Abstract
Epithelial cells line the lumen of tubular organs and are key players in their respective functions. They establish a unique luminal environment by providing a protective barrier and by performing vectorial transport of ions, nutrients, solutes, proteins, and water. Complex intercellular communication networks, specific for each organ, ensure their interaction with adjacent epithelial and non-epithelial cells, allowing them to respond to and modulate their immediate environment. In the epididymis, several epithelial cell types work in a concerted manner to establish a luminal acidic milieu that is essential for the post-testicular maturation and storage of spermatozoa. The epididymis also prevents autoimmune responses against auto-antigenic spermatozoa, while ensuring protection against ascending and blood pathogens. This is achieved by a network of immune cells that are in close contact and interact with epithelial cells. This review highlights the coordinated interactions between spermatozoa, basal cells, principal cells, narrow cells, clear cells, and immune cells that contribute to the maturation, protection, selection and storage of spermatozoa in the lumen of the epididymis.
INTRODUCTION
Epithelial cells that line the lumen of several tubular organs establish a unique luminal environment by executing vectorial transepithelial transport of ions, nutrients, solutes and water. This important process often involves intimate crosstalk and collaboration with neighboring cells, both epithelial and non-epithelial. In particular, luminal acidification is a key feature of organs such as the epididymis, kidney, lung, and inner ear (Breton and Brown, 2013). In the epididymis, the establishment of an acidic luminal environment participates in the maturation of spermatozoa by facilitating transfer of proteins from epithelial cells to sperm via extracellular vesicles called epididymosomes (Guyonnet et al., 2011, Sullivan, 2015, Yanagimachi et al., 1985, Zhou et al., 2018), and in the maintenance of sperm quiescence via inhibition of specific sperm channels and transporters (Kirichok et al., 2006, Navarro et al., 2008, Navarro et al., 2007, Visconti, 2009, Visconti et al., 2011).
In addition to their role in transepithelial transport, epithelia also provide a protective barrier and, as such, they are often the first sites of invasion by pathogens (Andrews et al., 2018). Furthermore, some epithelia have an even more complex function, because they also need to provide a balance between tolerance and immune activation. This relates in particular – but not exclusively – to the epithelium lining the lumen of the epididymis, where prevention of autoimmune response against auto-antigenic spermatozoa, while ensuring protection against ascending and blood pathogens, is a key determinant of male fertility (Da Silva and Barton, 2016, Da Silva et al., 2011, Fijak et al., 2018, Voisin et al., 2018).
This review describes the role of epithelial cells in epididymal functions, focusing on the creation of an optimal acidic luminal environment for sperm maturation and storage. The contribution of immune cells - which are in close proximity to the epithelium - to the maintenance of the blood-epididymis barrier, their potential role in the establishment of tolerance to spermatozoa, and their ability to mount an immune response against ascending and blood pathogens will also be discussed.
Composition and regulation of the epididymal epithelium
Epithelial cells in the epididymis form the blood-epididymis barrier, which ensures the maintenance of a unique luminal environment that is conducive to sperm maturation, protection and storage (Gregory and Cyr, 2014, Mital et al., 2011). The epididymal epithelium is pseudostratified and is composed of three main cell types, clear cells (CCs), principal cells (PCs) and basal cells (BCs). A fourth epithelial cell, known as narrow cell (NC), is located in the very proximal epididymal region, the so-called initial segments (IS) (Breton et al., 2016, Robaire B, 2006).
Clear Cells (CCs) and Narrow Cells (NCs):
Both CCs and NCs actively secrete protons into the lumen via a proton pump (V-ATPase), which is located in apical microplicae and sub-apical vesicles (Breton and Brown, 2013, Breton et al., 2016). This process requires the hydrolysis of ATP, and NCs and CCs contain a very large number of mitochondria compared to their neighboring cells. As such, they are part of the “mitochondria-rich cell” family, which features cells that have common phenotypic characteristics, including apical microplicae, an active endocytotic pathway, high V-ATPase expression, and cytosolic carbonic anhydrase type II, in addition to their high mitochondria content (Brown and Breton, 1996). The V-ATPase is a complex enzyme that is composed of several subunits that form a transmembrane complex called the V0 domain, and a catalytic complex called the V1 domain, which is attached to the V0 domain (Hinton et al., 2009). A particular set of subunit isoforms governs the expression of the V-ATPase in the plasma membrane of professional proton-secreting cells such as epididymal NCs and CCs, and kidney intercalated cells (Breton and Brown, 2013, Pietrement et al., 2006). These isoforms include the transmembrane V0 subunit a4, and the cytosolic V1 subunit B1. Defects in V-ATPase expression following deletion of the transcription factor Foxi1 results in abnormally elevated epididymal luminal pH and infertility of male mice due to the inability of their sperm to move up the female tract (Blomqvist et al., 2006, Vidarsson et al., 2009).
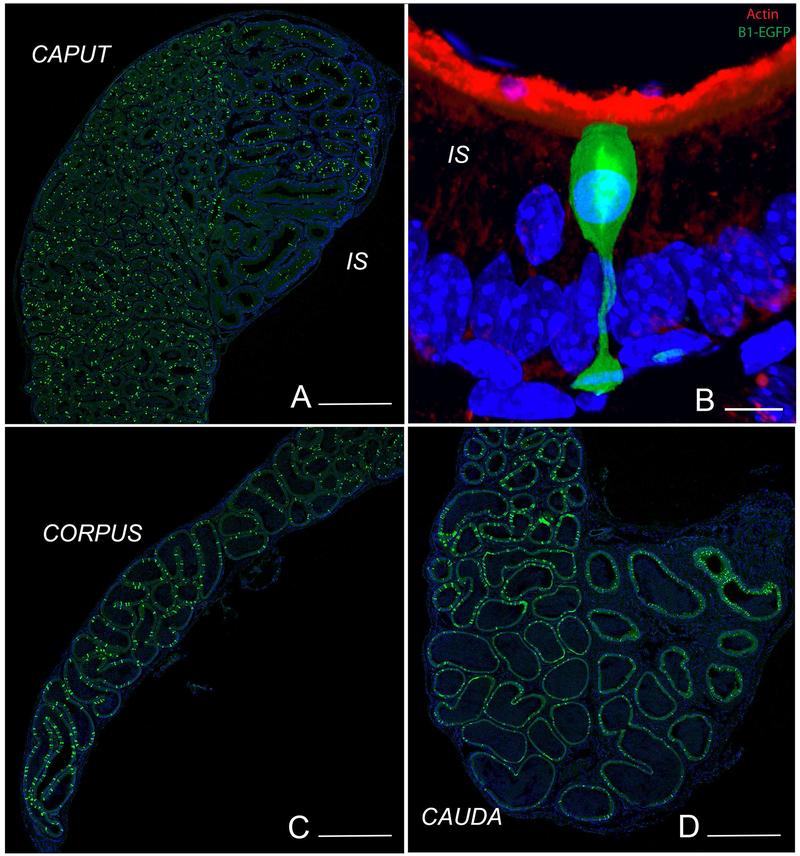
In order to better characterize CCs and NCs, we previously made a transgenic mouse that expresses EGFP driven by the V-ATPase B1 promoter in B1-expressing cells. These mice express high level of EGFP not only in epididymal CCs and NCs, but also in kidney intercalated cells and lung non-ciliated cells (Miller et al., 2005). These mice allowed the visualization of NCs in the IS, and CCs in all other epididymal regions (Fig. 1A, C, D). As previously described, NCs have a distinct “champagne glass” morphology: their nuclei are located more apically compared to adjacent PCs, and they have a very narrow body that widens as it connects to the basement membrane (Fig. 1B). In contrast, CCs have a more cuboidal appearance.
Figure 1). Visualization of NCs and CCs in the epididymis of transgenic mice expressing EGFP under the control of the promoter of the V-ATPase B1 subunit (B1-EGFP).
EGFP+ NCs (green) are located in the IS, and EGFP+ CCs (green) are located in the caput (A), corpus (C) and cauda (D) regions. B) In the IS, NCs have a “champagne glass” appearance and their nuclei are located in the apical region of the epithelium, compared to adjacent PCs. A dense network of filamentous actin is seen in the apical stereocilia of PCs (labeled in red using phalloidin). Nuclei are labeled in blue using DAPI. Bars: A, C, D = 500 μm, B = 5 μm.
