Abstract
Background:
Converging evidence implicates abnormal thalamocortical interactions in the pathophysiology of schizophrenia. This evidence includes consistent findings of increased resting-state functional connectivity MRI of the thalamus with somatosensory and motor cortex during wake and reduced spindle activity during sleep. We hypothesized that these abnormalities would be correlated, reflecting a common mechanism: reduced inhibition of thalamocortical neurons by the thalamic reticular nucleus (TRN), which is the major inhibitory nucleus of the thalamus and is abnormal in schizophrenia. Reduced TRN inhibition would be expected to lead to increased and less filtered thalamic relay of sensory and motor information to the cortex during wake and reduced burst firing necessary for spindle initiation during sleep.
Methods:
Twenty-six schizophrenia outpatients and 30 demographically matched healthy individuals had overnight polysomnography and resting-state functional connectivity MRI scans. We examined the relations of sleep spindle density during stage 2 non-rapid eye movement sleep with connectivity of the thalamus with the cortex during wakeful rest.
Results:
As in prior studies, schizophrenia patients exhibited increased functional connectivity of the thalamus with bilateral somatosensory and motor cortex, and reduced sleep spindle density. Spindle density inversely correlated with thalamocortical connectivity, including in somotosensory and motor cortex, regardless of diagnosis.
Conclusions:
These findings link two biomarkers of schizophrenia – the sleep spindle density deficit and abnormally increased thalamocortical functional connectivity – and point to deficient TRN inhibition as a plausible mechanism. If TRN-mediated thalamocortical dysfunction increases risk for schizophrenia and contributes to its manifestations, understanding its mechanism could guide the development of targeted interventions.
Keywords: Sleep, schizophrenia, functional connectivity, thalamus, thalamic reticular nucleus, sleep spindles
Converging lines of evidence implicate abnormal communication of the thalamus with the cortex in the pathophysiology of schizophrenia. Resting-state functional connectivity MRI (rs-fcMRI) studies, for example, consistently report increased thalamic connectivity with somatosensory and motor cortex in schizophrenia (1–8) and in individuals at clinical high risk for psychosis, in whom it predicts conversion to full-blown illness (9). Findings of decreased sleep spindle activity in patients with schizophrenia and their first-degree relatives also implicate abnormal thalamocortical interactions (for review, see 10). Sleep spindles are a defining electroencephalogram (EEG) oscillation of non-rapid eye movement stage II sleep (N2) and depend on thalamocortical circuitry for their expression (11–15). The goal of the present study was to determine whether abnormally increased thalamocortical functional connectivity correlated with sleep spindle deficits in schizophrenia, which would be consistent with the hypothesis that they reflect a common pathophysiology.
Sleep spindles are initiated in the thalamic reticular nucleus (TRN, 16), a thin net-like structure around the thalamus that receives collaterals from corticothalamic and thalamocortical neurons. Sleep spindles depend on these thalamocortical feedback loops for their propagation and synchronization across the cortex (12, 13, 17, 18). Sleep spindles facilitate the synaptic plasticity involved in memory (19, 20), correlate with sleep-dependent memory consolidation, learning efficiency and IQ in a large body of basic research (21) and can be manipulated to improve memory either pharmacologically (22, 23) or using transcranial stimulation (24–26)in healthy humans. Patients with schizophrenia show sleep spindle deficits (27–29) that are associated with impaired sleep-dependent memory consolidation (30–32). Reduced spindle activity that correlates with worse cognitive performance and lower IQ is also seen in early course antipsychotic-naive schizophrenia patients and young nonpsychotic first-degree relatives. Collectively, this evidence suggests that reduced spindle activity is an endophenotype of schizophrenia that reflects the functional integrity of thalamocortical networks and contributes to cognitive deficits (10, 33, 34).
We hypothesize that the abnormal thalamocortical interactions that give rise to both hyperconnectivity and sleep spindle deficits in schizophrenia reflect reduced TRN inhibition of the thalamus. The TRN, which is comprised entirely of gamma-amino butyric acid (GABA) neurons (35), is the major inhibitory nucleus of the thalamus. Strategically positioned between thalamus and cortex, it powerfully inhibits glutamatergic thalamocortical neurons to gate the relay of information to cortex during wake and to initiate spindles during sleep (14). Sleep spindle initiation depends on powerful and prolonged inhibition of thalamocortical neurons by the TRN (11, 36), particularly sensory projecting neurons (37). This inhibition is followed by rebound spike-bursts in thalamocortical neurons that entrain cortical neurons to oscillate at spindle frequency (12). Postmortem studies provide evidence of TRN abnormalities in schizophrenia, including a reduction of parvalbumin neurons (38–40), which predominate in sensory relay nuclei (41). A consequent impairment of TRN-mediated inhibition of thalamocortical neurons in schizophrenia may increase the forwarding of sensory and motor information to the cortex, resulting in thalamic hyperconnectivity, and reduce the burst firing necessary for sleep spindle initiation, resulting in spindle deficits.
