Abstract
The epithelial lining of the human epididymis is critical for sperm maturation. This process requires distinct specialized functions in the head, body and tail of the duct. These region-specific properties are maintained by distinct gene expression profiles which are governed by transcription factor networks, non-coding RNAs and other factors. Using RNA-seq we characterized the transcriptomes of the caput, corpus and cauda segments of adult human epididymis tissue and primary human epididymis epithelial (HEE) cell cultures derived from them. Among the many differentially expressed transcripts were epithelial-selective transcription factors (TFs), microRNAs, and genes involved in antiviral responses. Caput-enriched TFs, included hepatocyte nuclear factor 1 (HNF1) and the androgen receptor (AR), both of which were also predicted to occupy cis-regulatory elements identified as open chromatin in HEE cells. HNF1 targets were identified genome-wide using ChIP-seq, in HEE cells. Next the impact of HNF1 on the HEE cell transcriptional network was determined by siRNA-mediated depletion of the TF. The results showed that HNF1 coordinates epithelial water and solute transport in caput epithelium. The importance of AR in HEE cells was revealed by AR ChIP-seq, and by RNA-seq after synthetic androgen (R1881) treatment. AR has a distinct transcriptional program in the HEE cells and likely recruits different co-factors (RUNX1 and CEBPβ in comparison to those used in prostate epithelium. Our data identify many other transcription factors that also regulate the development and differentiation of HEE cells. Moreover, a comparison of genome-wide open chromatin from immature and adult HEE cells showed key TFs in the transition to fully differentiated function of this epithelium. These data may help identify new targets to treat male infertility and have the potential to open new avenues for male contraception.
Keywords: human epididymis epithelium, caput, corpus, cauda, RNA-seq, differential gene expression, sperm maturation, antimicrobial and antiviral, transcriptional network
1). The human epididymis epithelium
The epithelial lining of the human epididymis ensures spermatozoa acquire full motility and the ability to fertilize an egg. The epididymis consists of three regions; the caput and corpus direct early and late sperm maturation, respectively, and the cauda provides a storage compartment. The efferent ducts, which connect the testis to the epididymis are also functionally specialized, but will not be considered further here. The transcriptional networks responsible for the regional specialization in human epididymis are poorly characterized. Studies in rodents (Turner, et al., 2003) and small mammals (Guyonnet, et al., 2009) have greatly enhanced our understanding of epididymis biology. However, well-established differences in epididymis anatomy and physiology between species (Cornwall, 2009, Sullivan and Mieusset, 2016) necessitate the investigation of human epididymis tissues and cells to reveal critical aspects of their function.
2). Combining deep sequencing protocols to investigate transcriptional networks
Next generation sequencing (NGS) protocols have the capacity to provide precise, in-depth insights into gene regulatory networks in the human epididymis. To investigate human epididymis epithelial function we used tissue segments and also established differentiated epididymis epithelial cell cultures from the same tissue donors (Leir, et al., 2015). Tissue was obtained with IRB approval from patients undergoing inguinal radical orchiectomy for a diagnosis of testicular cancer (ages 19 – 55 years). None of the epididymides have extension of the testicular cancer. Figure 1 illustrates the pipeline whereby we combine data from different NGS protocols to build a regulatory map of a given transcription factor (TF). First DNase-seq (or ATAC-seq) reveals open (active) chromatin regions in human epididymis epithelial (HEE) cells that likely contain cis-regulatory elements coordinating gene expression in these cells. Subsequent in silico analysis identifies transcription factor-binding sites (TFBS) that are over-represented in HEE open chromatin (Bischof, et al., 2013, Browne, et al., 2016, Browne, et al., 2014). ChIP-seq using well-validated antibodies specific for individual TFs identifies their sites of occupancy genome-wide, and determines their target genes (Browne, et al., 2016, Yang, et al., 2018). Furthermore, the contribution of the TF to the transcriptome can be determined by RNA-seq following siRNA-mediated TF depletion, or in the case of ligand-activated TFs such as hormone receptors, upon their activation (Figure 1). We used this approach to map tissue-specific transcriptional networks in HEE cells, which were then validated by relevant functional assays (Browne, et al., 2016, Fossum, et al., 2017, Fossum, et al., 2014, Gosalia, et al., 2015, Yang, et al., 2016).
Figure 1.
