Abstract
Overuse-induced tendinopathy is highly prevalent in the general population. Percutaneous fenestration, or dry needling, techniques have been increasing in popularity, but despite their current use, there are no controlled laboratory studies to provide fundamental support for this practice. The objective of this study was to establish a model for percutaneous needling of the rat supraspinatus tendon using ultrasound guidance, and to evaluate the biological response of needling healthy tendon. A total of 44 male Sprague Dawley rats (477±39g) were used to evaluate the effect of dry needling on healthy supraspinatus tendon properties. 10 rats were reserved as un-needled control animals, and the remaining animals underwent either mild or moderate bilateral needling protocols and were sacrificed at 1 or 6 weeks post-needling (n=8–10/group). Color Doppler ultrasound imaging was performed to analyze blood flow within the tendon. Histological and immunohistochemical analyses were used to determine cellular, inflammatory, and extracellular matrix properties of the tissue. Finally, quasi-static tensile mechanical analysis was performed to obtain viscoelastic, structural, and material properties to evaluate the tendon healing outcome. Data was tested for normality, and then 2-way ANOVA tests were performed followed by post-hoc tests for multiple comparisons. Both the mild and moderate needling groups caused a transient healing response at early time points as shown by a statistically significant (p<0.05) reduction in mechanical properties, and increase in blood flow, inflammation, and production of collagen III and glycosaminoglycans as compared to the control. Furthermore, mild needling properties returned to or exceeded pre-needling values at the 6-week time point. Clinical Significance: Needling the rat supraspinatus tendon is a feasible technique that causes a transient healing response followed by a return to, or improvement of, normal tendon properties, indicating potential applicability in understanding the effects of current practices utilizing dry needling of tendons in humans.
Keywords: Ultrasound, Dry Needling, Fenestration, Supraspinatus, Tendon, Tendinosis
Introduction
Overuse injuries that lead to tendon degeneration, termed tendinosis, are prevalent in recreational and professional athletes and manual laborers.1–5 Tendinosis is characterized by pain, swelling, and impaired performance6; 7 and may result in tendon rupture.8–11 Originally thought to be an inflammatory condition, tendinosis is now understood to be a degenerative process, characterized by hypercellularity, vascular hyperplasia, and collagen disorganization.6–8 Common sites of tendinosis include the rotator cuff, common extensor, patellar, and Achilles tendons.
Tendinosis treatment begins with conservative measures including rest, activity modification, bracing, physical therapy, and non-steroidal anti-inflammatory medications. Although often effective, these methods sometimes have limited success in treating tendinopathy symptoms.12 Recently percutaneous and minimally-invasive treatments of chronic tendinosis have increased in popularity.13–18 Percutaneous dry needling involves repeat fenestration of diseased tissues using a needle, typically performed under ultrasound (US) guidance to provide real-time imaging and needle guidance to target the precise area of disease.19–21 Evidence suggests that micro-trauma to the diseased tendon may lead to disruption of pathological tissues, induction of bleeding, and release of factors to stimulate healing.13; 22 The theoretical aim of dry needling is to convert a chronic, quiescent lesion into an acute, activated lesion, which may have greater healing potential. This US-guided dry needling technique has been described alone and in combination with injection of platelet-rich plasma,23 autologous blood,24 or glucose,25 among other substances.25 These treatments are thought to promote the influx of growth factors involved in the inflammatory phase of healing.25 Several clinical studies have suggested this can be an effective treatment.13; 14; 26; 27 However, no controlled laboratory studies have been performed to provide a rigorous scientific evaluation of this commonly used clinical practice. With widespread clinical use, lack of such studies demonstrates a significant gap in supportive medical literature.
The objective of this study was to investigate the feasibility of dry needling the supraspinatus tendon using US guidance in an established rat model for studying rotator cuff injury and disease, and to determine its effects on tendon vascular, compositional, and mechanical properties in healthy tendon samples. The rat rotator cuff model provides a unique advantage because it is the only tendinosis animal model similar to the etiology of overuse in humans.28 While this study aims to evaluate dry needling method in healthy tendons, future studies will evaluate dry needling on the rat tendinosis model. We hypothesize that the needling procedure will cause consistent micro-injuries initiating a transient healing response, but without permanent damage to the tendon tissue. The results will provide a model system for evaluating the effect of dry needling.
Methods
This prospective study was performed in accordance with the University of Pennsylvania Institutional Animal Care and Use Committee.
