Abstract
Background:
Adolescence is a developmental period in which depression and related mood syndromes often emerge, but few objective markers exist to guide diagnosis or predict symptoms. One potential mood marker is the functioning of frontoinsular networks, which undergo substantial development in adolescence and have been implicated in adult depression. To test this hypothesis, we used task-based neuroimaging to evaluate whether frontoinsular network dysfunction was linked to current and prospective mood health in adolescents.
Methods:
Adolescents (n=40, ages 13–19) reporting varying levels of depressive symptom severity performed an emotional working memory task with neuroimaging. Next, teens completed a two-week follow-up consisting of a daily diary report of negative affect, and final report of depressive symptoms (n=28 adherent). Analyses tested associations between task-related functional connectivity in frontoinsular networks and baseline or prospective measures of mood health over two-week follow-up.
Results:
Frontoinsular task response was associated with higher current depression severity (p=0.049, η2p=0.12), increases in future depression severity (p=0.018, η2p=0.23), and more intense and labile negative affect in daily life (ps=0.015 to 0.040, η2p=0.22 to 0.30). In particular, hypoconnectivity between insula and lateral prefrontal regions of the frontoparietal network was related to both baseline and prospective mood health, and hyperconnectivity between insula and midline or temporal regions of the default network was related to prospective mood health.
Conclusion:
These findings indicate that frontoinsular imbalances are related to both current depression and changes in mood health in the near future, and suggest that frontoinsular markers may hold promise as translational tools for risk prediction.
Keywords: adolescent, depression, mood, functional connectivity, working memory, biomarker
Introduction
Adolescence is a critical period of vulnerability to depression and related mood syndromes, with approximately half of first-episode onset of depression occurring before the age of 20 (1) and evidence that subclinical symptoms in the teen years tend to precede adult-onset depression (2, 3). However, there are a number of reasons that diagnosing or predicting mood disturbances in adolescence is challenging. First, individual adolescents who experience depression may have very different symptom trajectories (e.g., chronicity, severity), and there are few reliable tools for predicting the course of mood health on an individual basis (4). Second, existing clinical tools tend to rely on a single modality for evaluating mood health, which may further limit diagnostic or predictive models. For example, over-reliance on self-report is especially problematic in adolescence because teen self-report is more vulnerable to confounding factors including social desirability or limited cognitive prospection (5, 6). Together, these issues underscore the need for objective diagnostic approaches.
Challenges in diagnosing and predicting adolescent depression have spurred interest in identifying biomarkers of mood health (7). One promising line of biomarker research is focused on the coordinated functioning of large-scale frontoinsular brain networks involved in cognitive regulation of attention, memory and emotion (8). According to frontoinsular network models, the anterior insula acts as a “network switch” that allocates resources towards or away from other large-scale networks, such as the frontoparietal network (FN) and the default network (DN) (9). The prototypical FN includes lateral prefrontal and posterior parietal regions that tend to be recruited together in the service of goal-directed attention, working memory manipulation, and other aspects of executive functioning (10). Meanwhile, the DN includes midline cortical regions and temporal areas that are involved in introspection and autobiographical memory (11). Thus, imbalances in the coordinated activity of insula with FN or DN are believed to underlie deficits regulating internally-oriented or externally-oriented attention (12) – cognitive deficits that are also a hallmark of depression (13).
In support of their relevance to mood health, prior research has shown that frontoinsular network functioning is disrupted in depressed individuals. A meta-analysis of resting-state functional connectivity in major depressive disorder revealed reliable patterns of hypoconnectivity within FN, hyperconnectivity within DN, and (bidirectional) abnormalities in functional connectivity between insula, cingulate, and areas of FN or DN (14). Independent resting-state studies have replicated these meta-analytic findings and have further linked intrinsic hyperconnectivity between insula and prefrontal regions of DN to maladaptive introspection (15) and attention biases towards negative, salient information (16). Thus, imbalances in frontoinsular network functioning may contribute to, or reflect, impairments in the ability to direct attention away from goal-irrelevant thoughts and towards goal-relevant information in working memory or the environment -impairments which characterize depression (17) and are exacerbated by the presence of salient emotional material (18). Task-based neuroimaging provides direct evidence for this idea: biases towards insula-DN coordination, and weakened insula-FN coordination, have been observed when depressed individuals are tasked with directing attention away from introspection or negative emotional material (19) and towards goals (20). The replication of frontoinsular abnormalities across neuroimaging modalities and research paradigms has prompted neurocognitive models proposing that frontoinsular imbalances are central to the cognitive and emotion regulatory failures that may fuel mood disorders (12, 14).
Despite consistent findings for frontoinsular dysfunction in depressed adults, research on such markers in adolescents is limited (see (21)). Of the studies that do address insula dysfunction in adolescent depression, some cross-sectional studies have documented abnormal functional connectivity between insula and regions of midline or temporal cortex either at rest (22, 23) or in response to emotion processing tasks (24–26). Meanwhile, longitudinal studies in this area have documented prospective associations between insula functional connectivity and future mood health (27, 28). However, findings have been mixed regarding the direction of effect for insula abnormalities, and both cross-sectional and longitudinal studies have tended to focus on affective networks linking insula with discrete limbic systems rather than frontoinsular circuits linking insula with large-scale networks such as DN or FN. This gap is notable because frontoinsular network functioning in adolescence may be especially relevant to mood health: it is during adolescence that such networks undergo striking reorganization (29, 30) coinciding with gains in executive functioning and emotion regulation (31, 32). Thus, in adolescence, frontoinsular coordination in response to tasks that challenge emotional executive functioning may be a particularly useful marker of vulnerability to problems in mood health, providing information that reflects the teen’s ability to engage with goals in the presence of salient emotional challenge.