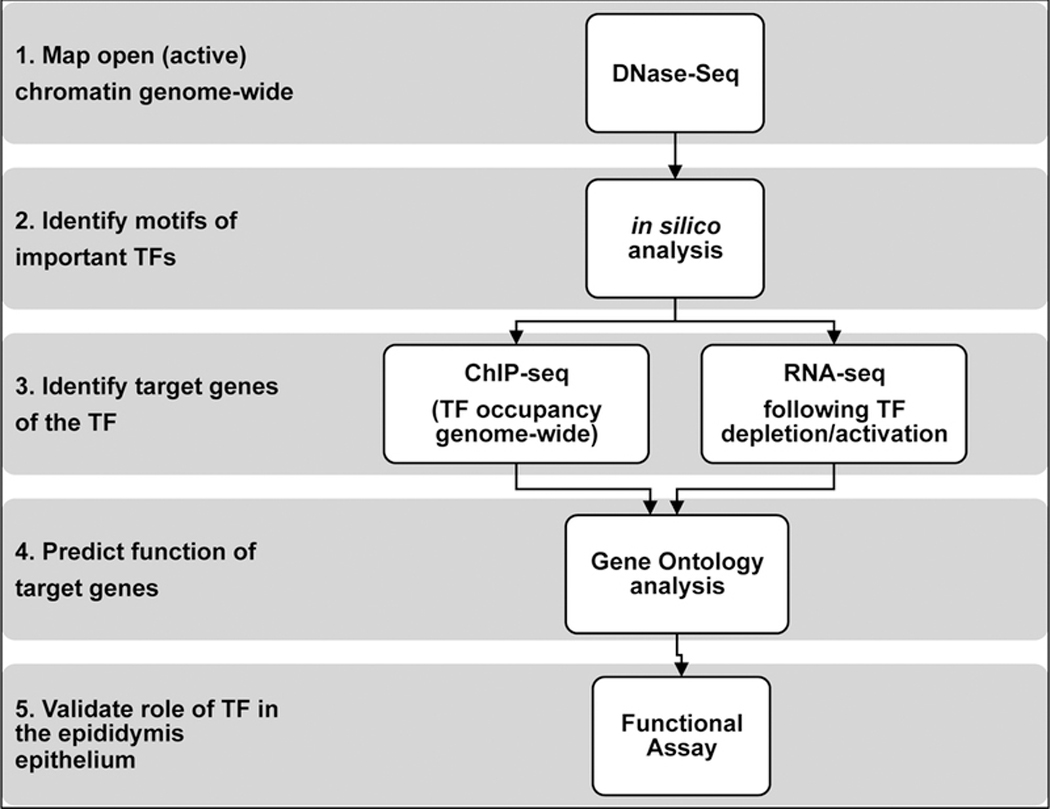
Combining different NGS-based experiments to build transcriptional regulatory networks in the epididymis epithelium. 1. DNase-seq maps open (active) regions of chromatin to identify potential regulatory elements. 2. ChIP-seq identifies transcription factor occupancy genome-wide and its target genes. 3. RNA-seq following TF activation/depletion determines the contribution of the TF to the transcriptome. 4. Gene ontology analysis to predict the function of target genes. 5. Validate identified role of TF with a functional assay.
3). The transcriptomes of human caput, corpus and cauda tissues and derivative HEE cells.
i). Tissue Regions.
We explored the transcriptome of caput, corpus and cauda tissues from human epididymis using RNA-seq (Browne, et al., 2016). Consistent with the microarray analysis of human epididymis tissue done by others, we observed that caput tissue is functionally distinct from the corpus and cauda tissues, which have similar transcriptomes (Dube, et al., 2007, Thimon, et al., 2007, Zhang, et al., 2006). RNA-seq has a higher sensitivity than is provided by microarrays. Hence, it was to be expected that our RNA-seq analysis revealed many more differentially expressed genes (DEGs) and associated gene ontology processes that diverged significantly between caput and corpus/cauda (Browne, et al., 2016).
ii). Caput cell-enriched genes and processes.
