Abstract
Botulinum neurotoxins (BoNTs) are potent neurotoxins and are the causative agent of botulism, as well as valuable pharmaceuticals. BoNTs are divided into seven serotypes that comprise over 40 reported subtypes. BoNT/A1 and BoNT/B1 are currently the only subtypes approved for pharmaceutical use in the USA. While several other BoNT subtypes including BoNT/A2 and /A6 have been proposed as promising pharmaceuticals, detailed characterization using in vivo assays are essential to determine their pharmaceutical characteristics compared to the currently used BoNT/A1 and /B1. Several methods for studying BoNTs in mice are being used, but no objective and quantitative assay for assessment of functional outcomes after injection has been described. Here we describe the use of CatWalk XT as a new analytical tool for the objective and quantitative analysis of the paralytic effect after local intramuscular injection of BoNT subtypes A1, A2, A6, and B1. Catwalk is a sophisticated gait and locomotion analysis system that quantitatively analyzes a rodent’s paw print dimensions and footfall patterns while traversing a glass plate during unforced walk. Significant changes were observed in several gait parameters in mice after local intramuscular injection of all tested BoNT subtypes, however, no changes were observed in mice injected intraperitoneally with the same BoNTs. While a clear difference in time to peak paralysis was observed between BoNT/A1 and /B1, injection of all four toxins resulted in a deficit in the injected limb with the other limbs functionally compensating and with no qualitative differences between the four BoNT subtypes. The presented data demonstrate the utility of CatWalk as a tool for functional outcomes after local BoNT injection through its ability to collect large amounts of quantitative data and objectively analyze sensitive changes in static and dynamic gait parameters.
Keywords: botulinum neurotoxin, BoNT, A1, A2, A6, B1, Catwalk, intramuscular injection
Introduction
Botulinum neurotoxins (BoNTs) are potent, naturally occurring toxins produced primarily by the gram-positive, spore forming bacterium Clostridium botulinum. BoNTs cause the long-lasting and potentially fatal paralytic disease botulism (Johnson and Montecucco, 2008). On the other hand, BoNTs are also widely used pharmaceuticals that have proven valuable in cosmesis and for alleviating debilitating symptoms of several neurological conditions (Dressler, 2012, 2016; Dressler et al., 2018). While BoNTs comprise a large family of protein toxins with seven immunologically distinct serotypes and over 40 reported subtypes (Gimenez and Gimenez, 1995; Hill and Smith, 2013; Hill et al., 2015; Montecucco and Rasotto, 2015; Peck et al., 2017), only two BoNT isotypes are currently being employed as pharmaceuticals: BoNT/A1 and BoNT/B1. Many BoNT subtypes have not been purified from their native hosts to allow detailed explorations of their characteristics and pharmaceutical properties. This is mainly due to the strict regulatory restrictions for producing and handling of BoNTs and C. botulinum, which are category A Tier 1 Select Agents because of their potential misuse as bioterrorist weapons. However, in recent years advances in genetic and molecular methods have allowed production and purification of several novel BoNT subtypes as well as recombinant mutated BoNTs, and subsequent functional characterizations. Limited functional studies conducted mostly on BoNT/A subtypes have suggested functional differences between the subtypes, with variations in potency, cell entry kinetics, onset and duration of action, and symptoms in mice.5-8 In particular, two BoNT/A subtypes, BoNT/A2 and BoNT/A6, have been found to have properties that may be beneficial for pharmaceutical use, such as greater potency in cultured neurons including human neurons, faster neuronal cell entry kinetics, and less systemic toxin spread after local intramuscular injection in mice (Akaike et al., 2013; Kroken et al., 2017; Mukai et al., 2014; Pellett et al., 2015; Pier et al., 2011; Shin et al., 2013; Torii et al., 2011a; Torii et al., 2014; Torii et al., 2011b; Whitemarsh et al., 2013). Since pharmaceutical BoNTs are injected locally, an essential aspect of evaluating novel BoNTs as potential pharmaceuticals is the functional analysis in animal models injected locally with the BoNTs. Examining in vivo distribution of BoNTs presents a challenge due to the high potency of these toxins, which results in extremely low amounts of toxin present in cells and in animal circulation and neurons, which limits direct detection of the BoNTs. Consequently, current in vivo models to evaluate pharmacologic properties of BoNTs after local injection are limited to only a few models that examine electrophysiological or functional parameters of BoNTs. Currently employed rodent BoNT assays include the mouse bioassay to determine potency (Hatheway, 1988; Schantz and Kautter, 1978), the DAS assay to determine local paralysis after intramuscular injection (Broide et al., 2013; Pellett et al., 2015; Sugiyama et al., 1975), the rotarod or voluntary wheel running to determine systemic motor-neuron deficiency after local injection (Keller, 2006; Pellett et al., 2015), local electrophysiological measurements (Kim et al., 2013; Mukai et al., 2014; Torii et al., 2014), and grip-strength assays (Torii et al., 2011b).
In an effort to objectively analyze the functional impact of locally injected BoNTs on locomotion and gait in mice, we examined the use of CatWalk XT as a new functional analytical tool. The CatWalk system is a sophisticated locomotor analysis tool capable of analyzing over 50 specific parameters related to paw prints and gait of the animal. Parameters are divided into several major categories: paw statistics, step sequence, base of support, print positions, phase dispersions, couplings, support, and other statistics. Catwalk XT has been employed and verified for studying several conditions in rodent models including arthritis (Masocha and Parvathy, 2009; Parvathy and Masocha, 2013), peripheral nerve damage (Bozkurt et al., 2008), spinal cord injury (Chen et al., 2014; Hamers et al., 2006), Parkinson’s (Frohlich et al., 2018), multiple sclerosis (Herold et al., 2016), and stroke (Caballero-Garrido et al., 2017; Cao et al., 2017). Such previous work has shown detectable differences in parameters such as intensity, print area, swing duration, and stance duration. These studies have demonstrated the CatWalk system’s ability to analyze various neurological conditions including predicting the onset of multiple sclerosis and observing the benefits of acupuncture on improvement of gait parameters after ischemic stroke. However, to our knowledge, CatWalk has not been previously used for the analysis of pathological or pharmacologic properties of any toxins including BoNTs, or for the analysis of local paralysis such as following intramuscular BoNT injection.
In this project, Catwalk XT was used to examine whether BoNT/A1, A2, A6 and B1 resulted in measurable variations in local and systemic functional activity after local intramuscular injection. Several differences among these BoNT sero- and subtypes have been previously described in mice using other analytical methods, as well as in neuronal cell models and in vitro (Akaike et al., 2013; Arndt et al., 2006; Benoit et al., 2017; Bradshaw et al., 2014; Kalb et al., 2014; Kalb et al., 2012; Kalb et al., 2009; Koizumi et al., 2014; Kroken et al., 2017; Kull et al., 2015; Ma et al., 2012; Moritz et al., 2018; Mukai et al., 2014; Pellett et al., 2018a; Pellett et al., 2016; Pellett et al., 2018b; Pellett et al., 2015; Pier et al., 2011; Przedpelski et al., 2018; Shin et al., 2013; Tepp et al., 2012; Torii et al., 2011a; Torii et al., 2011b; Wang et al., 2013; Whitemarsh et al., 2013; Whitemarsh et al., 2014). BoNT/A1 and /B1 are currently being used as pharmaceuticals. In human botulism cases, BoNT/B1 and BoNT/A1 both primarily affect the peripheral nervous system, while anecdotal evidence indicates that botulism caused by BoNT/B1 affects the autonomic nervous system to a greater extent than BoNT/A1 (Johnson and Montecucco, 2008). Comparisons of pharmaceutical uses of BoNT/A1 and /B1 suggest that both toxins result in local paralysis after intramuscular injection, but that BoNT/B1 may have slightly greater effects on autonomic functions such as sialorrhea or hyperhidrosis (Bentivoglio et al., 2015; Duarte et al., 2016). BoNT/A2 and /A6 have been shown to enter cells faster and more efficiently than A1 (Moritz et al., 2018; Pier et al., 2011), while A2 is currently being investigated in clinical trials in Japan and A6 has recently been suggested as a potential new pharmaceutical.