The present study was designed to address the above gaps by investigating frontoinsular markers of current depression, and mood health in the near future (two-week follow-up), in an adolescent sample. This study evaluated individual differences in frontoinsular network response to an emotional working memory task, and tested the associations between frontoinsular functioning and current depression or mood health over a two-week follow-up period (symptom severity, and intensity and lability of daily negative affect). Network response to an emotional working memory task was selected based on evidence that the ability to manipulate emotional material in working memory is a dimension of executive functioning that is impaired in depression and relevant to emotion regulation (17, 33, 34). We predicted that adolescents characterized by frontoinsular network imbalances (including hypoconnectivity among regions of insula and FN, and hyperconnectivity among regions of insula and DN) would report higher severity of depressive symptoms at baseline (hypothesis 1), more severe and labile negative affect over follow-up (hypothesis 2a, 2b) and larger increases in depression at follow-up (hypothesis 3).
Methods and Materials
Participants
The sample consisted of 40 right-hand-dominant adolescents (Table 1; power analysis in Supplement). Participants were recruited on the basis of either having no history of depression (n=21), or having a primary diagnosis of major depression (n=19), from the Boston metropolitan area and McLean Hospital programs. This approach was designed to enhance variance in depression severity, supporting dimensional analyses(35, 36) (analyses that consider categorical diagnosis of depression – yielding results consistent with dimensional analyses – are reported in the Supplement, together with descriptive statistics by diagnosis, Table S1). For evaluation of inclusion/exclusion criteria, information on psychiatric history was drawn from patient records or evaluated by a member of the research team using the MINI International Neuropsychiatric Interview (37). Across the sample, participants were excluded if they reported a history of mania or hypomania, moderate to severe substance use disorders, anorexia, bulimia, binge eating disorder, pervasive developmental disorders, psychosis, neurological impairment, head injury, MRI contraindications, cognitive or language impairments that interfered with the ability to complete behavioral testing, or current (past six weeks) use of benzodiazepines or stimulant medications (38, 39). Other psychoactive medication use is reported in Tables 1, S1; medication class was not related to measures of brain functioning, and did not moderate experimental effects, ps>0.10. (However, overall medication use covaried with depression and it was not possible to investigate experimental effects in the absence of medications). Depressive symptoms were not significantly associated with age, self-identified gender, race and ethnicity, or parent education or income (all ps>0.10). However, to control for potential developmental or gender effects, all analyses controlled for age and gender (40, 41).
Table 1.
Demographics and Mood Health
| Full Sample (n=40) | Follow-up Sample (n=28) | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Age (years) | 16.05 (1.57) | 16.07 (1.78) | ||
| Depressive Symptoms (CESD Score) At Baseline | 18.43 (16.27) | 14.82 (15.45) | ||
| Depressive Symptoms (CESD Score) At Follow-up (Day 15) | 11.18 (11.17) | |||
| Max Negative Affect (short PANAS-N) Over Follow-up | 12.25 (3.78) | |||
| SD in Negative Affect (short PANAS-N) Over Follow-up | 2.09 (1.00) | |||
| Max Positive Affect (short PANAS-P) Over Follow-up | 14.29 (3.54) | |||
| SD in Positive Affect (short PANAS-P) Over Follow-up | 2.43 (1.06) | |||
| % | % | |||
| Gender | ||||
| Female | 72.5% | 82.1% | ||
| Male | 22.5% | 14.3% | ||
| Non-binary | 5.0% | 3.6% | ||
| Medication Use | ||||
| Norepinephrine-dopamine reuptake inhibitor | 12.5% | 10.7% | ||
| Selective serotonin-norepinephrine reuptake inhibitor | 10.0% | 7.1% | ||
| Selective serotonin reuptake inhibitor | 35.0% | 25.0% | ||
| Tetracyclics | 0.0% | 0.0% | ||
| Anticonvulsants/Antipsychotics | 15.0% | 7.1% | ||
| Lithium | 7.5% | 10.7% | ||
| Anxiolytics (non-Benzodiazepine) | 7.5% | 7.1% | ||
| Any psychoactive medication use | 40.0% | 32.1% | ||
| Race | ||||
| African American | 2.5% | 0.0% | ||
| American Indian/Alaskan Native | 0.0% | 0.0% | ||
| Asian | 10.0% | 14.3% | ||
| Biracial or Other | 12.5% | 7.1% | ||
| White | 75.0% | 78.6% | ||
| Ethnicity | ||||
| Hispanic | 2.5% | 3.6% | ||
| Not Hispanic or Other | 97.5% | 96.4% | ||
| Education (Parent Highest) | ||||
| Without High School Diploma | 0.0% | 0.0% | ||
| High School Graduate Without College Degree | 2.5% | 3.6% | ||
| Some College Education | 5.0% | 3.6% | ||
| Degree from Four-Year College (or more) | 92.5% | 92.8% | ||
| Current % | Life % | Current % | Life % | |
| Major Depressive Disorder (MDD) | 47.5% | 47.5% | 35.7% | 35.7% |
| Anxiety Disorders secondary to MDD | 15.0% | 15.0% | 17.9% | 17.9% |
| Posttraumatic Stress Disorder | 0.0% | 0.0% | 0.0% | 0.0% |
| Generalized Anxiety Disorder | 15.0% | 15.0% | 17.9% | 17.9% |
| Panic Disorder | 5.0% | 5.0% | 7.1% | 7.1% |
| Agoraphobia | 0.0% | 0.0% | 0.0% | 0.0% |
| Social Phobia | 2.5% | 2.5% | 3.6% | 3.6% |
| Specific Phobia | 0.0% | 0.0% | 0.0% | 0.0% |
| (Mild) Substance Use Disorders | 0.0% | 0.0% | 0.0% | 0.0% |
Note: Center for Epidemiological Studies Depression Scale (CESD), Maximum (Max), Positive and Negative Affect Schedule -Negative affect subscale, short version (PANAS-N, possible score range of 5 to 25), Positive and Negative Affect Schedule -Positive affect subscale, short version (PANAS-P, possible score range of 4 to 20), standard deviation (SD).