Similarly, we used RNA-seq to define the transcriptome of cultured HEE cells from the caput, corpus and cauda regions. These cells, which are described in more detail elsewhere (Leir, et al., 2015), include many epithelial cell types that are present in the intact epididymis. They include principal cells, with high expression of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, together with basal cells expressing keratin 5 (KRT5) among other populations. A more detailed analysis of individual cell types in these cultures will be revealed by single cell RNA sequencing (scRNA-seq). As noted in the tissue analysis, very few transcripts were differentially expressed between the corpus and cauda HEE cells, while there were many DEGs between cells from these regions and from the caput. This demonstrated that caput HEE cells are functionally divergent from those of the corpus and cauda, which have similar transcriptomes (Browne, et al., 2016). Several ion and solute transport-related processes are predominant in caput epithelial cells. These include channels and transporters for potassium, chloride, bicarbonate and water. Also, highly over-represented in caput cells are pathways of response to hormone stimulus, and inspection of the genes within these pathways revealed a number of relevant hormone receptor genes including the androgen receptor (AR), the estrogen receptor (ESR) and the glucagon receptor (GCGR) (Browne, et al., 2016). The enrichment of the AR in the proximal epididymis is consistent with AR immunostaining of adult human epididymis sections (Ungefroren, et al., 1997). Also enriched in caput epithelial cells are the transcripts of several genes with a key role in urogenital tract development. These include paired-box-2 and −8 (PAX2 and PAX8), which are known to regulate kidney development (Bouchard, et al., 2002, Torres, et al., 1995) and hepatocyte nuclear factor 1 beta (HNF1β). HNF1β is also important for kidney development, as demonstrated by urogenital tract abnormalities in both kidney-specific Hnf1β null mice (Igarashi, et al., 2005) and humans with HNF1β mutations (Bellanne-Chantelot, et al., 2004).
iii). Corpus/cauda cell-enriched genes and processes.
Our RNA-seq analysis showed that relatively few genes are differentially expressed between corpus and cauda HEE cells. However, of note pathways of defense response were significantly more important in the corpus/cauda (Browne, et al., 2016).
4). Regional distribution of antibacterial and antiviral responses in the epididymis.
The differential expression of defense response genes warrants further discussion in part due to is complexity and also because of the marked regional distribution of the innate immune response.
i). Defensins.
Key components of the innate immune response in humans include α- and β-defensins, which are small cationic and cysteine-rich peptides that share similar tertiary structures but have different cellular distributions. β-defensins are produced by many mucosal epithelial cells in the body, though within the male reproductive tract the majority are abundant only in the testis (Hollox and Abujaber, 2017) and epididymis (Dorin and Barratt, 2014). In addition to their direct antimicrobial properties, defensins also possess chemotactic activity towards neutrophils, immature dendritic cells and memory T cells (Niyonsaba, et al., 2004, Yang, et al., 1999). β-defensins were reported to play important roles in human and rodent sperm function (Dorin and Barratt, 2014) however, there are species-specific differences (Bjorkgren, et al., 2016, Zhang, et al., 2018, Zhou, et al., 2013). In humans, several β-defensins including DEFB1 and DEFB126 are associated with male fertility (reviewed in (Dorin and Barratt, 2014). However, mutation of their mouse homologues (Defb1 and Defb22), respectively) did not impair fertility. Although β-defensins have intraregional segment-specific expression patterns in the rodent epididymis (Jelinsky, et al., 2007), we did not see marked regional distribution the human epididymis using RNA-seq or quantitative RT-PCR. This may in part be due to humans lacking the connective tissue septa that demarcate segments of the mouse epididymis (Jelinsky, et al., 2007). Human caput and corpus express multiple β-defensins, while the cauda mainly expresses DEFB1 and DEFB4 and low levels of DEFB108 and DEFB131 (Figure 2A). Also more abundant in caput and corpus are Sperm-Associated Antigens (SPAG) 11A and 11B, which show some regions of similarity with the β-defensins, and have antimicrobial properties. Of note, sperm isolated from within epididymis tissue express significantly higher levels of β-defensins than the tissue itself (Figure 2B). Interestingly DEFB4 transcripts, which are at very low abundance in epididymis tissue, are highly expressed in HEE cells from all three epididymis regions (Figure 2A).
Figure 2.