Validation of Needling Technique
13 male Sprague Dawley rats were used for four sets of studies to determine the most consistent needling procedure of the rat supraspinatus tendon. Live animals were not necessary for this, so procedures were performed within 30 minutes post-sacrifice to ensure that rigor mortis would not interfere with tissue properties. The procedure included injecting a small amount of dye during different dry needling approaches, followed by immediate dissection and evaluation of the location of the injected dye to confirm needle placement in the tissue. A musculoskeletal radiologist with experience in diagnostic ultrasound and ultrasound-guided injection procedures performed all studies. A high-frequency transducer and a clinical scanner were investigated. The animal’s shoulder was placed in external rotation and adduction to expose more of the supraspinatus tendon from under the acromion.29–31 The shoulder was imaged and needled either in the long axis of the supraspinatus tendon from lateral to medial or in the transverse plane from posterior to anterior. We investigated three needle sizes, 23, 25, and 27 gauge, and two needle lengths, 1” and 0.5”. To identify needle placement, Verhoeff stain (~1ul) was injected using US guidance into the tendon. The shoulder was immediately dissected to confirm the location of the dye within the tendon.
Study Design
A live animal study was then performed to determine the effect of dry needling on healthy rat supraspinatus tendons. 44 male Sprague Dawley rats (477±39g) were obtained from Charles River Laboratories (Wilmington, MA), housed in standard cages with 2 rats/cage, and provided food and water ad libitum. A subset (n=10) were reserved as un-needled control animals, and the remaining animals were randomized into mild or moderate bilateral needling protocols, and were sacrificed at 1 or 6 weeks post-needling (n=8–10/group). Animals sacrificed at 6 weeks post-needling also underwent color Doppler ultrasound imaging prior to needling, 5 and 24 hours post-needling, and 6 weeks post-needling prior to sacrifice. After sacrifice, one shoulder was used for histological and the other for mechanical evaluation. A power analysis was performed to determine sample sizes.
Dry Needling Procedure
Dry needling was performed based on the results of the validation procedures listed in the methods section above. Animals were anesthetized with isoflurane, and the shoulder was prepared by removing hair locally. Using the 14–5 MHz linear array transducer (Zonare, Mountain View, CA), the supraspinatus tendon and the surrounding landmarks (acromion and humeral head) were identified in the transverse plane. Under US guidance, a 27G ½” needle was introduced posteriorly and guided into the supraspinatus tendon in the superior aspect of the subacromial space. For the mild protocol, the needle was inserted 3 times, and for the moderate protocol the needle was inserted an additional 3 times fanned proximally and 3 times distally (9 times total). All animals were provided with Buprenorphine for pain management, and recovered from anesthesia under a heat lamp until they became fully responsive and demonstrated a return to normal behavior.
Doppler Ultrasound Imaging
Color Doppler ultrasound was performed using a 40 MHz center frequency transducer (MS550D, VisualSonics, Toronto, ON) in the same positioning. 8–10 images were acquired for each shoulder and blood flow within the tendon was analyzed using an IDL program (Harris Geospatial Solutions, Herndon, VA). Mean color level (average blood flow velocity), fractional area (% area of Doppler signal), and color weighted fractional area (weighted average of blood flow velocity/unit area) were quantified. Measures for each image within a specimen were averaged.
Histological Evaluation
Tendon samples for pre-needling control and 1 and 6 weeks post-needling (n=6–8/group) were dissected, processed, sectioned, and stained with hematoxylin-eosin (H&E), safranin-O and fast green, and immunohistochemical staining for type III collagen (Col III, Sigma-Aldrich, C7805, 1:500), interleukin-1 beta (IL-1β, Chemicon, AB1832, 1:400), and tumor necrosis factor alpha (TNFα, Novus Biologics, NBP1–19532, 1:750). Approximately 2–3 sections along the width of each specimen were blinded and imaged at 2 different locations along the tendon at 200x. H&E and safranin-O stains were semi-quantitatively evaluated by three blinded investigators and graded for cell shape (1=spindle to 3=round), cellularity (1=less cells to 4=more cells), and amount of safranin-O stain (1=less stain to 4=more stain). Immunohistochemical stains were quantitatively analyzed for percent area of positive stain using a MATLAB program (Mathworks, Natick, MA).32
Mechanical Evaluation
Tendons from pre-needling and 1 and 6 weeks post-needling (n=8–10/group) were blinded and dissected, leaving the bone insertion intact. Verhoeff stain lines were applied at the insertion and in 2, 4, and 8 mm increments to define the insertion and midsubstance regions. Cross-sectional area was measured using a laser-based device from the insertion up to the 8mm stain line.33 The proximal end of the tendon was fixed with sandpaper and adhesive at the 8mm stain line. The humerus and humeral head was secured in polymethylmethacrylate without obstructing the tendon motion. The specimen was attached between two fixtures and submerged in a 37°C phosphate-buffered saline bath. The protocol consisted of (1) preloading (0.08N), (2) preconditioning (10 cycles from 0.1–0.5N), (3) stress-relaxation (5% strain for 10 minutes), and (5) ramp to failure (0.3% strain/sec). Strain was measured optically.34 Percent relaxation was calculated from the stress-relaxation test and structural (max load, stiffness) and material (max stress, modulus) properties were determined from the ramp to failure test.