Procedures
The study consisted of a neurocognitive testing session, including a clinical interview, self-report measures of depression and state affect, and a functional magnetic resonance imaging (fMRI) scan including administration of an emotional working memory task (see Supplement and (42) for other procedures). After the experimental session, for a two-week follow-up period, a daily diary was delivered electronically via a HIPAA-compliant website (REDCap; https://www.project-redcap.org/) to the participant’s smartphone or preferred electronic device. The daily diary included evaluation of current positive and negative affect one time per day for 14 days, and was delivered at a time of day selected by the participant. On day 15, the participant reported on depressive symptoms. A total of n=28 participants were compliant with follow-up procedures (completed ≥7 days of the daily diary assessments; there were no differences in demographic or clinical characteristics as a function of follow-up compliance, see Table 1 and Supplement). Critically, all imaging findings were consistent when including subjects who completed 3+, 4+, 6+ or 7+ days of assessments (see Supplement). Participants were reimbursed for their time and offered referral information for local psychological services. Research procedures were approved by the Partners Institutional Review Board and conducted in accordance with the provisions of the World Medical Association Declaration of Helsinki.
Measures
Depressive symptom severity.
Severity of depressive symptoms was evaluated using the Center for Epidemiological Studies Depression Scale (CESD, (43–45)). The CESD was administered at the experimental session, and again on day 15 of the follow-up period.
Positive and negative affect.
To evaluate current mood state, participants completed a subset of items from the Positive and Negative Affect Schedule (PANAS; (46), see (47) for prior research using the same truncated scale). The PANAS was administered at the experimental session, and in each daily diary. Negative (PANAS-N) and positive (PANAS-P) subscales were scored for each date of collection. The primary affective measures over the follow-up were maximum PANAS-N (reflecting peak intensity of negative emotions) and standard deviation in PANAS-N (reflecting mood lability (48)). (See additional details in Supplement).
Emotional working memory task.
Brain and behavioral responses to task demands for emotional working memory were evaluated with the Emotion Face Sorting (EFS) task presented using EPrime 2.6 (Psychology Software Tools, Pittsburgh, PA). The EFS measures the ability to manipulate and attend to goal-relevant features of information in working memory in the presence of goal-irrelevant emotional features (Figure 1 and Supplement).
Figure 1. Emotional executive functioning task.
The Emotion Face Sorting (EFS) task was designed to challenge working memory in the presence of emotional distraction. The task was presented in blocks (task blocks interspersed with resting fixation blocks); the main contrast of interest was functional connectivity in response to task vs. resting blocks. Participants were instructed that responses should be given as quickly and accurately as possible. Within each task block, each trial included four components: Learn (2160 ms). The participant was presented with a set of either two fearful faces (“negative” condition) or two happy faces (“positive” condition). Face stimuli consisted of negative or positive emotional face images from 12 individuals (6 female) from the NimStim set available at http://www.macbrain.org (Tottenham et al., 2009). Cue (1440 ms). Next, the participant was presented with a cue to either maintain the same spatial position of the faces (“stay” condition), or to mentally reverse the spatial position of the faces (moving the left-side image to the right, and the right-side image to the left; “switch” condition). Working Memory Manipulation (2880 ms). After viewing the cue symbol, the screen became black and the participant had a period of time to accomplish the working memory manipulation (maintain, or spatially switch, the images). Probe (up to 2880 ms). Finally, the participant was presented with a set of face images (both original faces sorted correctly, both original faces sorted incorrectly, or one original face paired with a new face image of the same valence), and responded to indicate whether the content and spatial organization of the images matched the images held in working memory. After responding, the participant was presented with a fixation cross for a jittered inter-trial interval (720 to 8000 ms). Displayed are examples of (A) a “stay/fearful” trial, and (B) a “switch/happy” trial. All faces presented within an individual trial were of the same emotional valence. During functional scanning, participants completed four blocks of 20 trials/block, separated by four fixation blocks (26 seconds/block).
Functional imaging.
Participants completed scanning using a Siemens Tim Trio 3T scanner and 32-channel head coil. Anatomical scanning included a high-resolution T1-weighted anatomical image (TR=2100 ms, TE=2.25 ms, GRAPPA acceleration factor of 2, flip angle=12, 128 slices, field of view=256 mm, voxel size 1.0×1.0×1.3 mm). Functional scanning included sixteen minutes of functional images using a Human Connectome Project (49, 50) multiband sequence (TR=720 ms, TE=30 ms, GRAPPA acceleration factor of 2, flip angle=66, 66 slices, field of view=212 mm, voxel size 2.5×2.5×2.5 mm, total volumes=1347).
Analyses
Analyses included: (1) general image preprocessing; (2) calculation of motion parameters, artifacts, and outlier volumes; (3) denoising the timeseries; (4) first-level task-weighted general linear modelling to calculate changes in functional connectivity in response to task demands; and (5) group-level analyses. All analyses controlled for age and gender (40, 41). Before being entered in regression analyses, group-level continuous variables were z-transformed, and the categorical variable (gender) was contrast-coded.
Behavioral analyses.
In preparation for experimental analyses, we performed analyses to confirm adequate task performance (i.e., above-chance accuracy ≥ 55%), and evaluate task performance (Supplement). Behavioral data were processed using R (https://www.r-project.org/), and analyzed using SPSS (IBM; New York, NY).
Neuroimaging preprocessing and corrections.
See Supplement for information on image preprocessing, and corrections for motion, artifacts, and denoising using Matlab (version R2016a, Mathworks, Natick, MA) and SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), Artifact Detection Tools (ART, www.nitrc.org/projects/artifact_detect/), and the CONN toolbox (https://www.nitrc.org/projects/conn/; (51)). Motion and outlier volumes were not significantly associated with experimental variables (ps>0.10), and motion correction and volume censoring were performed on the first level of analysis (52–54).
First-level analysis.
