Abstract
Acylsugars are insecticidal plant specialized metabolites produced in the Solanaceae (nightshade family). Despite having simple constituents, these compounds are unusually structurally diverse. Their structural variations in phylogenetically closely related species enable comparative biochemical approaches to understand acylsugar biosynthesis and pathway diversification. Thus far, varied enzyme classes contributing to their synthesis were characterized in cultivated and wild tomatoes, including from core metabolism – isopropylmalate synthase (Leu) and invertase (carbon) – and a group of evolutionarily related BAHD acyltransferases known as acylsucrose acyltransferases. Gene duplication and neofunctionalization of these enzymes drove acylsugar diversification both within and beyond tomato. The broad set of evolutionary mechanisms underlying acylsugar diversity in Solanaceae make this metabolic network an exemplar for detailed understanding of the evolution of metabolic form and function.
Introduction
Plants collectively produce hundreds of thousands of low molecular mass molecules known as specialized or secondary metabolites. In contrast to the smaller collection of broadly distributed core metabolites such as proteogenic amino acids and enzyme cofactors, specialized metabolites are restricted to specific taxonomic groups [1] and cell or tissue types [2]. The wide variety of specialized metabolite structures and functions presumably result from millions of years of mediating both positive and defensive plant-microbe and plant-animal interactions [3,4]. While their lineage-specificity limits the number of biosynthetic pathways that can be studied in any specific species [5], recent advances in next generation sequencing and mass spectrometry technologies reduce our reliance on model species and pave the way for unravelling and exploiting metabolic diversity in all plants.
Acylsugars are structurally diverse protective metabolites sporadically detected in multiple plant families. For example, they are found in trichomes [6] of plants in the Solanaceae, Martyniaceae, Rosaceae, Geraniaceae, and Caryophyllaceae, and in roots of Brassicaceae plants [7]. Acylsugars from the Solanaceae family – including plants of the Solanum, Physalis, Nicotiana, Petunia, and Salpiglossis genera [8] – are extensively characterized. They are glycolipids consisting of a sugar core decorated by straight- and branched-chain acyl esters of lengths from C2 to C20 (Fig. 1), produced in glandular trichome tip cells. In cultivated tomatoes, they are produced in long type I/IV trichomes [9,10], but not in the type VI glands [10,11]. Acylsugars have documented insecticidal characteristics. For example, they mediate tritrophic interactions in plant defense by tagging the herbivore for predation through volatile acylsugar breakdown products [12] and also directly protect plants from fungal pathogen attacks [13]. In tomato, breeding efforts seek to manipulate acylsugar content and composition for better insect resistance and have generated improved breeding lines with increased trichome density [14], higher acylsugar amounts [14], varying proportions of sucrose or glucose based acylsugars [15,16], and different acyl chain profiles [15,17].
Figure 1.
Acylsugar structural diversity is built on simple design principles. More than one hundred known acylsugar structures are based on three varied components: sugar cores (top panel: sucrose, glucose and inositol are shown from left to right), acylation positions (middle panel shown for sucrose esters), and acyl chain types (bottom panel). The species are used as representative examples.
Acylsugar biosynthesis has characteristics that make it a compelling target for understanding the evolution of genotypic and phenotypic variation. First, trichome tip cell specific synthesis greatly facilitates gene discovery and has implications for pathway evolution. Second, structural variation of acylsugars within and across species and genera facilitate comparative biochemical approaches. Third, they are synthesized from simple components by soluble enzymes, facilitating in vitro analysis, including pathway reconstruction in a test tube. These, combined with functional genomics tools of transformation, CRISPR-Cas9 [18,19] and viral induced gene silencing (VIGS) [20] available in the Solanaceae, permit direct and detailed analysis of how genotypic variation affects phenotypic diversity.
Acylsugar structural diversity in the Solanaceae family and their biosynthesis in tomatoes
Acylsugars are remarkably structurally diverse, especially considering that they are built from simple components (Fig. 1) by a small number of core biosynthetic network enzymes (Fig. 2a). The disaccharide sucrose is the predominant sugar core and was identified in genera across the family, including Solanum [21], Petunia [22], and in Salpiglossis sinuata [8]. Acylsugars built on hexose cores were characterized in a small number of species dispersed across the family. For example, acylated inositols in Solanum lanceolatum [23] and hexoses of as yet uncharacterized structure in Solanum nigrum [8]. Glucose-based acylesters were demonstrated to be sporadically distributed in species of Solanum [24,25], Nicotiana [26], and Petunia [27]. Using sucrose as an example and taking all possible esterifications into consideration, the theoretical number of unique acylsugar structures approaches 815 million (138) – calculated based upon numbers of sucrose hydroxyl groups and different acyl moieties so far characterized from acylsugar producing species. This implies that even a single plant has the potential to generate vast acylsugar diversity. For example, Petunia axillaris leaf metabolites reveal numerous chromatographic peaks that are predicted as acylsugars based on LC/MS analyses [22], far beyond the smaller number of abundant acylsugars that were structurally resolved through NMR characterization. This point is reinforced by work on S. sinuata, where NMR structures are established for 16 of >400 acylsucroses annotated by LC/MS of leaf surface extracts (Steven Hurney, PhD thesis, Michigan State University, 2018). One key to elucidating the structural complexity of acylsugars produced in a plant species is to study the biochemical and genetic basis for acylsugar biosynthesis.