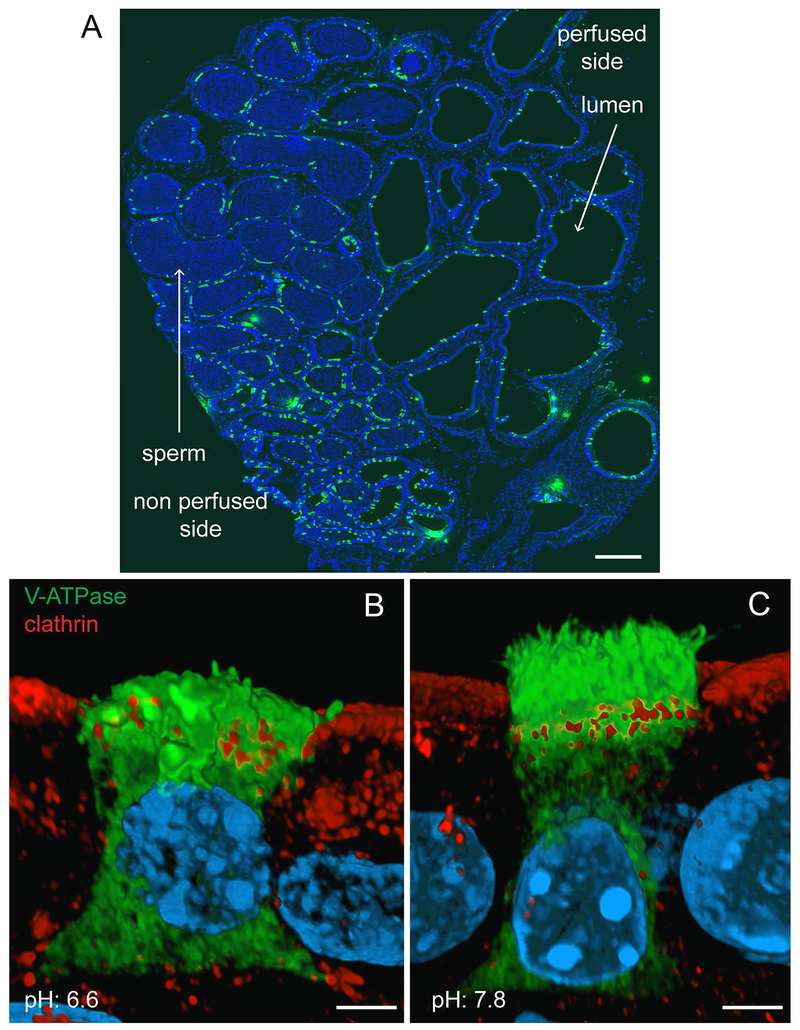
The larger lumen of the cauda epididymis is conducive to performing functional analysis of the epithelium through luminal perfusion in vivo (Fig. 2A). This procedure allows for the study of epithelial cells while they reside in their native environment, and to determine how they are modulated by luminal factors, without affecting the basolateral side of the epithelium. In this manner, we found that proton secretion in CCs is activated by an elevation of luminal pH, which induces the trafficking of V-ATPase-containing vesicles from the apical pole to the plasma membrane. This results in the formation and elongation of numerous apical projections (Battistone et al., 2019, Beaulieu et al., 2005, Park et al., 2017, Pastor-Soler et al., 2003). Based on their appearance on thin (5 µm) sections used for immunofluorescence labeling, we initially named these protrusions “microvilli” (Breton et al., 1996). However, we later visualized these membrane structures using Helium Ion Microscopy and found they instead appear as “microplicae” (Paunescu et al., 2014), in agreement with a previous scanning electron microscopy observation by the Cooper group (Hamilton et al., 1977). More recently, Airyscan confocal microscopy allowed the visualization of these microplicae after 3D reconstruction of optical z-plane serial sections of CCs labeled for the V-ATPase B1 subunit (Fig. 2B, C). While “resting” CCs perfused luminally at the control pH of 6.6 exhibited short V-ATPase-positive apical microplicae and showed mainly intracellular localization of the V-ATPase (Fig. 2B; green), “activated” CCs perfused at the alkaline pH of 7.8 had numerous microplicae labeled for the V-ATPase and less intracellular reactivity (Fig. 2C; green) (Battistone et al., 2019, Beaulieu et al., 2005, Park et al., 2017, Pastor-Soler et al., 2003). Addition of bicarbonate, angiotensin II (ANGII), ATP, or adenosine to the luminal perfusate all induced the redistribution of V-ATPase from intracellular vesicles to the apical membrane (Beaulieu et al., 2005, Belleannee et al., 2010, Pastor-Soler et al., 2003, Shum et al., 2008). Luminal pH, bicarbonate and adenosine all work by activating the cAMP/PKA pathway, either through the adenosine receptor ADORA2B, or in the case of luminal alkalinization and bicarbonate, by activating the bicarbonate-sensitive soluble adenylyl cyclase sAC (Battistone et al., 2018, Belleannee et al., 2010, Pastor-Soler et al., 2003). Intracellular calcium participates in the activation of CCs by luminal ATP, which binds the P2X4 receptors located in the apical membrane (Battistone et al., 2018, Belleannee et al., 2010). Stimulation of pH-activated ATP purinergic receptors such as P2X4 (Clarke et al., 2000, Holzer, 2003, Huang et al., 2014) could provide a feedback mechanism by which CCs would increase their rate of proton secretion in response to elevation in luminal pH. Alternatively, hydrolysis of ATP to form adenosine could play a role in pH sensing via pH-dependent ectonucleotidases that are also located on the apical surface of the epididymal epithelium. Indeed, we detected a higher luminal adenosine concentration and a lower ATP concentration when the cauda epididymis was perfused at the alkaline pH of 7.8 versus pH 6.6 (Battistone et al., 2019). This was attributed to activation by alkaline pH of the ectonucleotidases, ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP), and ectonucleoside triphosphate diphosphohydrolase (ENTPD), which hydrolyze ATP into ADP, as well as ecto-5’-nucleotidase (NT5E), which hydrolyzes AMP to produce adenosine (Yegutkin, 2008).
Figure 2). Effect of luminal pH on V-ATPase sub-cellular localization in CCs in the cauda epididymis.
Mouse cauda epididymidis was perfused in vivo, and cryostat sections of fixed tissues were stained for the V-ATPase B1 subunit (green). Nuclei are labeled in blue with DAPI. A) Luminal spermatozoa are absent from the perfused tubules. Luminal V-ATPase positive CCs are detected in the perfused and non-perfused regions. B) 3D reconstruction by Airyscan microscopy of a CC perfused with a solution adjusted to the control pH of 6.6. Labeling for the endocytic marker clathrin (red) was performed to identify the apical border of the cell. Short V-ATPase-labeled microplicae and intracellular V-ATPase labeling are observed in this “resting” cell (green). Adjacent PCs are seen with intracellular clathrin labeling. C) 3D reconstruction by Airyscan microscopy of a CC perfused with a solution adjusted to the alkaline pH of 7.8. Double-labeling for V-ATPase (green) and clathrin (red) was performed. Numerous long V-ATPase-labeled microplicae are observed in this “activated” cell, and less intracellular V-ATPase labeling is detected (green). Adjacent PCs are seen with intracellular clathrin labeling. Bars: A = 400 μm; B, C = 2.5 μm. (Modified from (Battistone et al., 2019).
Impairment of purinergic regulation leads to several diseases, including male infertility, and numerous purinergic receptors and ectonucleotidases have been described in the epididymis (Banks et al., 2010, Belleannee et al., 2010, Burnstock, 2014, Gorodeski, 2015, Kauffenstein et al., 2014, Martin-Satue et al., 2010, Martin-Satue et al., 2009, Mulryan et al., 2000, Robson et al., 2006). Thus, purinergic signaling would allow CCs to respond to variations in luminal pH in order to maintain an acidic lumen, which as discussed above, is essential for male fertility (Battistone et al., 2019). Importantly, our previous studies point toward a role for principal cells (PCs) in ATP secretion, and their communication with CCs via cell-cell crosstalk to regulate luminal pH. In the next section, we will describe the direct and indirect participation of PCs in luminal acidification.