Statistics
Normally distributed data (Doppler and mechanics) was analyzed using a 2-way ANOVA followed by Bonferroni tests. For parameters with significance over time, 1-way ANOVAs were performed within each group with Bonferroni tests across time point. Non-parametric data (histology) was analyzed using Kruskal-Wallis and Dunn’s tests. Significance was set at p<0.05.
Results
Dry Needling Validation
In our initial investigations, sacrificed animals were used to visualize the rat rotator cuff anatomy, learn how to best position the shoulder for the optimal view and access for US-guided injection, and determine the smallest needle size that can accurately be navigated to the location of the tendon. We determined that a 14–5 MHz transducer was the most appropriate for this application, since it provided both good resolution and penetration for imaging the rotator cuff tendons, and the hockey-stick probe shape allowed for ease of needle positioning.
When comparing the longitudinal and transverse approaches, it was determined that the transverse approach was the most consistent. With the longitudinal approach, where the subacromial space was visualized in the long axis of the supraspinatus tendon, only 1 out of 6 shoulders had proper placement of the needle with dye injected in the tendon. For the transverse approach, the US transducer was positioned transverse to the shoulder with the needle entering the shoulder posteriorly and directed anteriorly into the supraspinatus tendon. This proper anatomical location was identified by imaging medial to the joint and in the sagittal plane of the scapula and locating the “Y” of the scapula (which made up of the scapular body, coracoid process, and scapular spine) (Fig. 1a). By sliding the transducer laterally, the scapular spine can be followed to the acromion to where it is in the same plane as the humeral head. The hypoechoic region (space) between these two osseous structures is where the supraspinatus tendon lies (Fig. 1b and 1c). In these testing sessions, 12 out of the 14 shoulders that were injected with dye showed proper positioning of the needle, and the remaining 2 shoulders were inconclusive due to failure to visualize any dye injected in the tendon or surrounding area. It was also determined that the 27-gauge needle of 0.5” length was the smallest possible size while still being able to accurately guide the needle into the correct anatomical location.
Fig. 1.
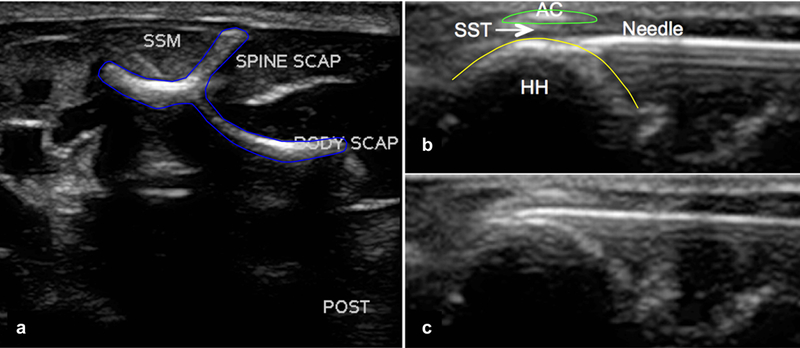
Ultrasound images of the rat shoulder in the sagittal plane (transverse approach). (a) Image medial to the shoulder joint depicting the “Y” of the scapula and the supraspinatus muscle (SSM). The acromion was identified by sliding the transducer laterally and following the scapular spine until the humeral head is in view. (b) Image of the humeral head (HH) and acromion (AC), with the needle (27 gauge) entering from posterior to anterior, and (c) the needle entering between the acromion and the humeral head into the supraspinatus tendon (SST).