Figure 2.
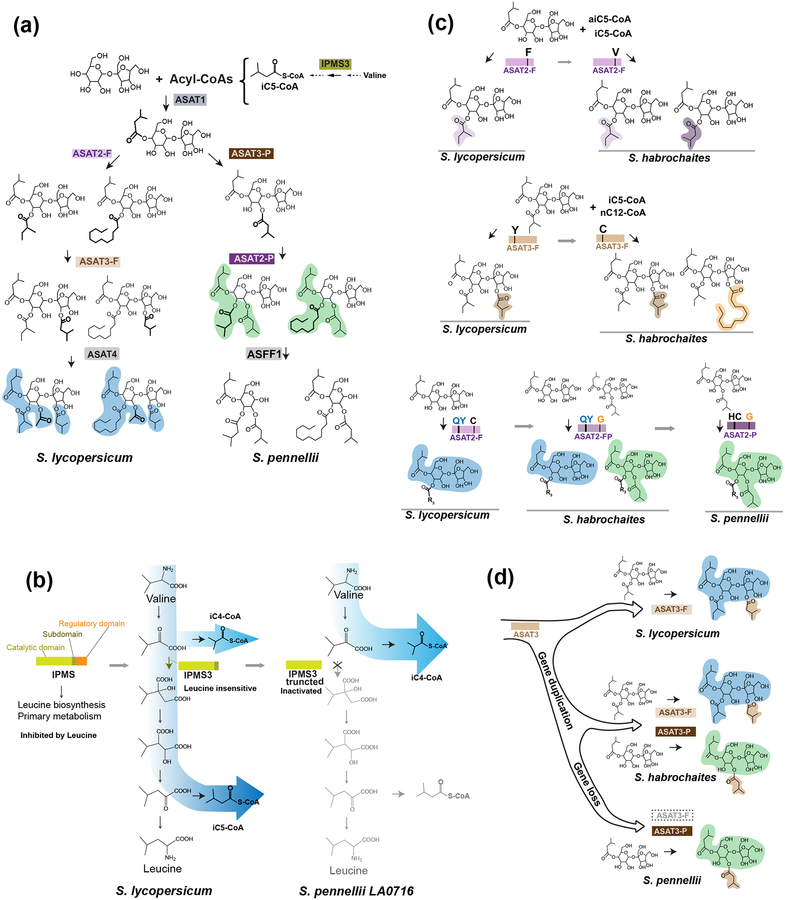
Acylsugar biosynthetic pathway diversity in tomato species. (a) The acylsugar biosynthetic pathways characterized from the cultivated tomato S. lycopersicum and wild relative S. pennellii. IPMS3, isopropylmalate synthase-like 3; ASAT, acylsucrose acyltransferase; ASFF1, acylsucrose fructofuranosidase 1. (b) Left, recruitment of the feedback insensitive and trichome gland cell expressed SlIPMS3 in cultivated tomato S. lycopersicum for biosynthesis of acylsugar isoC5-CoA acyl chain precursor. Right, the IPMS3 allele is further truncated at the C-terminus and inactivated in S. pennellii LA0716, diverting the acyl CoA precursor pathway to produce more isoC4-CoA derived acylsugars. (c) Examples of how small numbers of amino acid differences influence ASAT2 and ASAT3 enzyme acyl CoA donor and sugar core substrate preference and promiscuity. The tomato species from which the ASAT alleles are derived are labeled beneath the product(s). (d) Functional diversification of ASAT3 through gene duplication, neofunctionalization, and loss in the tomato clade.
Our understanding of acylsugar biosynthesis is most complete in the tomato clade of Solanum. Cultivated tomato (Solanum lycopersicum) makes tri- and tetra-acylated sucrose molecules, with acylations on both the sucrose pyranose and furanose rings (Fig. 2a, blue shaded, F-type acylsucroses). In contrast, trichomes of the wild tomato Solanum pennellii LA0716 produce both acylglucoses and pyranose acylated tri-acylsucroses (Fig. 2a, green shaded, P-type acylsucroses). Acylsugar biosynthetic enzyme gene discovery benefited enormously from two germplasm collections derived from crosses between these two species: isogenic introgression lines (ILs) [28] and backcross introgression lines (BILs) [29]. While these species are sexually compatible, their acylsugar metabolism diverge to the point that S. pennellii alleles act as loss of function mutations in cultivated tomato, which facilitated gene discovery through ‘forward’ genetic screening [30].
This strategy was used to identify three out of four of the core pathway acylsucrose acyltransferases (ASATs) [31–33], which belong to the rapidly evolving BAHD acyltransferase enzyme group [34,35]. In S. lycopersicum, four ASATs sequentially add acyl chains to the sucrose backbone to produce a small number of tri- and tetra-acylsucroses (Fig 2a) [31]. In addition to ASATs, allelic variation in the ILs led to discovery of a neofunctionalized enzyme of cultivated tomato Leu biosynthesis, which is responsible for production of isovaleryl coenzyme A (isoC5-CoA) (Fig. 2b) [36]. Although it catalyzes the same reaction leading to one carbon elongation as the canonical microbial and plant amino acid biosynthetic enzymes, isopropylmalate synthase like 3 (IPMS3) has unique regulation of enzymatic activity and gene expression, setting it apart. First, it lacks the inhibitory Leu-binding allosteric C-terminal domain, and thus is insensitive to Leu end-product feedback inhibition. Second, unlike the broad expression expected of amino acid biosynthetic enzymes, IPMS3 expression is limited to trichome secretory cells. In S. pennellii LA0716, the IPMS3 protein is further truncated at the C-terminus, losing detectable in vitro enzyme activity (Fig. 2b). This results in accumulation of acylsugars containing isobutyl (isoC4) chains in this and other S. pennellii accessions homozygous for this doubly deleted allele [36]. As more acylsugar biosynthetic genes were uncovered and pieced together to complete the biosynthetic pathways (Fig. 2a)[31–33,36,37], it became possible to study how gene duplication and enzyme variation contributed contribute to the evolution of a metabolic network and created acylsugar diversity.