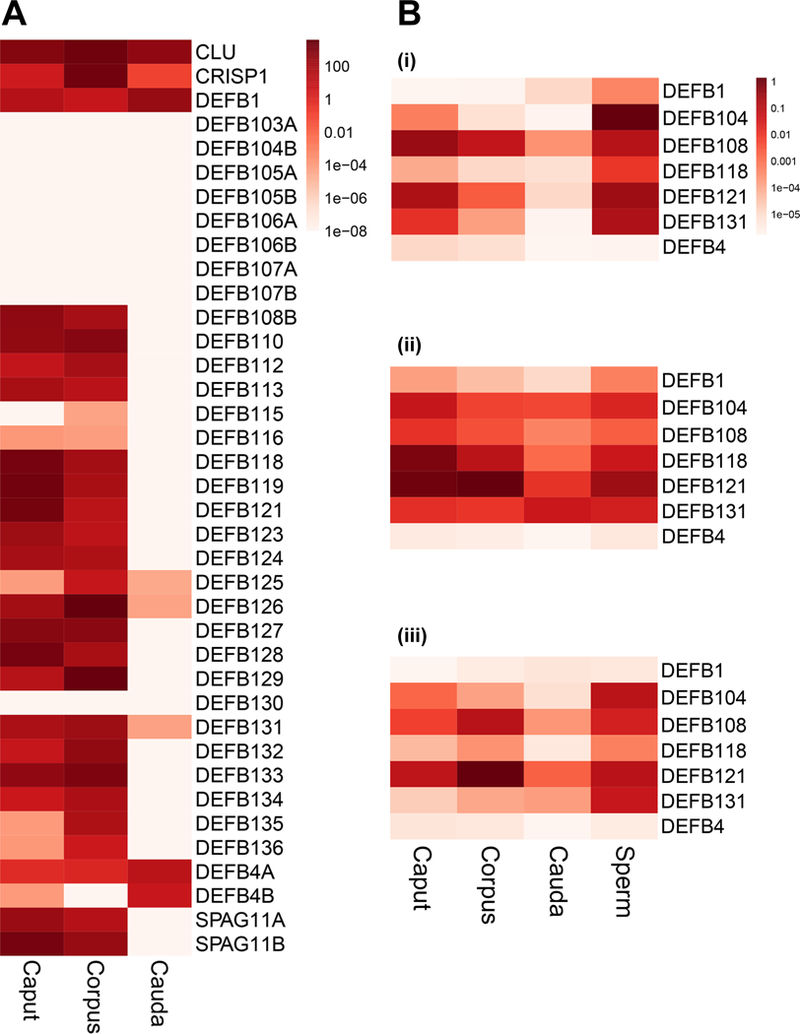
β-defensin expression in different regions (caput, corpus and cauda) of the human epididymis. (A) Heatmap showing β-defensin levels in RNA-seq data from human epididymis tissues (Log scale; 2 donors) (Browne, et al., 2016). Epididymis positive control markers: CLU= clusterin; CRISP1= cysteine rich secretory protein 1. (B) Expression levels of 6 β-defensins (DEFB1, DEFB104, DEFB108, DEFB121, DEFB131 and DEFB4) assayed by RT-qPCR relative to −2 microglobulin in epididymis tissue and sperm isolated from 3 different donors (i-iii) (none of the donors overlap with those shown in panel A).
Several β-defensins are known to inhibit the activity of lipopolysaccharide (LPS) (Ribeiro, et al., 2016), We found that in HEE cells from 5 of 9 donors, LPS or lipoteichoic acid (LTA) treatment of caput and corpus HEE cells increased (~ 65% for LPS and ~30% for LTA) DEFB1 and DEFB4 levels though cells from the remaining 4 donors were not responsive. Additionally, LPS or LTA exposure did not induce expression of the other β-defensins tested (DEFB104, DEFB108, DEFB118, DEFB121, DEFB126 and DEFB131), all of which were abundant in epididymis tissues.
ii). Antiviral responses.
Though epididymitis (inflammation of the epididymis) is more commonly caused by bacteria, viruses such as rubulavirus, Coxsackie-B, Herpesvirus (HSV) and Human papilloma viruses (HPV) are likely viral pathogens in the epididymis (Dejucq and Jegou, 2001, Emerson, et al., 2007, Kapranos, et al., 2003, Vuorinen, et al., 2014). While investigating the antiviral defense response mechanisms of HEE cells (Browne, et al., 2018), we found that the toll-like receptor (TLR) 3 and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) are enriched in cells from the corpus and cauda regions. HEE cells from corpus and cauda, are substantially responsive to antiviral ligands (poly(I:C) and HSV-60), as shown by increased IFN-β mRNA expression and IFN-β secretion, while caput cells show little response. Nuclear translocation of phosphorylated p65 occurs after poly(I:C) exposure. However, PAX2, which was implicated in regulating antiviral response pathways, is required for basal expression of the DNA sensor, Z-DNA binding protein (ZBP1) and type I interferon, in caput but not in cauda cells (Browne, et al., 2018).
