Abstract
Background:
The intestinal metabolome reflects biological consequences of diverse exposures and may provide insight into asthma pathophysiology.
Objective:
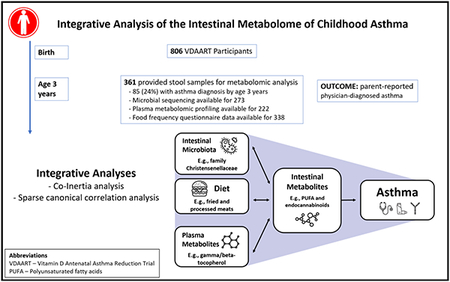
To perform an untargeted integrative analysis of the intestinal metabolome of childhood asthma in this ancillary study of the Vitamin D Antenatal Asthma Reduction Trial (VDAART).
Methods:
Metabolomic profiling was performed by mass spectrometry on fecal samples collected from 361 3-year-old subjects. Adjusted logistic regression analyses identified metabolites and modules of highly correlated metabolites associated with asthma diagnosis by age 3 years. Sparse canonical correlation analysis identified associations relevant to asthma between the intestinal metabolome and other “omics”: intestinal microbiome as measured by 16S rRNA sequencing, plasma metabolome as measured by mass spectrometry, and diet as measured by food frequency questionnaires.
Results:
Several intestinal metabolites were associated with asthma at age 3 years, including inverse associations between asthma and polyunsaturated fatty acids (adjusted logistic regression beta = −6.3, 95% CI −11.3, −1.4, p = 0.01) and other lipids. Asthma-associated intestinal metabolites were significant mediators of the inverse relationship between exclusive breastfeeding for the first 4 months of life and asthma (p for indirect association = 0.04), and the positive association between a diet rich in meats and asthma (p = 0.03). Specific intestinal bacterial taxa, including family Christensenellaceae, and plasma metabolites, including gamma-tocopherol/beta-tocopherol, were positively associated with asthma and with asthma-associated intestinal metabolites.
Conclusion:
Integrative analyses revealed significant interrelationships between the intestinal metabolome and the intestinal microbiome, plasma metabolome, and diet in association with childhood asthma. These findings require replication in future studies.
Keywords: asthma, microbiome, Christensenellaceae, metabolome, vitamin E, nutrition, diet, breastfeeding
Graphical Abstract
Capsule Summary:
Intestinal metabolites, including immune-modulating lipids, are associated with childhood asthma. Specific bacterial taxa, plasma metabolites and dietary factors, including lack of breastfeeding and meat intake, correlate with asthma-associated metabolites.
INTRODUCTION
Asthma is the most common chronic disease in children(1), and each year approximately 400,000 people die from asthma worldwide(2). Asthma is a complex disease, with dietary, genetic, microbial, and environmental factors all contributing to risk via mechanisms that remain incompletely understood(3). These diverse exposures are reflected by the intestinal metabolome(4), which provides a functional readout of their consequences(5). Intestinal metabolites are small molecules that may be derived from the host, microbiota, or exogenous sources including the diet. Metabolomics analysis has yielded novel insights into the molecular mechanisms of several human diseases(6). Integrative analyses of the human intestinal metabolome with the diet, the intestinal microbiome, and the circulating metabolome reveals intricate relationships, providing insights into the basic physiology of clinically relevant metabolites and their contributions to pathogenic changes(7). For example, in the context of cardiovascular disease, the metabolite trimethylamine-N-oxide is a product of intestinal microbial metabolism of dietary phosphatidylcholine that enters systemic circulation and has been linked to development of atherosclerosis(8). There may be similar undiscovered mechanisms that contribute to the pathophysiology of childhood asthma.
We sought to gain insight into asthma pathophysiology by performing an untargeted analysis of the intestinal metabolome of childhood asthma in this ancillary study of the Vitamin D Antenatal Asthma Reduction Trial (VDAART), hypothesizing that asthma is associated with perturbations to the intestinal metabolome. In addition to characterizing the asthma-associated intestinal metabolome at age 3 years, we conducted integrative analyses to investigate relationships between the intestinal metabolome and other asthma-related “omics” including the intestinal microbiome, plasma metabolome and diet.
METHODS
All methods are described in detail in the Supplemental Methods.
Study Design and Clinical Outcome Ascertainment
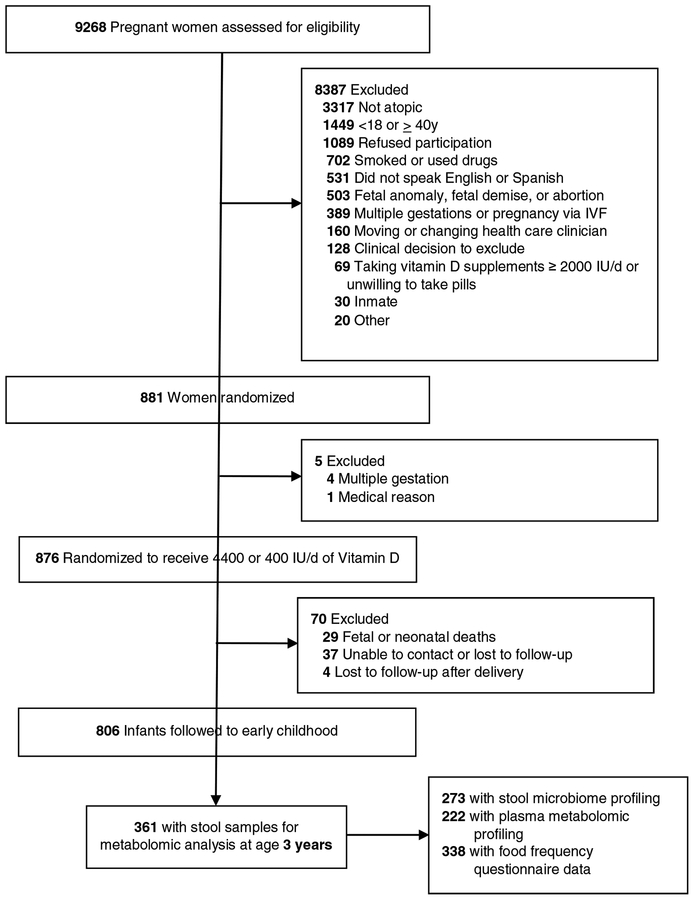
Subjects were offspring of participants in VDAART, a multi-site randomized, double-blind, placebo-controlled trial of Vitamin D supplementation during pregnancy for prevention of asthma and other allergic disease in offspring conducted in the United States (NCT00920621)(9). The study protocol was approved by the institutional review boards at each participating institution and at Brigham and Women’s Hospital. All participants provided written informed consent. The asthma outcome was based on parental report of physician diagnosis of asthma in the child’s first 3 years of life. A flow diagram is displayed in Figure 1.
Figure 1.
Flow diagram of subjects included in study. A portion of this figure has been published previously(56).
Metabolomic Profiling
VDAART participants followed after birth (n = 806) were asked to provide blood and stool samples at age 3 years. Stool samples were provided by 361 (45%) and blood samples by 411 (51%) for metabolomics analysis. Stool was not collected if the child had used antibiotics in the past 7 days. For both stool and plasma samples, metabolomic profiling was performed at Metabolon, Inc. (Research Triangle Park, NC) as previously described(10). Additionally, 245 subjects, 156 of whom had available stool metabolomics, were selected for a prior analysis of plasma metabolomics utilizing an additional quantitative lipids platform at Metabolon, Inc(11). These were included in limited correlation analyses of intestinal and plasma lipids.
