids for the most part are known to inhibit neurogenesis as nuclear receptor agonists, we identified a subgroup of them that bind Smoothened and are capable of stimulating neuron proliferation. This unusual finding resulted from a high throughput drug screening approach, was confirmed by secondary cell based assays, and formed the basis for a program of SAR analysis to characterize the chemical determinants underlying the binding. This subgroup of steroids present us with an unprecedented opportunity to jump start clinical trials assessing the therapeutic benefits of potentiating Hedgehog signaling, because these same glucocorticoid drugs have been used clinically by tens of millions and their safety profiles are well know. Areas in which they may provide immediate benefit and could be tested include Parkinson’s disease, spinal cord injury, myocardial repair post infarction, and wound healing.
References
- Bambakidis NC, Horn EM, Nakaji P, Theodore N, Bless E, Dellovade T, Ma C, Wang X, Preul MC, Coons SW, et al. (2009). Endogenous stem cell proliferation induced by intravenous hedgehog agonist administration after contusion in the adult rat spinal cord. J Neurosurg Spine 10, 171–176. [DOI] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, and Koyama T (2010). Glucocorticoids and lithium in adult hippocampal neurogenesis. Vitam Horm 82, 421–431. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, and Beachy PA (2002). Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A 99, 14071–14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran RB, and Scott MP (2001). A mouse model for medulloblastoma and basal cell nevus syndrome. J Neurooncol 53, 307–318. [DOI] [PubMed] [Google Scholar]
- Damsted SK, Born AP, Paulson OB, and Uldall P (2011). Exogenous glucocorticoids and adverse cerebral effects in children. Eur J Paediatr Neurol [DOI] [PubMed]
- Doucas H, Garcea G, Neal CP, Manson MM, and Berry DP (2005). Changes in the Wnt signalling pathway in gastrointestinal cancers and their prognostic significance. Eur J Cancer 41, 365–379. [DOI] [PubMed] [Google Scholar]
- Duax WL, Griffin JF, Weeks CM, and Wawrzak Z (1988). The mechanism of action of steroid antagonists: insights from crystallographic studies. J Steroid Biochem 31, 481–492. [DOI] [PubMed] [Google Scholar]
- Heine VM, and Rowitch DH (2009). Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. J Clin Invest 119, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, and McMahon AP (2001). Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15, 3059–3087. [DOI] [PubMed] [Google Scholar]
- Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, and Zhang SC (2008). Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells 26, 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, and Bovolenta P (2002). Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci 25, 89–96. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, and Ruiz i Altaba A (2005). Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proteau PJ (2004) Steroid Hormones and Therapeutically Related Compounds, in Organic Medicinal Chemistry and Pharmaceutical Chemistry (Block J. H. a. B. J., John M, Ed.), p 767, Lippincott Williams and Wilkins, Baltimore [Google Scholar]
- Ruiz i Altaba A, Palma V, and Dahmane N (2002). Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci 3, 24–33. [DOI] [PubMed] [Google Scholar]
- Shimmer BP, Parker KL (2006) Adrenocorticotropic hormone; Adrenocortical steroids and their synthetic analogs; Inhibitors of the synthesis and actions of adrenocorticoid hormones, in Goodman and Gilman’s: The Pharmacologic Basis of Therapeutics (Brunton LL, Lazo JS, Parker KL, Ed.) 11th ed., pp 1587–1612, McGraw-Hill, New York. [Google Scholar]
- Tsuboi K, and Shults CW (2002). Intrastriatal injection of sonic hedgehog reduces behavioral impairment in a rat model of Parkinson’s disease. Exp Neurol 173, 95–104. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E (2011). Stress, stress hormones, and adult neurogenesis. Exp Neurol doi: 10.1016/j.expneurol.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zheng H, Xiao HL, She ZJ, and Zhou GM (2007). Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun 363, 347–354. [DOI] [PubMed] [Google Scholar]
- Wang J, Lu J, Bond MC, Chen M, Ren XR, Lyerly HK, Barak LS, and Chen W (2010). Identification of select glucocorticoids as Smoothened agonists: potential utility for regenerative medicine. Proc Natl Acad Sci U S A 107, 9323–9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, and Scott MP (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114. [DOI] [PubMed] [Google Scholar]