5). Regional distribution of transcription factors and their role in the human epididymis epithelium
Consistent with the regional differences in epididymis function, we observed differential expression of TFs that establish and maintain unique transcriptional networks in the caput and corpus/cauda. Thirty- two TFs were significantly more abundant in caput cells and 58 TFs were more enriched in corpus and cauda cells (Browne, et al., 2016). Among caput cell-enriched TFs was the AR, consistent with data showing that caput epithelium plays a predominant role in AR-regulated processes of sperm maturation (Krutskikh, et al., 2011, O’Hara, et al., 2011). Mutations in PAX2, which is also differentially expressed in caput cells, are associated with kidney and urogenital tract abnormalities (Bower, et al., 2012, Eccles and Schimmenti, 1999). Other TFs of interest that were differentially expressed in caput cells include SRY (Sex Determining Region Y)-Box 17 and Spalt-like TF 1 (SALL1). Mutations in SOX17 cause autosomal dominant vesicoureteral reflux syndrome (OMIM:61374) (Gimelli, et al., 2010), which has a phenotype of functional abnormalities in the kidneys, bladder and ureters. SALL1 (previously named Epididymis Secretory Protein Li 89) mutations are associated with two inherited disorders, Townes–Brocks syndrome (TBS) and branchio-oto-renal syndrome (BOR) (Engels, et al., 2000, Kohlhase, et al., 1998), which are developmental defects affecting multiple organ systems including the anus (TBS) and the kidneys (BOR). Analysis of open chromatin data identified TFs that are predicted to occupy cis-regulatory elements in HEE cells (Yang, et al., 2016). Of those that were enriched in caput cells, we further investigated the role of the androgen receptor (AR) and hepatocyte nuclear factor 1 (HNF1).
i). The androgen receptor (AR) transcriptional network in HEE cells.
The transcriptional regulation of the AR in the human epididymis is poorly studied. We used our primary adult caput HEE cells (Leir, et al., 2015) to perform ChIP-seq for AR following activation by synthetic androgen (R1881). We also intersected these data with RNA-seq results from R1881-treated HEE cells to identify direct targets of AR. Inspection of over-represented motifs in AR ChIP-seq peaks identified several new potential AR cofactors including CCAAT/Enhancer binding protein-beta (CEBPβ) and Runt-related transcription factor-1 (RUNX1) (Yang, et al., 2018). Moreover, we showed that CEBPβ occupancy is required for AR binding at a subset of sites in HEE cells. In normal prostate tissue and the LNCaP prostate cancer cell line, FOXA1 is the key TF interacting with AR at peaks of occupancy (Jin, et al., 2014, Pomerantz, et al., 2015) suggesting a distinct AR transcriptional program in the epididymis and prostate epithelium. The importance of AR in the caput epididymis is consistent with knockout mouse models which demonstrate that AR is required for normal epididymis function (Krutskikh, et al., 2011, O’Hara, et al., 2011).
ii). The HNF1β transcriptional network in HEE cells.
We also investigated the role of another caput enriched TF, HNF1. HNF1α and HNF1β TFs recognize the same DNA consensus sequence, which they occupy as homodimers or heterodimers. As noted above (section 3) HNF1β is important for kidney development in mice (Igarashi, et al., 2005) and humans (Bellanne-Chantelot, et al., 2004) and is the predominant form of HNF1 in HEE cells. RNA-seq analysis of the impact of repression of HNF1α and β showed differential expression of 1892 transcripts (902 were downregulated and 990 upregulated) in comparison to a non-targeting siRNA (Browne, et al., 2016). Combining HNF1β ChIP-seq data in HEE cells with the RNA-seq data enabled a gene ontology process enrichment analysis of DEGs with HNF1 ChIP-seq peaks within 20kb. Among the most significant pathways associated with down-regulated genes were transport of water, phosphate and bicarbonate, all critical processes in epididymis epithelial function. Measurements of intracellular pH confirmed a role for HNF1 in regulating the epididymis luminal environment (Browne, et al., 2016). HNF1 is also part of a transcription factor complex that regulates expression of the CFTR gene in intestinal epithelial cells (Kerschner and Harris, 2012, Mouchel, et al., 2004) and likely plays a similar role in the caput epithelium, since we observe HNF1 ChIP-seq peaks overlapping known cis-regulatory elements in the locus.
6). Regional expression of microRNA
Region-specific expression of small noncoding RNAs can also regulate segment-specific gene expression in the epididymis epithelium. Tissue-derived microRNA (miRNA) signatures from human epididymis were reported by others (Belleannee, et al., 2012). We recently documented the microRNA (miRNA) repertoire of caput, corpus and cauda tissues and HEE cells (Browne, et al., 2018). We identified 324 epithelial cell-derived and 259 tissue-derived miRNAs, some of which were region-specific. MiRNAs that were abundant in the caput cells included miR-573 and miR-155 while miR-1204 and miR-770 were enriched in cauda cells. In the caput tissue abundant miRNAs included miR-1247 and miR-4461 while miR-146a, miR-135b and miR-3074 were enriched in the cauda tissue. These epithelial cell- and tissue-derived miRNAs may contribute to the regulation of regionalized gene expression and function of the human epididymis epithelium (Browne, et al., 2018). It is also probable that as in rodent epididymis, vesicles released from the human epididymis epithelial cells (epididymosomes, (Sullivan, et al., 2007)) contain specific miRNAs that may be recruited by sperm as part of their maturation process (Sharma, et al., 2018).
