Abstract
Background:
The recovery of motor function following stroke is largely dependent on motor learning-related neuroplasticity. It has been hypothesized that intensive aerobic exercise training as an antecedent to motor task practice may prime the central nervous system to optimize motor recovery post-stroke.
Objective:
The objective of this study was to determine the differential effects of forced or voluntary aerobic exercise combined with upper extremity repetitive task practice (RTP) on the recovery of motor function in adults with stroke.
Methods:
A combined analysis of two preliminary randomized clinical trials was conducted in which participants (N=40) were randomized into one of three groups: 1) Forced Exercise and RTP (FE+RTP), 2) Voluntary Exercise and RTP (VE+RTP) or 3) time-matched stroke-related education and RTP (Edu+RTP). Participants completed 24 training sessions over 8 weeks.
Results:
A significant interaction effect was found indicating that improvements in the Fugl-Meyer Assessment were greatest for the FE+RTP group (P=0.001). All three groups improved significantly on the Fugl-Meyer Assessment by a mean of 11, 6, and 9 points for the FE+RTP, VE+RTP and Edu+RTP groups, respectively. No evidence of a treatment-by-time interaction was observed for Wolf Motor Function Test outcomes; however, those in FE+RTP group did exhibit significant improvement on the total, gross motor, and fine motor performance times (P≤0.01 for all observations).
Conclusions:
Results indicate that FE administered prior to RTP enhanced motor skill acquisition greater than VE or stroke-related education. Aerobic exercise, FE in particular, should be considered as an effective antecedent to enhance motor recovery post-stroke.
Introduction
Stroke is a leading cause of long-term disability among older adults in the United States,1 with approximately 5.8 million survivors experiencing residual neurological deficits.2 Neuroplasticity is thought to underlie the relearning of lost motor function through adaptation and potentiation of neural connectivity.3, 4 Rehabilitation specialists aim to facilitate neuroplasticity by prescribing motor learning-based therapies, such as constraint-induced movement therapy and task-oriented motor practice.4, 5 Despite advances in rehabilitation, nearly two thirds of individuals do not recover sufficient motor function to incorporate the more affected hand into daily activities six months after stroke.6 To address this gap, field experts have called for novel rehabilitation approaches to improve outcomes and long-term functional recovery for individuals following stroke.7, 8
There is evidence in animal and human literature to suggest that aerobic exercise (AE) training may have a role in facilitating neuroplasticity.9–13 Animal models of stroke indicate that moderate to high intensity AE training primes the central nervous system (CNS) by creating a neural response that facilitates plasticity, thereby optimizing motor recovery.7, 13–16 Accumulating data suggest AE leads to an upregulation of brain-derived neurotrophic factor (BDNF), a neurotrophin which has been implicated as a mediator of motor recovery after stroke.7, 12–18
In healthy humans, AE training has been shown to influence factors associated with neuroplasticity, including increased peripheral levels of BDNF,14, 19 insulin-like growth factor-1,14, 20 cerebral blood flow, and enhanced synaptogenesis and neurogenesis.14, 15 In humans with stroke, Boyne and colleagues found elevated peripheral BDNF responses and increased corticospinal excitability following strenuous aerobic training.21 From a behavioral perspective, numerous studies have demonstrated that in healthy young adults, a single bout of moderate to high intensity AE completed prior to motor task practice enhances motor skill acquisition.22–24 Moderate intensity AE has also been shown to improve cognitive function and mood in healthy adults and those with chronic conditions.17, 25–30 Thus, abundant evidence exists demonstrating that AE modulates the CNS to influence brain function and motor performance.
Based on previous data linking AE interventions to improvements in brain function, we sought to determine whether the neuroplastic effects of AE could be exploited to improve motor recovery in individuals with chronic hemiparesis due to stroke. Two modes of AE training were evaluated: forced exercise (FE) and voluntary exercise (VE). Forced exercise is a novel exercise model that augments, but does not replace, the voluntary efforts of a participant to facilitate sustained AE training.31–34 Changes in central and peripheral function provide rationale to extend FE to this population, as stroke often leads to difficulty achieving or sustaining intensive AE exercise due to structural and metabolic changes in hemiparetic muscle,35 profound cardiovascular deconditioning36 and a general decrease in cortical activation.37 The FE approach allows participants to achieve and maintain a greater exercise rate than what they can achieve voluntarily by augmenting pedaling cadence on a stationary bicycle, thus overcoming stroke-related sensorimotor impairment. It is critical to note that FE is not passive exercise; participants must actively contribute to the activity in order to exercise within a prescribed target heart rate (HR) zone. By augmenting participants’ voluntary efforts, FE provides a consistent exercise pattern and rapid rate that may enhance afferent input and sensorimotor interactions to elicit the physiological response necessary for neuroplasticity.