Based on this previous work, it has been hypothesized that BoNT/A2 and /A6 enter neurons at the injection site faster and may therefore be more effective at lower concentrations. Objectively detecting the subtle functional impact in mice at low toxin concentrations can be difficult with current techniques. This study uses the comprehensive CatWalk XT analysis software in parallel with DAS and Rotarod to directly compare previously utilized methods and supply functional data that have previously not been obtained in BoNT research. The data presented suggest that all four toxin types similarly paralyzed the injected limb with no functional decline in all other limbs but instead compensatory behavior. Nerve functional indices indicated that the peroneal nerve is predominantly affected after intramuscular injection into the lateral head of the gastrocnemius muscle, with BoNT/A2 and A6 appearing to have less of an effect on the tibial nerve relative to their effect on the peroneal nerve compared to BoNT/A1. Overall, the data suggest that CatWalk is a sensitive, quantitative, and objective tool useful for analyzing the local paralysis of mice injected intramuscularly with botulinum neurotoxin.
Materials and Methods
Biosafety and biosecurity.
The Johnson laboratory and personnel are registered with the Federal Select Agent Program for research involving botulinum neurotoxins (BoNT) and BoNT-producing strains of clostridia. The research program, procedures, documentation, security, and facilities are monitored by the University of Wisconsin-Madison Biosecurity Task Force, the University of Wisconsin-Madison Office of Biological Safety, the University of Wisconsin Select Agent Program, and the Centers for Disease Control and Prevention (CDC) as part of the University of Wisconsin-Madison Select Agent Program. Personnel have undergone suitability assessments and completed rigorous and continuing biosafety training, including biosafety level 3 (BSL3) or BSL2 and select agent practices, before participating in laboratory studies involving BoNTs and neurotoxigenic C. botulinum. All animal experiments have been approved by the University of Wisconsin IACUC.
The CatWalk system.
The CatWalk XT version 10.6 (Noldus Information Technology) was used as a quantitative gait analysis system that allows for the objective analysis of static and dynamic gait parameters in mice or rats (Koopmans et al., 2007). The system does this using a unique footprint technology. Animals traverse a glass plate within which a green light is emitted and internally reflected. When the animals make contact with the glass plate, the green light is refracted. An increase in weight put on a certain area of a paw results in an increase in brightness of the green light. These images are captured by a high speed camera from underneath the glass plate. These resulting footprints are identified and analyzed using the accompanying hardware of the CatWalk system (Kappos et al., 2017).
Training mice on CatWalk.
Mice were marked for identification and began training on the CatWalk approximately 1 week prior to injections. A minimum of two training sessions were conducted each day and complexity of training increased with each day. At first, mice were placed on the walkway and allowed to freely move around for a few minutes to become familiar with the new environment. Next, mice were placed on the CatWalk, and once they traversed the entire glass plate to the other end and entered the ‘goal box’ or were manually placed into the ‘goal box’, they were then placed back into their home cage. For the initial sessions of training, each mouse had to make an uninterrupted run from one end to the other before it was placed back in its home cage. The final sessions required each mouse to complete 3 successful, uninterrupted runs to go back to its cage. To promote the association between being in the goal box and getting to go back into the home cage, throughout all training sessions, mice were allowed to enter the goal box at the end of the walkway before going back into their home cage. If an animal was hesitant to enter the goal box, it was picked up and placed in it followed by placement into its home cage.
Botulinum neurotoxins.
BoNTs /A1, /A2, /A6, and /B1 were purified from C. botulinum strains Hall A-hyper, Kyoto-F, CDC41370B2tox-, and OkraB as previously described (Lin et al., 2010; Malizio et al., 2000; Moritz et al., 2018; Prabakaran et al., 2001). The purity of the toxins was confirmed by spectroscopy and SDS-PAGE as previously published (Moritz et al., 2018; Prabakaran et al., 2001; Wang et al., 2013).The purified toxins were stored in 0.1 M sodium phosphate buffer, pH 7 with 40% glycerol at −20°C u ntil use. The specific activity of each subtype preparation was determined using an intraperitoneal mouse bioassay (MBA) as previously described (Hatheway, 1988; Schantz and Kautter, 1978). The specific activities of the BoNTs in mice were 5.6 pg/LD50 (A1), 3.5 pg/LD50 (A2), 5.9 pg/LD50 (A6), and 4.2 pg/LD50 (B1).
Hind limb injections for CatWalk analysis.
Toxin dilutions were prepared in Gelatin Phosphate buffer (GelPhos) (30 mM sodium phosphate [pH 6.3] and 0.2% gelatin). Each toxin was first diluted to 300 U / ml, and further dilutions were prepared from this working stock for intramuscularly (IM) and intraperitoneally (IP) injections. For the IM injections, the toxin was further diluted to 45 U /1 ml, 15 U/1 ml, 5 U /1 ml, and 1.7 U/1 ml in serial dilution steps. An equal volume of GelPhos buffer was used for the negative control (mock-injected control mice). Using a 0.3 cc insulin syringe (BD) with 5 μl markings, mice were injected IM with 10 μl of diluted BoNT/A1, A2, A6, or B1 in GelPhos buffer into the right gastrocnemius muscle. For parallel IP injections to confirm the toxin dose, the 300 U / ml working stocks were diluted to 30 U /5 ml, 15 U / 5 ml, and 7.5 U / 5 ml. Groups of 3 mice were injected IP with 0.5 ml of each dilution using a 0.5 cc insulin syringe (BD). The mice injected IP were used to perform a mouse bioassay (MBA) to confirm the toxin doses injected IM as previously described. Mice injected IM were analyzed by CatWalk as well as by observing the digit abduction score (DAS). A group of 5 mice injected IM with 10 μl of GelPhos were analyzed with CatWalk to serve as a negative control.
Intraperitoneal injections for CatWalk analysis.
Toxin dilutions were prepared in GelPhos buffer. Using a 0.5 cc insulin syringe, groups of 5 mice were injected IP with 2-fold serial dilutions of either BoNT/A1 or BoNT/B1, ranging from 3.2 to 0.1 U in 0.5 mL GelPhos buffer. Symptoms in mice injected with 0.8 U or lower were analyzed on the Rotarod and CatWalk at the indicated time points through 4 days post-injection. The mice injected with toxin concentrations greater than 0.8 U were observed for survival to verify toxin doses injected. A separate group of 5 mice injected with 500 μl of GelPhos (no toxin) were analyzed with CatWalk to serve as a negative control.
CatWalk setup and use.
In the experiment settings of the Catwalk analysis hardware, mice were selected as the animal species. A total of 6 time points were entered, and treatment groups were defined. The minimum number of compliant runs to acquire was set to 3. Prior to collecting data for each time point, trial lists were created and given the proper animal assignment.
All data collection was performed in a dark room. The glass walkway was cleaned and a background image was captured before each trial was collected. Additionally, the walkway was cleaned if a mouse left behind feces or urine but had yet to complete all 3 of the required runs. In this case, acquisition was stopped for cleaning, a new background image was obtained, and the acquisition was restarted. Runs were deleted manually if they were accepted as compliant during acquisition but the mouse made a prolonged pause on the walkway. Additionally, runs were deleted if a mouse changed directions during the run.
Five mice per group were analyzed at each time point, with each mouse completing 3 runs per time point. Each mouse was analyzed at 0 h (before injection), 4 h, 24 h, 48 h, 72 h, and 96 h post injection. The green intensity threshold was optimized for each run. Paw prints were then identified using the automatic footprint classification function. Sections of run footage were eliminated from analysis if the mouse paused during the run. All prints were verified by visually inspecting the automatically assigned labels, and any errors and unidentified prints were manually corrected. Non-automated measurements were performed by hand (toe spread, intermediate toe spread, and manual print length). All parameters were then statistically analyzed using the built-in software, and graphs of the results were produced. Results were divided into categories based on the specific parameter measured: paw statistics, step sequence, base of support, print positions, phase dispersions, couplings, support, and other parameters.