Color Doppler Ultrasound
Color Doppler ultrasound parameters were evaluated in the supraspinatus tendon prior to needling (Fig. 2a), 5 and 24 hours following needling (Fig. 2b and 2c), and 6 weeks post-needling (Fig 2d). All three parameters showed significant interactions between treatment and time point. The mean color level, representing blood flow velocity, was not significantly altered over time, but there was a significant increase in the mild group compared to the moderate group at the 24 hour time point (Fig. 2e). However, both fractional area and color weighted fractional area significantly increased 5 and 24 hours after needling in both groups compared to the pre-needling values. Only the mild group showed a significant decrease in the 6 week post-needling compared to these earlier time points. However, neither group showed differences between pre-needling and 6 weeks post-needling (Fig. 2f and 2g). Finally, color weighted fractional area was significantly increased in the mild group compared to the moderate group at 24 hours post needling (Fig. 2g).
Fig. 2.
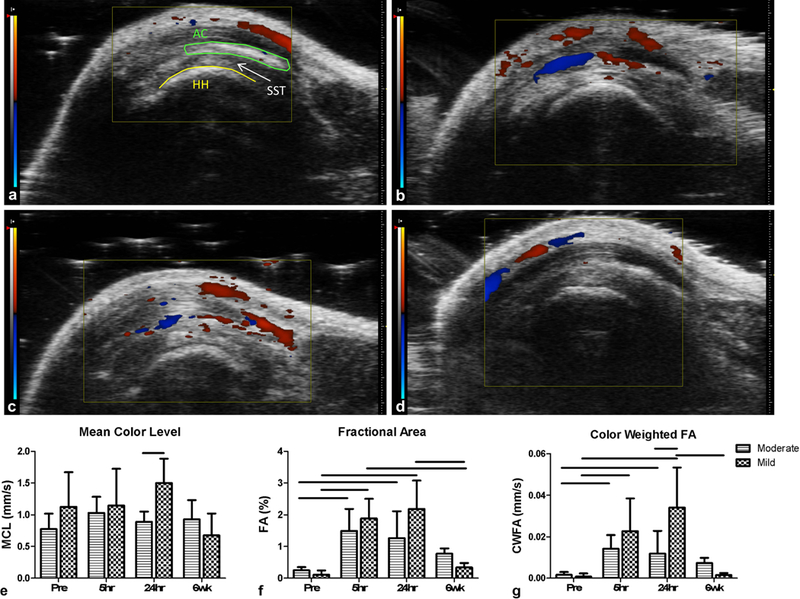
Color Doppler ultrasound representative images from the mild group showing (a) pre-needling, and (b) 5 hours, (c) 24 hours, and (d) 6 weeks post-needling. The pre-needling image has the humeral head (HH), acromion (AC) and supraspinatus tendon (SST, located between the HH and AC) labeled. Quantification of (e) mean color level (MCL), (f) fractional area (FA), and (g) color weighted fractional area (CWFA). Both the moderate and mild needling groups showed a large increase in fractional area measures at 5 and 24 hours post-needling, with a return to baseline values by week 6. Data is displayed as average and standard deviation, and all significance lines indicate p<0.05 between groups.
Histological Evaluation
The H&E cellular evaluation (representative images shown in Fig. 3a) demonstrated that there was a significant increase in cellularity and more rounded cell shape in the moderate needling group at week 1 compared to control. While the mild group qualitatively demonstrated increases in these properties as well, there were no statistically significant changes over time (Fig. 3b and 3c). Additionally, for the safranin-O staining (representative images shown in Fig. 3a), the moderate group showed a significant increase from control at week 1, and a significant increase compared to the mild group at week 6 (Fig. 3d).
Fig. 3.
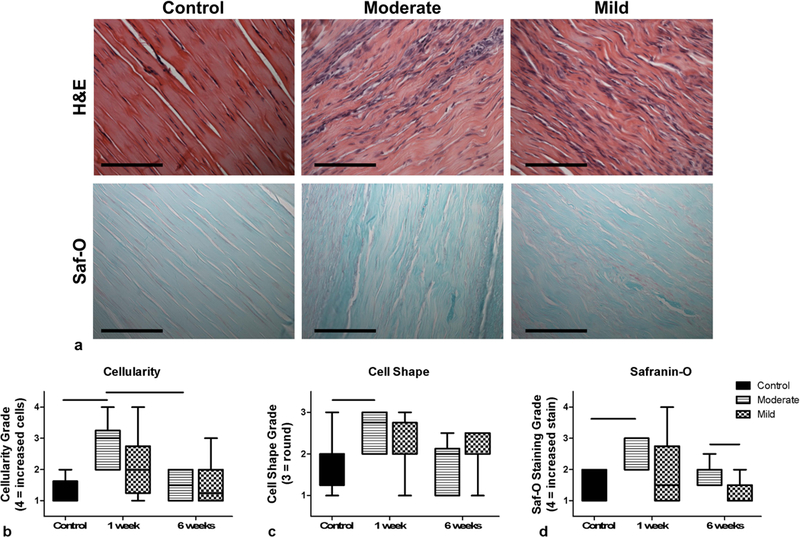
(a) Representative histological images of control (un-needled) and moderate and mild samples at 1 week post-needling (scale bar is 100µm). Purple hematoxylin staining in H&E indicates the presence of cell nuclei and pink/red staining in safranin-O images indicates the presence of proteoglycans. The plots display semi-quantitative histological grading of (b) H&E cellularity (1=less cells to 4=more cells) and (c) cell shape (1=spindle to 3=round), and (d) area of safranin-O staining (1=less stain to 4=more stain). The moderate group showed increases in all three parameters at 1 week post-needling. Data is displayed as median and interquartile range, and all significance lines indicate p<0.05 between groups.