Tomato acylsugar diversity via enzymatic promiscuity, flipping enzyme order and invertase ‘hijacking’
Acylsugars in the tomato clade show striking inter- and intraspecific structural diversity, which is associated with gene duplication and functional diversification of acylsugar biosynthetic genes. Wild tomato species differ in the types and amounts of acylsugars in comparison to cultivated tomato, and we found examples where product structural differences are associated with evolution of ASATs, leading to enzymes that vary in substrate specificity and degree of promiscuity [31,32,37].
Key amino acid differences that cause specific phenotypic variations were identified using a comparative biochemical approach [31,37,38]. For example, green-fruited tomato relatives have an ASAT2 – the second enzyme in the S. lycopersicum pathway – which promiscuously uses both anteisoC5-CoA (aiC5-CoA; 2-methylbutyryl-CoA) and isoC5-CoA donors, leading to products with either chain at the sucrose R3 position. In contrast, SlASAT2 of cultivated tomato and its closest relatives fail to use isoC5-CoA as donor due to a single Val408 to Phe amino acid substitution, leading to a less diverse set of acylsucrose structures accumulating in vivo (Fig. 2c, top panel) [21,31]. Another example of a single amino acid change modulating promiscuity and metabolic diversity was found by comparing ASAT3 of S. lycopersicum to those of Solanum habrochaites accessions. In this case, a single Tyr41 to Cys amino acid substitution in the S. lycopersicum protein changes an enzyme that only uses shorter chain C5 CoA esters to a more promiscuous one, which adds either isoC5 or lauroyl (nC12) group to the furanose ring at R3’ (Fig. 2c, middle panel) [31,32]. These in vitro activity changes correlate with both interspecific in vivo differences across the tomato group and intraspecific acylsugar variations in different S. habrochaites species [32]. Together with more global differences in C4 vs. C5 acyl chain abundance caused by allelic variation in S. pennellii IPMS3 (Fig. 2b) [36], ASAT acyl CoA substrate specificity and promiscuity influence tomato acylsugar acyl chain composition.
Differences in ASAT acyl acceptor substrate specificity have an even more profound influence on in vivo acylsugar product structural diversity in the S. pennellii/S. habrochaites tomato subclade [37]. Trichomes in this group produce ‘P-type’ triacylsucroses acylated only on the pyranose ring (Fig. 2a). This is the result of a reconfiguration of the biosynthetic network, where the ASAT3-P enzyme catalyzes the second step – utilizing monoacylated sucrose acceptor substrate – and ASAT2-P catalyzes the third step converting diacylsucroses to the final triacylated products. Detailed analysis of the amino acid differences responsible for this ‘flipped pathway’ revealed a small number of amino acids mediating the acceptor substrate specificity changes (Fig. 2a) [37]. In fact, a single Cys304 to Gly in vitro mutation in S. lycopersicum ASAT2, to an amino acid present in all tested S. pennellii ASAT2 enzymes, led to a promiscuous enzyme that accepts both mono- and di-acylsucrose substrates (Fig. 2c, bottom panel). Complete conversion to a P-type enzyme, without detectable F-type activity, requires an additional two amino acid changes. This strongly suggests that P-type ASAT2 activity evolved from an F-type enzyme through a promiscuous enzyme intermediate. In contrast, evolution of the seemingly ancestral ASAT3-F furanose ring acylating activity to S. pennellii ASAT3-P, which only acylates the pyranose ring, involved gene duplication, neofunctionalization, and gene loss (Fig. 2d) [32,37]. S. habrochaites appears to be an intermediate case, retaining both ASAT3-F and ASAT3-P paralogs, leading to accumulation of sucrose products of both the P- and F-types (Figs. 2a and 2d).
The S. pennellii LA0716 flipped pathway not only produces P-type triacylsucrose products, but also is associated with evolution of the unusual S. pennellii acylsucrose β-fructofuranosidase (SpASFF1), a trichome-specific invertase-like enzyme [24] (Fig. 2a). This enzyme specifically hydrolyzes P-type triacylsucroses, to produce the triacylglucoses found in abundance on the surface of S. pennellii LA0716, yet is inactive against furanose ring acylated tri- or tetraacylsucroses made by the cultivated tomato pathway. This evolutionary innovation is remarkable for several reasons. First, it was potentiated by the neofunctionalization of ASAT2 and ASAT3 that produces P-type triacylsucroses. Second, it involves modification of β-fructofuranosidase (invertase), an enzymatic activity long associated with core carbohydrate metabolism [39]. Finally, as with IPMS3 and ASAT enzymes, recruitment of this activity to specialized metabolism involved evolution of cell type specific expression.