Bacterial Microbiome Profiling
DNA extraction and sequencing of the bacterial 16S V4 hyper-variable region using the Illumina MiSeq platform (San Diego, CA) were performed at Partners Healthcare Personalized Medicine (Boston, MA). Additional processing of microbiome data was performed using Qiime(12) and Phyloseq package for R(13). As it was recently shown that quantitative microbiome profiling is preferable to relative abundance profiling in co-occurrence analyses and in seeking disease associations(14), quantitative PCR using universal 16S rRNA primers was performed at Partners Healthcare Personalized Medicine to estimate bacterial biomass concentration per stool sample for use in estimating quantitative species-level taxa abundances.
Dietary Ascertainment
Child diet was evaluated at age 3 years, when parents completed a modified version of a semi-quantitative 87-item food frequency questionnaire (FFQ) that was previously validated in preschool-age children(15). FFQ responses were used to estimate total daily calorie intake as detailed in the Supplemental Methods.
Statistics
Details of statistical analyses are provided in the Supplemental Methods. Statistical analyses were conducted using R version 3.5.0 (R Foundation for Statistical Computing). Modules of highly correlated and likely functionally related intestinal metabolites were identified using the weighted gene correlation network analysis (WGCNA) R package (version 1.61)(16). Eigenvalues summarizing relative abundances of metabolites of each module for each subject were used in subsequent analyses. Multivariable logistic regression was used to determine the associations of metabolites and metabolite module eigenvalues with asthma. Covariates in adjusted analyses were selected on the basis of significant (p < 0.05) bivariate associations between these variables and asthma (Table 1) and included sex, race/ethnicity, study center, maternal education and gestational age, which has reported associations both with early microbiome development(17,18) and asthma(19,20). Total IgE, allergic sensitization and eczema were associated with asthma, but were considered to be characteristic of the outcome rather than confounders and as such were not included as covariates.
Table 1. Baseline characteristics.
Tabulated for subjects who provided stool samples for metabolomics analysis and for the entire VDAART offspring cohort.
| Stool Metabolomics Available | Entire VDAART Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| All children (n = 361) | Asthma (n = 85) | No Asthma (n = 276) | P value | All children (n = 806) | Asthma (n = 125) | No Asthma (n = 623) | P value | |
| Gender - number (%) | 0.24 | 0.02 | ||||||
| Male | 203 (56) | 53 (62) | 150 (54) | 421 (52) | 79 (63) | 318 (51) | ||
| Female | 158 (44) | 32 (38) | 126 (46) | 385 (48) | 46 (37) | 305 (49) | ||
| Race/ethnicity - number (%) | 0.001 | <0.001 | ||||||
| Black, non-Hispanic | 161 (45) | 53 (62) | 108 (39) | 317 (39) | 73 (58) | 229 (37) | ||
| White, non-Hispanic | 71 (20) | 13(15) | 58 (21) | 161 (20) | 17 (14) | 130 (21) | ||
| Hispanic or Other | 129 (36) | 19 (22) | 110 (40) | 328 (41) | 35 (28) | 264 (42) | ||
| Gestational age (weeks) - mean (SD) | 38.7 (2.5) | 37.8 (2.8) | 39.0 (2.0) | <0.001 | 39.0 (2.0) | 38.3 (2.6) | 39.1 (1.8) | <0.001 |
| VDAART treatment group - number (%) | 0.87 | 0.64 | ||||||
| 4,400 lU/day Vitamin D | 182 (50) | 44 (52) | 138 (50) | 405 (50) | 60 (48) | 316 (51) | ||
| 400 lU/day Vitamin D | 179 (50) | 41 (48) | 138 (50) | 401 (50) | 65 (52) | 307 (49) | ||
| Study Center - number (%) | 0.006 | 0.001 | ||||||
| Boston | 82 (23) | 19 (22) | 63 (23) | 240 (30) | 39 (31) | 175 (28) | ||
| St. Louis | 178 (49) | 53 (62) | 125 (45) | 292 (36) | 62 (50) | 227 (36) | ||
| San Diego | 101 (28) | 13(15) | 88 (32) | 274 (34) | 24 (19) | 221 (35) | ||
| Maternal education - number (%) | 0.04 | 0.005 | ||||||
| < High school | 50 (14) | 16(19) | 34(12) | 100 (12) | 23 (18) | 73 (12) | ||
| High school or Technical School | 106 (29) | 27 (32) | 79(29) | 241 (30) | 40 (32) | 181(29) | ||
| Some college | 84 (23) | 24 (28) | 60 (22) | 192 (24) | 35 (28) | 137 (22) | ||
| College graduate or higher | 121 (34) | 18 (21) | 103 (37) | 273 (34) | 27 (22) | 232 (37) | ||
| Household Income - number (%) | 0.02 | 0.004 | ||||||
| < $30,000 | 118 (33) | 35 (41) | 83 (30) | 236 (29) | 47 (38) | 176 (28) | ||
| $30,000–$49,999 | 42 (12) | 10 (12) | 32(12) | 105 (13) | 17 (14) | 78 (13) | ||
| $50,000–$74,999 | 38 (11) | 9 (11) | 29(11) | 97 (12) | 12 (10) | 77 (12) | ||
| $75,000–$99,999 | 29 (8) | 3 (4) | 26 (9) | 79 (10) | 5 (4) | 68 (11) | ||
| $100,000–$149,999 | 38 (11) | 4 (5) | 34(12) | 62 (8) | 6 (5) | 53 (9) | ||
| > $ 150,000 | 16 (4) | 0 (0) | 16 (6) | 31 (4) | 0 (0) | 29 (5) | ||
| Refused to say or unknown | 80 (22) | 24 (28) | 56 (20) | 196 (24) | 38 (30) | 142 (23) | ||
| Total IgE - mean (SD) | 135 (494) | 286 (941) | 87(183) | <0.001 | 123 (434) | 273 (882) | 96 (271) | 0.003 |
| Allergic sensitization - number (%) | 145 (48) | 49 (67) | 96 (42) | <0.001 | 253 (47) | 55(63) | 198 (44) | 0.001 |
| Eczema - number (%) | 100 (28) | 38 (45) | 62 (22) | <0.001 | 172 (23) | 43 (36) | 117 (19) | <0.001 |
| Recent Steroid Use - number (%) | 31 (9) | 25(29) | 6 (2) | <0.001 | 48 (6) | 39 (31) | 9 (1) | <0.001 |
p values are for t tests for gestational age and total IgE; and for Chi square tests for all other comparisons. Total IgE was log-transformed before comparison by t test. Recent steroid use pertains to use of inhaled or systemic steroids in the three months prior to the age 3 years study visit, during which stool samples were collected.
Missing data: Asthma status was missing for 58 subjects, none of whom provided stool samples for metabolomics. Gestational age was unavailable for 2 children. Total and serum specific IgE was missing for 272 and 265 subjects, respectively; including 62 and 59 subjects with stool metabolomics. Eczema at age 3 years was unavailable for 55 subjects, none of whom provided stool samples for metabolomics.