The immunohistochemical evaluation (representative images shown in Fig. 4a) demonstrated that 1 week post-needling there were significant increases in all three targets Col III, TNFα, and IL-1β (Fig. 4b–4d). Both groups were also significantly decreased at 6 weeks compared to 1 week (except for the TNFα moderate group), and statistically not different from control values (Fig. 4b–4d).
Fig. 4.
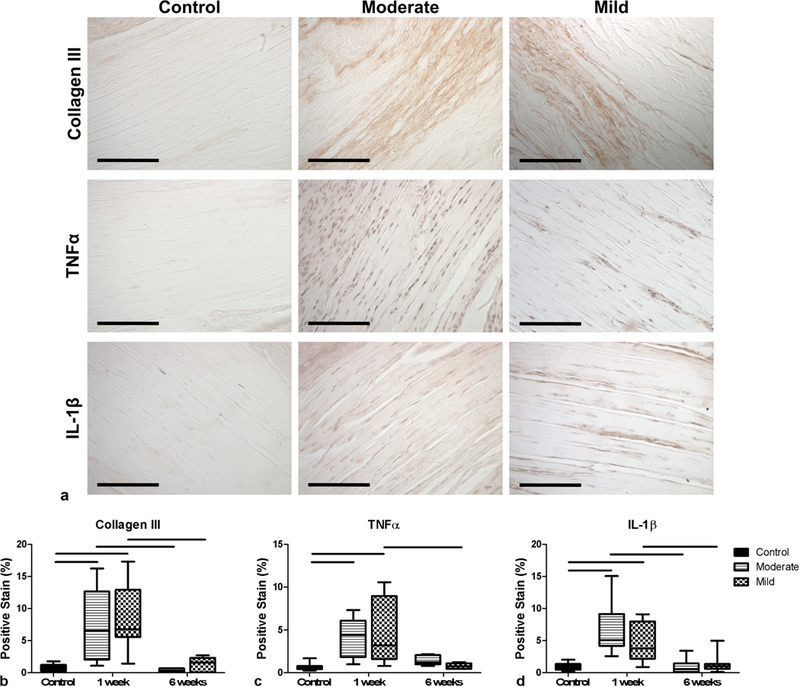
(a) Representative immunohistochemical images of control (un-needled) and moderate and mild samples at 1 week post-needling (scale bar is 100µm). Brown staining indicates the presence of the protein of interest, as indicated by the row labels. The plots display quantitative evaluation of the percent of positive (brown) staining of (b) Collagen III, (c) TNFα, and (d) IL-1β within the images. All groups showed significant increases in these proteins at 1 week post-needling. Data is displayed as median and interquartile range, and all significance lines indicate p<0.05 between groups.
Mechanical Evaluation
There was a significant increase in cross-sectional area in the moderate needling group by 1 week that returned to baseline by 6 weeks, whereas there were no significant changes in the mild group (Fig. 5a). Both needling groups caused a reduction in percent relaxation that continued out to 6 weeks post-needling (Fig. 5b). There was a statistically significant interaction between needling group and time point for both cross-sectional area and percent relaxation. Both groups also demonstrated a significant increase in max load at 6 weeks compared to both the un-needled control and the 1 week post-needling groups (Fig. 5c). The moderate group showed a decrease in max stress at 1 week that recovered to pre-needling values by 6 weeks (Fig. 5d). The tendon stiffness and modulus were reduced in both the moderate and mild groups at 1 week (Fig. 5e and 5f). While stiffness recovered to pre-needling values for both groups, only the mild group recovered pre-needling tendon modulus by week 6 (Fig. 5e and 5f). Additionally, the mild group modulus was significantly increased compared to the moderate group at week 6 post-needling (Fig. 5f).