Acylsugar diversity in a biogeographic context
Striking intraspecific acylsugar variation is seen among different accessions of S. habrochaites or S. pennellii species collected from habitats isolated by deep valleys or mountain ridges across the Andes (Kim et al., 2012; Schilmiller et al., 2015). With increasing understanding of acylsugar pathway diversification, these acylsugar biogeographical associations can be interpreted at the molecular level. For example, the mixed distribution of two IPMS3 alleles – one functional and the other non-functional due to two different C-terminal deletions (Fig. 2b) [36] – is associated with the varied ratio of acylsugar isoC4 and isoC5 acyl chains in different S. pennellii accessions from the northern to southern part of the range. Similarly, loss of ASAT4 R2 acetylation activity – through multiple presumably independent gene inactivation events – correlates with the lack of acylsugar acetylation in S. habrochaites populations from the northern part of the species range [40]. Different S. habrochaites ASAT3 alleles – varying by presence or absence of the ability to perform long chain furanose ring acylation (n ≥ 10) – provide another instance of ASAT variation shaping plant acylsugar acylation patterns across native geographies [32,37]. The diversification of acylsugars in tomato accessions dispersed across Peru and Ecuador is reminiscent of a recent study documenting that species in the genus Solanum experienced explosive diversification in the Neotropic region, shaped by geographical long-distance dispersal and past climate changes [41]. The acylsugar biosynthetic network and the pathway diversification could serve as a microcosm for mechanistic understanding of the rise of broad phenotypic diversity in the Neotropics.
Evolution of the acylsugar biosynthetic network beyond tomato
The analysis of metabolic evolution in the Solanum tomato clade described above reveals that phenotypic diversification over the past seven million years involved multiple mechanisms within and across species. These include ASAT3 gene duplication, varied examples of neofunctionalization of BAHD acyltransferase activities, ‘recruitment’ of an invertase and an isopropylmalate synthase from core metabolism, and ASAT4 gene function loss in accessions of S. habrochaites.
The steadily increasing genomics and metabolomics data across the Solanaceae provide opportunities to look deeper in time and develop hypotheses regarding the origin of this pathway and mechanisms of diversification across 50–100 million years [8]. The data suggest that the ASAT gene clade originated from an alkaloid biosynthetic BAHD ancestor, with the genes duplicating prior to Solanaceae and Convolvulaceae divergence (Fig. 3; phylogenetic tree on left) [8]. Comparison of tomato to Salpiglosis sinuata and Petunia – species representing earlier emerging lineages – revealed a major difference in the pathway: altered order of acylation catalyzed by non-orthologous ASATs. As schematized in Fig 3, ancestral ASAT1 (aASAT1) enzyme – catalyzing the first step of the ancestral pathway in S. sinuata and Petunia – acylates position R2 of the sucrose pyranose ring, with the next two acylations at R4 and R3 by aASAT2 and aASAT3, consecutively. This is in contrast to the ‘modern’ cultivated tomato pathway starting with sucrose R4 acylation by mASAT1, followed by mASAT2 R3 acylation, and then furanose ring R3’ acylation by mASAT3 (Fig. 3) [8,37,42]. The rise of the modern pathway seen in tomato is associated with changes in aASAT2 and aASAT3 ortholog activities to perform the first and second step in the modern pathway (mASAT1 and mASAT2, respectively); this served to ‘shift’ the order of orthologous enzyme action, associated with loss of ancestral aASAT1 following the Solanum-Capsicum lineage split (Fig. 3) [8].
Figure 3.
Variation in acylsucrose acyltransferases in a phylogenetic context. A combination of enzymology, metabolite profiling, genomics and transcriptomics approaches led to a model for evolution of the core acylsucrose biosynthetic network over the tens of millions of years since divergence from the last common ancestor with Convolvulaceae [8]. The rectangular boxes represent ASAT homologs found in the corresponding species. The same color represents the closest ASAT homologs across species. All ASATs shown were biochemically characterized except the ones in Solanum nigrum and Hyoscyamus niger. The triacylated sucroses with all acyl chains on the pyranose ring (P-type acylsucroses) produced by S. pennellii have the same acylation pattern as those produced in Petunia and Salpiglossis. However, the enzymes, and the order of acylation to produce these P-type acylsucroses, differ as depicted. In fact, ‘flipped pathway’ leading to synthesis of the S. pennellii P-type acylsucroses appears to be a metabolic innovation that originated after the last common ancestor of the Solanum tomato clade and before the divergence from the last common ancestor of S. pennellii and S. habrochaites. This model is consistent with P-type acylsucroses evolving multiple times in the Solanaceae.