The aim of this study was to determine the effects of AE combined with upper extremity (UE) motor task practice on the recovery of motor function in adults with stroke. To examine our aim, we compared the effects of FE, VE, and time-matched task practice without an AE component. It was hypothesized that the FE paradigm would be superior to VE or time-matched motor task practice, by enabling participants to attain and sustain a rate of exercise necessary to trigger a neuroplastic “priming” response,38 creating an environment in which motor recovery is optimized.
Materials and Methods
Study Design and Population
This report is a secondary analysis of data from two randomized clinical trials (clinicaltrials.gov registration numbers , National Institute of Health and , American Heart Association). Both studies were approved by the Cleveland Clinic Institutional Review Board and all participants completed the informed consent process. Individuals 18-85 years of age, >6 months post-unilateral stroke who met the following inclusion criteria were eligible for participation: 1) UE Fugl-Meyer Motor Score 19-55, and 2) physician approval to undergo cardiopulmonary exercise stress test. Primary exclusion criteria were as follows: 1) hospitalization for myocardial infarction, congestive heart failure, or heart surgery within 3 months of study enrollment, 2) serious cardiac arrhythmia, 3) hypertrophic cardiomyopathy, 4) severe aortic stenosis, 5) cardiac pacemaker, 6) pulmonary embolus, 7) other cardiovascular, medical, or musculoskeletal contraindication to exercise, 8) significant cognitive impairment or major psychiatric disorder resulting in difficulty to participate in study, and 9) anti-spasticity injection in the upper extremity within 3 months of study enrollment.
Outcomes of Interest
All participants underwent a cardiopulmonary exercise (CPX) test using an upright cycle ergometer (Lode Excalibur Sport with Pedal Force Measurement, Lode B.V., Groningen, Netherlands) interpreted by a cardiologist blinded to group allocation to determine safe cardiopulmonary response to maximum exertion. A 12-lead electrocardiogram was used to continuously monitor heart rate and rhythm five minutes prior to, during, and for five to ten minutes after CPX testing.
An incremental workload protocol was used beginning at 20 Watts (W) and increasing in 20 W stages every two minutes until attaining 100 W, at which time resistance was increased by 40 W every two minutes. Pedaling cadence was self-selected and participants were cued to maintain this cadence throughout the CPX test. The CPX test was terminated based on the American College of Sports Medicine maximal exertion criteria39 or volitionally by the participant due to fatigue.
Clinical outcomes evaluating motor recovery were obtained at baseline, EOT and 4 weeks following EOT (EOT+4) by the same trained occupational therapist blinded to group allocation. The primary motor outcome was the Fugl-Meyer Assessment (FMA), an impairment-based measure consisting of 33 items evaluating the ability of the individual to move out of synergistic patterns toward isolated movement control.40 Movement quality in the affected UE is compared to the non-affected UE, and scored using a 0-2 ordinal scale, in which 0 indicates no movement, 1 indicates partial movement of the affected UE, and 2 indicates movement comparable to the non-affected UE.
The secondary motor outcome was the Wolf Motor Function Test (WMFT).41 The WMFT is a validated measure of UE function post-stroke, consisting of 15 timed tasks and 2 strength tasks. Each timed task can be classified primarily as gross or fine motor in nature, and is also graded using a 0-5 ordinal scale rating movement quality, with higher scores indicating better movement quality.
Interventions
Following baseline testing, participants were randomized to one of three groups using a non-replenished sealed envelope pull method. The interventions arms, described in detail in our previous publication,42 were as follows: 1) Forced exercise + UE repetitive task practice (FE+RTP), 2) Voluntary exercise + UE repetitive task practice (VE+RTP), or 3) Stroke education + UE repetitive task practice (EDU+RTP). All groups engaged in 90-minute sessions 3 times per week for 8 weeks.
Forced Exercise + Repetitive Task Practice (FE+RTP)
Individuals randomized to the FE+RTP group exercised for 45 minutes on a recumbent stationary cycle ergometer equipped with an electric motor and control algorithm to mechanically augment pedaling rate by 30% greater than the participant’s voluntary pedaling rate achieved during the exercise stress test.33, 34, 43 Each 45-minute FE session was monitored by an exercise physiologist or physical therapist and included a five-minute warm-up, 35-minute main exercise set, and a five-minute cool-down. While assistance was provided in the FE mode via the motor to augment pedaling rate, it is important to note that the participant was actively contributing to the pedaling action. Participants were instructed to maintain their HR between 60-80% of their heart rate reserve (HRR) calculated using maximal HR achieved during the CPX with the Karvonen formula.44 Heart rate was continuously monitored and study staff cued participants as needed to ensure compliance with the target HR range. Blood pressure and rate of perceived exertion were obtained every 10 minutes, while cadence and power were recorded every five minutes. Following a 10-minute rest period, participants completed a 45-minute session of UE RTP.