Principal cells (PCs)
The contribution of PCs to the establishment of an acidic luminal environment in the epididymis is complex and relies on different mechanisms depending on their location within the organ. In the IS, PCs reabsorb bicarbonate via several transporters and enzymes that are also used by kidney proximal tubules (Breton, 2001, Caflisch and DuBose, 1990, Jensen et al., 1999a, Jensen et al., 1999b, Levine and Marsh, 1971). In the cauda, PCs have the ability to secrete either bicarbonate or protons depending on physiological cues. CFTR, located in the apical membrane of PCs, mediates bicarbonate secretion and is activated following stimulation with adenosine, genistein, adrenergic agonists, and neurotransmitters on the basolateral side of the epithelium (Carlin et al., 2003, Chan et al., 1994, Leung and Wong, 2000, Sedlacek et al., 2001). By monitoring the luminal perfusate after in vivo perfusion of the cauda epididymis, we measured a rapid pH recovery from an acidic pH of 5.8 towards the normal pH of 6.6, which was inhibited by CFTRinh172 indicating the participation of CFTR-dependent bicarbonate secretion (Park et al., 2017). Recovery of the luminal pH also occurred when the epididymis was perfused at the alkaline pH of 7.8, indicating proton secretion under these conditions. While this process was partially impaired by the V-ATPase inhibitor concanamycin A, showing the contribution of CCs, a substantial recovery was still detected. The remaining proton secretion was in fact due to proton secretion by PCs via the apical sodium/hydrogen exchanger NHE3 (Park et al., 2017), in agreement with earlier work showing that luminal acidification depends on the presence of luminal sodium in the rat and mouse cauda epididymis (Au and Wong, 1980, Bagnis et al., 2001, Kaunisto et al., 2001). Altogether, these results indicate the direct participation of both CCs and PCs in luminal acidification following perfusion at alkaline pH. Luminal addition of the permeant form of cAMP, cpt-AMP, partially inhibited this luminal recovery, a result that was surprising given the known stimulatory effect of cAMP on the V-ATPase in CCs. Interestingly, while luminal alkalinization induced the redistribution of NHE3 from intracellular vesicles to the apical membrane in PCs, similar to its action on the V-ATPase in CCs, luminal cpt-cAMP prevented this pH-induced response. Thus, either activation of CFTR-dependent bicarbonate secretion or inhibition of NHE3-dependent proton secretion could explain the partial inhibition of pH recovery from an alkaline pH observed in the presence of cpt-cAMP. The respective response of CCs and PCs to physiological cues might, therefore, depend on the origin of the extracellular stimulus, with a pro-acidification increase in intracellular cAMP occurring in CCs following activation by luminal factors, while a pro-alkalinization effect would occur in PCs upon stimulation by basolateral agonists.
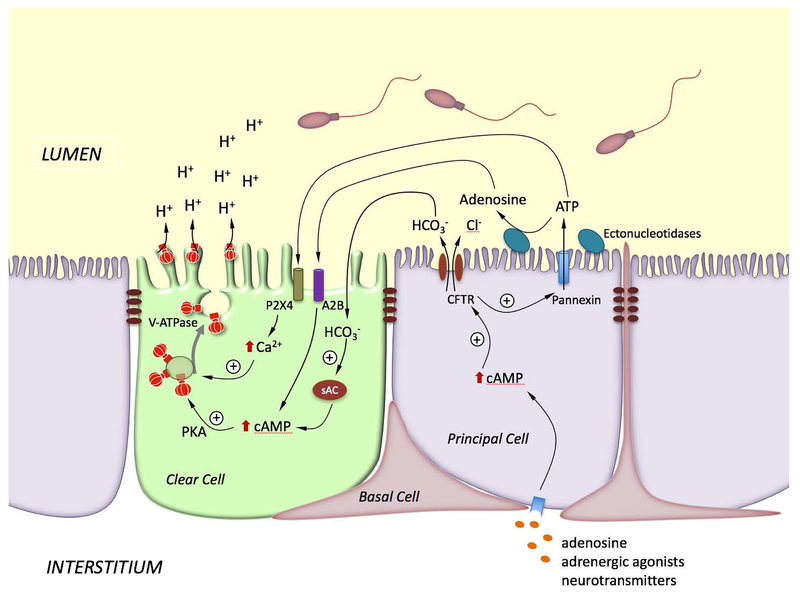
In addition to their direct role in modulating luminal pH, PCs also contribute to the regulation of proton secretion by CCs via paracrine signaling. Our PC-CC crosstalk model is illustrated in Fig. 3. Elevation of cAMP in PCs following stimulation on the basolateral side by circulating agonists would lead to an increase in CFTR-mediated bicarbonate secretion, which was proposed to occur during sexual arousal to “prime” spermatozoa before ejaculation (Carlin et al., 2003). In this setting, full activation of epididymal sperm would be prevented by the presence of inhibitory factors, as well as low sodium and high potassium luminal concentrations (Verma, 2001, Visconti et al., 2011). Nevertheless, prolonged elevation of bicarbonate might be detrimental to sperm viability. CCs would then re-acidify the lumen following activation of sAC by luminal bicarbonate and the subsequent increase in cAMP in CCs. In addition to its role in bicarbonate secretion by PCs, CFTR also participates in ATP secretion by these cells. Indeed, significant ATP secretion occurs in the perfused cauda, which was reduced by luminal perfusion with the CFTR inhibitor, CFTRinh (Ruan et al., 2012). The epididymal principal cell line (DC2) also secretes ATP, and this process is enhanced by adrenaline and forskolin, and is reduced by the pannexin inhibitor carbenozolone (CBX) (Ruan et al., 2012). In the perfused cauda, CBX partially prevented luminal pH recovery from an alkaline pH, and the alkaline pH-induced V-ATPase apical accumulation. While CBX did not modify the low ATP concentration detected at pH 7.8, it decreased adenosine concentration. Altogether, these results indicated that ATP secretion by PCs, followed by its rapid hydrolysis to produce adenosine, participates in the pH-induced activation of V-ATPase-dependent proton secretion by CCs (Battistone et al., 2019). In this model, CFTR is a key player in the PC-CC crosstalk as it mediates both bicarbonate and ATP transport.
Figure 3). Model showing the coordinated interaction between PCs and CCs for the activation of V-ATPase-dependent proton secretion in CCs.
Stimulation of PCs by basolateral factors such as adenosine, adrenergic agonists or neurotransmitters elevate intracellular cAMP, which in turns activates bicarbonate secretion via apical CFTR. Luminal bicarbonate then reaches CCs, where it activates the soluble adenylate cyclase (sAC) to produce cAMP. CFTR also stimulates ATP secretion via pannexin activation. ATP either activates P2X4 located on the apical membrane of CCs, or is hydrolyzed by extracellular ectonucleotidases to produce adenosine. Adenosine activates the GPCR receptor ADORA2B (A2B) located on the apical membrane of CCs, leading to elevation in intracellular cAMP. Activation of the cAMP/PKA pathway by bicarbonate or adenosine induces V-ATPase apical membrane accumulation. On the other hand, activation of P2X4 by luminal ATP in CCs induces an elevation of intracellular calcium, which also facilitates V-ATPase membrane accumulation. The redistribution of V-ATPase from intracellular vesicles to the apical membrane results in the formation and elongation of V-ATPase-enriched microplicae in CCs and stimulates proton secretion.
Most male patients with cystic fibrosis (CF), the disease caused by several CFTR mutations, or individuals carrying “silent” CFTR mutations that do not cause CF, are infertile due to congenital bilateral absence of the vas deferens (CBAVD) or epididymal morphological defects (Cuppens and Cassiman, 2004, Wosnitzer, 2014, Yefimova et al., 2018). While these male reproductive tract anomalies were reproduced in CFTR KO pigs and ferrets (Pierucci-Alves et al., 2011, Sun et al., 2010), the first CFTR KO male mice produced were reported to be fertile (O’Neal et al., 1993, Snouwaert et al., 1992). However, more detailed analysis subsequently demonstrated that CFTR KO mice are in fact sub-fertile, that they have vas deferens morphological abnormalities, and produce sperm that have significantly lower fertilizing capacity (Reynaert et al., 2000, Xu et al., 2007), reviewed in (Wilke et al., 2011)). While defects in the anion channel function of CFTR was originally considered to be the primary cause of CF, it is now generally accepted that CFTR has the ability to interact with and regulate several ion channels and transporters (Berdiev et al., 2009, Guggino and Stanton, 2006, Li and Naren, 2010, Lim et al., 2017, Pietrement et al., 2008), and can even modulate pro-inflammatory signaling pathways (Dong et al., 2015). In addition, CFTR regulate cell proliferation and differentiation via its interaction with tight junction proteins, a function that we will discuss later in this review. Therapeutic efforts to develop novel treatments for CF or other diseases caused by CFTR mutations have now started to consider the complex functions of CFTR, in addition to its “classical” role as an anion channel.