There are currently no consensus standards for multiple testing correction in metabolomics; methods such as the Bonferroni correction and even more liberal corrections are considered too stringent for metabolomics data due to the high correlation of functionally related metabolites. We employed a false discovery correction using a “number of effective tests” method that accounts for the highly collinear nature of metabolites(21, 22): we used principal components analysis of the metabolites to determine how many components are required to explain 50% of the variance in the data, with the rationale that correlated metabolites will load similarly to the same components. We consider the number of components, in this case 17, as the number of effective tests, and use this number to compute a multiple testing corrected p value (0.05 divided by 17 = 0.0029).
Multivariable linear regression analyses were performed to determine associations between potential predictors of the intestinal microenvironment (e.g., breastfeeding, antibiotics exposure, etc.) and asthma-associated metabolites and metabolite modules. Pearson correlation was used to analyze associations between plasma metabolites and corresponding intestinal metabolite module eigenvalues. Mediation analyses were performed for two purposes: (1) to estimate the direct association between breastfeeding and asthma, and the indirect association mediated through intestinal metabolites; and (2) to estimate the direct association between dietary variables and asthma, and the indirect association mediated through intestinal metabolites. All tests were 2-sided and the significance level was pre-specified at p < .05 except where otherwise specified.
Integrative Analyses
Coinertia analysis was used to compare global associations of the plasma metabolome, intestinal metabolome and diet with the intestinal metabolome. Sparse canonical correlation analysis was used to identify intestinal metabolites that are associated with features of other data types (plasma metabolites, intestinal microbes, and dietary items) and with asthma. These methods are described in detail in the Supplemental Methods.
RESULTS
Subject Characteristics
To assess the intestinal metabolome of childhood asthma, we analyzed stool samples collected from offspring of VDAART participants. Stool samples were provided by 361 3 year-old subjects, including 85 (24%) diagnosed with asthma by age 3 years (Table 1). Subjects with asthma differed from those without asthma on several baseline characteristics among the entire VDAART cohort and similar differences were seen among the subset who provided stool samples. Of subjects who provided stool samples, those with asthma vs. no asthma were significantly more likely to be male (62% vs 54%), black (62% vs 39%), born at earlier gestational ages (mean (SD) 37.8 (2.8) vs 39.0 (2.0) weeks), and born to mothers with lower education (21% vs 37% with college degree or higher) and lower-income households (41% vs 30% income below $30,000). Asthma status was associated with study site (Boston, St. Louis or San Diego) and this was likely due to differences in race/ethnicity and socioeconomic status between sites. Asthma was also associated with atopy, including elevated serum specific IgE (≥ 0.35 kU/l) to at least one common food or environmental allergen (67% vs 42%), diagnosis of eczema (45% vs 22%), and recent steroid use (29% vs 2%).
Early-Life Intestinal Metabolites are Associated with Childhood Asthma
A total of 737 intestinal metabolites were measured via mass spectrometry and analyzed from stool samples collected at age 3 years. Logistic regression analyses adjusted for sex, race/ethnicity, study center, maternal education and gestational age identified 45 metabolites that were significantly (p < 0.05) associated with asthma by age 3 years (Table E1). Of note, all but 3 of these 45 metabolites were inversely associated with asthma. Five of the 45 metabolites remained significant after correcting for multiple testing by a number of effective tests method, including a polyunsaturated fatty acid, omega-6 docosapentaenoic acid, and two diacylglycerols (Table E1). As steroid medication use could impact the intestinal metabolome and is expected be higher in subjects with asthma, we added a covariate to the model to account for use of either inhaled or systemic steroids in the three months prior to turning 3 years old (Table E1). Per parental report, 44 (12% of subjects) had used inhaled steroids, 15 (4%) had used systemic steroids; and a total of 48 (13% of subjects) had used either inhaled or systemic steroids. Associations between metabolites and asthma were not significantly changed after adjusting for steroid use (Table E1).
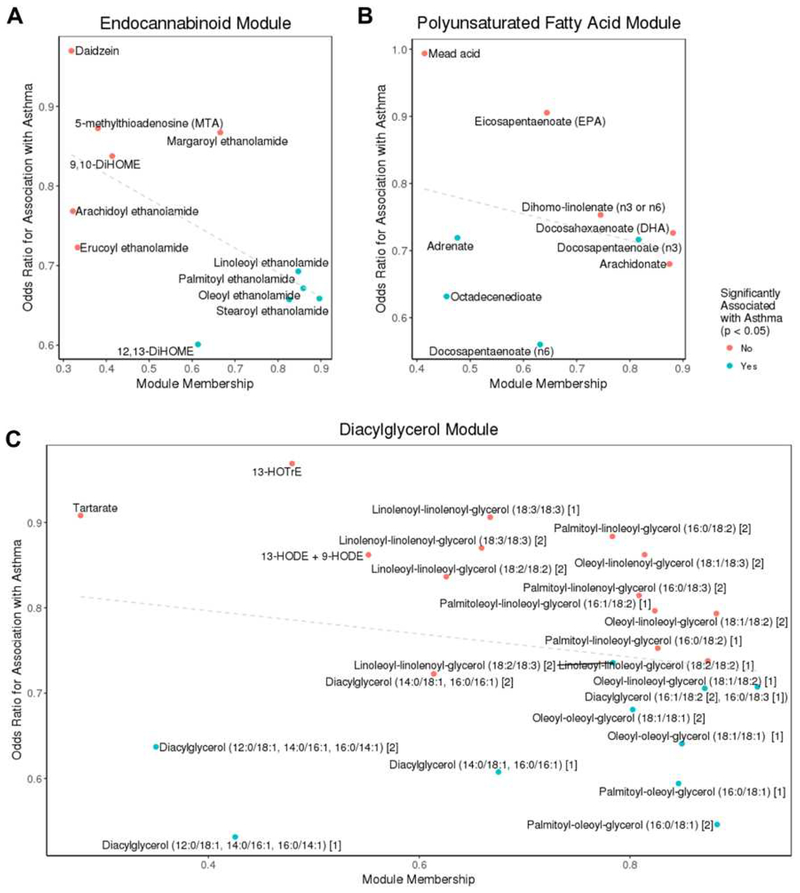
To query the functional significance of these metabolites, we constructed a network of highly correlated – and likely functionally related – intestinal metabolites. Network construction was performed on all metabolites with no filtering on association with asthma. The analyses identified 54 modules of highly correlated intestinal metabolites (Table E2). Three modules of predominantly lipid metabolites ranging in size from 9 to 24 metabolites were inversely associated with asthma based on their eigenvectors, including a module of primarily diacylglycerols (beta = −6.5, 95% CI = −11.8, −1.4, p = 0.01), a module of primarily endocannabinoids (beta = −6.5, 95% CI −11.6, −1.6, p = 0.01) and a module of primarily polyunsaturated fatty acids (PUFAs) (beta = −6.3, 95% CI −11.3, −1.4, p = 0.01) (Figure 2, Table E3).
Figure 2.
Members of the endocannabinoid (A), polyunsaturated fatty acid (B) and diacylglycerol (C) metabolite modules, which were inversely associated with asthma at age 3 years, are displayed by their association with asthma (y axis, adjusted odds ratio) and by module membership (correlation of individual metabolite with module eigenvalue, x axis).