7). Development and differentiation of HEE cells
We previously mapped open chromatin in immature primary HEE cells (Bischof, et al., 2013, Harris and Coleman, 1989) and more recently in caput HEE cells from adult donors (Leir, et al., 2015, Yang, et al., 2016). A similar number of DHS were identified in immature cells (132,814 sites) and adult cells (128,573 sites). To identify cis-regulatory elements that were selective for immature HEE cells, DHS from five different cell types, generated by the ENCODE consortium (Song, et al., 2011) were subtracted from the immature HEE cell signature and non-overlapping sites recorded. The five ENCODE data sets were from skin fibroblasts (FibroP), a lymphoblastoid cell line (GM12878), an erythroleukemia cell line (K562), a liver carcinoma cell line (HepG2) and human umbilical vein endothelial cells (HUVEC). An identical subtraction was performed to identify DHS that were selective for adult HEE cells. Of the total HEE cell DHS 25.8% (34,327/132,814) were selective to immature HEE cells and 30.1% (38,778/128,573) were selective to adult HEE cells, and about 50% were shared between the two.
8). In silico analysis of Transcription Factor Binding Sites (TFBS) enriched in DHS within immature and adult HEE cells
To search for enriched TFBS predicted in open chromatin peaks in immature and adult HEE cells, we performed de novo HOMER motif analyses (Heinz, et al., 2010) on both immature-selective and adult-selective DHS (Supplementary data, Tables SI-II). In silico prediction of TFBS enriched in immature- compared to adult-selective DHS include Basic Leucine Zipper ATF-Like Transcription Factor (BATF) and B Cell CLL/Lymphoma 6 (BCL6). In follicular T-helper cells, BATF regulates BCL6 expression (Ise, et al., 2011), though the relevance of this factor to HEE cell function is unclear. Among TFBS enriched in both immature- and adult-selective DHS is the TEA Domain Transcription Factor (TEAD) motif. Of note, TEAD motifs are also found within peaks of AR occupancy in caput HEE cells (Yang, et al., 2018). The TEAD TF family members (TEAD1–4) participate in diverse cellular processes such as cell growth, proliferation, homeostasis and also development (Johnson and Halder, 2014, Mo, et al., 2014). Of note, TEAD2 and TEAD4 are enriched in caput cells while TEAD3 is enriched in the corpus and cauda cells. The motif for the nuclear receptor 2E1 (NR2E1, also known as TLX) is also enriched in both immature- and adult-selective DHS. In mice, Nr2e1 can repress expression of Pax2 (Yu, et al., 2000), a TF that we have shown to play important roles in both immature and adult HEE cells (Browne, et al., 2018, Browne, et al., 2014). In addition, NR2E1 regulates the PAX6 expression and the PAX6 binding motif is also enriched in immature-selective DHS.
In silico prediction of TFBS enriched in adult- compared to immature-selective DHS identified multiple TFs relevant to the differentiated function of the adult epididymis epithelium. These include Fos-Related Antigen 1 (FRA1, also known as FOSL1), which is enriched in corpus and cauda cells. FRA1 is a component of the AP-1 transcription factor complex. Both AR ChIP-seq and HNF1ChIP-seq peaks identified AP-1 components as potential co-factors in adult HEE cells (Browne, et al., 2016, Yang, et al., 2018). The SRY-Box 9 (SOX9) motif was also enriched in adult-selective DHS. Sox9 regulates anti-Müllerian hormone gene expression (De Santa Barbara, et al., 1998).
9). Conclusions
In conclusion in this review we illustrate how a combination of NGS protocols, supported by relevant bioinformatics tools, can be used to advance our understanding of the regional differentiated properties of a highly complex organ, the human epididymis. Further mechanistic studies are underway to integrate these genome-wide analyses of the transcriptional networks, with a detailed understanding of the biology of the epididymis epithelium in man.
Supplementary Material
Acknowledgements
We are grateful to the anonymous men with testicular cancer who consented to provide epididymis tissue for these studies. We thank Drs A. Gillen, R. Yang, and G. Crawford for assistance in generating the DNase seq data published previously (GSE51002 and GSE74709).
Funding
This work was supported by NIH grant R01HD068901 (PI:A.H.) and the Cystic Fibrosis Foundation (Leir17G0).