Digit abduction scoring (DAS).
Local paralysis of the same mice analyzed by Catwalk was additionally observed with the digit abduction scoring (DAS) system. At the indicated time points, mice were briefly suspended in the air by the tail and the toe spread of the injected limb was rated on a 0-5 scale as previously described (Pellett et al., 2015). The DAS value was always determined before the CatWalk runs.
Rotarod training and analysis.
For mice that were injected IP, overall motor-neuron deficiency was examined by Rotarod as previously described (Pellett et al., 2015). While being trained on the CatWalk, mice were simultaneously trained on the Rotarod. Sorting of mice was initially done to separate runners from non-runners. Once groups were established, the mice ran on the Rotarod several times each day before analysis began. Mice ran for 5 minutes with the rod increasing in speed from 4 to 40 rpm. Any mice that fell off during training were placed back on the Rotarod to complete the session.
At the indicated times, groups of 5 mice were placed on the Rotarod. Mice attempted to complete a 5 min run with the rod increasing in speed from 4 to 40 rpm. Averages and standard deviations of Rotarod times for each group were calculated in Excel.
Statistics.
The CatWalk system automatically generates run, trial, and group statistics along with corresponding graphs of the data of runs for each group based on the footprint classification. The generated spreadsheet and graphs display the averages and standard error of the mean of each trial at each time point for each treatment group. To determine statistical relevance of the various parameters, the averages of all 3 runs of the 5 mice per group (15 runs total) at 48 h post injection were compared to those of the runs from the same mice before injection in a pairwise two-tailed t-test. Post-treatment differences with p-values below 0.05 were considered statistically relevant.
Rotarod and DAS data were both analyzed in Excel. Averages and standard deviations of data of each treatment group at the indicated time points were calculated. Graphs were prepared in Excel using these data.
Results
Intramuscular sub-lethal doses of BoNT/A1, /A2, and /A6 result in similar local paralysis as measured by DAS.
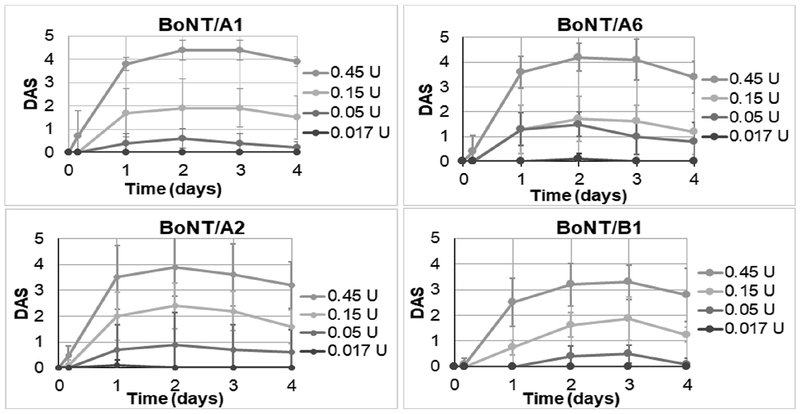
Mice were injected with sub-lethal concentrations of each BoNT (0.45 U, 0.15 U, 0.05 U, and 0.017 U), and in parallel a ‘mini-titer’ of the same toxin dilutions was conducted by mouse bio-assay to confirm the toxin dose. The results of the mini-titers showed that the dose for BoNT/A2, A6, and B1 was within the normal error range of the mouse bioassay, whereas the result for BoNT/A1 was ~1.7-fold lower than expected, indicating that slightly more BoNT/A1 was injected locally into mice (Table 1). In addition, the BoNT/A1 injected mice appeared to have more systemic symptoms at the highest toxin dose compared to BoNT/A2 and /A6 injected mice. To examine local paralysis after intramuscular injections with low concentrations of BoNTs/A1, /A2, /A6, or /B1, the DAS of the injected limb was observed through 4 days post-injection (Fig. 1). As described in previous studies,13 the DAS results were similar for all three BoNT/A subtypes and reached their maximum at about 48 h after injection. BoNT/B1 injected mice had a slower onset of maximum local paralysis and reached a peak DAS around 3 days post-injection. Additionally, the maximum DAS score for mice injected with BoNT/B1 was slightly lower compared to mice injected with the BoNT/A subtypes (~3.3 versus ~4-4.5).
Table 1:
Specific activity and results of mini-titer of the BoNTs used in this study
| BoNT Subtype | Specific activity mLD50 (pg/U) | Mini-titer mLD50 (pg/U) |
|---|---|---|
| A1 | 5.6 | 3.3 |
| A2 | 3.5 | 4.2 |
| A6 | 5.9 | 5.3 |
| B1 | 4.2 | 4.5 |
Figure 1: DAS of mice injected intramuscular with BoNT/A1, A2, A6, or B1.
Serial dilutions of BoNTs A1, A2, A6, or B1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The DAS was determined daily and the average and standard deviation at each day are shown for each toxin concentration.
Local intramuscular injection of BoNTs A1, A2, A6, and B1 results in the same overall pattern of static and dynamic paw functions.
The same mice that were observed for local paralysis by DAS were also analyzed by CatWalk at the same time points.
IM injections of all BoNTs (A1, A2, A6, and B1) resulted in significant changes in several static and dynamic paw parameters, with a maximum effect observed at 48 h for BoNT/A1, A2, and A6, and 72 h for BoNT/B1 (suppl. Fig 1–4). In order to determine which parameters were affected by all four toxin types tested, the increase or decrease of function for all parameters at 48 h versus 0 h was determined. This was achieved by setting the average values of pre-injection mice (5 mice per group, 3 runs per mouse) to 100% function, and determining the change in the same animals, respectively, at 48 h post injection. None or only minor changes were observed in mock-injected control mice, confirming that the needle stick and vehicle infection had no functional impact on the animals at 48 h post injection. A clear pattern of statistically significant changes in functional parameters was observed that was similar for all four toxin types (Fig. 2, see suppl. Table 1 and 2 for definitions of the parameters). Among the impacted static paw parameters, most measured parameters were significantly changed in the injected hindlimb in a dose dependent manner, with stand index, maximum contact, print width and area, swing and swing speed, and single and initial dual stance being the most affected. Several of the parameters that were decreased in the injected hindlimb were increased in the contralateral hindlimb, although to a lesser extent, indicating functional compensation by the contralateral limb (supplementary Figures 1–4). The decrease in print width, print area, maximum contact area, and maximum and mean intensity of the maximum contact area of the right hind paw correlated with the increase observed in DAS (Fig. 1). There were no major changes in these parameters in the front paws. Alterations in print length, time spent on a paw (stand), and time after maximum contact of a paw (max contact at %), on the other hand, were more variable and did not correlate to toxin dose for all toxins used, although a trend for a shorter time spent on the injected paw and correspondingly greater time spent on the contralateral paw was noted.
Figure 2: Functional changes in static and dynamic paw parameters in mice after intramuscular injection with BoNT/A1, A2, A6, or B1.
Serial dilutions of BoNTs A1, A2, A6, or B1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk, and the average run values for each mouse at 48 h post injection were compared to those of the same mouse before injection. The function of each parameter before injection was set to 100 (%), such that a value smaller than 100 indicates a decrease in function and a value greater than 100 indicates an increase in function. The average decrease or increase in function for each limb and for each group of mice is shown. Control shows the results for the mock-injected mice. Only values that were statistically relevant in a pairwise two-tailed t-test (p < 0.05) are shown.