From these analyses, we concluded that several intestinal metabolites were associated with childhood asthma, with the majority of associated metabolites decreased in asthma. Modules of highly correlated asthma-associated lipid metabolites included PUFAs, endocannabinoids and diacylglycerols
Breastfeeding Is a Potential Determinant of Asthma-Associated Intestinal Metabolites
To identify determinants of the asthma-associated intestinal metabolome, we examined potential predictors of the early-life intestinal microenvironment (Table 2). In bivariate analyses, those with asthma vs. no asthma were more likely to have been exposed to perinatal antibiotics (52% vs 40%), less likely to have been exclusively breastfed for the first 4 months of life (14% vs 37%) and more likely to have attended daycare by age 3 years (62% vs 50%). We identified no association between asthma and pet dog ownership, mode of delivery or having at least one older sibling. In a logistic regression analysis adjusted for all potential predictors of the early-life intestinal microenvironment and for sex, race/ethnicity, maternal education, study site and gestational age, the only independent predictor of asthma was exclusive breastfeeding for the first 4 months of life, which was inversely associated with asthma (odds ratio 0.36, 95% CI 0.18–0.67, p = 0.002).
Table 2. Bivariate analyses of the associations of asthma with potential determinants of the early-life intestinal metabolome.
Analyses were performed for subjects who provided stool samples for metabolomics analysis and for the entire VDAART offspring cohort.
| Stool Metabolomics Available | Entire VDAART Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| All children (n = 361) | Asthma (n = 85) | No Asthma (n = 276) | P value | All children (n = 806) | Asthma (n = 125) | No Asthma (n = 623) | P value | |
| Maternal antibiotics during or child antibiotics after delivery - number (%) | 157 (43) | 45 (53) | 112 (41) | 0.06 | 337 (42) | 65 (52) | 248 (40) | 0.01 |
| Birth by Cesarean section - number (%) | 113 (31) | 24 (28) | 89 (32) | 0.57 | 239 (30) | 40 (32) | 185 (30) | 0.65 |
| Exclusive breastfeeding until age 4 months - number (%) | 109 (31) | 12 (15) | 97 (36) | 0.001 | 247 (33) | 16 (14) | 218 (37) | <0.001 |
| Day care by age 3 years - number (%) | 178 (50) | 47 (57) | 131 (49) | 0.24 | 378 (53) | 73(62) | 290 (50) | 0.02 |
| Pet dog during infancy - number (%) | 88 (25) | 17 (20) | 71 (26) | 0.38 | 168 (22) | 21 (18) | 142 (24) | 0.20 |
| At least one living child previously born to mother - number (%) | 205 (57) | 50 (59) | 155 (56) | 0.76 | 435 (54) | 67 (54) | 344 (55) | 0.82 |
p values are for Chi square tests.
Unavailable/Missing Data: Perinatal antibiotics and mode of delivery were unavailable for 3 subjects. Exclusive breastfeeding until age 4 months was unavailable for 68 subjects, including 13 with available stool metabolomics. Daycare status was unavailable for 99 subjects, including 8 with available stool metabolomics. Household dog status was unavailable for 49 subjects, including 4 with available stool metabolomics.
To investigate the possibility that intestinal metabolites mediate the association between breastfeeding and asthma, we looked for associations between asthma-associated metabolites and breastfeeding in linear regression analyses adjusted for potential confounders. Twelve intestinal metabolites were associated with asthma and with breastfeeding in a directionally consistent manner (Table E4). Mediation analyses estimated that individual metabolites accounted for 9.0% to 23.2% of the association of breastfeeding with asthma, and 8 of the 12 metabolites were significant (p for indirect effect < 0.05) mediators of the association of breastfeeding with asthma (Table E4). Seven of the 12 were diacylglycerols, and accordingly, the diacylglycerol metabolite module from network analysis was inversely associated with asthma and positively associated with breastfeeding (beta 0.02, 95% CI 0.01–0.03, p = 0.004) in adjusted analyses. Mediation analysis estimated that 13% of the relationship between breastfeeding and asthma was mediated by the diacylglycerol intestinal metabolite module (p for indirect association = 0.04). In sum, this analysis identified lack of exclusive breastfeeding for the first 4 months of life as a risk factor for asthma that may act in part through intestinal metabolites including diacylglycerols.
The Intestinal Metabolome is Globally Associated with the Intestinal Microbiome, Plasma Metabolome, and Diet
We next sought to determine the extent to which the intestinal metabolome associates with other relevant “omics” including the intestinal microbiome, plasma metabolome and diet. The intestinal bacterial microbiome was profiled using quantitative 16S rRNA DNA sequencing on the same stool samples that underwent metabolomic profiling, plasma metabolomic profiling was performed via mass spectrometry, and diet was assessed via food frequency questionnaire responses. The number of subjects with each data type is shown in Figure 1.
Coinertia analysis revealed significant global similarity between the intestinal metabolome and other “omics” (p < 0.02 for all pairwise “omics” comparisons). This method generates an RV score, which ranges from 0 to 1 with higher scores indicating greater global similarity between a pair of data sets. Diet and the plasma metabolome had similar associations with the intestinal metabolome (RV scores 0.22 and 0.21, respectively), and the intestinal microbiome had the greatest similarity with the intestinal metabolome (RV score 0.35). These results suggest that the intestinal metabolome engages in the most significant interrelationships with the intestinal microbiome, compared to the diet or plasma metabolome.
Intestinal Bacterial Taxa Correlate with Intestinal Metabolites and with Childhood Asthma
We next sought to identify bacterial taxa that correlate with intestinal metabolites and with asthma using sparse canonical correlation analysis, a method that identifies features of two data types (i.e., intestinal metabolome and microbiome) available for the same set of subjects that are correlated with each other and with a clinical outcome (i.e., asthma). This method generates a canonical variate by assigning non-zero canonical loadings to a limited number of intestinal metabolites and taxa, with the direction and magnitude of each feature’s loading indicating the direction and strength of association with asthma and with other features with non-zero loadings. In this analysis, rather than seeking bacteria that are most strongly associated with asthma, we aim to identify highly or uniquely metabolically active bacteria that, by way of metabolic activity, associate with asthma.
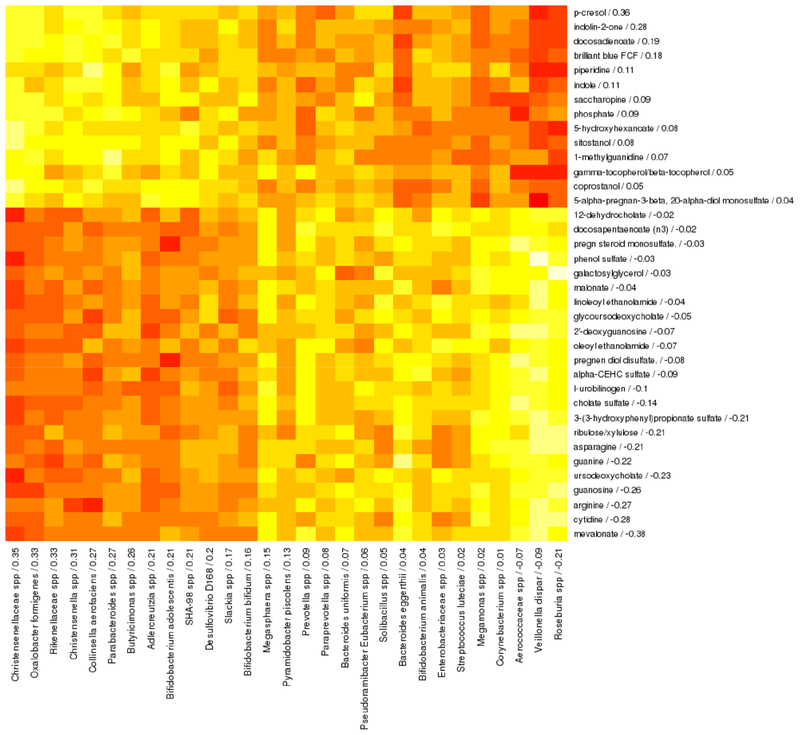
Among stool samples collected at age 3 years, a canonical variate was based on 37 intestinal metabolites and 29 bacterial taxa that were associated with each other and with asthma. Canonical loadings for these metabolites and taxa were used to calculate intestinal metabolite and bacterial taxa canonical scores for each subject. Intestinal metabolite and bacterial taxa scores were positively associated with asthma after adjustment for sex, race/ethnicity, study center, maternal education and gestational age (adjusted logistic regression beta = 0.20 and 0.0002; p = 0.005 and 0.047, respectively), and were correlated with each other (Pearson rho = 0.65, p < 0.001). Increased abundance of family Christensenellaceae spp was most strongly associated with asthma and asthma-associated intestinal metabolites (Figure 3).