Methods
Participants
Twenty-six schizophrenia outpatients, recruited from an urban mental health center, and 30 healthy individuals, recruited from the community through poster and website advertisements, participated. After exclusion for excessive motion during scanning (see description below), 22 patients and 29 controls were retained for group comparisons of functional connectivity. Group comparison of sleep spindle density was based on 26 patients and 29 controls after one control was excluded due to technical problems with the sleep recording. Correlations of connectivity and spindle density included 22 patients and 28 controls. Patient and control groups did not differ in age, sex, handedness, mean parental education or estimated premorbid verbal IQ (Table 1). Two patients were unmedicated and the rest had been maintained on stable doses of antipsychotic and adjunctive medications for at least six weeks prior to enrollment (Supplemental Table 1). Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV (42) and symptoms were rated with the Positive and Negative Syndrome Scale (PANSS, 43). Healthy participants were screened to exclude individuals with a personal history of mental illness (SCID-Non-patient edition, 44) or a family history of either schizophrenia spectrum disorder or psychosis.
Table 1. Participant Characteristics.
Means, standard deviations and group comparisons of demographic data. PANSS=Positive and Negative Syndrome Scale
| Schizophrenia (n=22) mean ± SD | Healthy Controls (n=29) mean ± SD | t | p | |
|---|---|---|---|---|
| Age | 31.7 ± 7.1 | 30.2 ± 6.3 | .80 | .43 |
| Sex | 5F/17M | 8F/21M | X2 =.64 | .75 |
| Handednessa | 80 ± 24 | 65 ± 54 | 1.16 | .25 |
| Mean Parental Education (yrs) | 14.3 ± 3 | 15.1 ± 3.4 | −.86 | .40 |
| Estimated Verbal IQb | 104 ± 9.3 | 108 ± 8.5 | 1.68 | .10 |
| Mean Residual Motionc | .24 ± .06 | .23 ± .07 | −.39 | .70 |
| PANSS Total | 69 ± 14.4 | |||
| PANSS Positive | 17 ± 5.2 | |||
| PANSS Negative | 19 ± 4.7 | |||
Based on the modified Edinburgh Handedness Inventory (21,22) Laterality scores of −100 and +100 denote exclusive use of left or right hand, respectively.
Based on standard scores on the reading subtest of the Wide Range Achievement Test -III (84).
Root mean square of translation in x, y and z directions averaged across the two resting state runs.
All participants were screened to exclude those with a diagnosed sleep disorder, treatment with sleep medications, a history of significant head injury or neurological disorder, a history of substance abuse or dependence within the past six months (based on interview, chart review, clinician report and urine toxicological screening) and contraindications for MRI (e.g., metal in the body, pregnancy). All participants gave written informed consent. The study was approved by the Partners Human Research Committee.
Procedures
Overview:
Participants had four nights of polysomnography (PSG) in the Massachusetts General Hospital Clinical Research Center as part of a double-blind, randomized, placebo controlled clinical trial that involved adding 3 mg of eszopiclone to ongoing medications for the two treatment nights. Placebo and treatment visits were separated by one week and took place on two consecutive weeknights, with the first night of each visit serving as the baseline night and the second the learning night. For the present study, we measured spindle activity during N2 on the baseline night of the placebo visit. MRI scans were acquired approximately one week after completion of the sleep visits.
Spindle measurement and analysis:
PSG was acquired at 400Hz using an Aura LTM64 acquisition system (Grass Technologies, Astro-Med Inc., RI) and Easycap EEG (electroencephalography) caps (Easycap GmbH, Herrsching, Germany) with 58 EEG, two submental EMG (electromyography), and two EOG (electrooculography) channels. Each 30s epoch of PSG was visually classified into stages (Wake, N1, N2, N3, REM) according to standard criteria (45) by raters blind to visit, group and night. N2 EEG data were preprocessed and analyzed using BrainVision Analyzer 2.0 (BrainProducts, Germany) and MATLAB R2014a (MathWorks, MA), filtered at 0.3-35Hz and notch filtered at 60Hz. Channels displaying significant artifacts for more than 30 minutes of the recording were interpolated with spherical splines. After referencing to the average of all EEG channels, data were visually inspected and 30s epochs with artifacts were removed. Sleep spindles were automatically detected at each channel using a wavelet-based algorithm that was previously validated against hand-counted spindles in both patients with schizophrenia and healthy individuals (31, 46). The threshold for spindle detection, 9 times the median signal amplitude of artifact-free epochs, was chosen to maximize between-class (‘spindle’ vs ‘non-spindle’) variance in the wavelet coefficient (47) in samples of schizophrenia patients and controls from a previous study (31). The outcome measure was spindle density (spindles per minute) during N2 sleep.