Changes in dynamic paw parameters were also observed for mice injected with BoNTs. One of the most significant changes was observed in the swing speed of the hind paws. Similar to the static paw parameters, a dose-dependent decrease in the swing speed and increase in swing duration of the injected limb was seen for all four BoNT subtypes, whereas a corresponding increase in swing speed and decrease in swing duration was observed for the contralateral hind leg. The ipsilateral front leg also showed a somewhat milder increase in swing speed and decrease in swing duration, indicating further compensatory behavior by the right front leg and limping of the animals. While the duration of ground contact of the right hind paw (single stance) decreased in a dose dependent manner, the duration of ground contact of the contralateral un-injected paw increased correspondingly. Other dynamic paw parameters including initial and terminal dual stance (ground contact of both hind paws), as well as body speed and variations in body speed, varied markedly with over 2.5 fold decreases or increases, but the changes were not consistently toxin dose dependent. Overall, at 48 h post IM injection, BoNT/A1, /A2, /A6, and /B1 resulted in a similar dose dependent reduction in injected paw contact area, intensity, and time as well as reduced swing speed, with a corresponding but milder increase in paw contact area, intensity, and time as well as increased swing speed of the contralateral hindleg. No significant changes were observed for the front paws.
Local intramuscular injection of BoNTs A1, A2, A6, and B1 results in the same overall pattern of gait parameters measured by Catwalk.
The impact of IM BoNT injections on static and dynamic paw parameters also resulted in alterations in interlimb coordination parameters, affecting the gait of the animals (Fig. 3 and suppl. Fig 1–4). While no significant changes were observed for each BoNT in the step sequence, average speed, total number of steps, or contact with body parts other than the paws, significant changes were observed for phase dispersions and couplings that involved the right hind paw. The overall pattern of these changes was similar for all four BoNTs tested (Fig. 3). Phase dispersion and couplings both measure inter-paw coordination by measuring the moment of initial contact of a target paw expressed as a percentage of the step cycle time of an anchor paw (phase dispersion) or the temporal relationship between placement of two paws within one step cycle (couplings). The greatest difference was observed for the interlimb coordination of the left front paw with the injected right hind paw, with over 2.5-fold increases at the highest toxin dose. However, the most consistent and dose dependent increase was observed for the right front-right hind leg coordination, although the fold difference was much less, up to 1.5 fold. Interlimb coordination parameters that did not include the injected limb did not exhibit significant changes. For the whole animals, diagonal support was decreased, whereas lateral support was increased. However, no clear toxin-dose response could be observed for these parameters. The overall pattern of changes in interlimb coordination further supports a limping of the right hindlimb with compensatory behavior of the right forelimb and left hindlimb.
Figure 3: Functional changes in gait parameters in mice after intramuscular injection with BoNT/A1, A2, A6, or B1.
Serial dilutions of BoNTs A1, A2, A6, or B1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk, and the average run values for each mouse at 48 h post injection were compared to those of the same mouse before injection. The function of each parameter before injection was set to 100 (%), such that a value smaller than 100 indicates a decrease in function and a value greater than 100 indicates an increase in function. The average decrease or increase in function for each group of mice is shown for each toxin and the mock injected mice (control). Only values that were statistically relevant in a pairwise two-tailed t-test (p < 0.05) are shown.
BoNT/A2 and /A6 appear to have less distal effects than BoNT/A1 and /B1
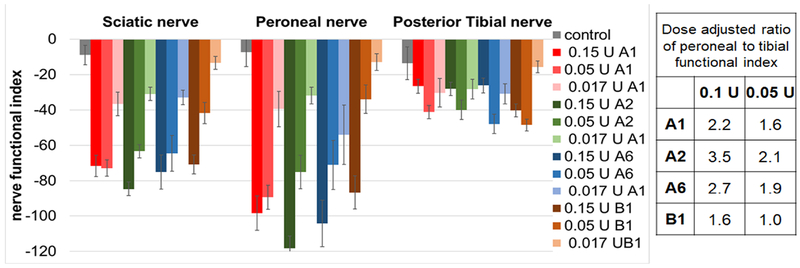
Nerve functional indices (NFIs) are a quantitative measure of specific nerve function. Formulas to calculate NFIs have been developed for mice and rats based on experiments measuring foot print patterns following induced nerve damage on specific nerve fibers. Catwalk determines the nerve functional indices for the peroneal, tibial, and sciatic nerves. Since manual measurements of the full paw print width and print length are required for these parameters, this could only be determined for toxin doses low enough to result in full paw prints. Thus, the NFIs of the right hindleg were determined after injection of 0.15 U, 0.05 U, and 0.017 U of BoNT/A1, A2, A6, and B1 (Fig. 4). The peroneal nerve was most affected for all toxins with a dose-dependent decrease in the peroneal functional index (PFI) for all toxins. The tibial functional index (TFI) decreased to a lesser extent, and there was no correlation with increasing toxin dose beyond the 0.05 U dose. As expected, the sciatic nerve NFI was the average of the NFI of the peroneal and tibial nerve. The dose-adjusted ratio of the PFI/TFI was greatest for BoNT/A2 and BoNT/A6, and lowest for BoNT/B1 (Fig 4), indicating possible differences in the local distribution of these toxin subtypes after intramuscular injection. However, further tests are required to evaluate statistical significance of these results.
Figure 4: Nerve functional indices of mice injected intramuscular with BoNT/A1, A2, A6, or B1.
Serial dilutions of BoNTs A1, A2, A6, or B1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk, and the nerve functional indices were determined at 48 h. The mean and standard error of the mean for each group of mice is shown. The figure shows the injected toxin doses, and the table shows the values at two doses adjusted to the results of the mini-titer of the injected toxin dilutions (Table 1).
Systemic toxicity from intraperitoneal injections of BoNTs does not result in altered CatWalk parameters.
To determine if CatWalk could be used as an analytical tool for the analysis of systemic symptoms caused by BoNTs, mice injected IP with either BoNT/A1 or /B1 (0.1 U – 0.8 U) were analyzed by CatWalk daily over a 4 day time period (data not shown). No significant changes were observed in nearly all CatWalk parameters. Only stride length appeared to decrease after IP injection of only the highest concentration used (0.8 U). Mice injected with 0.8 U of BoNT/A1 experienced an approximately 25% decrease in the average stride length of each paw, while mice injected with 0.8 U of BoNT/B1 experienced an approximately 15% decrease. The impact on stride length was similar for all paws. These data indicate that the functional parameters measured by Catwalk are not altered by systemic toxicity due to BoNTs at sub-lethal doses.
Discussion
Examining the impact of locally injected botulinum neurotoxins in rodent models has previously been limited to a few select methods. One of these methods for the analysis of local paralysis, digit abduction scoring (DAS) (Pellett et al., 2015; Wilder-Kofie et al., 2011), is subjective and therefore at risk for experimenter bias. A more objective measure is the grip strength test, but this test suffers from great mouse-to-mouse variability and habituation, making frequent measurements over a longer time period impractical. Electrophysiological methods (Mukai et al., 2014; Schulz et al., 2014) can directly assess neuronal transmission of a specific nerve, but this procedure is laborious, needs specialized equipment and training, and requires anesthesia of the animals, which makes it impractical for frequent analysis of multiple animals over a period of several days. Other methods such as Rotarod (Pellett et al., 2015), treadmill (Tsai et al., 2013), or voluntary wheel running (Keller, 2006) examine the whole animal rather than local effects. Expansion of experimental methods that yield consistent, quantitative data on local paralysis of rodent models would be beneficial to the BoNT research community. CatWalk has been previously used in other areas of neurological research (Bozkurt et al., 2008; Caballero-Garrido et al., 2017; Cao et al., 2017; Chen et al., 2014; Frohlich et al., 2018; Hamers et al., 2006; Herold et al., 2016; Masocha and Parvathy, 2009; Parvathy and Masocha, 2013) and has been verified as a method for the analysis of rodents with conditions such as spinal cord injury (Chen et al., 2014; Hamers et al., 2006) and multiple sclerosis (Herold et al., 2016). The use of this sophisticated piece of technology, however, has not been researched as a possible analytical tool for peripheral local paralysis in rodents, including after intramuscular injection of BoNT. The work presented here used CatWalk to quantitatively analyze the paralytic impact, both locally and systemically, of four different BoNT subtypes, BoNT/A1, A2, A6, and B1. BoNT/A1 and /B1 are currently used as pharmaceuticals, and BoNT/A2 is in clinical trials in Japan. BoNT/A6 has been suggested as an alternative pharmaceutical due its faster and more efficient cell entry (Moritz et al., 2018).