References
- Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140:510–7. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Calvo E, Thimon V, Cyr DG, Legare C, Garneau L, Sullivan R. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One. 2012;7:e34996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof JM, Gillen AE, Song L, Gosalia N, London D, Furey TS, Crawford GE, Harris A. A genome-wide analysis of open chromatin in human epididymis epithelial cells reveals candidate regulatory elements for genes coordinating epididymal function. Biol Reprod. 2013;89:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkgren I, Alvarez L, Blank N, Balbach M, Turunen H, Laajala TD, Toivanen J, Krutskikh A, Wahlberg N, Huhtaniemi I, Poutanen M, Wachten D, Sipila P. Targeted inactivation of the mouse epididymal beta-defensin 41 alters sperm flagellar beat pattern and zona pellucida binding. Mol Cell Endocrinol. 2016;427:143–54. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Moriniere V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L. Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific datab ase. Hum Mutat. 2012;33:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JA, Leir SH, Eggener SE, Harris A. Region-specific innate antiviral responses of the human epididymis. Mol Cell Endocrinol. 2018;473:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JA, Leir SH, Eggener SE, Harris A. Region-specific microRNA signatures in the human epididymis. Asian J Androl. 2018;20:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JA, Yang R, Eggener SE, Leir SH, Harris A. HNF1 regulates critical processes in the human epididymis epithelium. Mol Cell Endocrinol. 2016;425:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JA, Yang R, Leir SH, Eggener SE, Harris A. Expression profiles of human epididymis epithelial cells reveal the functional diversity of caput, corpus and cauda regions. Mol Hum Reprod. 2016;22:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JA, Yang R, Song L, Crawford GE, Leir SH, Harris A. Open chromatin mapping identifies transcriptional networks regulating human epididymis epithelial function. Mol Hum Reprod. 2014;20:1198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol. 1998;18:6653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65:208–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorin JR, Barratt CL. Importance of beta-defensins in sperm function. Mol Hum Reprod. 2014;20:821–6. [DOI] [PubMed] [Google Scholar]
- Dube E, Chan PT, Hermo L, Cyr DG. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis. Biol Reprod. 2007;76:1034–44. [DOI] [PubMed] [Google Scholar]
- Eccles MR, Schimmenti LA. Renal-coloboma syndrome: a multi-system developmental disorder caused by PAX2 mutations. Clin Genet. 1999;56:1–9. [DOI] [PubMed] [Google Scholar]
- Emerson C, Dinsmore WW, Quah SP. Are we missing mumps epididymo-orchitis? Int J STD AIDS. 2007;18:341–2. [DOI] [PubMed] [Google Scholar]
- Engels S, Kohlhase J, McGaughran J. A SALL1 mutation causes a branchio-oto-renal syndrome-like phenotype. J Med Genet. 2000;37:458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum SL, Mutolo MJ, Tugores A, Ghosh S, Randell SH, Jones LC, Leir SH, Harris A. Ets homologous factor (EHF) has critical roles in epithelial dysfunction in airway disease. J Biol Chem. 2017;292:10938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum SL, Mutolo MJ, Yang R, Dang H, O’Neal WK, Knowles MR, Leir SH, Harris A. Ets homologous factor regulates pathways controlling response to injury in airway epithelial cells. Nucleic Acids Res. 2014;42:13588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, Zuffardi O, Giorda R, Wells JM, Gimelli G, Ghiggeri GM. Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum Mutat. 2010;31:1352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalia N, Yang R, Kerschner JL, Harris A. FOXA2 regulates a network of genes involved in critical functions of human intestinal epithelial cells. Physiol Genomics. 2015;47:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet B, Marot G, Dacheux JL, Mercat MJ, Schwob S, Jaffrezic F, Gatti JL. The adult boar testicular and epididymal transcriptomes. BMC Genomics. 2009;10:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Coleman L. Ductal epithelial cells cultured from human foetal epididymis and vas deferens: relevance to sterility in cystic fibrosis. J Cell Sci. 1989;92 ( Pt 4):687–90. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollox EJ, Abujaber R. Evolution and Diversity of Defensins in Vertebrates In: Pontarotti P ed. Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts: Springer International Publishing AG, 2017:27–50. [Google Scholar]
- Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944–7. [DOI] [PubMed] [Google Scholar]
- Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, Murphy KM. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. 2011;12:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, Wilson E, Brown EL, Kopf GS, Johnston DS. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–70. [DOI] [PubMed] [Google Scholar]