Repetitive task practice emphasizes highly-repetitious blocked practice tasks that are functional, goal-oriented and relevant to the individual.45 The approach to RTP was modeled after Lang, Birkenemeier and colleagues.46–48 Tasks included gross and fine motor components involving proximal and distal motor control; for example, reaching to a shelf positioned at knee height, grasping a plastic cup, and placing it on a shelf positioned at shoulder height. The RTP activities were administered by a neurologic physical therapist who tailored each task to ensure appropriate difficulty and relevance. During the 45-minute RTP session, the therapist typically selected 3-5 tasks and targeted 70-100 repetitions of each task.
Voluntary Exercise + Repetitive Task Practice (VE+RTP)
The VE+RTP sessions were conducted in an identical manner to the FE+RTP sessions, except participants in the VE+RTP group exercised for 45 minutes on a stationary recumbent cycle ergometer at a self-selected cadence without assistance from a motor. Target heart rate range was identical to the FE+RTP group, at 60-80% of HRR. Exercise monitoring and RTP sessions were also conducted in an identical manner to the FE+RTP group.
Stroke Education +Repetitive Task Practice (Edu+RTP)
The control group underwent a time-matched intervention consisting of a 45-minute session of stroke-related education followed by a 45-minute session of UE RTP. Each education session covered a different stroke-related topic such as stroke pathology, nutrition, pharmacology, fatigue, etc. The RTP sessions were administered in an identical manner to the FE+RTP and VE+RTP groups.
Statistical Analysis
Randomized groups were compared on participant demographics, using ANOVA for normally distributed variables, the Kruskal-Wallis test for non-normally distributed continuous variables, or the chi-squared test for categorical variables. The effects of intervention were assessed using separate linear mixed effects models for each outcome, each including a random intercept, a main effect term for time, and a group-by-time interaction term. Each model adjusted for baseline differences between randomized group by including the baseline assessment as part of the outcome vector and by excluding the group main effect term from the model.49 For each outcome, the group-by-time interaction was assessed at the 0.05 significance. To meet model assumptions, WMFT total performance time, gross motor performance time, and fine motor performance time were log transformed.
Regardless of statistical significance, post hoc contrasts were performed for each model estimating the change from baseline to EOT and from baseline to EOT+4 within each randomized group. Within each outcome, pairwise comparisons were Bonferroni-corrected to maintain a 5% type I error rate per outcome. All analyses were conducted using R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 40 participants ranging from 6 to 101 months post-stroke were included in analyses. A flow diagram is presented in Figure 1. Participant demographic and medical characteristics are summarized in Table 1. Intervention-related variables including dosage of RTP (number of repetitions), cycling cadence in revolutions per minute (RPM), and AE intensity (% HRR and power) are also summarized in Table 1. All three groups were administered comparable RTP dosages. The two aerobic exercise groups exercised for similar times at comparable aerobic intensities, 60% HRR and 59% HRR for the FE+RTP and VE+RTP groups, respectively. Forty-four percent of the FE and 50% of the VE participants achieved 60% HRR or greater during the AE training. Cadence was significantly higher for the FE+RTP group compared to VE+RTP, at 74 versus 59 RPM, respectively.
Figure 1:
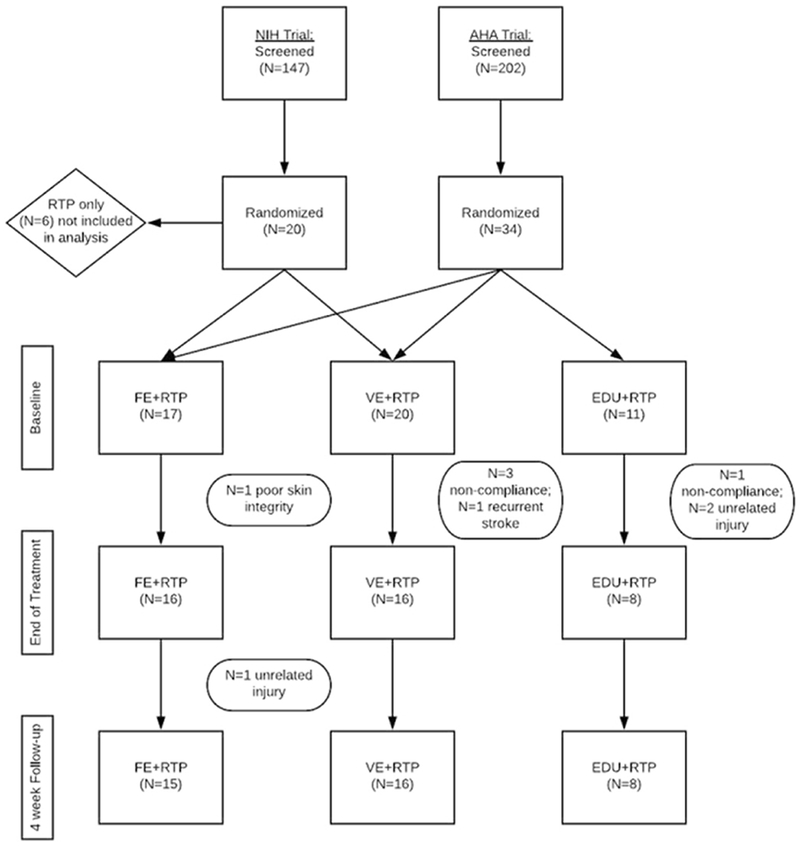
Flow chart depicting the two randomized clinical trials included in this analysis.