Group comparisons of sleep spindle density were based on t-tests at each electrode. A nonparametric clustering method optimized for EEG (48) and implemented in R (http://www.R-project.org/) was used to correct for multiple comparisons. Adjacent electrodes that met an uncorrected threshold of p≤.05 were clustered and within each cluster the t-values were summed across electrodes. Cluster-level corrected p-values were determined by estimating the likelihood that a cluster of that summed t-value would be found by chance under the null distribution derived from 10,000 permutations with random group assignment.
MRI Acquisition:
Scans were acquired with a 3T Siemens (Erlangen, Germany) Trio TIM whole body high speed imaging device equipped for echo planar imaging (EPI) and a 32-channel head coil. Head stabilization was achieved with cushioning and participants wore earplugs to attenuate noise. Autoalign was used for automatic slice positioning (49). Anatomical images were acquired with a 3D multiecho magnetization-prepared rf-spoiled rapid gradient-echo MEMPRAGE sequence (T1-weighted) with EPI based volumetric navigators for real time motion correction (50) (TR=2530ms, Flip Angle=7°, TEs=1.7ms/3.6ms/5.5ms/7.3ms, iPAT=2; FOV=256mm; 176 in-plane sagittal slices; voxel size=1mm3 isotropic, scan duration 6m 12s). Two rs-fcMRI scans were collected with a gradient echo T2*-weighted sequence for Blood Oxygen Level Dependent (BOLD) contrast (TR=3000 ms, Flip Angle=85° TE=30 ms, FOV=216mm, 47 contiguous horizontal slices parallel to the intercommissural plane, voxel size = 3mm3, interleaved, scan duration 6m 12s). Rs-fcMRI sequences included prospective acquisition correction (PACE) for head motion to adjust slice position and orientation during data acquisition (51). Participants were instructed to keep their eyes open for the duration of the resting state scans.
MRI Preprocessing:
Rs-fcMRI data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB. Anatomical images were segmented into white matter, gray matter and cerebrospinal fluid masks. Images were corrected for the time of slice acquisition, spatially realigned with respect to the reference image, resliced, and coregistered with the anatomical images. The volumes were normalized to the Montreal Neurological Institute (MNI) template and spatially smoothed using a Gaussian kernel with a full width at half maximum of 6 mm.
MRI Data Quality:
To minimize spurious correlations in rs-fcMRI data and to avoid artifactual group differences due to head motion (52), we excluded data from four patients and one control based on high levels of residual motion — greater than two standard deviations above the sample mean (root mean square of translation in x, y and z directions averaged across the two runs). In the remaining participants, we identified and removed artifactual volumes using Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect/) based on either head displacement in the x, y or z direction that was more than 1mm from the previous frame or if the global mean intensity of the volume was more than three standard deviations above that of the entire functional scan. There were no group differences in residual motion (t(49)=.39, p=.70) or the number of artifactual volumes (t(49)=1.49, p=.14) in the final sample.
Functional connectivity analyses:
Analyses were implemented in CONN (53) version 17 using a component base noise reduction method, Anatomical CompCor (54) rather than global signal regression, to remove physiological and other noise (55). Preprocessing involved applying a temporal band-pass filter of .008-.09 Hz to the time series. Residual head motion parameters (rotation and translations in x, y and z directions and their first-order temporal derivatives) and artifactual volumes (flagged by ART) were regressed out in the model. Functional connectivity maps were generated for each participant by extracting the average time course of the BOLD signal from the bilateral whole thalamus seed, which was defined using the probabilistic FSL Oxford thalamic connectivity atlas with a threshold of 25 (56, 57: Fig. S1), and correlating it with every other gray matter voxel. The resulting Pearson coefficients were transformed into Fisher’s z-values. This yielded a map for each resting-state run where the value at voxel indexed connectivity with the thalamus. The two runs for each subject were averaged.
We examined group differences in thalamocortical functional connectivity with t-tests at every voxel. We examined the relations of sleep spindle density (averaged across electrodes) with thalamocortical connectivity using regression with Group, Spindle Density and Group by Spindle Density as predictors. Whole brain correction for multiple comparisons was based on a voxel level uncorrected threshold of p≤.001 and a false discovery rate (FDR)-corrected cluster threshold of p≤.05.
Results
Thalamocortical hyperconnectivity in schizophrenia:
As in prior studies, schizophrenia patients exhibited significantly increased resting-state functional connectivity of the thalamus with bilateral motor and somatosensory cortex and with the left parahippocampal gyrus (Fig. 1A–B, Table 2). There were no regions of significantly reduced thalamic functional connectivity in schizophrenia (See Fig. S2 for unthresholded functional connectivity statistical maps for each group).