- Jin HJ, Zhao JC, Wu L, Kim J, Yu J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat Commun. 2014;5:3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranos N, Petrakou E, Anastasiadou C, Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil Steril. 2003;79 Suppl 3:1566–70. [DOI] [PubMed] [Google Scholar]
- Kerschner JL, Harris A. Transcriptional networks driving enhancer function in the CFTR gene. Biochem J. 2012;446:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18:81–3. [DOI] [PubMed] [Google Scholar]
- Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, Huhtaniemi I. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology. 2011;152:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leir SH, Browne JA, Eggener SE, Harris A. Characterization of primary cultures of adult human epididymis epithelial cells. Fertil Steril. 2015;103:647–54 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchel N, Henstra SA, McCarthy VA, Williams SH, Phylactides M, Harris A. HNF1alpha is involved in tissue-specific regulation of CFTR gene expression. Biochem J. 2004;378:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara L, Welsh M, Saunders PT, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152:718–29. [DOI] [PubMed] [Google Scholar]
- Pomerantz MM, Li F, Takeda DY, Lenci R, Chonkar A, Chabot M, Cejas P, Vazquez F, Cook J, Shivdasani RA, Bowden M, Lis R, Hahn WC, Kantoff PW, Brown M, Loda M, Long HW, Freedman ML. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat Genet. 2015;47:1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CM, Silva EJ, Hinton BT, Avellar MC. beta-defensins and the epididymis: contrasting influences of prenatal, postnatal, and adult scenarios. Asian J Androl. 2016;18:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev Cell. 2018;46:481–94 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Zhang Z, Grasfeder LL, Boyle AP, Giresi PG, Lee BK, Sheffield NC, Graf S, Huss M, Keefe D, Liu Z, London D, McDaniell RM, Shibata Y, Showers KA, Simon JM, Vales T, Wang T, Winter D, Zhang Z, Clarke ND, Birney E, Iyer VR, Crawford GE, Lieb JD, Furey TS. Open chromatin defined by DNaseI and FAIRE identifies regulatory elements that shape cell-type identity. Genome Res. 2011;21:1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–91. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Mieusset R. The human epididymis: its function in sperm maturation. Hum Reprod Update. 2016;22:574–87. [DOI] [PubMed] [Google Scholar]
- Thimon V, Koukoui O, Calvo E, Sullivan R. Region-specific gene expression profiling along the human epididymis. Mol Hum Reprod. 2007;13:691–704. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–65. [DOI] [PubMed] [Google Scholar]
- Turner TT, Bomgardner D, Jacobs JP, Nguyen QA. Association of segmentation of the epididymal interstitium with segmented tubule function in rats and mice. Reproduction. 2003;125:871–8. [DOI] [PubMed] [Google Scholar]
- Ungefroren H, Ivell R, Ergun S. Region-specific expression of the androgen receptor in the human epididymis. Mol Hum Reprod. 1997;3:933–40. [DOI] [PubMed] [Google Scholar]
- Vuorinen T, Osterback R, Kuisma J, Ylipalosaari P. Epididymitis caused by coxsackievirus A6 in association with hand, foot, and mouth disease. J Clin Microbiol. 2014;52:4412–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. [DOI] [PubMed] [Google Scholar]
- Yang R, Browne JA, Eggener SE, Leir SH, Harris A. A novel transcriptional network for the androgen receptor in human epididymis epithelial cells. Mol Hum Reprod. 2018;24:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Kerschner JL, Gosalia N, Neems D, Gorsic LK, Safi A, Crawford GE, Kosak ST, Leir SH, Harris A. Differential contribution of cis-regulatory elements to higher order chromatin structure and expression of the CFTR locus. Nucleic Acids Res. 2016;44:3082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Kerschner JL, Harris A. Hepatocyte nuclear factor 1 coordinates multiple processes in a model of intestinal epithelial cell function. Biochim Biophys Acta. 2016;1859:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RT, Chiang MY, Tanabe T, Kobayashi M, Yasuda K, Evans RM, Umesono K. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci U S A. 2000;97:2621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhou Y, Xie S, Yin Q, Tang C, Ni Z, Fei J, Zhang Y. CRISPR/Cas9-mediated genome editing reveals the synergistic effects of beta-defensin family members on sperm maturation in rat epididymis. FASEB J. 2018;32:1354–63. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Liu Q, Li YM, Hall SH, French FS, Zhang YL. Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol Cell Endocrinol. 2006;250:169–77. [DOI] [PubMed] [Google Scholar]
- Zhou YS, Webb S, Lettice L, Tardif S, Kilanowski F, Tyrrell C, Macpherson H, Semple F, Tennant P, Baker T, Hart A, Devenney P, Perry P, Davey T, Barran P, Barratt CL, Dorin JR. Partial deletion of chromosome 8 beta-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013;9:e1003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