Table 1.
Participant demographics and intervention variables by randomized group.
| Factor | FE + RTP (N=16) | VE + RTP (N=16) | RTP + Education (N=8) | p-value |
|---|---|---|---|---|
| Age | 51±12 | 60±14 | 58±12 | 0.14 |
| Male sex (versus female) | 12 (75%) | 10 (62%) | 7 (88%) | 0.43 |
| Race: | 0.11 | |||
| African American | 8 (50%) | 3 (19%) | 4 (50%) | |
| Asian | 1 (6%) | 1 (6%) | 0 (0%) | |
| Other | 2 (12%) | 0 (0%) | 1 (12%) | |
| White | 5 (31%) | 12 (75%) | 3 (38%) | |
| Hispanic ethnicity | 1 (6%) | 0 (0%) | 1 (12%) | 0.67 |
| Dominant side affected (versus non-dominant) | 10 (62%) | 7 (44%) | 5 (62%) | 0.65 |
| Months since stroke | 12 [7,16] | 16 [11,32] | 17 [12,35] | 0.23 |
| Baseline Fugl Meyer score | 37±8 | 33±11 | 33±9 | 0.40 |
| Average cadence (RPM) | 74 [68,82] | 59 [52,66] | -- | 0.008 |
| Aerobic Exercise Intensity (% of HRR) | 60 [48, 65] | 59 [46, 62] | -- | 0.50 |
| Beta Blocker usage, N (percentage) | 8 (50%) | 6 (37%) | 5 (62%) | 0.50 |
| Aerobic exercise time (min/session) | 43.0 [39.7, 44.2] | 43.3 [42.1, 44.6] | 0.36 | |
| Power (watts) | 32.4 [24.1, 41.8] | 33.9 [28.3, 54.9] | -- | 0.72 |
| Average number of repetitions/session | 276 [248,322] | 264 [232,328] | 306 [268,440] | 0.50 |
Summary statistics presented as mean ± standard deviation for normally distributed data, median [Q1, Q3] for skew data, or N (%) for categorical data.
FE+RTP Group Exhibits Greatest Improvement in Fugl-Meyer Assessment
Group by time and within group results for all motor outcomes at three time points (baseline, EOT, and EOT+4) are shown in Table 2. There was a significant group-by-time interaction in the FMA score favoring the FE+RTP group. (p=0.001). Post-hoc pairwise comparisons revealed that improvements by the FE+RTP group were significantly greater than the VE+RTP group from baseline to EOT and baseline to EOT+4 (P<0.05). All three groups improved significantly by a mean of 11, 6, and 9 points for the FE+RTP, VE+RTP and Edu+RTP groups, respectively. Changes in FMA scores are shown in Figure 2.
Table 2.
Outcomes at each evaluation time point by randomized group (i.e., FE + RTP, VE + RTP, and RTP + Education).