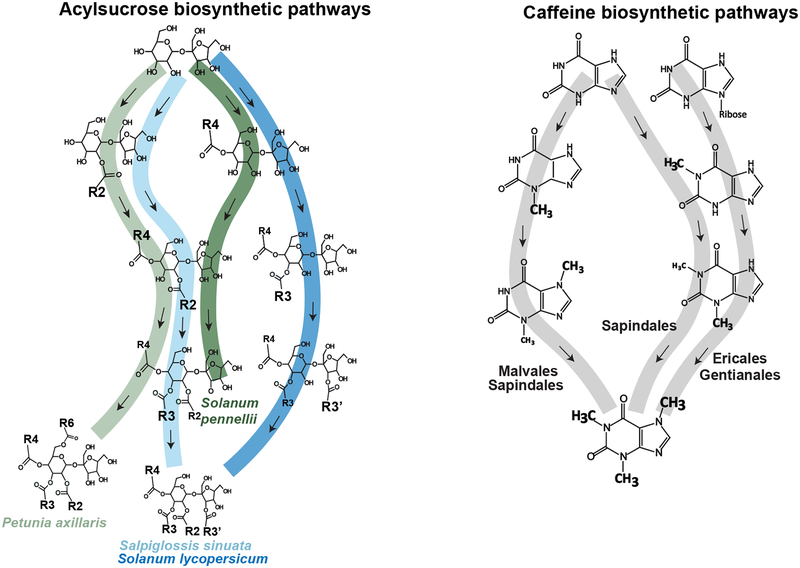
The different acylation orders to produce the P-type acylsucroses in S. pennellii and P. axillaris (Fig. 4, left panel, green ribbon) [37,42] and F-type acylsucroses in S. lycopersicum and S. sinuata (Fig. 4, left panel, blue ribbon) [8,31] are reminiscent of convergent evolution in caffeine biosynthesis (Fig. 4, right panel) [43]. Synthesis of caffeine requires three sequential methylations of a xanthine backbone, but the order of methylation varies across the four plant orders studied (Fig. 4, right panel). Although the convergent evolution of caffeine is achieved through different biosynthetic routes, the methyltransferases involved share the same evolutionary origin [44,45]. Similarly, ASATs appear to share a common ancestor involved in alkaloid biosynthesis [8]. For both caffeine and acylsugar biosynthesis, the promiscuity of ancestral enzymes primed pathway divergence and eventually led to similar but distinct derived pathways [37,45]. The factors that drove the multiple origins of P-type acylsucroses seem unclear, whereas they may have set the stage for phytochemical diversity – probably through ASFF hydrolysis activity [24] – that leads to mixed acylsucroses and acylglucoses accumulation in several Solanaceae species [25–27].
Figure 4.
Comparison of acylsucrose and caffeine biosynthesis: different biosynthetic routes generate similar products. Different orders of sucrose acylation in S. pennellii and P. axillaris (left panel, indicated by green ribbon) produce similarly structured acylsucroses with all acyl chains on the pyranose ring (P-type acylsucroses). In S. lycopersicum and S. sinuata, distinct biosynthetic routes (left panel, indicated by blue ribbons) generate acylsucroses with acyl chains on both pyranose and furanose ring (F-type acylsucroses). This theme of multiple biosynthetic routes to similar acylsucroses is reminiscent of caffeine biosynthesis, which convergently evolved in different plant orders (right panel). Both acylsugar and caffeine biosyntheses require sequential chemical modifications on the backbone structures that were ‘hijacked’ from primary metabolism. Each arrow on the left or right panels represents a biochemical reaction catalyzed by an acylsucrose acyltransferase for acylsugars or xanthine methyltransferase for caffeine.
Conclusion – future directions
Acylsugars are a group of related but structurally diverse plant specialized metabolites, which were reported in six plant families so far [6,7]. The structures and biosynthesis of acylsugars are best characterized in trichomes of the Solanaceae, where they are synthesized from commonly available sucrose and acyl-CoA primary metabolites via short biosynthetic pathways. As biosynthesis of these compounds is analyzed in other families, it will be interesting to explore the details of their parallel evolution and to learn whether interactions between plants, microorganisms, and herbivores drove their evolution and structural diversification.
While our understanding of acylsugar biosynthesis increases as more enzymes in the biosynthetic network are characterized, rational modification of these biotic stress tolerance molecules will also improve with knowledge in areas not discussed in this review. Acylsugar acylhydrolases (ASHs), which remove acyl chains from specific acylsugar positions in vitro [46], may be involved in acylsugar degradation or editing in vivo. However, these enzymes appear to create a futile cycle by degrading ASAT acylsugar products. The enigmatic ASH activities suggest the possible existence of unidentified mechanisms for generating acylsugar diversity. For example, acylsugar structures could be remodeled by ASH activities, creating alternative ASAT acyl acceptor substrates. Given ASAT enzyme promiscuity, manipulation of trichome tip cell acyl CoA pools should be an effective way to modify chain types. Proof of concept for this approach was seen when the inactive SpIPMS enzyme replaced the catalytically active version in cultivated tomato IL8–1, leading to isoC4 acylsucrose accumulation instead of isoC5. Understanding the mechanisms by which longer straight- and branched-chain acyl CoA substrates are synthesized for acylsugar biosynthesis in the tip cells should lead to strategies for manipulating acylsugar acyl chain length. Such engineering could improve insect tolerance, as seen in the tomato breeding lines with different fatty acid ester profiles [47,48] or sugar cores [49], which show synergistic effects against insects. The ‘toolbox’ for manipulating diverse acylsugar structures will promote new strategies to improve plant insect defense through breeding or genetic engineering of desired plant acylsugar phenotypes.
Highlights.