Figure 1:
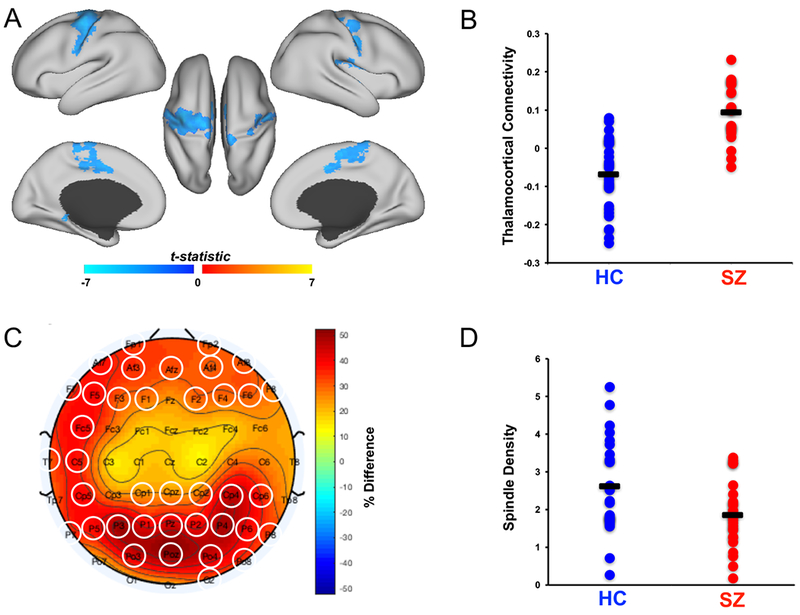
Group differences. (A) Statistical map of group differences in thalamocortical functional connectivity displayed on the cortical surface of the template brain at pcorrected≤05. Greater connectivity in schizophrenia (SZ) is depicted in blue. There were no regions of significantly greater connectivity in healthy controls (HC). (B) Dot plot of averaged thalamocortical connectivity in the group difference mask. Black bars represent group means. (C) Topographical map of group differences in sleep spindle density, warm colors represent higher spindle density in healthy controls. The electrodes circled in white form a significant cluster. (D) Dot plot of averaged sleep spindle density in the cluster of electrodes with significantly reduced spindle density. Black bars represent group means.
Table 2. Maxima and locations of clusters showing significant group differences in functional connectivity with the thalamus.
All reported clusters have pcorrected≤.05 based on correction for the entire brain. There were no clusters where controls showed significantly greater functional connectivity than patients. Local maxima within the clusters (indented) are listed only if they fell in a different Brodmann area (BA) than the global maximum.
| Region | Voxels | MNI Coordinates | BA | z-stats (max) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L Postcentral Gyrus | 3624 | −38 | −20 | 46 | 2 | −5.49 |
| L Precentral Gyrus | −28 | −20 | 70 | 4 | ||
| L Postcentral Gyrus | −37 | −17 | 46 | 3 | ||
| L Postcentral Gyrus | −51 | −6 | 35 | 6 | ||
| L Cingulate Gyrus | −10 | −1 | 45 | 24 | ||
| L Medial Frontal Gyrus | −3 | −18 | −52 | 6 | ||
| L Paracentral Lobule | −3 | −7 | 46 | 31 | ||
| R Insula | 1640 | 36 | −34 | 22 | 13 | −5.54 |
| R Precentral Gyrus | 42 | −12 | 31 | 6 | ||
| R Precentral Gyrus | 36 | −13 | 37 | 4 | ||
| R Postcentral Gyrus | 36 | −16 | 41 | 3 | ||
| R Precentral Gyrus | 207 | 54 | −2 | 4 | 6 | −5.17 |
| L Parahippocampal Gyrus | 194 | −20 | −46 | −8 | 19 | −4.52 |
| L Parahippocampal Gyrus | −23 | −49 | −8 | 37 | ||
Reduced spindle density in schizophrenia:
We also replicated findings of reduced sleep spindle density in schizophrenia. High density overnight PSG recordings revealed globally reduced spindle density (number per minute) during N2 sleep that reached significance in a large cluster (38 electrodes, pcorrected=.009; Fig. 1C–D).
Spindle density correlates with thalamocortical functional connectivity:
To test the hypothesis that thalamocortical connectivity and spindle density both reflect the functional integrity of TRN-mediated thalamocortical circuitry, we examined their relations. A regression model with factors for spindle density (averaged across all electrodes), group and their interaction showed that lower spindle density was significantly associated with greater thalamic connectivity in left motor and somatosensory cortex and left superior temporal gyrus (Fig. 2A, Table 3). These relations did not differ by group (Fig. 2B).
Figure 2:

Relations of sleep spindle density with thalamocortical connectivity. (A) Group differences in thalamocortical connectivity (blue), regions showing significant (pcorrected≤.05) inverse correlations of average spindle density with thalamocortical connectivity (yellow) and their overlap (green) displayed on the cortical surface of the template brain. No regions showed significant positive correlations. (B) Thalamocortical connectivity in regions showing a significant inverse correlation (yellow and green in 2A) is plotted against average spindle density.
Table 3. Maxima and locations of clusters showing significant relations of thalamocortical functional connectivity with spindle density.