Several parameters were significantly impacted in a dose-dependent manner after local IM injection of all BoNT subtypes, indicating CatWalk could be reliable for obtaining unbiased, quantitative data on local paralysis (Fig. 2, 3, suppl. Fig 1–4). Not surprisingly, the parameters that were most affected were parameters related to the functional use of the paw of the injected hindlimb including stand index, maximum contact, print width and area, swing and swing speed, single and initial dual stance, and phase dispersion and coupling involving the injected hindlimb. The overall pattern of functional defect was similar for all toxin types examined, except that the maximum effect for BoNT/B1 was observed a day later than for BoNT/A1, A2, and A6. Previous studies have shown faster and more efficient cell entry by BoNT/A2 and A6 compared to A1 (Kroken et al., 2017; Moritz et al., 2018; Pier et al., 2011; Whitemarsh et al., 2013), and indicated an earlier onset of paralysis in mice after local injection (Moritz et al., 2018; Pellett et al., 2015; Torii et al., 2011b). In this study, not enough time points were analyzed to either confirm or contradict these observations, as the main focus was on analyzing the functional patterns in mice injected with the tested BoNTs and the utility of CatWalk as an analytical tool.
For all BoNTs tested, the functional defect was only observed in the injected hindlimb, and in fact, the contralateral hindlimb showed functional compensation. This was observed even at the highest toxin dose of 0.45 U, at which some minor systemic symptoms were evident, indicating that some of the toxin had spread away from the local injection site. In recent years, several research groups have demonstrated spread of BoNTs from the injection site by retrograde transport or diffusion through the tissue and transport in body fluids (Alexiades-Armenakas, 2008; Antonucci et al., 2008; Cai et al., 2017; Filipovic et al., 2012; Koizumi et al., 2014; Lawrence et al., 2012; Matak et al., 2012; Mazzocchio and Caleo, 2015; Ramachandran et al., 2015; Restani et al., 2011; Restani et al., 2012a; Restani et al., 2012b; Simpson, 2013). The data presented here indicate that at physiologically relevant concentrations to cause local paralysis after peripheral intramuscular injection, even within the high end of the dose range, the functional effects remain localized. This was emphasized even more by analysis of the nerve functional indices for the peroneal and tibial nerve branches (Fig. 4). For all toxins, the peroneal nerve was affected to a greater extent than the tibial nerve, which is likely due to the BoNTs being injected laterally into the apical part of the gastrocnemius muscle, thus closer to the peroneal nerve. The dose-adjusted ratio of peroneal versus tibial functional nerve index was greater for BoNT/A2 and A6 compared to BoNT/A1, and greater for all BoNT/A subtypes compared to BoNT/B1. This indicates potentially lower functional effects away from the injection site for BoNT/A2 and /A6, which would be consistent with previous observations (Moritz et al., 2018; Mukai et al., 2014; Pellett et al., 2015; Torii et al., 2011a; Torii et al., 2014; Torii et al., 2011b; Whitemarsh et al., 2013). However, the nerve functional indices were also toxin dose dependent, and small differences in the toxin dilutions could alter the results. Even though this study controlled for small errors in dilution by calculating a dose adjusted PFI/TFI ratio, the mouse bioassay itself has a relatively large error and thus further repeat analyses will be required to examine statistical relevance of these results.
While the presented results suggest CatWalk as a useful tool to examine functional defects over time after local injection with BoNTs, results from studies of intraperitoneal injections with BoNT/A1 and /B1 indicated that CatWalk is likely not a useful tool to reliably quantify symptoms caused by IP injected BoNTs. The range in mice that causes symptoms but does not kill mice or completely prevents walking across the CatWalk is very narrow, and within this range no major changes were observed.
There are several obstacles to overcome when using the CatWalk system. As with other methods assessing local effects of BoNTs in mice, one difficult aspect is the narrow range of toxin that can be injected. In such a small animal, the amount of toxin injected quickly changes from having little to no systemic effects to being lethal (Torii et al., 2014).In this study, extra sets of mice were injected with the same dilutions IP to confirm the toxin dose (Table 1). One consideration for continued research would be to consider using rats instead of mice to get a larger concentration range for analysis. This would allow for an increased possibility to observe variations among subtypes. Another difficulty with this analytical system is the amount of time required to collect and analyze data and ensure enough time points and runs are collected to reveal representative data. Due to the complexity of the system and the amount of time required to train animals, perform experiments, and analyze the data, we would primarily recommended this analysis for focused experiments using up to a moderate number of animals. Finally, the data analysis software of the CatWalk system provides graphs of the mean and standard error of the mean for each group of mice as well as for each individual mouse, but no statistical test is included. Since the individual values for footprints of each run of each mouse are not accessible, it is difficult to evaluate statistical significance of the data. In this study we used a statistical assessment (by a paired student’s t-test) of the mean values of each mouse per group (n=5) before and after injection. However, this method was not considering the variations in footprints within and between runs. Future improvements of the software associated with the CatWalk system would improve such analyses.
Based on our findings, CatWalk is effective, quantitative, and objective at detecting static and dynamic paw parameters as well as gait parameters impacted by the injection of BoNTs in mice. CatWalk may be a beneficial addition to studies on BoNTs when quantitative data is desired to expand beyond the limitations of other methods for the analysis of local paralysis and provide a functional assessment observing normal unforced behavior. This method may be particularly useful to further examine the potency, first onset, time to maximum onset, and duration of various BoNT subtypes on motorneuron functioning as well as sensory functions, and to quantitatively and objectively examine functional effects of novel BoNTs or homologs. In addition, this method would be a good way to assess the functional impact after various injection routes and methods. Finally, Catwalk analysis is being employed to study several conditions in rodent models including arthritis (Masocha and Parvathy, 2009; Parvathy and Masocha, 2013), peripheral nerve damage (Bozkurt et al., 2008), spinal cord injury (Chen et al., 2014; Hamers et al., 2006), Parkinson’s (Frohlich et al., 2018), multiple sclerosis (Herold et al., 2016), and stroke (Caballero-Garrido et al., 2017; Cao et al., 2017). For many of these conditions, symptoms can be alleviated with local BoNT treatment (Bach-Rojecky et al., 2010; Brashear, 2010; Brown et al., 2014; Cameron et al., 2014; Dressler et al., 2018; Fabregat et al., 2013; Marchand-Pauvert et al., 2013; Singh and Fitzgerald, 2011). It would be great interest to utilize Catwalk to study the effects of BoNT treatments in rodent models of these conditions on gait of the treated animals.