Figure 3. Spearman correlation heatmap of intestinal metabolites (rows) and intestinal bacterial taxa (columns) that are correlated with each other and with asthma at age 3 years in sparse canonical correlation analysis.
Canonical loadings are given for each feature after the feature name, with higher loadings indicating greater contribution to the canonical variate and sign indicating direction of association. Red indicates negative correlations and yellow indicates positive correlations. This analysis included 273 subjects with both plasma and stool metabolomics data available.
When modules of highly correlated metabolites were analyzed instead of individual metabolites, the canonical variate included the same 29 bacterial taxa with similar loadings (Table E5) in association with 8 modules of highly correlated metabolites. These 8 modules included the PUFA and endocannabinoid modules that were previously identified as associated with asthma, and which loaded negatively on the canonical variate, consistent with their inverse association with asthma.
These analyses show that there is a strong association between the intestinal metabolome and microbiome that influences asthma risk at age 3 years. We found evidence that PUFAs, endocannabinoids and Christensenellaceae spp participate in relevant microbiomemetabolome interactions.
The Intestinal and Plasma Metabolomes Associate with Childhood Asthma
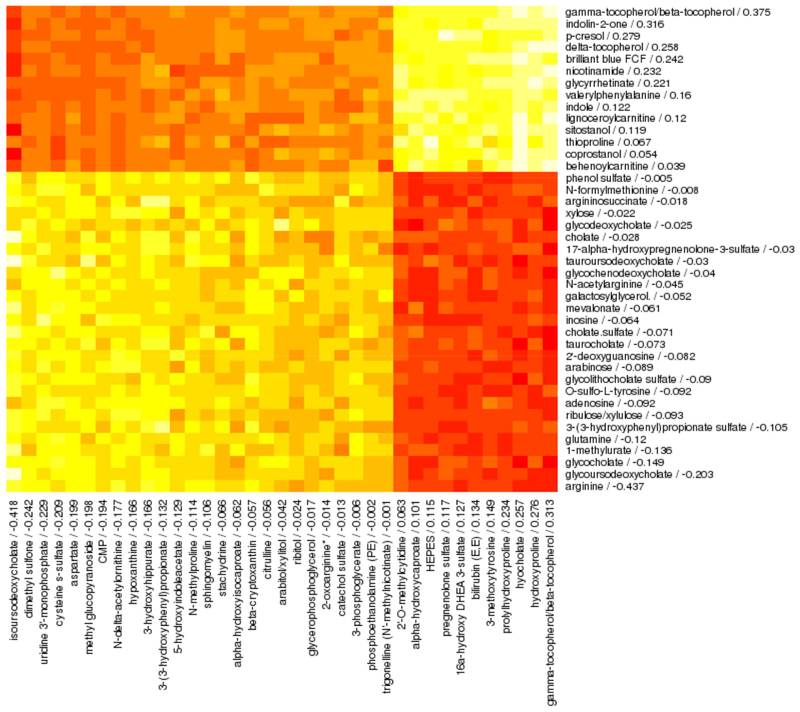
We also applied sparse canonical correlation analysis to identify 41 intestinal and 37 plasma metabolites from age 3 years that correlated with each other (Pearson rho = 0.61, p < 0.001) and with asthma (adjusted logistic regression beta = 0.25 and 1.65; p = 0.02 and 0.003, respectively) (Figure 4). Interestingly, gamma-tocopherol/beta-tocopherol was the highest-loading feature among both intestinal (loading = 0.31) and plasma (loading = 0.38) metabolites. However, this form of vitamin E, analyzed as an individual metabolite, was not significantly associated with asthma in adjusted logistic regression analyses either in stool (OR 1.43, 95% CI 0.83–2.56, p = 0.21) or plasma (OR 1.05, 95% CI 0.11–9.39, p = 0.97).
Figure 4. Spearman correlation heatmap of intestinal metabolites (rows) and plasma metabolites (columns) that are correlated with each other and with asthma at age 3 years in sparse canonical correlation analysis.
Canonical loadings are given for each feature after the feature name, with higher loadings indicating greater contribution to the canonical variate and sign indicating direction of association. Red indicates negative correlations and yellow indicates positive correlations. This analysis included 222 subjects with both plasma and stool metabolomics data available.
We recently found that plasma PUFAs were inversely associated with asthma at age 3 years in VDAART participants (23). Given the present findings of inverse associations between intestinal PUFA and other lipid metabolite modules and asthma, we hypothesized that plasma and intestinal lipids may be correlated. However, we found no correlation between plasma PUFAs (total, omega-3 or omega-6), endocannabinoids or diacylglycerols and their corresponding intestinal metabolite module eigenvalues (Table E6). These findings suggest that intestinal and plasma lipids may relate to asthma risk through different mechanisms.
From these analyses, we concluded that interrelationships between the intestinal and plasma metabolomes associate with asthma at age 3 years. Vitamin E in the form of gamma-tocopherol/beta-tocopherol may, in concert with co-exposures, have a significant influence on the asthma-associated metabolome.