| Outcome | FE + RTP (N = 16) | VE + RTP (N = 16) | RTP + Education (N = 8) | Interaction p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | EOT | EOT + 4* | Baseline | EOT | EOT + 4 | Baseline | EOT | EOT + 4 | ||
| FMA score | 37±8 | 48±9‡ | 49±9‡ | 33±11 | 38±12‡ | 40±13‡ | 33±9 | 42±9‡ | 42±9‡ | 0.001 |
| WMFT – affected side | ||||||||||
| Total performance time, s | 3.0 [2.4,5.4] | 2.4 [2.1,3.6]‡ | 2.6 [1.9,4.0]‡ | 7.1 [3.9,19] | 5.5 [2.9,31] | 4.4 [3.9,20] | 5.0 [3.7,5.7] | 3.5 [2.6,9.3] | 3.1 [2.7,5.4] | 0.42 |
| Gross motor performance time, s | 1.1 [0.9,1.8] | 0.9 [0.8,1.1]‡ | 0.9 [0.7,1.1]‡ | 1.9 [1.3,4.7] | 1.3 [1.0,2.8]‡ | 1.6 [0.9,3.1] | 1.2 [0.9,1.5] | 0.8 [0.7,1.1] | 0.9 [0.8,1.1] | 0.79 |
| Fine motor performance time, s | 4.6 [3.8,7.9] | 3.7 [3.3,6.0]‡ | 3.9 [3.0,6.7]‡ | 11 [5.7,34] | 7.7 [4.6,56] | 6.9 [6.3,36] | 8.0 [5.8,9.7] | 5.9 [4.2,16] | 5.0 [4.3,8.8] | 0.42 |
| Weight-to-box, lbs. | 16±4 | 18±3‡ | 18±3 | 10±7 | 14±6‡ | 14±5‡ | 14±6 | 16±6 | 17±5 | 0.78 |
| Grip strength, kg | 22±10 | 24±12 | 26±14‡ | 12±5 | 15±6 | 14±5 | 15±5 | 18±7 | 18±5 | 0.72 |
| Functional Ability Score | 3.9±0.5 | 4.5±0.5‡ | 4.5±0.4‡ | 3.3±0.9 | 3.7±0.9‡ | 3.8±0.9‡ | 3.5±0.6 | 4.2±0.5‡ | 4.2±0.5‡ | 0.10 |
Summary statistics presented as mean ± standard deviation for normally distributed data, median [Q1, Q3] for skew data, or N (%) for categorical data.
One FE patient was missing their assessments at EOT + 4 and was thus excluded from analyses.
Significant change from baseline (p < 0.05)
Figure 2:
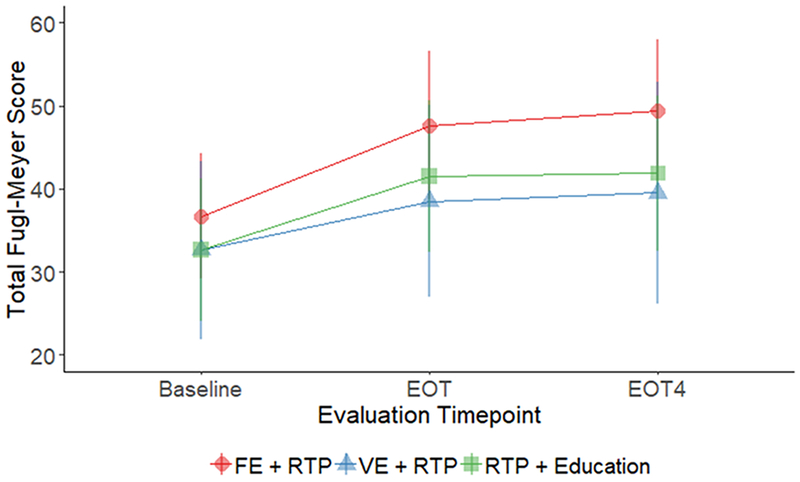
Line plot depicting mean and standard deviations of Fugl-Meyer Scores at baseline, end of treatment (EOT) and four weeks following end of treatment (EOT+4). A significant interaction effect was found indicating that improvements were greatest for the FE+RTP group (p=0.001).
Improved Motor Function for All Groups Measured by Wolf Motor Function Test
No evidence of a treatment-by-time interaction was observed for any of the WMFT outcomes (Table 2). However, total, gross motor, and fine motor performance time for the WMFT significantly improved from baseline to EOT and from baseline to EOT+4 for the FE+RTP group (P≤0.01 for all observations). The VE+RTP group experienced a significant improvement in gross motor performance time from baseline to EOT (P=0.03). Functional ability score significantly improved from baseline to EOT and from baseline to EOT+4 for each of the 3 randomized groups (P<0.001 for all observations). Grip strength significantly increased from baseline to EOT+4 for the FE+RTP group (P=0.01), but did not change in the other groups.
Discussion
The benefits of AE training in individuals post-stroke have almost exclusively been evaluated using cardiovascular endpoints.8, 36, 50 Improving cardiovascular fitness is clearly important and critical in overall health and in secondary stroke prevention. However, understanding how AE could be used to facilitate motor recovery has not been systematically evaluated and reflects a missed opportunity in rehabilitation practice. Greater improvements in clinical measures of motor recovery indicate that FE may be optimal in “priming” the CNS for motor skill acquisition associated with a RTP intervention.