Reported clusters have pcorrected≤.05 based on correction for the entire brain. No clusters showed a significant positive correlation. Local maxima within the clusters (indented) are listed only if they fell in a different Brodmann area (BA) than the global maximum.
| Region | Voxels | MNI Coordinates | BA | z-value (max) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L Precentral Gyrus | 181 | −24 | −16 | 64 | 4 | −4.21 |
| L Postcentral Gyrus | −40 | −19 | 64 | 3 | ||
| L Superior Temporal Gyrus | 169 | −50 | −10 | −8 | 22 | −4.88 |
| L Middle Temporal Gyrus | −56 | −12 | −8 | 21 | ||
| L Postcentral Gyrus | 143 | −30 | −32 | 54 | 3 | −5.20 |
Exploratory and control analyses:
Based on recent evidence that thalamic input regulates local cortical functional connectivity (58), we questioned whether abnormally increased thalamic input in schizophrenia would disrupt local cortical interactions. To address this, we investigated the relation of thalamocortical connectivity with intra-cortical connectivity in the regions that showed hyperconnectivity in schizophrenia. We quantified intra-cortical connectivity within the thalamocortical group difference mask by computing the average connectivity of each voxel with the averaged connectivity of the entire mask. We then correlated this measure of intra-cortical connectivity with the averaged thalamocortical connectivity of the mask. Intra-cortical connectivity within the group difference mask was significantly weaker in patients than controls (t(49)=2.8, p=.007; Fig. 3A) and only controls showed a strong reciprocal relation of intra-cortical connectivity with thalamocortical connectivity (r=−.75, p=2E-6; Patients: r =.19, p=.41; Fig. 3B), a difference that was significant (β=.84, p=5E-6).
Figure 3.

Relations of thalamocortical connectivity with cortical connectivity. (A) Dot plot of the averaged cortico-cortical connectivity in the regions that show thalamocortical hyperconnectivity in schizophrenia. Black bars represent group means. (B) Thalamocortical connectivity is plotted against cortico-cortical connectivity within the same mask. The slopes of the relations differ significantly between groups.
Several studies have examined correlations of thalamocortical hyperconnectivity with symptoms in schizophrenia, but the consistency and direction of these findings varies (59). In the present study, thalamic connectivity did not correlate with either positive or negative symptoms. In addition, hallucinations did not correlate with the connectivity of the thalamus with the superior temporal gyri, which are thought to be involved in their generation (60).
Antipsychotic dosage measured in chlorpromazine equivalents (61) did not significantly correlate with either sleep spindle density (r=−.30, p=.17) or thalamocortical connectivity (r=−.22, p=.35).
Discussion
We replicated previous findings of decreased spindle density and thalamic hyperconnectivity in schizophrenia and found that these two abnormalities were correlated. Both abnormalities reflect thalamocortical circuit dysfunction and their correlation supports the hypothesis of a common underlying pathophysiology. We propose that these abnormalities reflect reduced inhibition of thalamocortical neurons by the TRN. This would be expected to lead to increased and less filtered relay of sensory and motor information during wake, corresponding to stronger functional connectivity of the thalamus with sensory and motor cortex, and to decreased sleep spindles. The present findings link two biomarkers of schizophrenia – the spindle density deficit and abnormally increased thalamocortical functional connectivity – and suggest deficient TRN inhibition as a mechanism.
The relations of thalamocortical connectivity with spindle density were seen in both healthy controls and schizophrenia patients, and the slopes of these relations were almost identical. Patients simply had lower spindle density that corresponded to higher connectivity. These findings support the hypothesis that both thalamocortical connectivity and spindle density index functional variation in TRN-mediated thalamocortical circuitry, which lies on a continuum, with patients having less robust TRN-mediated inhibition. This seemingly quantitative rather than qualitative difference, along with previous findings of normal spindle morphology in schizophrenia (e.g., 31) may bode well for the prospects of therapy to normalize circuit function, increase spindles and improve outcome. The relationship observed in controls, who were not taking medications, suggests that medication is unlikely to be a confounding factor in these correlations.
As in prior studies, thalamocortical hyperconnectivity was seen primarily in motor and somatosensory cortex (1–6, 9). This selectivity may reflect the organization of thalamocortical circuitry, which can be divided into core and matrix pathways (41). Thalamocortical neurons of the matrix pathway exhibit immunoreactivity to the calcium binding protein calbindin, are widespread throughout the thalamus and project diffusely to multiple cortical regions. Core neurons, in contrast, react to parvalbumin, are primarily found in sensory and motor nuclei and have restricted, topographically organized projections to sensory cortical regions. The core pathway is thought to initiate focal spindles in sensory and motor regions, which have been associated with memory consolidation (62, 63), while the matrix pathway is thought to play a greater role in initiating widely distributed spindles and in synchronizing spindles across the cortex (64, 65). Thus, thalamocortical hyperconnectivity in sensory regions and a correlated reduction in spindle density in schizophrenia are most consistent with abnormalities of the core pathway.