Meat Intake Correlates with Intestinal Metabolites and with Childhood Asthma
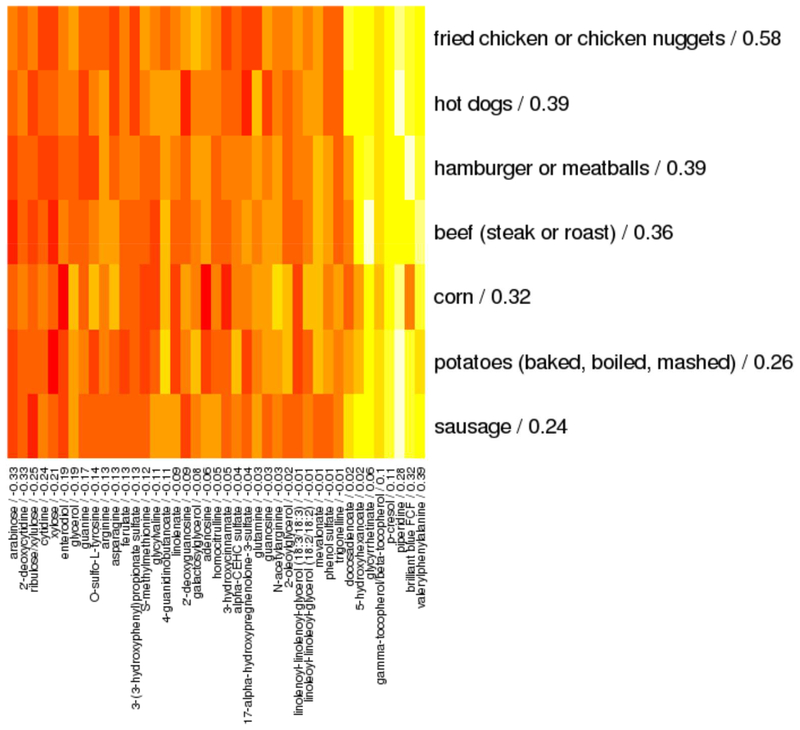
Finally, sparse canonical correlation analysis identified 7 foods in the diet and 41 intestinal metabolites that correlated with each other (Pearson rho = 0.40, p < 0.001) and with asthma (adjusted logistic regression beta = 0.19 and 0.50; p = 0.01 and 0.02, respectively) (Figure 5). Asthma associations were preserved after adding BMI and estimated total daily calorie intake to regression models (beta = 1.06, p = 0.003 for foods and beta = 0.22, p = 0.002 for metabolites). Foods with the highest positive loadings, indicating the most robust positive associations with asthma and intestinal metabolites, included fried and processed meats such as fried chicken, chicken nuggets, hot dogs and hamburgers (Figure 5). When modules of highly correlated metabolites were analyzed instead of individual metabolites, the canonical variate included the same 7 foods with similar loadings (Table E7) in association with 8 modules of highly correlated metabolites. These 8 modules included the diacylglycerol module that was previously identified as associated with asthma and which loaded negatively on the canonical variate, consistent with its inverse association with asthma. Mediation analysis estimated that 17% of the relationship between dietary score on these 7 foods and asthma was mediated by intestinal metabolite score (p value for indirect association = 0.04). These analyses highlighted an association between a diet rich in meats, especially fried or processed meats, and asthma, with evidence that this association is mediated in part by intestinal metabolites.
Figure 5. Spearman correlation heatmap of foods (rows) and intestinal metabolites (columns) that are correlated with each other and with asthma at age 3 years in sparse canonical correlation analysis.
Canonical loadings are given for each feature after the feature name, with higher loadings indicating greater contribution to the canonical variate and sign indicating direction of association. Red indicates negative correlations and yellow indicates positive correlations. This analysis included 338 subjects with food frequency questionnaire and stool metabolomics data available.
Few Intestinal Metabolites Correlate with the Diet, Intestinal Microbiome, Plasma Metabolome and Asthma
Two metabolites were inversely associated with asthma and had negative loadings in all three sparse canonical correlation analyses (of associations between the intestinal metabolome and the diet, plasma metabolome, and intestinal microbiome): phenol sulfate and galactosylglycerol. These may be central metabolites in asthma pathophysiology; however, given that only 2 such metabolites were found, in general, the intestinal metabolome is likely perturbed in different ways by asthma-associated plasma, dietary and microbiome changes.
Of note, intestinal gamma-tocopherol/beta-tocopherol had positive loadings in all three sparse canonical correlation analyses, and plasma gamma-tocopherol/beta-tocopherol also had a positive loading; however, this form of vitamin E was not associated with asthma when analyzed as an individual metabolite. These findings suggest that this metabolite may act in concert with co-exposures to influence systems-wide effects on asthma-associated “omics.”
DISCUSSION
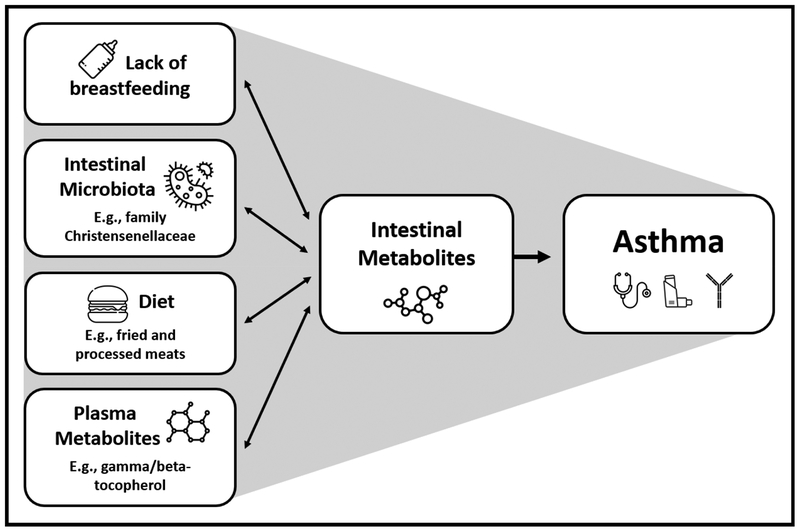
In this analysis of a relatively large and diverse sample of 3 year-old children, we characterized the intestinal metabolome of asthma. To our knowledge, this is the first report to integrate dietary, microbiome and plasma metabolomics into an evaluation of the intestinal metabolome during childhood. As the intestinal metabolome can be viewed as the functional readout of a variety of exposures, including the diet, the microbiome, and host physiology, we conducted integrative analyses to show that asthma-relevant interactions occur between the intestinal metabolome and the intestinal microbiome, plasma metabolome and diet (Figure 6). Our findings, though biologically plausible, require replication in future studies.
Figure 6. Schematic of results of integrative analyses of the asthma-associated intestinal metabolome.
Note that a proposed causal pathway is depicted here and is one of several potential causal pathways that could explain the observed associations.
Most intestinal metabolites that were associated with asthma were reduced in subjects with asthma, consistent with prior literature showing early-life depletions of specific microbes, microbe functions and metabolites in children with asthma and/or atopy (24, 25). Network analysis revealed inverse associations between asthma and three groups of intestinal lipids: PUFAs, diacylglycerols and endocannabinoids. The role of PUFAs as precursors to immune-modulating eicosanoids and pro-resolving mediators lends biological plausibility to an association with asthma(26). Indeed, dietary and circulating PUFAs have been associated with asthma, including in the VDAART cohort(23). However, we found no association between plasma PUFA and intestinal PUFA in the present analysis. While members of the human intestinal microbiota are not known to produce PUFAs, some participate in metabolism of PUFAs(27, 28). So, intestinal PUFA likely represents dietary PUFA that is both in excess of the amount absorbed in the small intestine, and that is not metabolized by the microbiota; that is, intestinal PUFA abundance is determined both by dietary intake and microbiota composition. Accordingly, we found that intestinal PUFA correlated with the asthma-associated intestinal microbiome. These findings suggest that intestinal and plasma PUFAs relate to asthma risk through different mechanisms, with the former more reflective of bacterial dysbiosis.