Fig. 5.
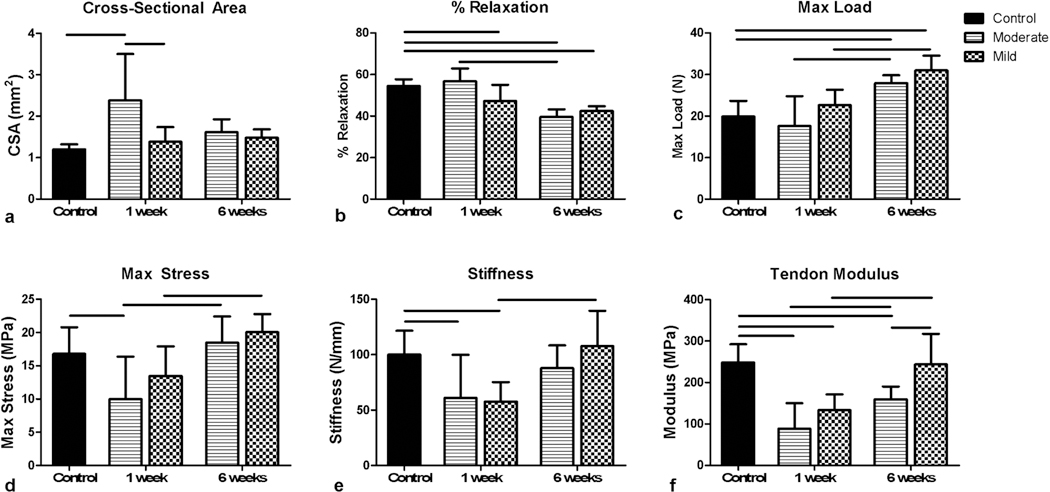
Mechanical evaluation of moderate and mild dry needling. (a) Only the moderate group showed an increase in cross-sectional area at 1 week. (b) Both groups decreased in percent relaxation by 6 weeks. (c) Max load was increased in both groups by 6 weeks. (d) Max stress, (e) stiffness, and (f) modulus all show reductions at 1 week. Both Max stress and stiffness recovered to baseline values by 6 weeks, whereas tendon modulus only recovered to baseline for the mild group. Data is displayed as average and standard deviation, and all significance lines indicate p<0.05 between groups.
Discussion
While several clinical studies suggest that dry needling, with or without injection of various substances, provides effective pain relief for tendinosis,13; 14; 26; 27 the effect on the tendon tissue health and pre-disposition to rupture remains unknown. Additionally, there have been no controlled laboratory studies to provide scientific support for this increasingly common clinical practice. Additionally, it is also unknown how the tendon responds to varying amounts of dry needling and if it may cause permanent damage to tendon tissue despite potential improvements in pain.
This study presents the development of a consistent method for US-guided needling of a rat supraspinatus tendon in order to determine the response that tendons have to dry needling, and to validate this method for future use in tendinopathy models. Validation procedures confirmed the sonographic anatomy of the rat rotator cuff, determined positioning of the animal and the probe, and established the optimal needling parameters and approach. It was determined that dry needling caused a transient increase in vascularity and an increase in inflammation, deposition of type III collagen, and in the case of the moderate needling group an increase in cellularity and glycosaminoglycan content. Additionally, qualitative observation of both the moderate and mild needling groups demonstrated more disorganized tissue structure at 1 week post-needling. The compositional and vascular changes returned to baseline values 6 weeks after treatment in both needling groups. Additionally, both needling procedures induced a minor injury response, with the moderate group showing more significant alterations. Specifically, only the moderate group demonstrated an increase in cross-sectional area and a decrease in max stress. However, both groups caused reductions in stiffness and modulus at 1 week. While the mild group recovered properties, the moderate group did not. Interestingly, both groups had reduced percent relaxation and increased max load at 6-weeks, indicating a more permanent beneficial change. Results suggest that both mild and moderate needling induced microdamage that elicited a healing response at early time points, and that the mild group completely recovered properties back to or better than pre-needling values. Since these results were performed in healthy tendon, this indicates that a mild amount of dry needling of the tendon could be considered safe in this model, with the potential for improvement in a tendinopathic tendon.