Both spindle deficits and thalamocortical hyperconnectivity may reflect abnormal TRN function. Postmortem studies give evidence of TRN abnormalities in schizophrenia including decreased nicotinic receptor binding (38), increased expression of excitatory amino acid transporters (39) and reduced parvalbumin neurons and perineuronal nets (40). These abnormalities may have a genetic origin. Risk genes for neurodevelopmental disorders, both schizophrenia and autism, affect TRN function and spindle expression suggesting the possibility of a pathogenic role (66–69). During gestation, axons that connect the cortex and the thalamus pass through the TRN, which helps guide them to their terminations (70). As early as the first postnatal week in rodents, spindle bursts, a precursor to adult sleep spindles that are similar in shape, frequency and origin (71), refine these reciprocal thalamocortical glutamatergic connections, particularly in somatosensory and motor cortex (72–75). These findings suggest mechanisms by which risk genes that affect the TRN early in neurodevelopment could disrupt the establishment of thalamocortical circuitry and contribute to vulnerability to schizophrenia and other neurodevelopmental disorders. While the neurodevelopmental literature strongly links TRN-generated spindle bursts to thalamocortical connectivity, we cannot exclude the possibility that a third factor mediates their relationship.
Dysfunction of TRN-mediated thalamocortical circuitry may contribute more broadly to the manifestations of schizophrenia. Thalamocortical hyperconnectivity has been correlated with cognitive impairment, negative symptoms and positive symptoms including hallucinations in larger studies (4, 6, 7). Abnormal perceptual experiences, which are also hypothesized to underlie delusions (76), may arise from increased and less filtered thalamic forwarding of sensory information to the cortex. Sensory gating deficits, impaired attentional filtering and abnormal corollary discharge (i.e., reduced suppression of sensations resulting from one’s own actions (2, 77–80)) could similarly be attributed to deficient TRN inhibition of sensory relay. Reduced spindle activity also correlates with positive symptoms and cognitive dysfunction in chronic, medicated and early course antipsychotic-naïve patients with schizophrenia (27–32) as well as non-psychotic first-degree relatives (33, 34). In healthy young individuals, reduced spindle density correlates with both elevated psychosis proneness and increased thalamic glutamine/glutamate levels supporting a mechanistic link between spindles, symptoms and heightened thalamic excitation (81). These findings are consistent with the hypothesis that both thalamocortical hyperconnectivity and spindle deficits reflect thalamocortical circuit dysfunction that may impair cognition and contribute to symptoms (9, 10).
Motivated by recent evidence that in addition to relaying information, thalamic input regulates local cortical functional connectivity to enhance information processing (58), we examined whether regions with abnormally increased thalamic input in schizophrenia would have disrupted local cortical interactions. While controls showed a strong reciprocal relation of thalamocortical connectivity with intra-cortical connectivity in these regions, patients showed no relationship and significantly reduced intra-cortical connectivity. Primary sensory and motor areas preferentially display local as opposed to long-range functional connectivity, which is thought to optimize area-specific information processing (82). The reduced intracortical connectivity in motor and somatosensory cortices seen in patients and its lack of correlation with thalamocortical connectivity, suggests that the balance between thalamic and local communication is disrupted. These are intriguing, but unexpected findings that require replication and functional correlation to understand their significance.
Several limitations of the present study merit consideration. First, the relatively modest sample sizes limited our statistical power and this may account for our failure to replicate previous findings of reduced connectivity of the thalamus with prefrontal cortex in schizophrenia seen in larger studies (c.f., 4). Relatedly, to maximize power in our analyses, we used the entire thalamus as a seed, which does not allow us to implicate specific thalamic regions in aberrant connectivity. Second, we used BOLD fMRI during wakeful rest to make inferences about connectivity. The strengths and weaknesses of this approach have been discussed elsewhere (83) and while fcMRI is not a direct measure of connectivity, it provides information about interregional communication. Although we found no correlations between antipsychotic dosage and our outcome variables, almost all the schizophrenia participants were taking medications that affect brain function. Sleep spindle deficits have also been reported in antipsychotic-naive patients and first-degree relatives and thalamocortical hyperconnectivity is also present in clinical high-risk individuals and is greater in those who eventually convert to psychosis. These findings indicate that neither sleep spindle deficits nor thalamocortical hyperconnectivity are a consequence of medications or chronicity. Nor is the relation of spindles with thalamocortical connectivity likely to be due to medications, since it was also seen in controls. That spindle and connectivity abnormalities are also present in medicated patients suggests that they are not corrected by current medication regimens for schizophrenia, and to the degree that they impair function, treating them remains an unmet need. Finally, we relied on indirect measures to make inferences about TRN function. The TRN is challenging to study directly in humans since its size and location make it mostly inaccessible to neuroimaging.
By linking two biomarkers of schizophrenia, this work suggests that they share a common mechanism and neurodevelopmental origin. If spindle deficits and thalamocortical hyperconnectivity reflect a common TRN-mediated thalamocortical pathophysiology that increases risk for schizophrenia and contributes to its cognitive deficits and symptoms, understanding its mechanism could guide the development of interventions targeting TRN dysfunction to treat schizophrenia and possibly even prevent its onset.