Diacylglycerols are crucial intracellular signaling second messengers involved in functions including immune cell signaling and cell growth(29). Intestinal extracellular diacylglycerols influence colonic epithelial cell growth in the setting of malignancy(30) and may be taken up from the intestinal lumen by host cells in other contexts. We report a novel association between intestinal diacylglycerols and childhood asthma, and provide evidence that intestinal diacylglycerols mediate associations between dietary factors and asthma. Specifically, fecal diacylglycerols were mediators of the inverse association between breastfeeding and asthma. Breastfeeding has long-term influences on the intestinal microenvironment lasting at least to school age (31). Though there have been several reports of inverse associations between breastfeeding and asthma, this association has been inconsistent(32–35). Some of this inconsistency could be explained by differences in other determinants of the intestinal microenvironment, such as the microbiota and subsequent diet, both of which influence fecal diacylglycerol abundances(36). Consistent with this, we found that intestinal diacylglycerols were associated with an asthma-associated diet rich in fried and processed meats, providing evidence that both breastfeeding and subsequent diet may influence asthma risk by way of their effects on the intestinal metabolome. Diacylglycerols are used as emulsifiers in a wide range of foods, though we cannot determine if the fecal diacylglycerols in our sample are of dietary, microbial or host origin.
Endocannabinoids have both dietary and endogenous sources and are involved with broad systemic processes including regulation of immune and metabolic functions(37). In addition to an inverse association between intestinal endocannabinoids and asthma, we found that intestinal endocannabinoids correlated with the asthma-associated microbiome. The mechanism of these novel potential associations is unclear, and one could speculate involvement of effects on mast cell activation(38), PPAR-alpha activity(39), or eicosanoid mediators(40).
We found that the intestinal microbiome had a strong global association with the intestinal metabolome, in keeping with other reports(5). In particular, bacteria of the family Christensenellaceae were associated with asthma and asthma-associated intestinal metabolites. Christensenellaceae has been most strongly associated with lean (vs obese) weight(41, 42). To our knowledge, Christensenellaceae spp. have not been previously associated with asthma, and this may be in part because prior analyses of the intestinal microbiome of asthma have focused on the microbiome in infancy(24, 25, 43–44) or adulthood(45), in contrast to our study of early childhood. Interestingly, an analysis of TwinsUK data found that the family Christensenellaceae was the most heritable taxon analyzed(41), raising the possibility that it could be a marker or consequence of genetic variants that associate with asthma.
We identified a potential multi-systems role for intestinal gamma-tocopherol/beta-tocopherol, which had positive loadings in all sparse canonical correlation analyses; however, effects are likely indirect, as this form of vitamin E was not associated with asthma when analyzed as an individual metabolite. Vitamin E has anti-oxidant properties and has been inconsistently linked to reduced risk of asthma(46). In contrast and consistent with our findings, the gamma-tocopherol form – which has increased in Western diets over recent decades(47) – has been associated with increased asthma risk or severity(47–49), though some analyses find anti-inflammatory effects(50). The systems effects of dietary vitamin E intake on the microbiome and metabolome are worthy of further investigation and may have relevance to asthma.
Finally, we found that a diet rich in fried and processed meats was associated with asthma and with asthma-associated intestinal metabolites. Several prior studies have found positive associations between processed or fried meat consumption and asthma(51–53), and one recent systematic review found that hamburger consumption had a particularly strong association with asthma(54). Our findings support a causal pathway whereby intestinal metabolites mediate at least part of the association between fried and processed meat consumption and asthma.
Some study limitations occurred. The outcome of asthma was based on parent-reported asthma diagnosis by age 3 years. Though preschool asthma is clinically important, this outcome may not accurately capture subjects whose asthma persists later in life(55). We expect this potential misclassification to result in a bias toward the null and reduced power to detect relevant associations. Generalizability may be limited by the VDAART study inclusion criteria, which included parental history of asthma, eczema or allergic rhinitis; and maternal non-smoking status. Intestinal metabolomics were not available for all VDAART subjects, though baseline characteristics were similar between subjects with available intestinal metabolomics and the overall cohort (Table 1). Although subjects who had used antibiotics in the week prior to stool sample collection were excluded and we have performed sensitivity analyses to account for steroid use, we cannot rule out residual confounding by medication use. While we adjusted for maternal education, we acknowledge the possibility of residual confounding by other socioeconomic factors. Current metabolomics analysis techniques do not reflect the entire intestinal chemical repertoire. Some metabolites, including most short chain fatty acids, are too volatile or low-concentration to be measured by the utilized metabolomic profiling method. Dietary analysis by food frequency questionnaire, though widely used, is subject to measurement bias. Causality cannot be inferred from these cross-sectional associations in 3 year-old subjects, and identified associations may not be relevant earlier or later in life. As VDAART participants continue to age, it will be important to follow up our findings with longitudinal analyses.
In summary, we found that intestinal metabolites are associated with asthma at age 3 years. Lack of exclusive breastfeeding for the first 4 months of life was associated with increased risk of asthma and may act via perturbation of the intestinal metabolome. Integrative analyses revealed that the intestinal metabolome is strongly associated with the intestinal microbiome, and with regard to asthma, species of the family Christensenellaceae may play a role in this relationship. A diet rich in fried or processed meats correlates with asthma in an association that may be mediated in part by intestinal metabolites. And finally, vitamin E in the form of gamma-tocopherol/beta-tocopherol may have indirect but influential systems-wide effects on asthma risk. These findings are situated within a prior literature supporting their biological relevance, and are worthy of further exploration to generate novel asthma biomarkers and treatment targets.
Supplementary Material
Key Messages:
Intestinal metabolites are associated with childhood asthma.
The intestinal metabolome and microbiome correlate strongly.
Associations of lack of breastfeeding and meat-rich diet with asthma may be mediated by the intestinal microbiome via intestinal metabolites.
Funding:
VDAART was funded by U01HL091528 from the National Heart, Lung, and Blood Institute. Additional funding came from NIH grants R01HL108818, R01HL123915, R01HL141826-01, 5T32AI007306-30 and ECHO grant OD023268.
Abbreviations:
- CI
confidence interval
- DNA
deoxynucleic acid
- FFQ
food frequency questionnaire
- IgE
Immunoglobulin E
- IVF
in vitro fertilization
- PCR
polymerase chain reaction
- rRNA
ribosomal ribonucleic acid
- SD
Standard deviation
- VDAART
Vitamin D Antenatal Asthma Reduction Trial
- WGCNA
Weighted gene correlation network analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: ClinicalTrials.gov NCT00920621
Conflict of Interest Statement: AAL has received author royalties from UpToDate, Inc. and consultant fees from AstraZeneca, LP. LBB participates on the Data Safety Monitoring Board of DBV Technologies. AB holds stock from DBV Technologies. RSZ is a consultant for AstraZeneca, DBV Technologies, Genentech, Inc., GlaxoSmithKline, Novartis, Patara Pharma, Regeneron, TEVA Pharmaceuticals, and Theravance Biopharma, and has received research support from Aerocrine, AstraZeneca, Genentech, Inc., GlaxoSmithKline, NHLBI, MedImmune, and Merck. JL-S is a consultant to Metabolon Inc. GTO is a co-investigator on a grant from Janssen Pharmaceuticals to Boston University that funds a study of the pathogenesis of chronic obstructive pulmonary disease. KL-S, RSK, MS, NL, DRG and STW have nothing to disclose.