Forced aerobic exercise facilitates motor recovery
Numerous questions remain regarding what exercise parameters induce the optimal effect in terms of facilitating motor recovery post-stroke. As such, it is important to differentiate among the variables that define exercise intensity. In our study, we classified exercise intensity according to aerobic intensity (measured as %HRR), rate intensity (speed or cadence), and power intensity (watts), in addition to duration (min/session). As it relates to AE, both groups exercised at similar aerobic intensities: 60% HRR for the FE group and 59% HRR for the VE group. Furthermore, power produced for both AE groups was similar at 32.4 and 33.9 watts for the FE and VE groups, respectively, as was exercise duration. The only training variable that differed between the two AE groups was cadence, or the rate of pedaling. On average, the FE group cycled 22 percent faster than the VE group, 74 compared to 59 RPM respectively. In animal models of ischemic stroke, treadmill training and wheel running are predominant modes of AE intervention. When investigating the optimal rate of exercise, moderate rate exercise (30-50% of maximal running speed) has been found most efficacious in reducing lesion size, decreasing inflammation, and facilitating neurogenesis.51 Moderate to high rate running speeds for shorter durations using a FE mode optimized ipsilesional and striatal angiogenesis.51 Additionally, we have found that increased pedaling rate was positively correlated with CNS cortical and subcortical functional connectivity and improved motor function in individuals with PD.33, 43, 52, 53 The FE approach augments the voluntary efforts of individuals with stroke-related sensorimotor impairment, enabling participants to maintain a consistent exercise pattern and rapid rate that appears to be critical in motor recovery. The precise mechanism responsible for improved motor recovery in the FE+RTP group is unclear, however, it is plausible that moderate to high rate exercise induces neurophysiological and vascular changes in the CNS comparable to those seen in animal models to facilitate neuroplasticity.
The impact of RTP dose and intensity
Examining the motor task practice aspect of our intervention, all three groups completed comparable dosages of RTP, with the FE+RTP, VE+RTP and Edu+RTP groups averaging 276, 264, and 306 repetitions per 45-min session, respectively. In a recent report, Lang and colleagues unexpectedly found no dose response effect and only modest improvements in motor function when administering 100, 200, 300, or individual maximal repetitions of RTP, 4 times/week over 8 weeks.48 While a direct comparison of outcomes is not appropriate due to variations in study design, it is noteworthy to mention that while our RTP intervention involved 25% fewer sessions and 45% less time, we employed a more rate-intensive RTP intervention, achieving a rate of ~350-400 reps/hour. This more intensive approach to RTP may have contributed to the improvements observed in clinical measures of motor function. Lang and others have acknowledged that the intensity of RTP training is a potentially important variable that has not been systematically investigated but should be considered in the motor recovery equation.48, 54
Considerations for Clinical Translation
An important consideration in the development of the intervention evaluated was its potential for clinical translatability and adoption. Our criteria for participation included individuals with broad ranges of UE function and did not exclude those with stable cardiovascular comorbidities. Given that we did not observe a relationship between baseline level of motor function and change in impairment, this combined AE+RTP approach appears to be efficacious for individuals with mild to moderate hemiparesis. The 45-minute duration of RTP was chosen because it is clinically feasible; however, we strongly recommend considering the rate of RTP administration as an important variable.54 Our approach included minimal rest between sets and tasks, resulting in relatively continuous engagement of task practice while achieving ~350-400 repetitions/hour.
With proper screening and monitoring, individuals with chronic stroke and cardiovascular comorbidities were able to safely exercise at moderate to high aerobic intensities for 45 minutes. Our protocol included cardiopulmonary exercise testing to determine the participant’s response to maximal activity. According to the American Heart Association/American Stroke Association physical activity recommendations for stroke survivors, graded exercise testing is recommended to assess functional capacity and cardiovascular response to exercise.50 Lastly, current reimbursement models in the US do not consider AE training as a skilled, reimbursable treatment for individuals with stroke. Nonetheless, we seek to advocate for patients and challenge payers by presenting scientific evidence that exercise is not only good for one’s health, but can facilitate brain function and potentially reduce disability.18, 55
Limitations
There are limitations associated with the current study; namely, results reflect a compilation of two smaller-scale randomized clinical trials. The RTP dosages were not identical for the three groups and the VE group exercised just under the prescribed aerobic intensity of 60% HRR, achieving 59% HRR. Additionally, the FE group as a whole was younger and presented with higher baseline FMA scores. While these demographic and intervention-related characteristics were not statistically different among groups, it is possible the characteristics of the FE group were ideally suited to respond to a FE intervention. Influence of lesion size, location, or the effect of genetic characteristics on neuroplasticity post-stroke were not considered in the randomization of participants.7, 56 Future larger studies are planned to determine if demographic factors may influence response to FE. Lastly, while we hypothesize potential mechanisms (e.g.: increased peripheral levels of BDNF, insulin-like growth factor-1, cerebral blood flow, and enhanced synaptogenesis14, 19, 20) that may be responsible for the changes in motor recovery evident with the FE+RTP group, neurotrophic factors were not measured. Future studies will utilize imaging and other modalities to elucidate the mechanism underlying improvement with FE and VE.