Supplementary Material
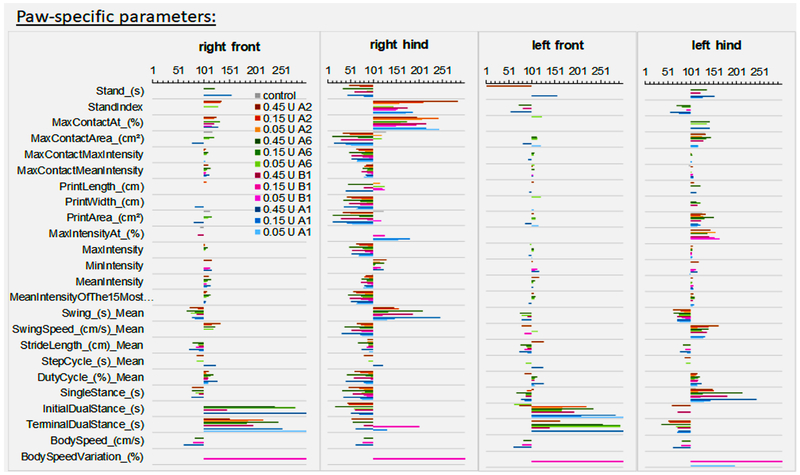
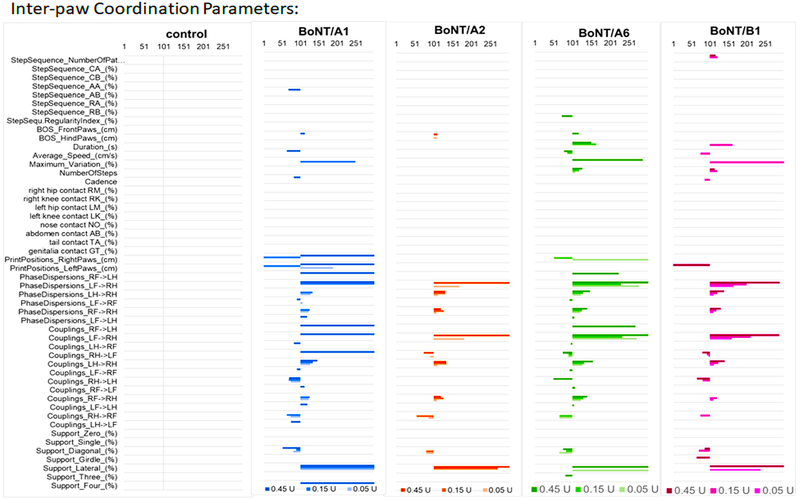
Supplementary Figure 1: Group data for mice injected intramuscular with BoNT/A1. Serial dilutions of BoNT/A1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Supplementary Figure 2: Group data for mice injected intramuscular with BoNT/A2. Serial dilutions of BoNT/A2 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Supplementary Figure 3: Group data for mice injected intramuscular with BoNT/A6. Serial dilutions of BoNTs/A6 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Supplementary Figure 4: Group data for mice injected intramuscular with BoNT/B1. Serial dilutions of BoNT/B1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Highlights:
CatWalk gait analysis quantitatively determined functional changes in mice injected in the gastrocnemius muscle with BoNT/A1, /A2, /A6, or /B1.
Injection of BoNTs A1, A2, A6, and B1 resulted in a similar overall pattern of static and dynamic paw functions.
BoNT/A2 and /A6 appeared to have less distal effects than BoNT/A1 and /B1
All work described in this manuscript was approved by the University of Wisconsin-Madison Institutional Biosafety Committee.
The Johnson laboratory and personnel are registered with the Federal Select Agent Program for research involving botulinum neurotoxins (BoNT) and BoNT-producing strains of clostridia. The research program, procedures, documentation, security, and facilities are monitored by the University of Wisconsin-Madison Biosecurity Task Force, the University of Wisconsin-Madison Office of Biological Safety, the University of Wisconsin Select Agent Program, and the Centers for Disease Control and Prevention (CDC) as part of the University of Wisconsin-Madison Select Agent Program.
All animal experiments were approved by and conducted according to guidelines by the University of Wisconsin Animal Care and Use Committee.
Acknowledgements:
This work was supported by the National Institute of Allergy and Infectious Diseases R01 AI095274, R56 AI095274. The authors would like to acknowledge the University of Wisconsin Madison Biosecurity Task Force for helping to ensure compliance with Select Agent Regulations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
⊠ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akaike N, Shin MC, Wakita M, Torii Y, Harakawa T, Ginnaga a., Kato K, Kaji R, Kozaki S, 2013. Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J Physiol 591, 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiades-Armenakas M, 2008. Retrograde transport and transcytosis of botulinum toxin serotypes to the brain: analysis of potential neurotoxicity. Journal of drugs in dermatology : JDD 7, 1006–1007. [PubMed] [Google Scholar]
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M, 2008. Long-distance retrograde effects of botulinum neurotoxin A. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 3689–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt JW, Jacobson MJ, Abola EE, Forsyth CM, Tepp WH, Marks JD, Johnson EA, Stevens RC, 2006. A structural perspective of the sequence variability within botulinum neurotoxin subtypes A1-A4. J Mol Biol 362, 733–742. [DOI] [PubMed] [Google Scholar]
- Bach-Rojecky L, Salkovic-Petrisic M, Lackovic Z, 2010. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injection. European Journal of Pharmacology 633, 10–14. [DOI] [PubMed] [Google Scholar]
- Benoit RM, Scharer MA, Wieser MM, Li X, Frey D, Kammerer RA, 2017. Crystal structure of the BoNT/A2 receptor-binding domain in complex with the luminal domain of its neuronal receptor SV2C. Scientific Reports 7, 43588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentivoglio AR, Del Grande A, Petracca M, lalongo T, Ricciardi L, 2015. Clinical differences between botulinum neurotoxin type A and B. Toxicon 107, 77–84. [DOI] [PubMed] [Google Scholar]
- Bozkurt A, Deumens R, Scheffel J, O’Dey DM, Weis J, Joosten EA, Fuhrmann T, Brook GA, Pallua N, 2008. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J Neurosci Methods 173, 91–98. [DOI] [PubMed] [Google Scholar]
- Bradshaw M, Tepp WH, Whitemarsh RC, Pellett S, Johnson EA, 2014. Holotoxin Activity of Botulinum Neurotoxin Subtype A4 Originating from a Nontoxigenic Clostridium botulinum Expression System. Appl Environ Microbiol 80, 7415–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear A, 2010. Botulinum toxin type A: Exploring new indications. Drugs of today (Barcelona, Spain : 1998) 46, 671–682. [DOI] [PubMed] [Google Scholar]
- Broide RS, Rubino J, Nicholson GS, Ardila MC, Brown MS, Aoki KR, Francis J, 2013. The rat Digit Abduction Score (DAS) assay: a physiological model for assessing botulinum neurotoxin-induced skeletal muscle paralysis. Toxicon 71, 18–24. [DOI] [PubMed] [Google Scholar]
- Brown EA, Schutz SG, Simpson DM, 2014. Botulinum toxin for neuropathic pain and spasticity: an overview. Pain Manag 4, 129–151. [DOI] [PubMed] [Google Scholar]
- Caballero-Garrido E, Pena-Philippides JC, Galochkina Z, Erhardt E, Roitbak T, 2017. Characterization of long-term gait deficits in mouse dMCAO, using the CatWalk system. Behavioural Brain Research 331, 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai BB, Francis J, Brin MF, Broide RS, 2017. Botulinum neurotoxin type A-cleaved SNAP25 is confined to primary motor neurons and localized on the plasma membrane following intramuscular toxin injection. Neuroscience 352, 155–169. [DOI] [PubMed] [Google Scholar]
- Cameron MH, Bethoux F, Davis N, Frederick M, 2014. Botulinum toxin for symptomatic therapy in multiple sclerosis. Curr Neurol Neurosci Rep 14, 463. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun N, Yang JW, Zheng Y, Zhu W, Zhang ZH, Wang XR, Shi GX, Liu CZ, 2017. Does acupuncture ameliorate motor impairment after stroke? An assessment using the CatWalk gait system. Neurochem Int 107, 198–203. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Cheng FC, Sheu ML, Su HL, Chen CJ, Sheehan J, Pan HC, 2014. Detection of subtle neurological alterations by the Catwalk XT gait analysis system. Journal of Neuroengineering and Rehabilitation 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D, 2012. Clinical applications of botulinum toxin, Curr Opin Microbiol, England, pp. 325–336. [DOI] [PubMed] [Google Scholar]
- Dressler D, 2016. Botulinum toxin drugs: brief history and outlook, J Neural Transm (Vienna) 123, 277–279. [DOI] [PubMed] [Google Scholar]
- Dressler D, Bhidayasiri R,, Bohlega S, Chana P,, Chien HF, Chung TM, Colosimo C, Ebke M, Fedoroff K, Frank B, Kaji R, Kanovsky P, Kocer S, Micheli F, Orlova O, Paus S, Pirtosek Z, Relja M, Rosales RL, Sagastegui-Rodriguez JA, Schoenle PW, Shahidi GA, Timerbaeva S, Walter U, Saberi FA, 2018. Defining spasticity: a new approach considering current movement disorders terminology and botulinum toxin therapy. Journal of Neurology 265, 856–862. [DOI] [PubMed] [Google Scholar]
- Duarte GS, Castelao M, Rodrigues FB, Marques RE, Ferreira J, Sampaio C, Moore AP, Costa J, 2016. Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. The Cochrane Database of Systematic Reviews 10, Cd004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabregat G, De Andres J, Villanueva-Perez VL, Asensio-Samper JM, 2013. Subcutaneous and Perineural Botulinum Toxin Type A For Neuropathic Pain: A Descriptive Review. The Clinical Journal of Pain. 29, 1006–12. [DOI] [PubMed] [Google Scholar]