Supplementary Material
Acknowledgments:
Support from: NHLBI T32HL007901-17 (BB); SNF P2ELP2_158891 (FIK); R01-MH048832 (RS); R01 MH67720 (DSM & RS); K24MH099421 (DSM, MV); 1S10RR023401, 1S10RR019307, 1S10RR023043; NCRR UL1TR001102-01 to Harvard Clinical and Translational Science Center. The authors would like to acknowledge Dr. Randy Buckner for consultation and Ben Seicol, Tessa Vuper, David Correll, Rachel Fowler, Elaine Parr and Cameron Callahan for data acquisition and EEG preprocessing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, Nenadic I. Thalamocortical connectivity during resting state in schizophrenia. European archives of psychiatry and clinical neuroscience. 2014;264:111–119. [DOI] [PubMed] [Google Scholar]
- 3.Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, Pearlson GD, Glahn DC. Mediodorsal and Visual Thalamic Connectivity Differ in Schizophrenia and Bipolar Disorder With and Without Psychosis History. Schizophr Bull 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skatun KC, Kaufmann T, Brandt CL, Doan NT, Alnaes D, Tonnesen S, Biele G, Vaskinn A, Melle I, Agartz I, Andreassen OA, Westlye LT. Thalamo-cortical functional connectivity in schizophrenia and bipolar disorder. Brain Imaging Behav 2017. [DOI] [PubMed] [Google Scholar]
- 6.Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O’Leary D, Mueller BA, Lim KO, McEwen SC, Calhoun Vd, Diaz M, Glover G, Greve D, Wible CG, Vaidya JG, Potkin SG, Mathalon DH. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Ye E, Jin X, Zhu Y, Wang L. Association between Thalamocortical Functional Connectivity Abnormalities and Cognitive Deficits in Schizophrenia. Scientific reports. 2019;9:2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HL, Rau CL, Li YM, Chen YP, Yu R. Disrupted thalamic resting-state functional networks in schizophrenia. Front Behav Neurosci 2015;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan tH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TG, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA psychiatry. 2015;72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry. 2016;80:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steriade M The corticothalamic system in sleep. Front Biosci 2003;8:d878–899. [DOI] [PubMed] [Google Scholar]
- 12.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol 1996;490 (Pt 1): 159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. [DOI] [PubMed] [Google Scholar]
- 14.Pinault D The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev 2004;46:1–31. [DOI] [PubMed] [Google Scholar]
- 15.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci 2011;14:1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol 2003;463:360–371. [DOI] [PubMed] [Google Scholar]
- 17.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev 2003;7:423–440. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. Journal of Neurophysiology. 2001;86:1365–1375. [DOI] [PubMed] [Google Scholar]
- 19.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res 2000;886:208–223. [DOI] [PubMed] [Google Scholar]
- 20.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience. 2005;25:9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogel SM, Smith CT. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev 2011;35:1154–1165. [DOI] [PubMed] [Google Scholar]
- 22.Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci 2013;25:1597–1610. [DOI] [PubMed] [Google Scholar]
- 23.Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci 2013;33:4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lustenberger C, Boyle M, Alagapan S, Mellin J, Vaughn B, Frolich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Current Biology. 2016;August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. [DOI] [PubMed] [Google Scholar]
- 26.Del Felice A, Magalini A, Masiero S. Slow-oscillatory Transcranial Direct Current Stimulation Modulates Memory in Temporal Lobe Epilepsy by Altering Sleep Spindle Generators: A Possible Rehabilitation Tool. Brain stimulation. 2015;8:567–573. [DOI] [PubMed] [Google Scholar]
- 27.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. [DOI] [PubMed] [Google Scholar]
- 28.Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic Dysfunction in Schizophrenia Suggested by Whole-Night Deficits in Slow and Fast Spindles. Am J Psychiatry. 2010;167:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manoach dS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res 2010;44:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, Prehn-Kristensen A. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med 2015;16:564–569. [DOI] [PubMed] [Google Scholar]
- 31.Wamsley E, Tucker MA, Shinn AK, Ono KE, McKinley S, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Goder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res 2010;44:42–47. [DOI] [PubMed] [Google Scholar]
- 33.Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, Kupfer D, Buysse D, Stickgold R, Keshavan MS. Sleep spindle deficits in antipsychotic-naive early course schizophrenia and in non-psychotic first-degree relatives. Frontiers in Human Neuroscience. 2014;8:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schilling C, Schlipf M, Spietzack S, Rausch F, Eisenacher S, Englisch S, Reinhard I, Haller L, Grimm O, Deuschle M, Tost H, Zink M, Meyer-Lindenberg A, Schredl M. Fast sleep spindle reduction in schizophrenia and healthy first-degree relatives: association with impaired cognitive function and potential intermediate phenotype. European archives of psychiatry and clinical neuroscience. 2016. [DOI] [PubMed] [Google Scholar]
- 35.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res 1980;200:341–354. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Wimmer RD, Wilson MA, Halassa MM. Thalamic Circuit Mechanisms Link Sensory Processing in Sleep and Attention. Front Neural Circuits. 2015;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, Wang F, Brown EN, Wilson MA. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158:808–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem 1999;73:1590–1597. [DOI] [PubMed] [Google Scholar]