Currently, the only supraspinatus tendinosis animal model similar to the etiology of overuse in humans is in the rat.28 While it was necessary to first establish a non-detrimental dry needling method in healthy tendons, future studies will evaluate dry needling on a tendinosis model which is the primary pathology treated by needling techniques clinically. The rat is a well-established model for the rotator cuff and has similar anatomical architecture to the human.35 Additionally, the repetitive motion of the supraspinatus tendon passing under the acromion is similar to that which occurs in the human shoulder during overhead activities, which can be a cause of rotator cuff tendinosis.35 The rat model similarly creates degenerative tendinosis following an overuse treadmill running protocol.28 This model is well accepted as there are now over 200 full-length peer-reviewed publications in the literature using the rat supraspinatus model. Other animal models of tendinosis include injections (collagenase, prostaglandins, fluoroquinolones, cytokines), but these chemical methods do not mimic naturally-occurring initiation of degeneration. We therefore chose the rat supraspinatus tendon for our dry needling procedure using US guidance so that we will be able to implement this procedure in a well-established tendinosis model that mimics the clinical condition.
This study has several limitations. First, the use of an animal model does not directly parallel the human condition. However, the rat supraspinatus tendon model mimics the human condition well. Additionally, the same size scales of the tendon and needle are not easily achieved in an animal model. While we used the smallest needle possible while maintaining the capability to guide the needle through tissue, it is still large relative to the small rat tendon compared to human tendons. We compensated for this by reducing the number of times that the tendon is needled (3–9x in the rat vs. ~30x in a human). Finally, this current study was performed on healthy tissue. This was an important first step to determine if this dry needling procedure is not detrimental to healthy tendons. Additionally, this study on healthy tendons will provide historical control data for future experiments on pathologic tendons. Despite these limitations, we have demonstrated that a rat model allows for controlled, repeatable needling that elicits a healing response but does not cause long-term tissue damage. Unlike clinical studies where the outcomes are limited to functional measures, animal models allow the study of biological and mechanical mechanisms behind tendon healing after treatment. The comprehensive analysis offered by an animal model is necessary to fully understand the implications of current dry needling treatment techniques. This motivation for an animal model outweighs the small limitations and differences in procedure.
This study shows that ultrasound-guided dry needling of the rat supraspinatus tendon is a feasible technique allowing the specific targeting of a normal animal tendon in vivo. We found that dry needling causes a significant mechanical and biological response initially after needling, but that this response is transient and recovers over time. While dry needling is frequently performed clinically and has been shown to reduce pain, there is no research evaluating the either beneficial or harmful tissue-level changes occurring after dry needling and how that may affect the tendon over time. This work supports future studies evaluating the effects of dry needling on a tendinosis condition, providing evidence to better inform the underlying mechanism and utility of this already common procedure.
Acknowledgements
The authors acknowledge Kerrie Tiedemann and Tonia Tsinman for their contributions. This research was funded by RSNA Research & Education Foundation Grant (RSD1524), NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (P30 AR050950), Rheumatology training grant (4T32AR007442–29), and an NSF Graduate Research Fellowship. The authors do not have any conflicts of interest to disclose.
References
- 1.Baquie P, Brukner P. 1997. Injuries presenting to an Australian sports medicine centre: a 12-month study. Clin J Sport Med 7:28–31. [DOI] [PubMed] [Google Scholar]
- 2.Frost P, Bonde JP, Mikkelsen S, et al. 2002. Risk of shoulder tendinitis in relation to shoulder loads in monotonous repetitive work. Am J Ind Med 41:11–18. [DOI] [PubMed] [Google Scholar]
- 3.Kujala UM, Sarna S, Kaprio J. 2005. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med 15:133–135. [DOI] [PubMed] [Google Scholar]
- 4.Murray IR, Murray SA, MacKenzie K, et al. 2005. How evidence based is the management of two common sports injuries in a sports injury clinic? Br J Sports Med 39:912–916; discussion 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka S, Petersen M, Cameron L. 2001. Prevalence and risk factors of tendinitis and related disorders of the distal upper extremity among U.S. workers: comparison to carpal tunnel syndrome. Am J Ind Med 39:328–335. [DOI] [PubMed] [Google Scholar]