Basal cells (BCs)
BCs are located at the base of pseudostratified epithelia and were originally believed to never contact the lumen. However, a subpopulation of BCs in several organs such as the epididymis, the prostate and the upper airway tract send long and slender projections between adjacent epithelial cells in the direction of the lumen (Shum et al., 2008, Veri et al., 1993). We named these projections axiopodia and showed that they can cross the tight junction barrier and reach the epididymal lumen (Roy et al., 2016, Shum et al., 2008). Interestingly, BCs with axiopodia are mainly located in the proximal cauda epididymis in the rat and in the IS of the mouse epididymis, indicating that their formation might depend on local stimuli. To visualize BCs in vivo, we generated transgenic mice that express the fluorescent protein tdTomato under the control of the promoter of the BC specific gene keratin 5. Using intravital multi-photon microscopy we found that BC axiopodia periodically extend and retract over time, that this movement is controlled by c-Src and MEK1/2-ERK1/2, and that inhibition of tyrosine kinase activity induces axiopodia retraction (Roy et al., 2016). We proposed that this unexpected cellular plasticity – called Periodic Axial Motility (PAM)-may reflect a unique mechanism by which BCs in pseudostratified epithelia send out “antennae” to sample the luminal environment, and subsequently influence the behavior of adjacent cells.
Overall, the function of BCs in the epididymis still remains poorly characterized. Previous studies indicated their role as endocrine mediators and protection against reactive oxygen species (Hermo et al., 1994, Nonogaki et al., 1992, Wong et al., 1999). They were originally believed to express macrophage markers and have immunological properties (reviewed in (Da Silva and Smith, 2015). However, later studies showed the presence of a dense network of immune cells (collectively referred to as mononuclear phagocytes; MPs) that are located alongside BCs at the base of the epithelium, and that BCs and MPs represent distinct populations of cells (Shum et al., 2014) (Fig. 4). More recently, genomic profiling showed enrichment of genes that encode proteins involved in cell adhesion, cytoskeletal arrangement, ion transport, cellular signaling, and inflammatory responses in BCs (Mandon et al., 2015). Of note some of these genes, such as TP63, have been previously reported in adult stem cells in other tissues (Mandon et al., 2015, Murashima et al., 2011), and it was suggested that BCs may represent an epididymal stem cell population. Indeed, cultures of BCs isolated from rat epididymis showed characteristics of adult progenitor cells with a decrease in keratin 5 expression, together with an increase in keratin 8 expression (Mandon et al., 2015).
Figure 4). Confocal microscopy visualization of BCs and MPs in the IS of CD11c-EYFP transgenic mice.
Immunofluorescence labeling for the BC specific marker keratin 5 (KRT5; red) shows abundant BCs with luminal reaching axiopodia in the IS. CD11c-EYFP positive MPs (green) also send luminal reaching projections, but they are clearly distinct from BCs. Nuclei are labeled in blue with DAPI. Bar = 15 μm.
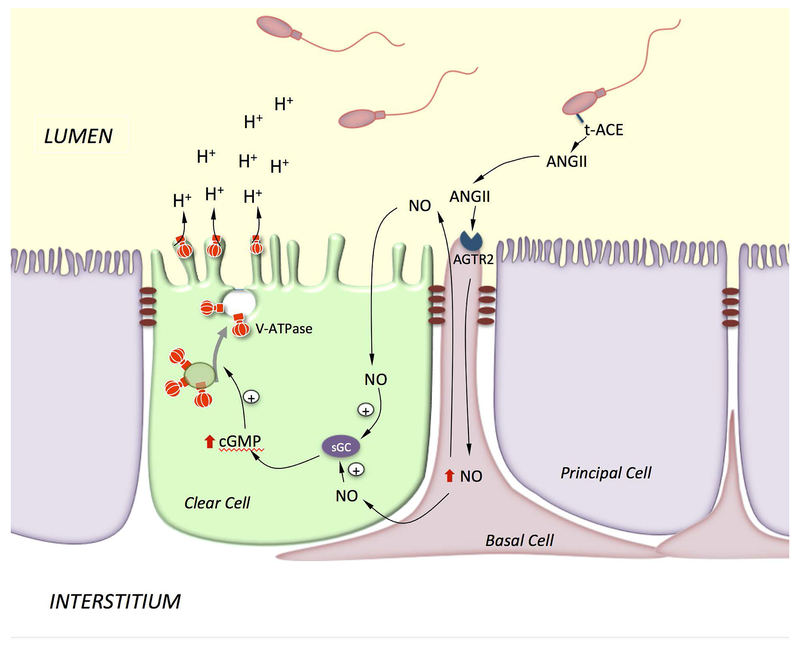
BCs were proposed to participate in transepithelial transport either directly, or indirectly via the paracrine regulation of PCs following basolateral stimulation (Cheung et al., 2005, Hermo et al., 2004, Leung et al., 2004). In addition, we previously showed that, in the rat epididymis, BCs also regulate the function of CCs via their axiopodia that can cross the blood/epididymis barrier to sample the luminal environment (Shum et al., 2008). In this way, activation of the ANGII type II receptor (AGTR2) in BCs by luminal ANGII stimulates proton secretion in adjacent CCs via activation of the NO/cGMP pathway. Our proposed cell-cell crosstalk model for activation of CCs following stimulation of AGTR2 in BCs is illustrated in Figure 5.
Figure 5). Model showing BC-CC crosstalk via the ANGII signaling pathway.
Axiopodia in BCs cross the TJs and are in contact with the lumen. Luminal ANGII stimulates AGTR2 in BCs and induces the production of NO, which then diffuses out to reach CCs, where it produces cGMP via activation of soluble guanylate cyclase (sGC). cGMP triggers V-ATPase apical accumulation and increases proton secretion by CCs. ANGII is produced by testicular ACE (t-ACE), which is attached to the sperm membrane via a GPI linker.
As mentioned above, the formation of BC axiopodia is dependent upon their location within the epididymis indicating their regulation by local cues. Treatment of mice with the anti-androgen flutamide did not affect the number of BCs with axiopodia in the mouse IS, indicating that systemic androgens do not regulate their formation/elongation. This observation was supported by the fact that BC axiopodia appear early after birth, before the elevation of circulating androgens that accompanies puberty. We then explored the possibility that they might be regulated by luminal factors that are secreted by the testis which, together with androgens, contribute to activating the MEK1/2-ERK1/2 pathway in the IS (Xu et al., 2011, Xu et al., 2013, Xu et al., 2010). As such, we performed efferent duct ligation (EDL) to block the entry of lumicrine factors into the epididymal lumen (Kim and Breton, 2016). There was a dramatic retraction of BC axiopodia after EDL, as well as a wave of apoptosis in BCs that occurred 1 day after EDL. This was followed by an increase in proliferation of a subset of BCs after 2 days. Therefore, lumicrine factors seem to maintain the luminal-sensing property of BCs in the mouse IS. In contrast, a marked reduction in the number of BCs with axiopodia was induced in the corpus epididymidis of rats treated with flutamide for 2 weeks (Shum et al., 2013). More distal regions might, therefore, rely on circulating androgens for the maintenance of epithelial differentiation, in agreement with the known dependence on 5α-reductase in epithelial homeostasis in the rat distal epididymis (Henderson et al., 2006). Thus, BCs have the remarkable ability of changing their shape in response to physiological cues in both the mouse and rat epididymis. This unexpected, highly dynamic property of BC axiopodia might ensure regular and efficient sampling and decoding of luminal signals. Indeed, because BCs are not always in contact with the luminal environment, receptors are less likely to become desensitized and would, therefore, provide a uniform response in the presence of a constant luminal signal (Roy et al., 2016). In the rat epididymis, luminal ANGII can be produced either by epithelial cells located upstream of the epithelium or by spermatozoa through the activity of the testicular form of angiotensin converting enzyme (t-ACE), which is linked to the sperm membrane via a GPI linker (Kondoh et al., 2005). During epididymal maturation t-ACE is released from spermatozoa into the luminal environment (Gatti et al., 1999). It is, therefore, likely that spermatozoa communicate with surrounding epithelial cells via the production of ANGII. By analogy with other cell types, activation of ANGII in BCs stimulates the production of nitric oxide, which then diffuses out to reach adjacent CCs, where it initiates production of cGMP by soluble guanylate cyclase located in CCs (Shum et al., 2008). This leads to the redistribution of V-ATPase in the apical membrane and stimulation of proton secretion by CCs. In agreement with this notion, infertility in ACE KO males is markedly reduced due to low sperm quality and their inability to fertilize an egg, which is a consequence of the absence of t-ACE (Esther et al., 1996, Hagaman et al., 1998). Thus, the lack of luminal ANGII in ACE KO males might result in impairment of luminal acidification, which would have detrimental consequences on sperm quality, similar to the male infertility phenotype induced in FOXI-1 KO male mice (Blomqvist et al., 2006).