A task weighted general linear model (GLM) (55, 56) was performed using CONN to evaluate task-related changes in functional connectivity among regions of interest (ROIs) in insula, FN, and DN. Regions of interest were defined on the basis of an a priori resting-state functional network parcellation (57) and included 4 insula ROIs, 26 ROIs in the FN, and 24 ROIs in the DN (Figure S1). The GLM analysis computed a non-parametric estimation of task-specific connectivity effects, by calculating weighted beta estimates for ROI-to-ROI regressions for each condition weighting the scans associated with that condition only (with average task activation effects controlled in denoising, above). Two conditions were defined: a task condition (task blocks) and a resting condition (fixation blocks). Within each condition, regression analyses between each pair of ROIs estimated the extent to which variance in activation in one ROI could be predicted by variance in activation of the second ROI, yielding a beta estimate for each ROI-to-ROI regression. The difference in beta estimates for a given ROI-to-ROI pair between task and rest (ßtask_A-to-B – ßrest_A-to-B) yielded an estimate of task-related changes in functional connectivity from region A to B. Four sets of ROI-to-ROI associations were calculated and compared between task and rest: (a) beta estimates in which activation in insula ROIs predicted activation in FN ROIs, averaged across all insula-to-FN ROI pairs, [insula-FN], (b) beta estimates in which activation in insula ROIs predicted activation in DN ROIs, averaged across all insula-to-DN ROI pairs, [insula-DN], (c) beta estimates in which activation in FN ROIs predicted activation in other FN ROIs, averaged across all FN-to-FN ROI pairs [within FN], and (d) beta estimates in which activation in DN ROIs predicted activation in other DN ROIs, averaged across all DN-to-DN ROI pairs [within DN]. (See Supplement for analyses aimed at localizing subnetworks that contributed to significant effects).
Group-level analyses.
Group-level multiple regressions tested associations between brain network functioning and (1) baseline depressive symptoms, or between brain network functioning and (2) negative affect intensity, (3) negative affect lability, or (4) depressive symptoms, over two-week follow-up. In the first regression, four predictor variables representing task-related functional connectivity in insula-FN, insula-DN, FN, and DN, were together regressed on baseline depressive symptoms (CESD). In the second and third regressions, the same network measures were regressed, together with baseline PANAS-N and PANAS-P, on maximum PANAS-N or on standard deviation in PANAS-N over follow-up. In the fourth regression, network measures were regressed, together with baseline CESD, on follow-up CESD. Thus, all prospective analyses also controlled for baseline mood measures.
Results
Functional network response to emotional working memory is associated with current depressive symptom severity.
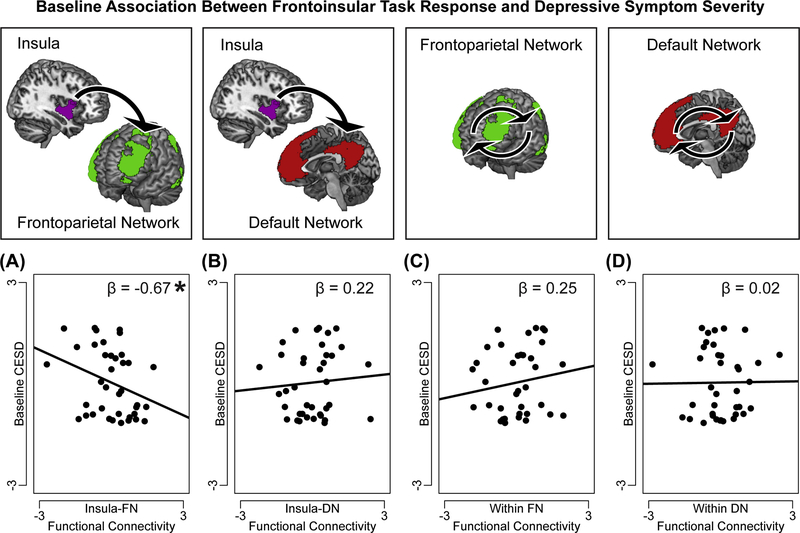
Task-related connectivity between insula-FN, between insula-DN, within FN, and within DN, were together regressed (with demographic covariates) on baseline depressive symptoms (CESD). Result showed that teens exhibiting lower task-related functional connectivity between insula-FN reported higher severity of depression at baseline, F(1,31)=4.04, p=0.049, η2p=0.12 (Figure 2). Task-related functional connectivity in other networks (between insula-DN, within FN, within DN) was not associated with baseline depression, ps>0.10.
Figure 2. Frontoinsular response to task is associated with current depressive symptom severity.
One multiple regression analysis tested the associations between task-related functional connectivity in a priori frontoinsular networks and baseline depressive symptoms (Center for Epidemiological Studies- Depression Subscale, CESD). In this regression, (A) task-related hypoconnectivity between insula and regions of frontoparietal network (FN) was associated with higher depression severity, but (B) task-related connectivity between insula and regions of default network (DN), or (C) among regions within FN, or (D) among regions within DN, was not significantly associated with depression severity. Note: On y-axis, baseline CESD scores are normalized and residualized for demographic covariates (age and gender); on x-axis, task-related network functional connectivity is normalized and residualized for covariates. Reported are standardized ß from regression analyses. A significant ß represents a significant independent variable in the regression, *p<0.05.
Functional network response to emotional working memory prospectively predicts negative affect.