- 6.Riley G 2004. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 43:131–142. [DOI] [PubMed] [Google Scholar]
- 7.Riley GP, Goddard MJ, Hazleman BL. 2001. Histopathological assessment and pathological significance of matrix degeneration in supraspinatus tendons. Rheumatology (Oxford) 40:229–230. [DOI] [PubMed] [Google Scholar]
- 8.Kannus P, Jozsa L. 1991. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am 73:1507–1525. [PubMed] [Google Scholar]
- 9.Tempelhof S, Rupp S, Seil R. 1999. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 8:296–299. [DOI] [PubMed] [Google Scholar]
- 10.Worland RL, Lee D, Orozco CG, et al. 2003. Correlation of age, acromial morphology, and rotator cuff tear pathology diagnosed by ultrasound in asymptomatic patients. J South Orthop Assoc 12:23–26. [PubMed] [Google Scholar]
- 11.Murrell GA, Walton JR. 2001. Diagnosis of rotator cuff tears. Lancet 357:769–770. [DOI] [PubMed] [Google Scholar]
- 12.Khan K, Cook J. 2003. The painful nonruptured tendon: clinical aspects. Clin Sports Med 22:711–725. [DOI] [PubMed] [Google Scholar]
- 13.Housner JA, Jacobson JA, Misko R. 2009. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med 28:1187–1192. [DOI] [PubMed] [Google Scholar]
- 14.Rha DW, Park GY, Kim YK, et al. 2013. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil 27:113–122. [DOI] [PubMed] [Google Scholar]
- 15.McShane JM, Shah VN, Nazarian LN. 2008. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow: is a corticosteroid necessary? J Ultrasound Med 27:1137–1144. [DOI] [PubMed] [Google Scholar]
- 16.Cardinal E, Chhem RK, Beauregard CG. 1998. Ultrasound-guided interventional procedures in the musculoskeletal system. Radiol Clin North Am 36:597–604. [DOI] [PubMed] [Google Scholar]
- 17.Lee KS, Rosas HG. 2010. Musculoskeletal ultrasound: how to treat calcific tendinitis of the rotator cuff by ultrasound-guided single-needle lavage technique. AJR Am J Roentgenol 195:638. [DOI] [PubMed] [Google Scholar]
- 18.Kane D, Greaney T, Bresnihan B, et al. 1998. Ultrasound guided injection of recalcitrant plantar fasciitis. Ann Rheum Dis 57:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson JA, Rubin J, Yablon CM, et al. 2015. Ultrasound-Guided Fenestration of Tendons About the Hip and Pelvis: Clinical Outcomes. J Ultrasound Med 34:2029–2035. [DOI] [PubMed] [Google Scholar]
- 20.Chiavaras MM, Jacobson JA. 2013. Ultrasound-guided tendon fenestration. Semin Musculoskelet Radiol 17:85–90. [DOI] [PubMed] [Google Scholar]
- 21.Housner JA, Jacobson JA, Morag Y, et al. 2010. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med 20:488–490. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand KA, Woo SL, Smith DW, et al. 1998. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med 26:549–554. [DOI] [PubMed] [Google Scholar]
- 23.Ferrero G, Fabbro E, Orlandi D, et al. 2012. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J Ultrasound 15:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James SL, Ali K, Pocock C, et al. 2007. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med 41:518–521; discussion 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabago D, Best TM, Zgierska AE, et al. 2009. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med 43:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McShane JM, Nazarian LN, Harwood MI. 2006. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med 25:1281–1289. [DOI] [PubMed] [Google Scholar]
- 27.Fader RR, Mitchell JJ, Traub S, et al. 2014. Platelet-rich plasma treatment improves outcomes for chronic proximal hamstring injuries in an athletic population. Muscles Ligaments Tendons J 4:461–466. [PMC free article] [PubMed] [Google Scholar]
- 28.Soslowsky LJ, Thomopoulos S, Tun S, et al. 2000. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg 9:79–84. [PubMed] [Google Scholar]
- 29.Thomopoulos S, Williams GR, Soslowsky LJ. 2003. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng 125:106–113. [DOI] [PubMed] [Google Scholar]
- 30.Gimbel JA, Van Kleunen JP, Mehta S, et al. 2004. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech 37:739–749. [DOI] [PubMed] [Google Scholar]
- 31.Reuther KE, Thomas SJ, Tucker JJ, et al. 2014. Disruption of the anterior-posterior rotator cuff force balance alters joint function and leads to joint damage in a rat model. J Orthop Res 32:638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riggin CN. 2018. Development and Utilization of Ultrasound Imaging Techniques to Evaluate the Role of Vascularity in Adult and Aged Rat Achilles Tendon Healing University of Pennsylvania, Philadelphia. [Google Scholar]
- 33.Favata M 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair University of Pennsylvania, Philadelphia, PA. [Google Scholar]
- 34.Derwin KA, Soslowsky LJ, Green WD, et al. 1994. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J Biomech 27:1277–1285. [DOI] [PubMed] [Google Scholar]
- 35.Soslowsky LJ, Carpenter JE, DeBano CM, et al. 1996. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 5:383–392. [DOI] [PubMed] [Google Scholar]