Summary
Our findings indicate that the completion of FE prior to UE RTP enhanced motor skill acquisition greater than VE or stroke-related education. These initial results provide rationale to utilize high-rate aerobic exercise, FE in particular, into models of stroke rehabilitation to improve motor recovery.
Acknowledgements
This study was supported by the National Institute of Neurological Disorders and Stroke (R03HD073566) and the American Heart Association (15MCPRP25700312). JLA has authored intellectual property associated with the algorithm used in the control of the forced exercise cycle. The remaining authors declare no conflicts of interest.
References
- 1.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the american heart association. Stroke. 2010;41:2402–2448 [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146 [DOI] [PubMed] [Google Scholar]
- 3.Dobkin BH, Dorsch A. New evidence for therapies in stroke rehabilitation. Current atherosclerosis reports. 2013;15:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2016;47:e98–e169 [DOI] [PubMed] [Google Scholar]
- 5.Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: The icare randomized clinical trial. JAMA. 2016;315:571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: Is the intensity adequate to induce a training effect? Archives of Physical Medicine and Rehabilitation. 2002;83:1378–1383 [DOI] [PubMed] [Google Scholar]
- 9.Singh AM, Neva JL, Staines WR. Aerobic exercise enhances neural correlates of motor skill learning. Behavioural brain research. 2016;301:19–26 [DOI] [PubMed] [Google Scholar]
- 10.Singh AM, Duncan RE, Neva JL, Staines WR. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci Med Rehabil. 2014;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh AM, Staines WR. The effects of acute aerobic exercise on the primary motor cortex. J Mot Behav. 2015;47:328–339 [DOI] [PubMed] [Google Scholar]
- 12.Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neuroscience and biobehavioral reviews. 2013;37:2243–2257 [DOI] [PubMed] [Google Scholar]
- 13.Alcantara CC, Garcia-Salazar LF, Silva-Couto MA, Santos GL, Reisman DS, Russo TL. Post-stroke bdnf concentration changes following physical exercise: A systematic review. Front Neurol. 2018;9:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knaepen K, Goekint M, Heyman EM, R. M Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor. Sports medicine. 2010;40:765–801 [DOI] [PubMed] [Google Scholar]
- 15.Ploughman M, Austin MW, Glynn L, Corbett D. The effects of poststroke aerobic exercise on neuroplasticity: A systematic review of animal and clinical studies. Transl Stroke Res. 2015;6:13–28 [DOI] [PubMed] [Google Scholar]
- 16.Mang CS, Brown KE, Neva JL, Snow NJ, Campbell KL, Boyd LA. Promoting motor cortical plasticity with acute aerobic exercise: A role for cerebellar circuits. Neural Plast. 2016;2016:6797928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30:464–472 [DOI] [PubMed] [Google Scholar]
- 18.Ploughman M, Kelly LP. Four birds with one stone? Reparative, neuroplastic, cardiorespiratory, and metabolic benefits of aerobic exercise poststroke. Curr Opin Neurol. 2016;29:684–692 [DOI] [PubMed] [Google Scholar]
- 19.Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (bdnf): A systematic review of experimental studies in the elderly. Arch Gerontol Geriatr. 2013;56:10–15 [DOI] [PubMed] [Google Scholar]
- 20.Gatti R, De Palo EF, Antonelli G, Spinella P. Igf-i/igfbp system: Metabolism outline and physical exercise. J Endocrinol Invest. 2012;35:699–707 [DOI] [PubMed] [Google Scholar]
- 21.Boyne P, Meyrose C, Westover J, Whitesel D, Hatter K, Reisman DS, et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. Journal of applied physiology. 2019;126:431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Statton MA, Encarnacion M, Celnik P, Bastian AJ. A single bout of moderate aerobic exercise improves motor skill acquisition. PloS one. 2015;10:e0141393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. Journal of applied physiology. 2014;117:1325–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AE, Goldsworthy MR, Garside T, Wood FM, Ridding MC. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp Brain Res. 2014;232:1875–1882 [DOI] [PubMed] [Google Scholar]
- 25.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: Interactions between exercise, depression, and bdnf. Neuroscientist. 2012;18:82–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rand D, Eng JJ, Liu-Ambrose T, Tawashy AE. Feasibility of a 6-month exercise and recreation program to improve executive functioning and memory in individuals with chronic stroke. Neurorehabil Neural Repair. 2010;24:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker L FL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of aerobic exercise on mild cognitive impairment. Archives of neurology. 2010;67:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological science. 2003;14:125–130 [DOI] [PubMed] [Google Scholar]