Modulation of tight junctions (TJs)
Epithelial cells are attached to each other by TJs. TJs are constituents of the blood-epididymis barrier, and contribute to the establishment and maintenance of the unique luminal environment in which epididymal spermatozoa transit (Cyr et al., 2018, Cyr and Gregory, 2018, Gregory and Cyr, 2014, Kim and Breton, 2016, Mital et al., 2011). TJs ensure the polarity of epithelial cells by separating their apical and basolateral domains and by acting like a fence that prevents membrane proteins from diffusing from one membrane domain to the other; they also regulate the paracellular transport of ions, other molecules and water (Rowart et al., 2018). The integrity of the blood-epididymis barrier is reduced with ageing, and it is modulated by androgens and the TGFβ pathway (Cyr et al., 2018, Gregory et al., 2001, Stammler et al., 2013). In addition, environmental toxins alter the function of epididymal TJs with impairment of male fertility (Cyr et al., 2018).
TJs are multiprotein complexes composed of several transmembrane proteins, including claudins, occludin and Junctional Adhesion Molecules, as well as intracellular zona-occludens (ZO) proteins that interact with the actin cytoskeleton. Claudin (Cldn) depletion in mice induced the disappearance of TJ strands visualized by electron microscopy, illustrating their role in the structural integrity of TJs. Occludin KO mice have TJs that appear morphologically intact (Saitou et al., 2000), but show abnormalities such as chronic inflammation of the gastric epithelium and male infertility indicating the regulatory role of occludin. In adult mice, occludin is present in TJs of the caput, corpus and cauda epididymidis, but it is absent from the IS, despite the presence of extensive TJs in this segment (Cyr et al., 2007). Several claudins, including Cldn-1, Cldn-3 and Cldn-4 have been localized in all epididymal regions (Cyr et al., 2018, Cyr et al., 2007, Kim and Breton, 2016). Interestingly these proteins are not restricted to TJs, but they are also located along the lateral membrane of PCs (Gregory et al., 2001, Kim and Breton, 2016, Shum et al., 2008). In addition, Cldn-1 and Cldn-4, but not Cldn-3, showed enrichment in the basolateral membrane of BCs (Dube et al., 2010, Kim and Breton, 2016). In the human epididymis, Cldn -2, Cldn-7 and Cldn-8 were observed in addition to Cldn-1, Cldn-3 and Cldn-4, and siRNA mediated knock-down of Cldn-1, Cldn-3, Cldn-4 and Cldn-7 reduced transepithelial resistance in a human caput epididymal cell line, further indicating their functional role in the establishment of the blood-epididymis barrier (Dube et al., 2010). The significance of the localization of claudins outside of TJs remains unknown, but we previously proposed that their expression in the membrane of BCs might provide a “molecular ladder” that would support the elongation of BC axiopodia along the membrane of adjacent epithelial cells during the process of Periodic Axial Motility (Kim and Breton, 2016, Roy et al., 2016, Shum et al., 2008).
In contrast to claudins, all three members of the ZO family, ZO-1, ZO-2, and ZO-3 are restricted to TJs in the epididymis (Gregory and Cyr, 2014, Kim and Breton, 2016). Inhibition of MEK1/2 reduced the expression of Cldn-1, Cldn-4 and ZO-2, but increased expression of ZO-3 and occludin without affecting ZO-1 and Cldn-3 expression (Kim and Breton, 2016). Expression of these proteins was not affected after MEK1/2 inhibition in all other epididymal segments. Interestingly, occludin mRNA was detected in the control mouse IS, and its level was not affected after MEK1/2 inhibition. Expression of occludin mRNA together with absence of protein in the IS indicate that maintenance of the MAPK/ERK pathway in an activated state in this segment prevents occludin mRNA translation. The functionality of this regulation remains obscure.
In addition to their role in the barrier function of epithelia, TJs also control cell proliferation and differentiation during embryonic development and tissue repair (Balda and Matter, 2000, Lima et al., 2010, Sourisseau et al., 2006). This occurs via modulation of gene expression following the recruitment of the transcription factor ZONAB from nuclei to the TJs, a process that is mediated by ZO-1. During development, as tubules elongate, the subcellular localization of ZONAB determines when epithelial cells switch from a proliferative (with ZONAB located in nuclei) to a differentiated state (ZO-1-bound ZONAB at TJs). We recently showed that CFTR interacts with ZO-1 and participates in the retention and/or recruitment of ZONAB at TJS. Thus, CFTR is a central element for the regulation of epithelial cell plasticity during development (Ruan et al., 2014). We found that CFTR precedes ZO-1 expression in the Wolffian duct, further indicating its participation in tubulogenesis. This notion was supported by the absence of tubular structures in a 3D epididymal DC2 cell culture model after CFTR inhibition or knockdown, in sharp contrast with the formation of well-defined tubular structures in control cells revealed by 3D confocal microscopy. In addition, the epididymis of CFTR KO mice had several features typical of an immature organ, including a smaller organ weight, reduction in tubule diameter, increase and decrease in epithelial cell height in the cauda and IS regions, respectively, and reduction in markers of epithelial cell differentiation.
As mentioned above, mutations in the CFTR gene are major causes of male infertility in humans (Wosnitzer, 2014, Yefimova et al., 2018). We proposed that this might be attributed to impairment of epithelial development and repair secondary to deregulation of the ZO1-ZONAB pathway, which would lead to the down-regulation of differentiation-associated genes and upregulation of proliferation-associated genes. This would then induce progressive weakening of tissues such as the epididymis, rendering them more susceptible to progressive stress and injury. This cascade of events would occur in addition to the well-recognized role of CFTR in the transepithelial transport of chloride and secondary water movement that was discussed earlier in this review.
Epididymal immunology
In addition to their barrier function, epithelia represent the first line of defense against infections. In some epithelia, this immunological response must be balanced with tolerance against non-pathogenic entities (Gunther and Seyfert, 2018). In particular, the epididymal tubule must ensure the prevention of autoimmune responses against auto-antigenic spermatozoa, while providing protection against ascending and blood pathogens (Da Silva and Barton, 2016, Da Silva and Smith, 2015, Fijak et al., 2018, Voisin et al., 2018). Epididymal innate immunity is partially controlled by beta defensins, which are abundant in the luminal compartment (Ribeiro et al., 2016), and is governed by several types of immune cells that populate the epididymal epithelium and interstitium. Interestingly, epididymal carcinomas are extremely rare indicating a finely-tuned regulation of tolerance against sperm cells, while allowing protection against cancer cells and pathogens.
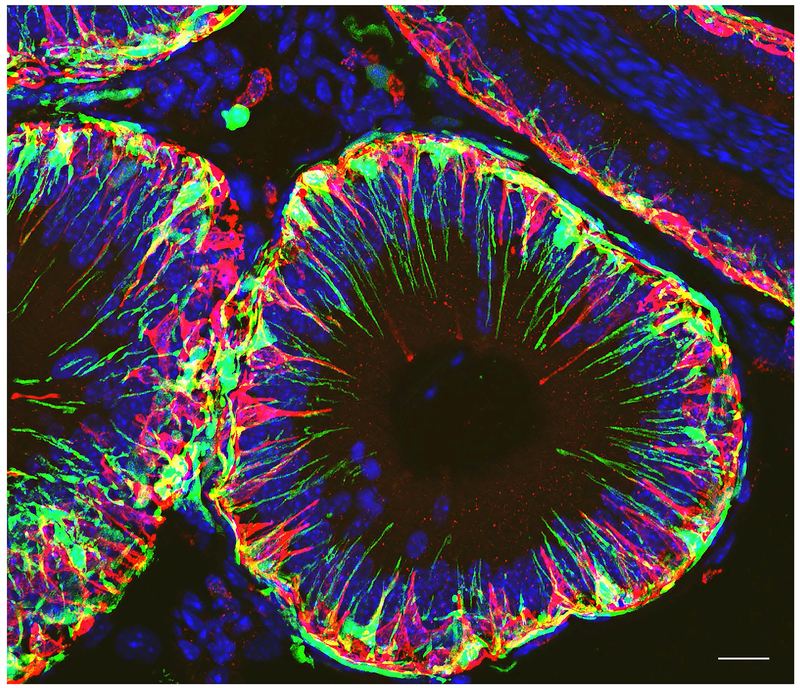
The immune environment of the epididymis is composed of a variety of cells including monocytes, macrophages, and dendritic cells, as well as B cells and T cells (Da Silva and Barton, 2016, Da Silva et al., 2011, Da Silva and Smith, 2015, Pierucci-Alves et al., 2018, Voisin et al., 2018). Transgenic mice expressing fluorescent surface markers specific for immune cells, such as CD11c (integrin alpha X) and CX3CR1 (a G-protein coupled chemokine receptor), have allowed their morphological and functional characterization in several organs, including the epididymis (Da Silva and Barton, 2016, Da Silva et al., 2011, Smith et al., 2014). These markers are expressed by sub-sets of immune cells referred to as mononuclear phagocytes (MPs) that include monocytes, dendritic cells and macrophages (Jakubzick et al., 2017). For example, CX3CR1-GFP transgenic mice revealed a dense cellular network in all epididymal segments (Fig. 6). Interestingly, similar to BCs, CX3CR1+ MPs showed distinct morphological features depending on their location within the organ. While CX3CR1+ cells are intimately interconnected with epithelial cells in the IS and send numerous luminal reaching projections (Fig. 6A), they simply cover the base of the epithelium in the cauda (Fig. 6G). In the caput region, only a few MPs had short intraluminal projections (Fig. 6D, arrow). CX3CR1+ cells were also located in the interstitium in all epididymal segments. Double-labeling for the macrophage marker F4/80 revealed a functional diversity in the proximal versus distal epididymal regions. While a large fraction of CX3CR1+ cells also expressed F4/80 in the IS (Fig. 6A-C) and caput (Fig. 6D-F), some F4/80+ cells were negative for CX3CR1 in the interstitium of the cauda region (Fig. 6G-I). The functional significance of this regional diversity is currently being investigated in our laboratory.