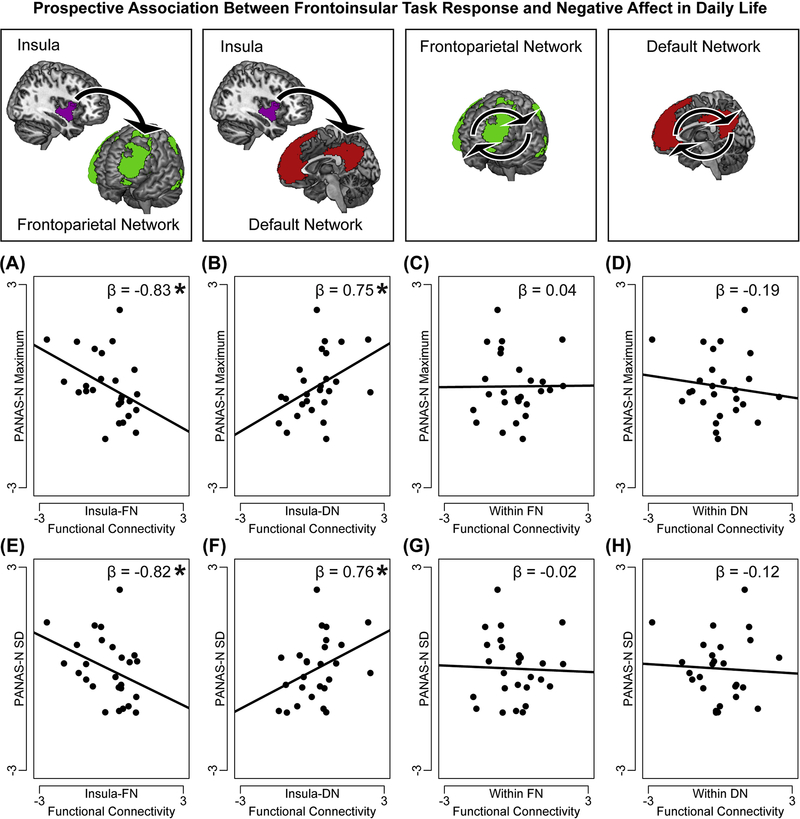
Two multiple regressions were performed in which task-related connectivity between insula-FN, between insula-DN, within FN, and within DN, demographic covariates, and baseline positive and negative affect (baseline PANAS-N and PANAS-P) were regressed on scores reflecting either future negative mood intensity (maximum PANAS-N over two-week follow-up) or lability (standard deviation in PANAS-N over two-week follow-up). In the first regression, both insula-FN hypoconnectivity, F(1,17)=7.37, p=0.015, η2p=0.30, and insula-DN hyperconnectivity, F(1,17)=6.14, p=0.024, η2p=0.27, were associated with higher intensity of negative affect in the next two weeks (Figure 3). In the second regression, both insula-FN hypoconnectivity, F(1,17)=5.58, p=0.030, η2p=0.25, and insula-DN hyperconnectivity, F(1,17)=4.79, p=0.040, η2p=0.22, were associated with increased lability of negative affect in the next two weeks (Figure 3). Together, these results suggest that weaker task-related connectivity between insula and FN (particularly lateral prefrontal regions of FN; see post-hoc analyses in Supplement), and amplified task-related connectivity between insula DN (especially medial prefrontal areas of DN; see Supplement), constitute markers of vulnerability to negative mood in daily life. Task-related functional connectivity among regions of networks excluding insula (within FN, within DN) was not associated with intensity or lability of future negative affect, ps>0.10. Controlling for future positive affect in these regressions did not alter these effects (see Supplement).
Figure 3. Frontoinsular response to task predicts future negative affect.
Two multiple regression analyses tested the associations between task-related functional connectivity in a priori frontoinsular networks and future negative affect intensity and lability (maximum score on, or standard deviation in, daily diary report of negative affect on the Positive and Negative Affect Scale-Negative Subscale, PANAS-N, over follow-up). In the first regression, task-related (A) hypoconnectivity between insula and regions of frontoparietal network (FN), and (B) hyperconnectivity between insula and regions of default network (DN), were each associated with higher intensity of future negative affect, but task-related functional connectivity (C) among regions of FN or (D) among regions of DN, was not associated with intensity of negative affect. In the second regression, task-related (E) hypoconnectivity between insula-FN and (F) hyperconnectivity between insula-DN were also associated with higher lability of future negative affect, but associations failed to emerge between (G) within-FN or (H) within-DN connectivity and negative affect lability. Note: On y-axis, PANAS-N measures are normalized and residualized for demographic covariates (age and gender) and baseline positive and negative affect (PANAS-N, PANAS-P); on x-axis, task-related network functional connectivity is normalized and residualized for covariates. Reported are standardized ß from regression analyses. A significant ß represents a significant independent variable in the regression, *p<0.05.
Functional network response to emotional working memory prospectively predicts severity of depression.
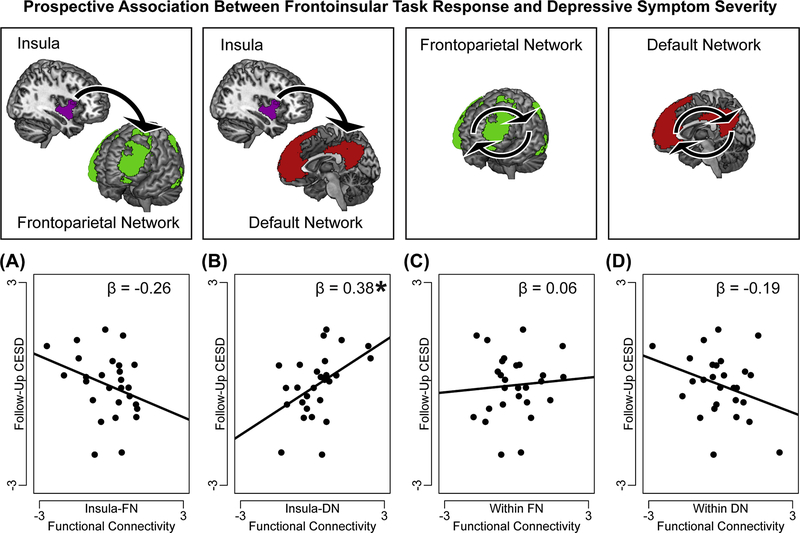
Task-related connectivity between insula-FN, between insula-DN, within FN, and within DN, demographic covariates, and baseline depression severity (CESD at baseline) were together regressed on future depression severity (CESD at two-week follow-up). Teens exhibiting insula-DN hyperconnectivity (especially in ventromedial and temporal areas) in response to the task at baseline reported more severe depression at follow-up, F(1,19)=5.73, p=0.018, η2p=0.23. The association between task-related functional connectivity between insula-FN and future depression did not reach significance, F(19)=2.15, p=0.154, η2p=0.10. (Figure 4).
Figure 4. Frontoinsular response to task predicts future depressive symptom severity.