REFERENCES
- 1.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis 2014;18:1269–1278. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: Is prevention possible? Lancet 2015;386:1075–1085. [DOI] [PubMed] [Google Scholar]
- 4.Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, et al. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J 2013;7:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zierer J, Jackson MA, Kastenmüller G, Mangino M, Long T, Telenti A, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet 2018;50(6):790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 2016;17:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome 2013;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): Rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin trials 2014;38:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, et al. Metabolomics: Open access high resolution mass spectrometry improves data auantity and auality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014;4:1–7. [Google Scholar]
- 11.Blighe K, Chawes BL, Kelly RS, Mirzakhani H, McGeachie M, Litonjua AA, et al. Vitamin D prenatal programming of childhood metabolomics profiles at age 3 y. Am J Clin Nutr 2017;106:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMurdie PJ, Holmes S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandeputte D, Kathagen G, D’Hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017;551:507–511. [DOI] [PubMed] [Google Scholar]
- 15.Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, et al. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J 1999;3:167–72. [DOI] [PubMed] [Google Scholar]
- 16.Langfelder P, Horvath S, Fisher R, Zhou X, Kao M, Wong W, et al. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsgren M, Isolauri E, Salminen S, Rautava S. Late preterm birth has direct and indirect effects on infant gut microbiota development during the first six months of life. Acta Paediatr. 2017;106:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chernikova DA, Madan JC, Housman ML, Zain-Ul-Abideen M, Lundgren SN, Morrison HG, et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr Res. 2018;84:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Källén B, Finnström O, Nygren KG, Otterblad Olausson P. Association between preterm birth and intrauterine growth retardation and child asthma. Eur Respir J. 2013;41:671–6. [DOI] [PubMed] [Google Scholar]
- 20.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823. [DOI] [PubMed] [Google Scholar]
- 21.Li MX, Yeung JM, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet 2012;131:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 2004;74:765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee-Sarwar KA, Kelly RS, Lasky-Su J, Kachroo P, Zeiger RS, O’Connor GT, et al. Dietary and plasma polyunsaturated fatty acids are inversely associated with asthma and atopy in early childhood. J Allergy Clin Immunol In Pract. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016;22:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrieta M-C, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015;7:307ra152–307ra152. [DOI] [PubMed] [Google Scholar]
- 26.Miles E, Calder P. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017; 9(7).E784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moi IM, Leow ATC, Ali MSM, Rahman RNZRA, Salleh AB, Sabri S. Polyunsaturated fatty acids in marine bacteria and strategies to enhance their production. Appl Microbiol Biotechnol 2018;102:5811–26. [DOI] [PubMed] [Google Scholar]
- 28.Kishino S, Takeuchi M, Park S-B, Hirata A, Kitamura N, Kunisawa J, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci 2013;110:17808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichmann TO, Lass A. DAG tales: The multiple faces of diacylglycerol – stereochemistry, metabolism, and signaling. Cell Mol Life Sci 2015;72(20):3931–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman E, Isaksson P, Rafter J, Marian B, Winawer S, Newmark H. Fecal diglycerides as selective endogenous mitogens for premalignant and malignant human colonic epithelial cells. Cancer Res 1989;49:544–548. [PubMed] [Google Scholar]
- 31.Zhong H, Penders J, Shi Z, Ren H, Cai K, Fang C, et al. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school-age children. Microbiome 2019;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol 2014;179:1153–67. [DOI] [PubMed] [Google Scholar]
- 33.Lodge C, Tan D, Lau M, Dai X, Tham R, Lowe A, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr 2015;104:38–53. [DOI] [PubMed] [Google Scholar]
- 34.Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Hoekstra MO, Gerritsen J, et al. Breast feeding, parental allergy and asthma in children followed for 8 years. The PIAMA birth cohort study. Thorax 2009;64:604–609. [DOI] [PubMed] [Google Scholar]
- 35.Oddy WH, Holt PG, Sly PD, Read AW, Lazndau LI, Stanley FJ, et al. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. Br Med J 1999;319:815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vulevic J, McCartney AL, Gee JM, Johnson IT, Gibson GR. Microbial species involved in production of 1,2-sn-diacylglycerol and effects of phosphatidylcholine on human fecal microbiota. Appl Environ Microbiol 2004;70:5659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iannotti FA, Di Marzo V, Petrosino S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog Lipid Res 2016;62:107–28. [DOI] [PubMed] [Google Scholar]
- 38.Roviezzo F, Rossi A, Caiazzo E, Orlando P, Riemma MA, Iacono VM, et al. Palmitoylethanolamide supplementation during sensitization prevents airway allergic symptoms in the mouse. Front Pharmacol 2017;8:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delayre-Orthez C, Becker J, Auwerx J, Frossard N, Pons F. Suppression of allergen-induced airway inflammation and immune response by the peroxisome proliferator-activated receptor-alpha agonist fenofibrate. Eur J Pharmacol 2008;581:177–184. [DOI] [PubMed] [Google Scholar]
- 40.Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoylethanolamide, and their metabolites. J Leukoc Biol 2015;97:1049–1070. [DOI] [PubMed] [Google Scholar]
- 41.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell 2014;159:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep 2018;8:9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 2018;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiemsma LT, Arrieta M-C, Dimitriu PA, Cheng J, Thorson L, Lefebvre DL, et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci 2016;130:2199–2207. [DOI] [PubMed] [Google Scholar]
- 45.Hevia A, Milani C, López P, Donado CD, Cuervo A, González S, et al. Allergic patients with long-term asthma display low levels of Bifidobacterium adolescentis. PLoS One 2016;11(2):e0147809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Zhang C, Wang Y, Li Y. Does vitamin E prevent asthma or wheeze in children: A systematic review and meta-analysis. Paediatr Respir Rev 2018;27:60–8. [DOI] [PubMed] [Google Scholar]
- 47.Cook-Mills JM, Abdala-Valencia H, Hartert T. Two faces of vitamin E in the lung. Am J Respir Crit Care Med 2013;188:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchese ME, Kumar R, Colangelo LA, Avila PC, Jacobs DR, Gross M, et al. The vitamin E isoforms α-tocopherol and γ-tocopherol have opposite associations with spirometric parameters: The CARDIA study. Respir Res 2014;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook-Mills JM, Gebretsadik T, Abdala-Valencia H, Green J, Larkin EK, Dupont WD, et al. Interaction of Vitamin E isoforms on asthma and allergic airway disease. Thorax 2016;71:954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J Allergy Clin Immunol 2018;141:1231–1238.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomes De Luna MDF, Gomes De Luna JR, Fisher GB, De Almeida PC, Chiesa D, Carlos Da Silva MG. Factors associated with asthma in adolescents in the city of Fortaleza, Brazil. J Asthma 2015;52:485–491. [DOI] [PubMed] [Google Scholar]
- 52.Cepeda AM, Thawer S, Boyle RJ, Villalba S, Jaller R, Tapias E, et al. Diet and respiratory health in children from 11 Latin American countries: Evidence from ISAAC Phase III. Lung 2017;195:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melo B, Rezende L, Machado P, Gouveia N, Levy R. Associations of ultra-processed food and drink products with asthma and wheezing among Brazilian adolescents. Pediatr Allergy Immunol 2018;29:504–11. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Wang J, Zhang X, Zhang L, Zhang H, Wang L, et al. Is the consumption of fast foods associated with asthma or other allergic diseases? Respirology 2018;23:901–13. [DOI] [PubMed] [Google Scholar]
- 55.Bacharier LB, Guilbert TW. Diagnosis and management of early asthma in preschool-aged children. J Allergy Clin Immunol 2012;130:287–96. [DOI] [PubMed] [Google Scholar]
- 56.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART randomized clinical trial. JAMA 2016;315:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.