Figure 6). Confocal microscopy showing a dense network of mononuclear phagocytes in all epididymal segments.
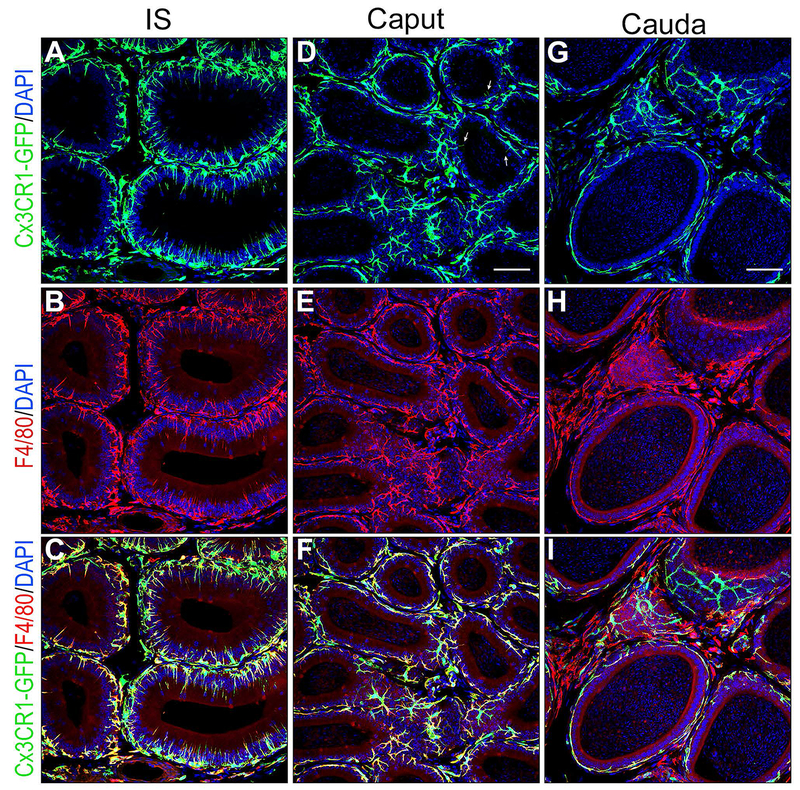
Epididymis of CX3CR1-EGFP transgenic mice was labeled for the macrophage marker F4/80 (red). A,B,C) In the IS, most CX3CR1 positive MPs (green) are also labeled for F4/80 identifying them as macrophages. The majority of macrophages are in close proximity with the epithelium and show numerous intraepithelial projections extending toward the lumen. D,E,F) In the caput, most CX3CR1 positive MPs (green) are also labeled for F4/80 identifying them as macrophages. These macrophages are in close proximity with the epithelium but only a few rare cells now send intraluminal projections towards the lumen (arrows in D). A significant number of cells positive for both CX3CR1 and F4/80 are located in the interstitium. G, H, I) In the cauda, cells positive for CX3CR1 and F4/80 are located next to the epithelium but they do not extend intraepithelial projections. In the interstitium, a mixed population of cells double positive for CX3CR1 and F4/80, and cells positive for F4/80 but negative for CX3CR1 are detected. Nuclei are labeled in blue with DAPI.
In the IS, CX3CR1+ cells have a remarkable morphological and phenotypical plasticity, which was revealed following EDL (Smith et al., 2014). As mentioned above, EDL induces a wave of apoptosis in epithelial cells of the IS. CD11c+ and CX3CR1+ MPs readily engulfed these apoptotic cells, allowing their rapid clearance, while the epithelium preserved its overall architecture and maintained ZO-1 staining in TJs. Flow cytometry analysis performed two days after EDL showed that the most affected subsets of MPs in the IS were CD11b+ cells expressing intermediate levels of CD11c and F4/80. These results are in agreement with the fact that IS CX3CR1-EGFP intraepithelial cells also express the macrophage marker F4/80 (Fig. 6A-C). It was then proposed that these cells may also clear defective epithelial cells and abnormal spermatozoa in the steady-state epididymis, as well as luminal pathogens after an infection (Da Silva and Smith, 2015, Smith et al., 2014). This raised the question of whether these cells can also induce tolerance by directly acquiring material originating from spermatozoa. A sperm quality control has previously been proposed (Guiton et al., 2013, Jrad-Lamine et al., 2013, Sutovsky, 2003), and MPs in the IS are well positioned to exert this function. The concept that spermatozoa undergo a selection process in the epididymis is attractive, particularly in the IS where they are still at low concentration when they enter this segment. In addition, the lumen of the IS tubule is small allowing close interaction between each sperm cell and the epithelium, making the process of sampling via MP luminal reaching projections possible. This process would require that they have the ability to present antigens to T cells. In fact, we previously showed that double positive CD11c+CD11b+ dendritic cells isolated by FACs (after negative selection of T cells, B cells, natural killer cells and granulocytes) present antigens to naïve CD4+ and CD8+ T cells in vitro (Da Silva et al., 2011). Whether they also have this antigen presenting ability in vivo is currently being investigated in our laboratory. The presence of several dendritic cell populations in the epididymis was recently confirmed (Voisin et al., 2018), where they were shown to be more abundant in the caput versus the cauda region.
Most CX3CR1-EGFP and CD11c-YFP MPs in the IS returned to their normal dendriform morphology 4 days after EDL, indicating that the presence of spermatozoa and testicular factors in the luminal compartment is not required for the formation and extension of their intraepithelial dendrites. This is in contrast to BCs, which permanently lose their axiopodia after EDL, indicating that luminal factors do play an important role in their ability to sample the luminal compartment in the mouse IS (Kim et al., 2015). This result further demonstrated that MPs and BCs are distinct cell types in the epididymis (Shum et al., 2014).
Both pathogen-induced and sterile inflammation in the epididymis contribute to male infertility. Inconsistency in medical terminology associated with inflammation of the male reproductive system, including the post-testicular tract, has led to poor understanding of the overall impact of genital tract infection and inflammatory conditions on male reproductive health and fertility (Fijak et al., 2018, Voisin et al., 2018). Nevertheless, it is estimated that between 15 and 30% of male infertility cases are attributed to infections, depending on geographical locations and access to appropriate medical care. Thus, there is a clear unmet need for a better understanding of epididymal (and testicular) immuno-pathophysiology.
SUMMARY
The concerted interactions between spermatozoa, basal cells, principal cells, narrow cells, clear cells, and immune cells represent a complex process by which the epididymal epithelium establishes and modulates the appropriate environment for the maturation, protection, selection, and storage of sperm. Decoding this intricate intercellular communication system will help understand how male fertility is established and maintained, and how dysfunctions in the post-testicular environment may lead to male infertility due to the impairment of spermatozoa to reach and fertilize an egg.
Acknowledgments
We would like to thank Dr. Winnie Shum for the generation of Figure 4. This work was supported by a Lalor Foundation Fellowship (to M.A.B.), and National Institutes of Health grants HD040793 (to S.B.), HD069623 (to S.B.) and DK097124 (to S.B.). The Microscopy Core facility of the Massachusetts General Hospital (MGH) Program in Membrane Biology receives support from Boston Area Diabetes and Endocrinology Research Center grant DK57521 and Center for the Study of Inflammatory Bowel Disease grant DK43351. S.B. is a recipient of the Richard Moerschner Endowed MGH Research Institute Chair in Men’s Health.