One multiple regression analysis tested the associations between task-related functional connectivity in a priori frontoinsular networks and future depressive symptom severity (Center for Epidemiological Studies- Depression Subscale, CESD, at 15-day follow-up). In this regression, task-related (A) hypoconnectivity between insula and regions of frontoparietal network (FN) did not reach a significant association with future depression (p=0.15), but (B) hyperconnectivity between insula and regions of default network (DN), was associated with higher severity of future depression. Task-related connectivity (C) among regions within FN, or (D) among regions within-DN, was not associated with future depression. Note: On y-axis, CESD scores are normalized and residualized for demographic covariates (age and gender) and baseline depression severity (CESD); on x-axis, task-related network functional connectivity is normalized and residualized for covariates. Reported are standardized ß from regression analyses. A significant ß represents a significant independent variable in the regression, *p<0.05.
Associations between task-related functional connectivity within other networks (within FN, within DN) were not significantly associated with depression at follow-up, ps>0.10. To examine the specificity of insula-FN or insula-DN task responses as markers of current symptoms versus prospective increases in symptom severity, a Meng test (58) was performed to compare the strength of the associations (partial correlations, including all variables in the original regression as covariates) between frontoinsular measures and either baseline or follow-up CESD. There was no significant difference in the associations between insula-FN task response and either current or prospective measures of depression, z=0.25, p=0.805. However, insula-DN task response had a significantly stronger relationship with prospective depressive symptom severity than with baseline symptom severity, z=4.52, p<0.001.
Discussion
The discovery of objective biomarkers to understand and forecast the course of depression in adolescence is a public health goal with clinical implications. Results of the present study align with this mission, suggesting that frontoinsular network functioning in adolescence may predict current and prospective mood health. Abnormalities in the coordinated recruitment of frontoinsular networks in response to task demands for emotional working memory were associated with current depression severity, increases in depressive symptoms two weeks later, and more intense and labile negative affect in daily life. Of note, prospective analyses isolated the contribution of frontoinsular markers in predicting mood health, over and above baseline symptoms. This distinction is critical: based on these results, in theory, frontoinsular functioning could distinguish which of two teens with the same current level of depression is more vulnerable to escalating negative mood and depression in the near future. More broadly, these findings highlight the potential utility of neurobiological biomarkers for risk prediction.
In this study, current and prospective mood health were associated with frontoinsular network imbalances including weaker task-related functional connectivity between insula and frontoparietal systems, and excessive task-related connectivity between insula and areas of the default network. These patterns are consistent with research in depressed adults that linked frontoinsular imbalances to attention biases towards self-referential emotional material (15, 16, 19). According to frontoinsular models of cognitive regulation, the insula is involved in allocating resources towards or away from other large-scale networks on the basis of salient internal or environmental events. In a cognitively demanding task, insula recruitment of lateral prefrontal regions of the FN may be important for goal-directed attention (here, towards goal-relevant information in working memory), whereas insula downregulation of midline and temporal regions of the DN may minimize task-interfering introspection (9). Consistent with this model, in the present study, weaker functional connectivity between insula and frontoparietal (especially lateral prefrontal) regions, and increased functional connectivity between insula and default network (especially medial prefrontal and inferior temporal) regions, were each associated with poorer task performance. The former pattern of insula-FN hypoconnectivity was specifically related to slower responding on low-working-memory-load trials, hence hypoconnectivity in this network may reflect depression-related impairments in the ability to regulate attention towards goals when task demands are less inherently salient. Meanwhile, the latter pattern of insula-DN hyperconnectivity was generally associated with slower response speed, possibly reflecting deficits downregulating bottom-up interference of self-focused thoughts or shifting away from introspection – which may make the individual vulnerable to rumination and depression. Future research aimed at disentangling the relative contributions of these overlapping frontoinsular networks to emotional executive functioning may provide additional information to guide such interpretations. For now, these research results converge with prior cross-sectional evidence in adults (20), and suggest that frontoinsular network abnormalities reflect deficits in emotional attention regulation that characterize teens who are currently experiencing, or are especially vulnerable to, poor mood health.
There was some divergence in this study between network abnormalities that were associated with current versus prospective mood health. Namely, insula-DN hyperconnectivity was only prospectively associated with mood health, whereas insula-FN hypoconnectivity was associated with both current and prospective mood health. This divergence suggests the possibility that distinct profiles of frontoinsular functioning serve as markers of vulnerability and illness. For example, insula-DN hyperconnectivity may reflect impairments in self-regulation that emerge in the face of emotional challenge, making individuals especially vulnerable to deteriorating mood health in response to stressors in daily life. In contrast, insula-FN hypoconnectivity may reflect deficits in self-regulation that both underlie stable (trait-like) dimensions of current symptoms and are amplified by environmental stressors. Together, it will be important to replicate the present results in independent longitudinal research that tracks associations between brain network functioning, daily life events, and mood health.
The results of this study have potential clinical implications. Teen depression can be difficult to predict because there are few objective tools for evaluating risk. Therefore, the identification of risk biomarkers has special appeal (8). Critically, risk biomarkers must be informative over and above other (less expensive) methods in order to be clinically useful. Here, frontoinsular task response predicted future mood health over and above baseline mood health, implying that such metrics may distinguish among teens with similar levels of depression which teen is more vulnerable to escalating negative mood and depression in the near future. Thus, frontoinsular imbalances may help guide preventive interventions, for example, if frontoinsular screening were integrated into clinical intake in a model similar to other diagnostic imaging (e.g., to diagnose sources of back pain). However, clinical translation will require robust replication of these results. In addition, future efforts to replicate and expand these results should consider other symptom dimensions; in light of evidence for frontoinsular dysfunction in other psychopathologies (e.g., mania, anxiety, psychosis), the specificity of frontoinsular imbalances is unknown and could constitute a non-specific biomarker for transdiagnostic increases in symptom severity. Although beyond the scope of the present study, these questions should be explored in future research with large samples that can distinguish specific and transdiagnostic relationships between frontoinsular functioning and psychiatric health.