Solanaceae glandular trichomes produce structurally diverse insecticidal acylsugars
Diversity is achieved by enzyme promiscuity and core metabolic enzyme ‘recruitment’
In vitro pathway reconstruction facilitates genotype to phenotype analysis
These pathways are amenable to evolutionary reconstruction over 10’s of millions of years
Acknowledgements
This work was supported by National Science Foundation grant IOS-PGRP-1546617 to RLL and National Institute of General Medical Sciences of the National Institutes of Health MSU Plant Biotechnology for Health and Sustainability graduate training grant T32–GM110523 to BJL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Chae L, Kim T, Nilo-Poyanco R, Rhee SY: Genomic signatures of specialized metabolism in plants. Science 2014, 344:510–513. [DOI] [PubMed] [Google Scholar]
- 2.Colinas M, Goossens A: Combinatorial transcriptional control of plant specialized metabolism. Trends Plant Sci 2018, 23:324–336. [DOI] [PubMed] [Google Scholar]
- 3.Kessler A, Kalske A: Plant secondary metabolite diversity and species interactions. Annu Rev Ecol Evol Syst 2018, 49:115–138. [Google Scholar]
- 4.Pichersky E, Raguso RA: Why do plants produce so many terpenoid compounds? New Phytol 2016, 220:692–702. [DOI] [PubMed] [Google Scholar]
- 5.Moghe GD, Kruse LH: The study of plant specialized metabolism: Challenges and prospects in the genomics era. Am J Bot 2018, 105:959–962. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Jing S-X, Luo S-H, Li S-H: Non-volatile natural products in plant glandular trichomes: chemistry, biological activities and biosynthesis. Nat Prod Rep 2019, doi: 10.1039/C8NP00077H. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Cho JG, Lee DS, Lee DY, Song NY, Kim YC, Lee KT, Chung HG, Choi MS, Jeong TS, et al. : Carbohydrate derivatives from the roots of Brassica rapa ssp. campestris and their effects on ROS production and glutamate-induced cell death in HT-22 cells. Carbohydr Res 2013, 372:9–14. [DOI] [PubMed] [Google Scholar]
- 8.Moghe GD, Leong BJ, Hurney S, Jones AD, Last RL: Evolutionary routes to biochemical innovation revealed by integrative analysis of a plant-defense related specialized metabolic pathway. eLife 2017, 6:e28468.Identified several ASATs of the acylsucrose biosynthetic pathway in Salpiglossis sinuata using in vitro biochemistry and in vivo virus induced gene silencing. Proposed that the evolutionary precursors of ASATs were most likely alkaloid biosynthetic genes and that there was a shift in enzymatic activities that facilitated the emergence of the Solanum pathway from the more ancestral Salpiglossis pathway. This study delves into the evolution of acylsugar biosynthesis over the course of 10’s of millions of years in the Solanaceae family.
- 9.Li C, Wang Z, Jones AD: Chemical imaging of trichome specialized metabolites using contact printing and laser desorption/ionization mass spectrometry. Anal Bioanal Chem 2014, 406:171–182. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima T, Wada H, Morita S, Erra-Balsells R, Hiraoka K, Nonami H: Single-cell metabolite profiling of stalk and glandular cells of intact trichomes with internal electrode capillary pressure probe electrospray ionization mass spectrometry. Anal Chem 2016, 88:3049–3057.This study described the first in situ single-cell metabolite profiling technique that differentiates the metabolic variations between two adjacent cell types of a glandular trichome – the tip and stalk cells. It provides the direct evidence that acylsugars are secreted from tip cells of type I/IV trichomes of tomatoes and corroborates the tissue specific expression of the acylsugar biosynthetic genes described in the review.
- 11.Balcke GU, Bennewitz S, Bergau N, Athmer B, Henning A, Majovsky P, Jiménez-Gómez JM, Hoehenwarter W, Tissier A: Multi-omics of tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell 2017, 29:960–983.Using a multi-omics approach and 13C-labeling experiments to characterize type VI glandular trichomes of two tomato species, the authors identified metabolic processes that drive the high production of specialized metabolites, such as flavonoids and terpenes. Their research aimed at understanding the under-examined link between central carbon metabolism and specialized metabolism – how core metabolic network is organized to deliver carbon, energy, and reducing power to achieve high productivity of protective secondary metabolites in tomato trichomes.
- 12.Weinhold A, Baldwin IT: Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc Natl Acad Sci USA 2011, 108:7855–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luu VT, Weinhold A, Ullah C, Dressel S, Schoettner M, Gase K, Gaquerel E, Xu S, Baldwin IT: O-acyl sugars protect a wild tobacco from both native fungal pathogens and a specialist herbivore. Plant Physiol 2017, 174:370–386.The authors studied effects of acylsugars from Nicotiana attenuata plants on two native fungal pathogens and the specialist herbivore, Manduca sexta. The authors used natural variation in acylsugar levels from different accessions to reveal a positive association between acylsugar levels and resistance to these pests and pathogens. In vitro assays revealed that both acylsugars and the free branch chain fatty acids negatively affected growth of M. sexta, and germination of the fungal pathogens.