Footnotes
CONFLICT OF INTEREST
SB has a financial interest in Kantum Pharma a company developing a diagnostic and therapeutic combination to prevent and treat Acute Kidney Injury. SB’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- Andrews C, McLean MH, Durum SK. Cytokine Tuning of Intestinal Epithelial Function. Front Immunol 2018;9:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au CL, Wong PY. Luminal acidification by the perfused rat cauda epididymidis. J Physiol 1980;309:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnis C, Marsolais M, Biemesderfer D, Laprade R, Breton S. Na+/H+-exchange activity and immunolocalization of NHE3 in rat epididymis. Am J Physiol Renal Physiol 2001;280:F426–36. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J 2000;19:2024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks FC, Calvert RC, Burnstock G. Changing P2X receptor localization on maturing sperm in the epididymides of mice, hamsters, rats, and humans: a preliminary study. Fertil Steril 2010;93:1415–20. [DOI] [PubMed] [Google Scholar]
- Battistone MA, Merkulova M, Park YJ, Peralta MA, Gombar F, Brown D, Breton S. Unraveling purinergic regulation in the epididymis: Activation of V-ATPase-dependent acidification by luminal ATP and adenosine. J Physiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistone MA, Nair AV, Barton CR, Liberman RN, Peralta MA, Capen DE, Brown D, Breton S. Extracellular Adenosine Stimulates Vacuolar ATPase-Dependent Proton Secretion in Medullary Intercalated Cells. J Am Soc Nephrol 2018;29:545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+ATPase (V-ATPase) recycling. J Biol Chem 2005;280:8452–63. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol 2010;298:C817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst 2009;5:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 2006;25:4131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S The cellular physiology of carbonic anhydrases. Jop. 2001;2:159–64. [PubMed] [Google Scholar]
- Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda). 2013;28:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S, Ruan YC, Park YJ, Kim B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J Androl 2016;18:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 1996;2:470–2. [DOI] [PubMed] [Google Scholar]
- Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 1996;199:2345–58. [DOI] [PubMed] [Google Scholar]
- Burnstock G Purinergic signalling in the reproductive system in health and disease. Purinergic Signal. 2014;10:157–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caflisch CR, DuBose TD Jr., Direct evaluation of acidification by rat testis and epididymis: role of carbonic anhydrase. Am J Physiol 1990;258:E143–50. [DOI] [PubMed] [Google Scholar]
- Carlin RW, Lee JH, Marcus DC, Schultz BD. Adenosine stimulates anion secretion across cultured and native adult human vas deferens epithelia. Biol Reprod 2003;68:1027–34. [DOI] [PubMed] [Google Scholar]
- Chan HC, Fu WO, Chung YW, Zhou TS, Wong PY. Adrenergic receptors on cultured rat epididymal cells: regulation of Cl-conductances. Biol Reprod 1994;51:1040–5. [DOI] [PubMed] [Google Scholar]
- Cheung KH, Leung GP, Leung MC, Shum WW, Zhou WL, Wong PY. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol 2005;125:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CE, Benham CD, Bridges A, George AR, Meadows HJ. Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol 2000;523 Pt 3:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppens H, Cassiman JJ. CFTR mutations and polymorphisms in male infertility. Int J Androl 2004;27:251–6. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Dufresne J, Gregory M. Cellular junctions in the epididymis, a critical parameter for understanding male reproductive toxicology. Reprod Toxicol 2018;81:207–19. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Gregory M. A special issue on the effects of toxicants on cellular junctions in development and reproduction. Reprod Toxicol 2018. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Androl 2007;9:463–75. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Barton CR. Macrophages and dendritic cells in the post-testicular environment. Cell Tissue Res 2016;363:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Smith TB. Exploring the role of mononuclear phagocytes in the epididymis. Asian J Androl 2015;17:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZW, Chen J, Ruan YC, Zhou T, Chen Y, Chen Y, Tsang LL, Chan HC, Peng YZ. CFTR-regulated MAPK/NF-kappaB signaling in pulmonary inflammation in thermal inhalation injury. Sci Rep 2015;5:15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube E, Dufresne J, Chan PT, Hermo L, Cyr DG. Assessing the role of claudins in maintaining the integrity of epididymal tight junctions using novel human epididymal cell lines. Biol Reprod 2010;82:1119–28. [DOI] [PubMed] [Google Scholar]
- Esther CR Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 1996;74:953–65. [PubMed] [Google Scholar]
- Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V, Tung KSK, Schuppe HC, Meinhardt A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update. 2018;24:416–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti JL, Druart X, Guerin Y, Dacheux F, Dacheux JL. A 105- to 94-kilodalton protein in the epididymal fluids of domestic mammals is angiotensin I-converting enzyme (ACE); evidence that sperm are the source of this ACE. Biol Reprod 1999;60:937–45. [DOI] [PubMed] [Google Scholar]
- Gorodeski GI. Purinergic Signalling in the Reproductive System. Auton Neurosci 2015;191:82–101. [DOI] [PubMed] [Google Scholar]
- Gregory M, Cyr DG. The blood-epididymis barrier and inflammation. Spermatogenesis. 2014;4:e979619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology. 2001;142:854–63. [DOI] [PubMed] [Google Scholar]
- Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 2006;7:426–36. [DOI] [PubMed] [Google Scholar]
- Guiton R, Henry-Berger J, Drevet JR. The immunobiology of the mammalian epididymis: the black box is now open! Basic Clin Androl. 2013;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther J, Seyfert HM. The first line of defence: insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin Immunopathol 2018;40:555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet B, Dacheux F, Dacheux JL, Gatti JL. The epididymal transcriptome and proteome provide some insights into new epididymal regulations. J Androl 2011;32:651–64. [DOI] [PubMed] [Google Scholar]
- Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O’Brien DA. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95:2552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW, Olson GE, Cooper TG. Regional variation in the surface morphology of the epithelium of the rat ductuli efferentes, ductus epididymidis and vas deferens. Anat Rec 1977;188:13–27. [DOI] [PubMed] [Google Scholar]
- Henderson NA, Cooke GM, Robaire B. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5alpha-reductase inhibition. J Endocrinol 2006;190:779–91. [DOI] [PubMed] [Google Scholar]
- Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl 2004;25:494–505. [DOI] [PubMed] [Google Scholar]
- Hermo L, Papp S, Robaire B. Developmental expression of the Yf subunit of glutathione S-transferase P in epithelial cells of the testis, efferent ducts, and epididymis of the rat. Anat Rec 1994;239:421–40. [DOI] [PubMed] [Google Scholar]
- Hinton A, Bond S, Forgac M. V-ATPase functions in normal and disease processes. Pflugers Arch 2009;457:589–98. [DOI] [PubMed] [Google Scholar]
- Holzer P Acid-sensitive ion channels in gastrointestinal function. Curr Opin Pharmacol 2003;3:618–25. [DOI] [PubMed] [Google Scholar]
- Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, Murrell-Lagnado R, Dong XP. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem 2014;289:17658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017;17:349–62. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Schmitt BM, Berger UV, Nsumu NN, Boron WF, Hediger MA, Brown D, Breton S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol Reprod 1999a;60:573–9. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Stuart-Tilley AK, Peters LL, Lux SE, Alper SL, Breton S. Immunolocalization of AE2 anion exchanger in rat and mouse epididymis. Biol Reprod 1999b;61:973–80. [DOI] [PubMed] [Google Scholar]
- Jrad-Lamine A, Henry-Berger J, Damon-Soubeyrand C, Saez F, Kocer A, Janny L, Pons-Rejraji H, Munn DH, Mellor AL, Gharbi N et al. Indoleamine 2,3-dioxygenase 1 (ido1) is involved in the control of mouse caput epididymis immune environment. PLoS One. 2013;8:e66494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Pelletier J, Lavoie EG, Kukulski F, Martin-Satue M, Dufresne SS, Frenette J, Ribas Furstenau C, Sereda MJ, Toutain B et al. Nucleoside triphosphate diphosphohydrolase-1 ectonucleotidase is required for normal vas deferens contraction and male fertility through maintaining P2X1 receptor function. J Biol Chem 2014;289:28629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunisto K, Moe OW, Pelto-Huikko M, Traebert M, Rajaniemi H. An apical membrane Na+/H+ exchanger isoform, NHE-3, is present in the rat epididymal epithelium. Pflügers Archiv 2001;442:230–6. [DOI] [PubMed] [Google Scholar]
- Kim B, Breton S. The MAPK/ERK-Signaling Pathway Regulates the Expression and Distribution of Tight Junction Proteins in the Mouse Proximal Epididymis. Biol Reprod 2016;94:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Roy J, Shum WW, Da Silva N, Breton S. Role of testicular luminal factors on Basal cell elongation and proliferation in the mouse epididymis. Biol Reprod 2015;92:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–40. [DOI] [PubMed] [Google Scholar]