There are several limitations to the present study. First, longitudinal tracking of mood health was brief, comprising two weeks of daily diary assessment and a single follow-up evaluation of depression severity. Therefore, it was not statistically possible to investigate other aspects of mood health such as nonlinear symptom changes over time, or track nuanced interactions between discrete life events (e.g., stressors, achievements) and mood fluctuations. Second, neurocognitive testing was administered at a single (baseline) timepoint. Longitudinal assessment of frontoinsular functioning may reveal deviations from normative development that provide other information for predicting mood health. Third, the present analyses were restricted to frontoinsular network relationships. However, other neural systems involved in monitoring emotional salience or believed to play a role in regulating internally- or externally-oriented attention(59) may also be of interest when predicting adolescent mood and daily functioning. Fourth, depression covaried with general use of psychoactive medications in this study. Although controlling for specific classes of medications did not alter experimental effects, we could not evaluate experimental effects in the absence of medication use. Research that evaluates brain network functioning in unmedicated teens may provide additional information. Fifth, there may be individual differences in other dimensions that moderate or clarify these findings. For example, the present findings suggest that weaknesses in emotional working memory may be reflected in dysfunctional circuits; however, this study did not evaluate related constructs such as general executive functioning or IQ. Therefore, it is unknown whether frontoinsular imbalances are evoked only when manipulating emotional material in working memory, or also with neutral material or by other executive functioning tasks. Taking this a step further, it is possible that network dysfunction contributes to depression via broad cognitive deficits or impairments in IQ. These questions should be addressed in future research designed to evaluate the cognitive contexts in which frontoinsular imbalances emerge.
In conclusion, this study provides support for frontoinsular measures to evaluate and predict mood health in adolescence. Here, teens with higher levels of depression at the time of neuroimaging showed hypoconnectivity between insula and frontoparietal regions, and hyperconnectivity between insula and areas of the default network, in response to a task that challenged emotional working memory. Task-related imbalances in frontoinsular network functioning were also prospectively associated with mood health in the near (two-week) future, even after taking into account baseline mood health. Frontoinsular functioning may have potential for clinical translation, although replication in large samples and over time are critical next steps.
Supplementary Material
Funding
Supported by the Phyllis and Jerome Lyle Rappaport Fellowship (R.H.K.), and NIMH grants R56 MH117131 (R.H.K.), F32 MH106262 (R.H.K.), K23 MH097786 (R.P.A.), R37 MH095809 (D.A.P.) and R01 MH101521 (D.A.P.). A subset of analyses reported here were presented (by R.H.K.) at the 2018 annual meetings of the American College of Neuropsychopharmacology (ACNP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests
Over the past three years, D.A.P received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, CompassPathway, Posit Science and Takeda Pharmaceuticals USA and honoraria from Alkermes for activities unrelated to the current study. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. (2012) Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research. 21(3):169–84. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertha EA, Balazs J. Subthreshold depression in adolescence: a systematic review. (2013) European Child & Adolescent Psychiatry. 22(10):589–603. doi: 10.1007/s00787-013-0411-0. [DOI] [PubMed] [Google Scholar]
- 3.Pine DS, Cohen E, Cohen P, Brook J. (1999) Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? American Journal of Psychiatry. 156(1):133–5. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Thapar A, Collishaw S, Pine DS, Thapar AK. (2012) Depression in adolescence. Lancet. 379(9820):1056–67. doi: 10.1016/s0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brener ND, Billy JOG, Grady WR. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: Evidence from the scientific literature. (2003) Journal of Adolescent Health. 33(6):436–57. doi: 10.1016/s1054-139x(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 6.King CA, Hill RM, Wynne HA, Cunningham RM. Adolescent Suicide Risk Screening: The Effect of Communication About Type of Follow-Up on Adolescents’ Screening Responses. (2012) Journal of Clinical Child and Adolescent Psychology. 41(4):508–15. doi: 10.1080/15374416.2012.680188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama MS, Di Martino A, Castellanos FX, Ho EJ, Marcelle E, Leventhal B, et al. Imaging the “At-Risk” Brain: Future Directions. (2016) Journal of the International Neuropsychological Society. 22(2):164–79. doi: 10.1017/s1355617715001356. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser RH. Neurocognitive Markers of Depression. (2017) Biological psychiatry. 81(4):e29–e31. doi: 10.1016/j.biopsych.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. (2010) Brain Structure & Function. 214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. (2013) Trends in Cognitive Sciences. 17(12):602–3. doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews-Hanna JR, Smallwood J, Spreng RN, editors. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Holtzheimer PE, Mayberg HS. Stuck in a rut: Rethinking depression and its treatment. (2011) Trends in Neurosciences. 34(1):1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder A Meta-analysis of Resting-State Functional Connectivity. (2015) Jama Psychiatry. 72(6):603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, et al. Dynamic Resting-State Functional Connectivity in Major Depression. (2016) Neuropsychopharmacology. 41(7):1822–30. doi: 10.1038/npp.2015.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser RH, Snyder HR, Goer F, Clegg R, Ironside M, Pizzagalli DA. (2018) Attention Bias in Rumination and Depression: Cognitive Mechanisms and Brain Networks. Clinical Psychological Science. 6 (6):765–782. Doi: 10.1177/2167702618797935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder HR. (2013) Major Depressive Disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. (2011) Journal of Cognitive Psychology. 23(6):669–94. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. (2015) Social Cognitive and Affective Neuroscience. 10(5):654–63. doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Ongur D, Auerbach RP, Yao SQ. Cognitive Vulnerability to Major Depression: View from the Intrinsic Network and Cross-network Interactions. (2016) Harvard Review of Psychiatry. 24(3):188–201. doi: 10.1097/hrp.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. (2014) Functional brain imaging studies of youth depression: A systematic review. Neuroimage-Clinical. 4:209–31. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. (2013) Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry. 74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. (2009) A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 460(3):227–31. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blom EH, Connolly CG, Ho TC, LeWinn KZ, Mobayed N, Han L, et al. (2015) Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. Journal of Affective Disorders. 178:215–23. doi: 10.1016/j.jad.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, et al. (2014) Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, et al. (2012) Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. Journal of Affective Disorders. 139(1):75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. (2017) Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. Journal of Affective Disorders. 207:86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin J, Narayanan A, Perlman G, Luking K, DeLorenzo C, Hajcak G, et al. (2017) Orbitofrontal cortex activity and connectivity predict future depression symptoms in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2(7):610–8. Epub 2017/02/27. doi: 10.1016/j.bpsc.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. (2010) Prediction of Individual Brain Maturity Using fMRI. Science. 329(5997):1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosenbach NUF, Petersen SE, Schlaggar BL. (2013) The Teenage Brai: Functional Connectivityn. Current Directions in Psychological Science. 22(2):101–7. doi: 10.1177/0963721412474297. [DOI] [Google Scholar]
- 31.Best JR, Miller PH. (2010) A Developmental Perspective on Executive Function. Child Development. 81(6):1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey BJ, Tottenham N, Liston C, Durston S. (2005) Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 9(3):104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser RH, Andrews-Hanna JR, Metcalf CA, Dimidjian S. (2015) Dwell or Decenter? Rumination and Decentering Predict Working Memory Updating After Interpersonal Criticism. Cognitive Therapy and Research. 39(6):744–53. doi: 10.1007/s10608-015-9697-1 [DOI] [Google Scholar]
- 34.Joormann J, Gotfib IH. (2008) Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 117(1):182–92. doi: 10.1037/0021-843x.117.1.182. [DOI] [PubMed] [Google Scholar]
- 35.Liu RT. (2016) Taxometric evidence of a dimensional latent structure for depression in an epidemiological sample of children and adolescents. Psychological Medicine. 46(6):1265–75. doi: 10.1017/s0033291715002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widiger TA, Samuel DB. (2005) Diagnostic categories or dimensions? A question for the diagnostic and statistical manual of mental disorders-fifth edition. Journal of Abnormal Psychology. 114(4):494–504. doi: 10.1037/0021-843x.114.4.494. [DOI] [PubMed] [Google Scholar]
- 37.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. (2010) Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). Journal of Clinical Psychiatry. 71(3):313–26. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 38.Hoiseth G, Andas H, Bachs L, Morland J. Impairment due to amphetamines and benzodiazepines, alone and in combination. (2014) Drug and Alcohol Dependence. 145:174–9. doi: 10.1016/j.drugalcdep.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Marraccini ME, Weyandt LL, Rossi JS, Gudmundsdottir BG. (2016) Neurocognitive Enhancement or Impairment? A Systematic Meta-Analysis of Prescription Stimulant Effects on Processing Speed, Decision-Making, Planning, and Cognitive Perseveration. Experimental and Clinical Psychopharmacology. 24(4):269–84. doi: 10.1037/pha0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenroot RK, Giedd JN. (2006) Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 30(6):718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Lenroot RK, Giedd JN. (2010) Sex differences in the adolescent brain. Brain and Cognition. 72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaiser R, Kang M, Lew Y, Van der Feen J, Aguirre B, Clegg R, et al. Abnormal Frontoinsular-Default Network Dynamics in Adolescent Depression and Rumination: A Resting-State Co-Activation Pattern Analysis [DOI] [PMC free article] [PubMed]
- 43.Radloff LS. (1991) The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of Youth and Adolescence. 20(2):149–66. doi: 10.1007/bf01537606. [DOI] [PubMed] [Google Scholar]
- 44.Bradley KL, Bagnell AL, Brannen CL. (2010) Factorial validity of the Center for Epidemiological Studies Depression 10 in adolescents. Issues in mental health nursing. 31(6):408–12. doi: 10.3109/01612840903484105. [DOI] [PubMed] [Google Scholar]
- 45.Phillips GA, Shadish WR, Murray DM, Kubik M, Lytle LA, Birnbaum AS. (2006) The Center for Epidemiologic Studies Depression scale with a young adolescent population: A confirmatory factor analysis. Multivariate Behavioral Research. 41(2):147–63. doi: 10.1207/s15327906mbr4102_3. [DOI] [PubMed] [Google Scholar]
- 46.Watson D, Clark LA, Tellegen A. (1988) Development and validation of brief measures of positive and negative affect – the PANAS scales. Journal of Personality and Social Psychology. 54(6):1063–70. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 47.Wallace ML, McMakin DL, Tan PZ, Rosen D, Forbes EE, Ladouceur CD, et al. (2017) The role of day-to-day emotions, sleep, and social interactions in pediatric anxiety treatment. Behaviour Research and Therapy. 90:87–95. doi: 10.1016/j.brat.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovacs M, Yaroslavsky I. (2014) Practitioner Review: Dysphoria and its regulation in child and adolescent depression. Journal of Child Psychology and Psychiatry. 55(7):741–57. doi: 10.1111/jcpp.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. (2013) Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage. 80:169–89. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, et al. (2010) Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. Plos One. 5(12):11. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitfield-Gabrieli S, Nieto-Castanon A. (2012) Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 52.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–41. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power JD, Schlaggar BL, Petersen SE. (2015) Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 105:536–51. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ismaylova E, Levesque ML, Pomares FB, Szyf M, Nemoda Z, Fahim C, et al. (2018) Serotonin transporter promoter methylation in peripheral cells and neural responses to negative stimuli: A study of adolescent monozygotic twins. Translational Psychiatry. 8:9. doi: 10.1038/s41398-018-0195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poletti S, Riberto M, Vai B, Ghiglino D, Lorenzi C, Vitali A, et al. (2018) A Glutamate Transporter EAAT1 Gene Variant Influences Amygdala Functional Connectivity in Bipolar Disorder. Journal of Molecular Neuroscience. 65(4):536–45. doi: 10.1007/s12031-018-1138-7. [DOI] [PubMed] [Google Scholar]
- 57.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 106(3):1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng XL, Rosenthal R, Rubin DB. (1992) Comparing correlated correlation coefficients. Psychological Bulletin. 111(1):172–5. doi: 10.1037/0033-2909.111.1.172. [DOI] [Google Scholar]
- 59.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 100(6):3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.