- 14.Leckie BM, De Jong DM, Mutschler MA: Quantitative trait loci increasing acylsugars in tomato breeding lines and their impacts on silverleaf whiteflies. Mol Breed 2012, 30:1621–1634. [Google Scholar]
- 15.Smeda JR, Schilmiller AL, Last RL, Mutschler MA: Introgression of acylsugar chemistry QTL modifies the composition and structure of acylsugars produced by high-accumulating tomato lines. Mol Breed 2016, 36:160. [Google Scholar]
- 16.Leckie BM, De Jong DM, Mutschler MA: Quantitative trait loci regulating sugar moiety of acylsugars in tomato. Mol Breeding 2013, 31:957–970. [Google Scholar]
- 17.Leckie B, Halitschke R, De Jong D, Smeda J, Kessler A, Mutschler M: Quantitative trait loci regulating the fatty acid profile of acylsugars in tomato. Mol Breed 2014, 34:1201–1213. [Google Scholar]
- 18.Lemmon ZH, Reem NT, Dalrymple J, Soyk S, Swartwood KE, Rodriguez-Leal D, Van Eck J, Lippman ZB: Rapid improvement of domestication traits in an orphan crop by genome editing. Nat Plants 2018, 4:766–770. [DOI] [PubMed] [Google Scholar]
- 19.Yin K, Gao C, Qiu JL: Progress and prospects in plant genome editing. Nat Plants 2017, 3:17107. [DOI] [PubMed] [Google Scholar]
- 20.Dommes AB, Herbert DB, Gross T, Becker A, Kivivirta KI: Virus-induced gene silencing: empowering genetics in non-model organisms. J Exp Bot 2018, 70:757–770. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh B, Westbrook TC, Jones AD: Comparative structural profiling of trichome specialized metabolites in tomato (Solanum lycopersicum) and S. habrochaites: acylsugar profiles revealed by UHPLC/MS and NMR. Metabolomics 2014, 10:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Enright M, Barry CS, Jones AD: Profiling, isolation and structure elucidation of specialized acylsucrose metabolites accumulating in trichomes of Petunia species. Metabolomics 2017, 13:1–10.27980501This study used LC-MS and NMR to identify 28 acylsucroses produced in 3 different Petunia species. The authors identified acylsugars containing malonate esters, not reported in tomato species. NMR analysis revealed that the aliphatic acyl chains (non-malonate) are located on the pyranose ring of purified sucrose esters.
- 23.Herrera-Salgado Y, Va L, Rios Y, Alvarez L: Myo-inositol-Derived glycolipids with anti-inflammatory activity from Solanum lanceolatum. J Nat Prod 2005, 68:1031–1036. [DOI] [PubMed] [Google Scholar]
- 24.Leong BJ, Lybrand D, Lou Y-R, Fan P, Schilmiller AL, Last RL: Evolution of metabolic novelty: a trichome-expressed invertase creates specialized metabolic diversity in wild tomato. bioRxiv 2018, doi: 10.1101/502971.Identified an invertase-like enzyme that is responsible for acylglucose biosynthesis in S. pennellii. Used a combination of in vitro and in vivo approaches to identify this enzyme and determine that P-type acylsucroses are the substrates for this co-opted invertase. This paper demonstrates a three-enzyme epistatic interaction between ASATs and the invertase-like enzyme responsible for producing a novel type of acylsugar in a wild relative of tomato.
- 25.Shapiro JA, Steffens JC, Mutschler MA: Acylsugars of the wild tomato Lycopersicon pennellii in relation to geographic distribution of the species. Biochem Syst Ecol 1994, 22:545–561. [Google Scholar]
- 26.Matsuzaki T, Shinozaki Y, Suhara S, Tobita T, Shigematsu H, Koiwai A: Leaf surface glycolipids from Nicotiana acuminata and Nicotiana pauciflora. Agric Biol Chem 1991, 55:1417–1419. [PubMed] [Google Scholar]
- 27.Chortyk OT, Kays SJ, Teng Q: Characterization of insecticidal sugar esters of Petunia. J Agric Food Chem 1997, 45:270–275. [Google Scholar]
- 28.Eshed Y, Abu-Abied M, Saranga Y, Zamir D: Lycopersicon esculentum lines containing small overlapping introgressions from L. pennellii. Theor Appl Genet 1992, 83:1027–1034. [DOI] [PubMed] [Google Scholar]
- 29.Ofner I, Lashbrooke J, Pleban T, Aharoni A, Zamir D: Solanum pennellii backcross inbred lines (BILs) link small genomic bins with tomato traits. Plant J 2016, 87:151–160.This work described development of the back cross inbred line (BIL), genetic resource for fine mapping phenotypic traits that are derived from the wild tomato Solanum pennellii (LA0716). As an important addition to the classic 76 introgression lines (ILs), BILs have multiple chromosome introgressions in each line, which not only increase mapping resolution, but facilitate identification of traits associated with epistatic interactions of multiple genetic loci.
- 30.Schilmiller A, Shi F, Kim J, Charbonneau AL, Holmes D, Daniel Jones A, Last RL: Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J 2010, 62:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan P, Miller AM, Schilmiller AL, Liu X, Ofner I, Jones AD, Zamir D, Last RL: In vitro reconstruction and analysis of evolutionary variation of the tomato acylsucrose metabolic network. Proc Natl Acad Sci 2016, 113:E239–E248.The authors identified multiple enzymes involved in the acylsucrose biosynthetic pathway of cultivated tomato and reconstructed the full biosynthetic pathway in vitro. A comparative biochemical and phylogenetically-driven approach was used to identify key residues involved in changes in ASAT substrate specificity. This paper describes the first reconstitution of the major acylsugars from any plant.