- 39.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–1399. [DOI] [PubMed] [Google Scholar]
- 40.Steullet P, Cabungcal JH, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, Salt TE, Cuenod M, Do KQ, Berretta S. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN). New York, Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 43.Sr Kay, Fiszbein A Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Nonpatient Edition. New York, Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 45.Iber C, Ancoli-Israel S, Chesson AL, Quan SF: The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, Ill, American Academy of Sleep Medicine; 2007. [Google Scholar]
- 46.Warby SC, Wendt SL, Welinder P, Munk EG, Carrillo O, Sorensen HB, Jennum P, Peppard PE, Perona P, Mignot E. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nature methods. 2014;11:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otsu N: Time Frequency and Wavelets in Biomedical Signal Processing. Piscataway, NJ, Wiley-IEEE Press; 1979. [Google Scholar]
- 48.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. [DOI] [PubMed] [Google Scholar]
- 49.van der Kouwe AJ, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorensen AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. [DOI] [PubMed] [Google Scholar]
- 50.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med 2012;68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med 2000;44:457–465. [DOI] [PubMed] [Google Scholar]
- 52.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 54.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 57.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003;50:1077–1088. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017;545:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.!!! INVALID CITATION !!!.
- 60.Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: A review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev 2015;55:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 62.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett 2006;403:52–56. [DOI] [PubMed] [Google Scholar]
- 63.Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci U S A. 2012;109:18583–18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonjean M, Baker T, Bazhenov M, Cash S, Halgren E, Sejnowski T. Interactions between core and matrix thalamocortical projections in human sleep spindle synchronization. J Neurosci 2012;32:5250–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piantoni G, Halgren E, Cash SS. The Contribution of Thalamocortical Core and Matrix Pathways to Sleep Spindles. Neural Plast. 2016;2016:3024342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Astori S, Wimmer RD, Prosser HM, Corti C, Corsi M, Liaudet N, Volterra A, Franken P, Adelman JP, Luthi A. The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc Natl Acad Sci U S A. 2011;108:13823–13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells MF, Wimmer RD, Schmitt LI, Feng G, Halassa MM. Thalamic reticular impairment underlies attention deficit in Ptchd1(Y/-) mice. Nature. 2016;532:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrade A, Hope J, Allen A, Yorgan V, Lipscombe D, Pan JQ. A rare schizophrenia risk variant of CACNA11 disrupts CaV3.3 channel activity. Scientific reports. 2016;6:34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krol A, Wimmer RD, Halassa MM, Feng G. Thalamic Reticular Dysfunction as a Circuit Endophenotype in Neurodevelopmental Disorders. Neuron. 2018;98:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci 1993;16:240–245. [DOI] [PubMed] [Google Scholar]
- 71.Lindemann C, Ahlbeck J, Bitzenhofer SH, Hanganu-Opatz IL. Spindle Activity Orchestrates Plasticity during Development and Sleep. Neural Plast. 2016;2016:5787423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. [DOI] [PubMed] [Google Scholar]
- 73.Cirelli C, Tononi G. Cortical Development, Electroencephalogram Rhythms, and the Sleep/Wake Cycle. Biol Psychiatry. 2015;77:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evrard A, Ropert N. Early development of the thalamic inhibitory feedback loop in the primary somatosensory system of the newborn mice. J Neurosci 2009;29:9930–9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinault D Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull 2011;37:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maher B, Abherrant Utterance and Delusions of Control: The Disconnection of Speech and Thought, and the Connection of Experience and Belief. Mind & Language. 2003;18:1–22. [Google Scholar]
- 77.Mathalon DH, Ford JM. Corollary discharge dysfunction in schizophrenia: evidence for an elemental deficit. Clin EEG Neurosci 2008;39:82–86. [DOI] [PubMed] [Google Scholar]
- 78.Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res 2017;180:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vukadinovic Z Sleep abnormalities in schizophrenia may suggest impaired trans-thalamic cortico-cortical communication: towards a dynamic model of the illness. Eur J Neurosci 2011;34:1031–1039. [DOI] [PubMed] [Google Scholar]
- 80.Vukadinovic Z Schizophrenia as a disturbance of cortical sensory maps. Translational Neurosci 2012;3:388–398. [Google Scholar]
- 81.Lustenberger C, O’Gorman RL, Pugin F, Tushaus L, Wehrle F, Achermann P, Huber R. Sleep spindles are related to schizotypal personality traits and thalamic glutamine/glutamate in healthy subjects. Schizophr Bull 2015;41:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol 2010;6:e1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 2010;103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilkinson GJ: The Wide Range Achievement Test-Revision 3. Wilmington, DE, Jastak Associates; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