- Filipovic B, Matak I, Bach-Rojecky L, Lackovic Z, 2012. Central action of peripherally applied botulinum toxin type a on pain and dural protein extravasation in rat model of trigeminal neuropathy. PloS One 7, e29803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich H, Claes K, De Wolf C, Van Damme X, Michel A, 2018. A Machine Learning Approach to Automated Gait Analysis for the Noldus Catwalk System. IEEE Transactions on Biomedical Engineering 65, 1133–1139. [DOI] [PubMed] [Google Scholar]
- Gimenez DF, Gimenez JA, 1995. The typing of botulinal neurotoxins. International Journal of Food Microbiology 27, 1–9. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Koopmans GC, Joosten EA, 2006. CatWalk-assisted gait analysis in the assessment of spinal cord injury. Journal of Neurotrauma 23, 537–548. [DOI] [PubMed] [Google Scholar]
- Hatheway CL, 1988. Botulism, in: Balows A, Hausler WH, Ohashi M, Turano MA (Eds.), Laboratory Diagnosis of Infectious Diseases: Principles and Practice. Springer-Verlag, New York, pp. 111–133. [Google Scholar]
- Herold S, Kumar P, Jung K, Graf I, Menkhoff H, Schulz X, Bahr M, Hein K, 2016. CatWalk gait analysis in a rat model of multiple sclerosis. BMC Neuroscience 17, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KK, Smith TJ, 2013. Genetic diversity Within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Current Topics in Microbiology and Immunology 364, 1–20. [DOI] [PubMed] [Google Scholar]
- Hill KK, Xie G, Foley BT, Smith TJ, 2015. Genetic diversity within the botulinum neurotoxin-producing bacteria and their neurotoxins. Toxicon 107, 2–8. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Montecucco C, 2008. Chapter 11 Botulism, in: Andrew GE (Ed.), Handbook of Clinical Neurology. Elsevier, pp. 333–368. [DOI] [PubMed] [Google Scholar]
- Kalb SR, Baudys J, Smith TJ, Smith LA, Barr JR, 2014. Three enzymatically active neurotoxins of Clostridium botulinum strain Af84: BoNT/A2, /F4, and /F5. Analytical Chemistry 86, 3254–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb SR, Baudys J, Webb RP, Wright P, Smith TJ, Smith LA, Fernandez R, Raphael BH, Maslanka SE, Pirkle JL, Barr JR, 2012. Discovery of a novel enzymatic cleavage site for botulinum neurotoxin F5. FEBS Letters 586, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb SR, Lou J, Garcia-Rodriguez C, Geren IN, Smith TJ, Moura H, Marks JD, Smith LA, Pirkle JL, Barr JR, 2009. Extraction and inhibition of enzymatic activity of botulinum neurotoxins/Al, /A2, and /A3 by a panel of monoclonal anti-BoNT/A antibodies, PloS One 4, e5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos EA, Sieber PK, Engels PE, Mariolo AV, D’Arpa S, Schaefer DJ, Kalbermatten DF, 2017, Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models, Brain and Behavior 7, e00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JE, 2006, Recovery from botulinum neurotoxin poisoning in vivo, Neuroscience 139, 629–637, [DOI] [PubMed] [Google Scholar]
- Kim CS, Jang WS, Son IP, Nam SH, Kim YI, Park KY, Kim BJ, Kim MN, 2013, Electrophysiological study for comparing the effect of biological activity between type A botulinum toxins in rat gastrocnemius muscle, Human & Experimental Toxicology 32, 914–920, [DOI] [PubMed] [Google Scholar]
- Koizumi H,, Goto S, Okita S, Morigaki R, Akaike N, Torii Y, Harakawa T, Ginnaga A, Kaji R, 2014, Spinal Central Effects of Peripherally Applied Botulinum Neurotoxin A in Comparison between Its Subtypes A1 and A2, Front Neurol 5, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Brook G, Gerver J, Honig WM, Hamers FP, Joosten EA, 2007, Strain and locomotor speed affect over-ground locomotion in intact rats, Physiology & Behavior 92, 993–1001, [DOI] [PubMed] [Google Scholar]
- Kroken AR, Blum FC, Zuverink M, Barbieri JT, 2017, Entry of Botulinum Neurotoxin Subtypes A1 and A2 into Neurons, Infect Immun 85, pii: e00795-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull S,, Schulz KM, Weisemann J, Kirchner S, Schreiber T, Bollenbach A, Dabrowski PW, Nitsche A, Kalb SR, Dorner MB, Barr JR, Rummel A, Dorner BG, 2015, Isolation and functional characterization of the novel Clostridium botulinum neurotoxin A8 subtype, PLoS One, United States, p, e0116381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence GW, Ovsepian SV, Wang J, Aoki KR, Dolly JO, 2012, Extravesicular intraneuronal migration of internalized botulinum neurotoxins without detectable inhibition of distal neurotransmission, Biochem J 441, 443–452, [DOI] [PubMed] [Google Scholar]
- Lin G, Tepp WH, Pier CL, Jacobson MJ, Johnson EA, 2010, Expression of the Clostridium botulinum A2 neurotoxin gene cluster proteins and characterization of the A2 complex, Applied and Environmental Microbiology 76, 40–47, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Nagai J, Sekino Y, Goto Y, Nakahira S, Ueda H, 2012, Single application of A2 NTX, a botulinum toxin A2 subunit, prevents chronic pain over long periods in both diabetic and spinal cord injury-induced neuropathic pain models, Journal of Pharmacological Sciences 119, 282–286, [DOI] [PubMed] [Google Scholar]
- Malizio CJ, Goodnough MC, Johnson EA, 2000, Purification of Clostridium botulinum type A neurotoxin, Methods in Molecular Biology (Clifton, NJ,) 145, 27–39, [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Aymard C, Giboin LS, Dominici F, Rossi A, Mazzocchio R, 2013, Beyond muscular effects: depression of spinal recurrent inhibition after botulinum neurotoxin A, J Physiol 591, 1017–1029, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masocha W, Parvathy SS, 2009, Assessment of weight bearing changes and pharmacological antinociception in mice with LPS-induced monoarthritis using the Catwalk gait analysis system, Life Sciences 85, 462–469, [DOI] [PubMed] [Google Scholar]
- Matak I, Riederer P, Lackovic Z, 2012, Botulinum toxin’s axonal transport from periphery to the spinal cord, Neurochemistry International 61, 236–239, [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Caleo M, 2015. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry 21, 44–61. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Rasotto MB, 2015. On botulinum neurotoxin variability. MBio 6, pii: e02131-02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz MS, Tepp WH, Bradshaw M, Johnson EA, Pellett S, 2018. Isolation and Characterization of the Novel Botulinum Neurotoxin A Subtype 6. mSphere 3, pii: e00466-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Shimatani Y, Sako W, Asanuma K, Nodera H, Sakamoto T, Izumi Y, Kohda T, Kozaki S, Kaji R, 2014. Comparison between botulinum neurotoxin type A2 and type A1 by electrophysiological study in healthy individuals. Toxicon 81, 32–36. [DOI] [PubMed] [Google Scholar]