- 32.Schilmiller AL, Moghe GD, Fan P, Ghosh B, Ning J, Jones AD, Last RL: Functionally divergent alleles and duplicated loci encoding an acyltransferase contribute to acylsugar metabolite diversity in Solanum trichomes. Plant Cell 2015, 27:1002–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schilmiller AL, Charbonneau AL, Last RL: Identification of a BAHD acetyltransferase that produces protective acyl sugars in tomato trichomes. Proc Natl Acad Sci USA 2012, 109:16377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Auria JC: Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 2006, 9:331–340. [DOI] [PubMed] [Google Scholar]
- 35.Tuominen LK, Johnson VE, Tsai C-J: Differential phylogenetic expansions in BAHD acyltransferases across five angiosperm taxa and evidence of divergent expression among Populus paralogues. BMC Genomics 2011, 12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning J, Moghe GD, Leong B, Kim J, Ofner I, Wang Z, Adams C, Jones AD, Zamir D, Last RL: A feedback insensitive isopropylmalate synthase affects acylsugar composition in cultivated and wild tomato. Plant Physiol 2015, 160:1821–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan P, Miller AM, Liu X, Jones AD, Last RL: Evolution of a flipped pathway creates metabolic innovation in tomato trichomes through BAHD enzyme promiscuity. Nat Commun 2017, 8:2080.This study used a phylogenetic approach to identify and characterize ASATs involved in acylsucrose biosynthesis in Solanum pennellii. They then identified residues responsible for altered substrate specificity that result in changes to the catalytic order of ASAT2 and ASAT3, which leads to production of P-type acylsucroses in S. pennellii. Further, the authors used site-directed mutagenesis to ‘flip’ the substrate specificity of each of these ASATs, recreating the S. pennellii ASAT activities with a small number of residue changes.
- 38.Fan P, Moghe GD, Last RL: Comparative biochemistry and in vitro pathway reconstruction as powerful partners in studies of metabolic diversity. Methods Enzymol 2016, 576:1–17. [DOI] [PubMed] [Google Scholar]
- 39.Wan H, Wu L, Yang Y, Zhou G, Ruan YL: Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends Plant Sci 2018, 23:163–177. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Kang K, Gonzales-Vigil E, Shi F, Jones AD, Barry CS, Last RL: Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the acyltransferase2 locus in the wild tomato Solanum habrochaites. Plant Physiol 2012, 160:1854–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Echeverría-Londoño S, Särkinen T, Fenton IS, Knapp S, Purvis A: Dynamism and context dependency in the diversification of the megadiverse plant genus Solanum L. (Solanaceae). bioRxiv 2018, doi: 10.1101/348961. [DOI] [Google Scholar]
- 42.Nadakuduti SS, Uebler JB, Liu X, Jones AD, Barry CS: Characterization of trichome-expressed BAHD acyltransferases in Petunia axillaris reveals distinct acylsugar assembly mechanisms within the Solanaceae. Plant Physiol 2017, 175:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denoeud F, Carretero-Paulet L, Dereeper A, Droc G, Guyot R, Pietrella M, Zheng C, Alberti A, Anthony F, Aprea G, et al. : The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 2014, 345:1181–4. [DOI] [PubMed] [Google Scholar]
- 44.Huang R, Hippauf F, Rohrbeck D, Haustein M, Wenke K, Feike J, Sorrelle N, Piechulla B, Barkman TJ: Enzyme functional evolution through improved catalysis of ancestrally nonpreferred substrates. Proc Natl Acad Sci USA 2012, 109:2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang R, O’Donnell AJ, Barboline JJ, Barkman TJ: Convergent evolution of caffeine in plants by co-option of exapted ancestral enzymes. Proc Natl Acad Sci USA 2016, 113:10613–8.This report studied the evolution of caffeine biosynthesis across four plant orders using ancestral sequence reconstruction. The authors reveal that caffeine biosynthesis has convergently evolved multiple times through co-option of enzymes that were maintained for other biochemical purposes over the course of 100 million years. It provides insight into how the repeated evolution of specialized metabolites composed of simple components can occur over millions of years.
- 46.Schilmiller AL, Gilgallon K, Ghosh B, Jones AD, Last RL: Acylsugar acylhydrolases: carboxylesterase-catalyzed hydrolysis of acylsugars in tomato trichomes. Plant Physiol 2016, 170:1331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leckie BM, D’Ambrosio DA, Chappell TM, Halitschke R, De Jong DM, Kessler A, Kennedy GG, Mutschler MA: Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS One 2016, 11:e0153345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Mahmoud S, Smeda JR, Chappell TM, Stafford-Banks C, Kaplinsky CH, Anderson T, Mutschler MA, Kennedy GG, Ullman DE: Acylsugar amount and fatty acid profile differentially suppress oviposition by western flower thrips, Frankliniella occidentalis, on tomato and interspecific hybrid flowers. PLoS One 2018, 13:e0201583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeda JR, Schilmiller AL, Anderson T, Ben-Mahmoud S, Ullman DE, Chappell TM, Kessler A, Mutschler MA: Combination of acylglucose QTL reveals additive and epistatic genetic interactions and impacts insect oviposition and virus infection. Mol Breed 2018, 38:3.This study used marker-assisted selection to identify plants containing three different QTL that affect acylglucose content on chromosomes 3, 4, and 11, and identified them as necessary for high acylglucose accumulation. The authors also tested the efficacy of their multiple S. pennellii-introgressed breeding line against beet armyworm and western flower thrips, revealing decreased oviposition of the breeding line compared to leaf disks containing no acylsugars.