- Kondoh G, Tojo H, Nakatani Y, Komazawa N, Murata C, Yamagata K, Maeda Y, Kinoshita T, Okabe M, Taguchi R et al. Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization. Nat Med 2005;11:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung GP, Cheung KH, Leung CT, Tsang MW, Wong PY. Regulation of epididymal principal cell functions by basal cells: role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1). Mol Cell Endocrinol 2004;216:5–13. [DOI] [PubMed] [Google Scholar]
- Leung GP, Wong PY. Activation of cystic fibrosis transmembrane conductance regulator in rat epididymal epithelium by genistein. Biol Reprod 2000;62:143–9. [DOI] [PubMed] [Google Scholar]
- Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol 1971;213:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Naren AP. CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr Biol (Camb) 2010;2:161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Legere EA, Snider J, Stagljar I. Recent Progress in CFTR Interactome Mapping and Its Importance for Cystic Fibrosis. Front Pharmacol 2017;8:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima WR, Parreira KS, Devuyst O, Caplanusi A, N’Kuli F, Marien B, Van Der Smissen P, Alves PM, Verroust P, Christensen EI et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J Am Soc Nephrol 2010;21:478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandon M, Hermo L, Cyr DG. Isolated Rat Epididymal Basal Cells Share Common Properties with Adult Stem Cells. Biol Reprod 2015;93:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Satue M, Lavoie EG, Fausther M, Lecka J, Aliagas E, Kukulski F, Sevigny J. High expression and activity of ecto-5’-nucleotidase/CD73 in the male murine reproductive tract. Histochem Cell Biol 2010;133:659–68. [DOI] [PubMed] [Google Scholar]
- Martin-Satue M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, Sevigny J. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol 2009;131:615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 2005;288:C1134–44. [DOI] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 2011;84:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–9. [DOI] [PubMed] [Google Scholar]
- Murashima A, Miyagawa S, Ogino Y, Nishida-Fukuda H, Araki K, Matsumoto T, Kaneko T, Yoshinaga K, Yamamura K, Kurita T et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology. 2011;152:1640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol 2008;52:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A. 2007;104:7688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki T, Noda Y, Narimoto K, Shiotani M, Mori T, Matsuda T, Yoshida O. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum Reprod 1992;7:81–5. [DOI] [PubMed] [Google Scholar]
- O’Neal WK, Hasty P, McCray PB Jr., Casey, Rivera-Perez J, Welsh MJ, Beaudet AL, Bradley A A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet 1993;2:1561–9. [DOI] [PubMed] [Google Scholar]
- Park YJ, Battistone MA, Kim B, Breton S. Relative contribution of clear cells and principal cells to luminal pH in the mouse epididymisdagger. Biol Reprod 2017;96:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 2003;278:49523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu TG, Shum WW, Huynh C, Lechner L, Goetze B, Brown D, Breton S. High-resolution helium ion microscopy of epididymal epithelial cells and their interaction with spermatozoa. Mol Hum Reprod 2014;20:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Alves F, Akoyev V, Stewart JC 3rd, Wang LH, Janardhan KS, Schultz BD. Swine models of cystic fibrosis reveal male reproductive tract phenotype at birth. Biol Reprod 2011;85:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Alves F, Midura-Kiela MT, Fleming SD, Schultz BD, Kiela PR. Transforming Growth Factor Beta Signaling in Dendritic Cells Is Required for Immunotolerance to Sperm in the Epididymis. Front Immunol 2018;9:1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrement C, Da Silva N, Silberstein C, James M, Marsolais M, Van Hoek A, Brown D, Pastor-Soler N, Ameen N, Laprade R et al. Role of NHERF1, CFTR and cAMP in the regulation of aquaporin 9. J Biol Chem 2008;284:2986–96. [DOI] [PubMed] [Google Scholar]
- Pietrement C, Sun-Wada GH, Da Silva N, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct Expression Patterns of Different Subunit Isoforms of the V-ATPase in the Rat Epididymis. Biol Reprod 2006;74:185–94. [DOI] [PubMed] [Google Scholar]
- Reynaert I, Van Der Schueren B, Degeest G, Manin M, Cuppens H, Scholte B, Cassiman JJ. Morphological changes in the vas deferens and expression of the cystic fibrosis transmembrane conductance regulator (CFTR) in control, deltaF508 and knock-out CFTR mice during postnatal life. Mol Reprod Dev 2000;55:125–35. [DOI] [PubMed] [Google Scholar]
- Ribeiro CM, Silva EJ, Hinton BT, Avellar MC. beta-defensins and the epididymis: contrasting influences of prenatal, postnatal, and adult scenarios. Asian J Androl 2016;18:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B HB, Orgebin-Crist M. The epididymis. Knobil and Neill’s Physiology of Reproduction (Third Edition). 2006;1:1071–148. [Google Scholar]
- Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowart P, Wu J, Caplan MJ, Jouret F. Implications of AMPK in the Formation of Epithelial Tight Junctions. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J, Kim B, Hill E, Visconti P, Krapf D, Vinegoni C, Weissleder R, Brown D, Breton S. Tyrosine kinase-mediated axial motility of basal cells revealed by intravital imaging. Nat Commun 2016;7:10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YC, Shum WW, Belleannee C, Da Silva N, Breton S. ATP secretion in the male reproductive tract: essential role of CFTR. J Physiol 2012;590:4209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, Brown D, Chan HC, Breton S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci 2014;127:4396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000;11:4131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek RL, Carlin RW, Singh AK, Schultz BD. Neurotransmitter-stimulated ion transport by cultured porcine vas deferens epithelium. Am J Physiol Renal Physiol 2001;281:F557–70. [DOI] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Hill E, Brown D, Breton S. Plasticity of basal cells during postnatal development in the rat epididymis. Reproduction. 2013;146:455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod 2014;90:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TB, Cortez-Retamozo V, Grigoryeva LS, Hill E, Pittet MJ, Da Silva N. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology. 2014;2:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–8. [DOI] [PubMed] [Google Scholar]
- Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol 2006;26:2387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammler A, Muller D, Tabuchi Y, Konrad L, Middendorff R. TGFbetas modulate permeability of the blood-epididymis barrier in an in vitro model. PLoS One. 2013;8:e80611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R Epididymosomes: a heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J Androl 2015;17:726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 2010;120:3149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microsc Res Tech 2003;61:88–102. [DOI] [PubMed] [Google Scholar]
- Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl 1993;14:23–44. [PubMed] [Google Scholar]
- Verma RJ. Sperm quiescence in cauda epididymis: a mini-review. Asian J Androl 2001;3:181–3. [PubMed] [Google Scholar]
- Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS One. 2009;4:e4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A. 2009;106:667–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Krapf D, de la Vega-Beltran JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl 2011;13:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin A, Whitfield M, Damon-Soubeyrand C, Goubely C, Henry-Berger J, Saez F, Kocer A, Drevet JR, Guiton R. Comprehensive overview of murine epididymal mononuclear phagocytes and lymphocytes: Unexpected populations arise. J Reprod Immunol 2018;126:11–7. [DOI] [PubMed] [Google Scholar]
- Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10 Suppl 2:S152–71. [DOI] [PubMed] [Google Scholar]
- Wong PY, Chan HC, Leung PS, Chung YW, Wong YL, Lee WM, Ng V, Dun NJ. Regulation of anion secretion by cyclo-oxygenase and prostanoids in cultured epididymal epithelia from the rat. J Physiol 1999;514 (Pt 3):809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosnitzer MS. Genetic evaluation of male infertility. Transl Androl Urol 2014;3:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Abdel-Fattah R, Yang L, Crenshaw SA, Black MB, Hinton BT. Testicular lumicrine factors regulate ERK, STAT, and NFKB pathways in the initial segment of the rat epididymis to prevent apoptosis. Biol Reprod 2011;84:1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Yang L, Hinton BT. The Role of fibroblast growth factor receptor substrate 2 (FRS2) in the regulation of two activity levels of the components of the extracellular signal-regulated kinase (ERK) pathway in the mouse epididymis. Biol Reprod 2013;89:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Yang L, Lye RJ, Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biol Reprod 2010;83:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi Liu G, Zhu H, Ma ZG, Wang XF et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci U S A. 2007;104:9816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat 1985;172:317–30. [DOI] [PubMed] [Google Scholar]
- Yefimova M, Bourmeyster N, Becq F, Burel A, Lavault MT, Jouve G, Veau S, Pimentel C, Jegou B, Ravel C. Update on the cellular and molecular aspects of cystic fibrosis transmembrane conductance regulator (CFTR) and male fertility. Morphologie. 2018. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 2008;1783:673–94. [DOI] [PubMed] [Google Scholar]
- Zhou W, De Iuliis GN, Dun MD, Nixon B. Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front Endocrinol (Lausanne). 2018;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]