- Parvathy SS, Masocha W, 2013. Gait analysis of C57BL/6 mice with complete Freund’s adjuvant-induced arthritis using the CatWalk system. BMC Musculoskeletal Disorders 14, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MW, Smith TJ, Anniballi F, Austin JW, Bano L, Bradshaw M, Cuervo P, Cheng LW, Derman Y, Dorner BG, Fisher A, Hill KK, Kalb SR, Korkeala H, Lindstrom M, Lista F, Luquez C, Mazuet C, Pirazzini M, Popoff MR, Rossetto O, Rummel A, Sesardic D, Singh BR, Stringer SC, 2017. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 9, pii: E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S, Bradshaw M, Tepp WH, Pier CL, Whitemarsh RCM, Chen C, Barbieri JT, Johnson EA, 2018a. The Light Chain Defines the Duration of Action of Botulinum Toxin Serotype A Subtypes. MBio 9, pii: e00089-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S, Tepp WH, Bradshaw M, Kalb SR, Dykes JK, Lin G, Nawrocki EM, Pier CL, Barr JR, Maslanka SE, Johnson EA, 2016. Purification and Characterization of Botulinum Neurotoxin FA from a Genetically Modified Clostridium botulinum Strain. mSphere 1, pii: e00100-00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S, Tepp WH, Lin G, Johnson EA, 2018b. Substrate cleavage and duration of action of botulinum neurotoxin type FA (“H, HA”). Toxicon 147, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S, Tepp WH, Whitemarsh RC, Bradshaw M, Johnson EA, 2015. In vivo onset and duration of action varies for botulinum neurotoxin A subtypes 1-5. Toxicon 107, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier CL, Chen C, Tepp WH, Lin G, Janda KD, Barbieri JT, Pellett S, Johnson EA, 2011. Botulinum neurotoxin subtype A2 enters neuronal cells faster than subtype Al. FEBS Letters 585, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Tepp W, DasGupta BR, 2001. Botulinum neurotoxin types B and E: purification, limited proteolysis by endoproteinase Glu-C and pepsin, and comparison of their identified cleaved sites relative to the three-dimensional structure of type A neurotoxin. Toxicon : official journal of the International Society on Toxinology 39, 1515–1531. [DOI] [PubMed] [Google Scholar]
- Przedpelski A, Tepp WH, Zuverink M, Johnson EA, Pellet S, Barbieri JT, 2018. Enhancing toxin-based vaccines against botulism. Vaccine 36, 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Lam C, Yaksh TL, 2015. Botulinum toxin in migraine: Role of transport in trigemino-somatic and trigemino-vascular afferents. Neurobiol Dis 79, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M, 2011. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 15650–15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Giribaldi F, Manich M, Bercsenyi K, Menendez G, Rossetto O, Caleo M, Schiavo G, 2012a. Botulinum neurotoxins a and e undergo retrograde axonal transport in primary motor neurons. PLoS Pathogens 8, e1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Novelli E, Bottari D, Leone P, Barone I, Galli-Resta L, Strettoi E, Caleo M, 2012b. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic (Copenhagen, Denmark) 13, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Schantz EJ, Kautter DA, 1978. Standardized assay for Clostridium botulinum toxins. Journal - Association of Official Analytical Chemists 61, 96–99. [Google Scholar]
- Schulz A, Walther C, Morrison H, Bauer R, 2014. In vivo electrophysiological measurements on mouse sciatic nerves. Journal of Visualized Experiments : JoVE, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MC, Yukihira T, Ito Y, Akaike N, 2013. Antinociceptive effects of A1 and A2 type botulinum toxins on carrageenan-induced hyperalgesia in rat. Toxicon 64, 12–19. [DOI] [PubMed] [Google Scholar]
- Simpson L, 2013. The life history of a botulinum toxin molecule. Toxicon 68, 40–59. [DOI] [PubMed] [Google Scholar]
- Singh JA, Fitzgerald PM, 2011. Botulinum toxin for shoulder pain: a cochrane systematic review. The Journal of Rheumatology 38, 409–418. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Brenner SL, Dasgupta BR, 1975. Detection of Clostridium botulinum toxin by local paralysis elicited with intramuscular challenge. Applied Microbiology 30, 420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepp WH, Lin G, Johnson EA, 2012. Purification and characterization of a novel subtype a3 botulinum neurotoxin. Appl Environ Microbiol 78, 3108–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii Y, Akaike N, Harakawa T, Kato K, Sugimoto N, Goto Y, Nakahira S, Kohda T, Kozaki S, Kaji R, Ginnaga A, 2011a. Type A1 but not type A2 botulinum toxin decreases the grip strength of the contralateral foreleg through axonal transport from the toxin-treated foreleg of rats. Journal of Pharmacological Sciences 117, 275–285. [DOI] [PubMed] [Google Scholar]
- Torii Y, Goto Y, Nakahira S, Kozaki S, Kaji R, Ginnaga A, 2014. Comparison of Systemic Toxicity between Botulinum Toxin Subtypes A1 and A2 in Mice and Rats. Basic Clin Pharmacol Toxicol. 116, 524–8. [DOI] [PubMed] [Google Scholar]
- Torii Y, Kiyota N, Sugimoto N, Mori Y, Goto Y, Harakawa T, Nakahira S, Kaji R, Kozaki S, Ginnaga A, 2011b. Comparison of effects of botulinum toxin subtype A1 and A2 using twitch tension assay and rat grip strength test. Toxicon 57, 93–99. [DOI] [PubMed] [Google Scholar]
- Tsai SW, Tung YT, Chen HL, Shen CJ, Chuang CH, Tang TY, Chen CM, 2013. Treadmill running upregulates the expression of acetylcholine receptor in rat gastrocnemius following botulinum toxin A injection. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 31, 125–131. [DOI] [PubMed] [Google Scholar]
- Wang D, Krilich J, Pellett S, Baudys J, Tepp WH, Barr JR, Johnson EA, Kalb SR, 2013. Comparison of the catalytic properties of the botulinum neurotoxin subtypes A1 and A5. Biochim Biophys Acta 1834, 2722–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitemarsh RC, Tepp WH, Bradshaw M, Lin G, Pier CL, Scherf JM, Johnson EA, Pellett S, 2013. Characterization of botulinum neurotoxin A subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect Immun 81, 3894–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitemarsh RC, Tepp WH, Johnson EA, Pellett S, 2014. Persistence of botulinum neurotoxin a subtypes 1-5 in primary rat spinal cord cells. PLoS One 9, e90252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Kofie TD, Luquez C, Adler M, Dykes JK, Coleman JD, Maslanka SE, 2011. An alternative in vivo method to refine the mouse bioassay for botulinum toxin detection. Comparative Medicine 61, 235–242. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Group data for mice injected intramuscular with BoNT/A1. Serial dilutions of BoNT/A1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Supplementary Figure 2: Group data for mice injected intramuscular with BoNT/A2. Serial dilutions of BoNT/A2 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Supplementary Figure 3: Group data for mice injected intramuscular with BoNT/A6. Serial dilutions of BoNTs/A6 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.
Supplementary Figure 4: Group data for mice injected intramuscular with BoNT/B1. Serial dilutions of BoNT/B1 in GelPhos buffer were injected into the gastrocnemius muscle of mice (n=5) at the indicated concentrations. The animals were analyzed by Catwalk for up to 4 days with 3 runs per animal at each indicated time point. The graphs were generated by the Catwalk analysis software and show the mean and standard error of the mean values for each parameter and each paw where applicable.