- 29.Basso JC, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2017;2:127–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan JA, Singhal G, Corrigan F, Jaehne EJ, Jawahar MC, Baune BT. The effects of aerobic exercise on depression-like, anxiety-like, and cognition-like behaviours over the healthy adult lifespan of c57bl/6 mice. Behavioural brain research. 2018;337:193–203 [DOI] [PubMed] [Google Scholar]
- 31.Linder SM, Rosenfeldt AB, Rasanow M, Alberts JL. Forced aerobic exercise enhances motor recovery after stroke: A case report. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2015;69:6904210010p6904210011–6904210018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linder SM, Rosenfeldt AB, Dey T, Alberts JL. Forced aerobic exercise preceding task practice improves motor recovery post-stroke American Journal of Occupational Therapy. 2017;70:in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike, it is about the pedaling: Forced exercise and parkinson’s disease. Exercise and sport sciences reviews. 2011;39:177–186 [DOI] [PubMed] [Google Scholar]
- 34.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23:600–608 [DOI] [PubMed] [Google Scholar]
- 35.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707 [DOI] [PubMed] [Google Scholar]
- 36.Billinger SA, Coughenour E, Mackay-Lyons MJ, Ivey FM. Reduced cardiorespiratory fitness after stroke: Biological consequences and exercise-induced adaptations. Stroke research and treatment. 2012;2012:959120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain : a journal of neurology. 2008;131:1381–1390 [DOI] [PubMed] [Google Scholar]
- 38.Thacker JS, Middleton LE, Mcllroy WE, Staines WR. The influence of an acute bout of aerobic exercise on cortical contributions to motor preparation and execution. Physiol Rep. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American College of Sports Medicine. Acsm’s resource manual for guildlines for exercise testing and prescription. Wolters Kluwer and Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 40.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240 [DOI] [PubMed] [Google Scholar]
- 41.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639 [DOI] [PubMed] [Google Scholar]
- 42.Rosenfeldt AB, Linder SM, Davidson S, Clark C, Zimmerman N, Lee J, et al. Combined aerobic exercise and task practice improve health-related quality of life after stroke: A preliminary analysis. Arch Phys Med Rehabil. 2018;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah C, Beall EB, Frankemolle AM, Penko A, Phillips MD, Lowe MJ, et al. Exercise therapy for parkinson’s disease: Pedaling rate is related to changes in motor connectivity. Brain connectivity. 2016;6:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American college of sports medicine. Resource manual for guidelines for exercise testing and prescription. Wolters Kluwer; 2018. [Google Scholar]
- 45.Hubbard IJ, Parsons MW, Neilson C, Carey LM. Task-specific training: Evidence for and translation to clinical practice. Occup Ther Int. 2009;16:175–189 [DOI] [PubMed] [Google Scholar]
- 46.Lang CE, Birkenmeier RL. Upper-extremity task-specific training after stroke or disability. Bethesda, MD: AOTA Press; 2014. [Google Scholar]
- 47.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabil Neural Repair. 2010;24:620–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang CE, Strube MJ, Bland MD, Waddell KJ, Cherry-Allen KM, Nudo RJ, et al. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase ii, single-blind, randomized, controlled trial. Ann Neurol. 2016;80:342–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzmaurice G, Laird N, JH W. Applied longitudinal analysis. Hoboken, NJ: Wiley; 2011. [Google Scholar]
- 50.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014 [DOI] [PubMed] [Google Scholar]
- 51.Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: A systematic review and perspective. Neurosci Res. 2014;87:8–15 [DOI] [PubMed] [Google Scholar]
- 52.Alberts JL, Phillips M, Lowe MJ, Frankemolle A, Thota A, Beall EB, et al. Cortical and motor responses to acute forced exercise in parkinson’s disease. Parkinsonism & related disorders. 2016;24:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beall EB, Lowe MJ, Alberts JL, Frankemolle AM, Thota AK, Shah C, et al. The effect of forced-exercise therapy for parkinson’s disease on motor cortex functional connectivity. Brain connectivity. 2013;3:190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly LP, Devasahayam AJ, Chaves AR, Wallack EM, McCarthy J, Basset FA, et al. Intensifying functional task practice to meet aerobic training guidelines in stroke survivors. Front Physiol. 2017;8:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins M, Clifton E, Van Wijck F, Mead G. Cost-effectiveness of physical fitness training for stroke survivors. Journal of Royal College of Physicians of Edinburgh. 2018;48:62–68 [DOI] [PubMed] [Google Scholar]
- 56.French MA, Morton SM, Pohlig RT, Reisman DS. The relationship between bdnf val66met polymorphism and functional mobility in chronic stroke survivors. Top Stroke Rehabil. 2018;25:276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]


