Abstract
Background:
The mechanisms by which the rete testis joins the efferent ducts, which joins the Wolffian duct during development, are not known. Mouse and chick models have been helpful in identifying genes that are important for the development of each part, but genes have not been identified as to those that play a role in the joining of each part. Clinical implications of the failure of the male reproductive tract to form a fully functional conduit for spermatozoa are not trivial. Epididymal disjunction, the failure of the efferent ducts to join the testis, is one of several epididymal anomalies that have been observed in some boys who were cryptorchid at birth.
Objective:
A systematic review of studies focusing on the morphogenesis of the mesonephric duct and mesonephric tubules in different species, and identification of clinical issues should there be failure of these tissues to develop.
Design:
PubMed and GUDMAP databases, and review of books on kidney development were searched for studies reporting on the mechanisms of morphogenesis of the kidney and epididymis.
Main outcomes measure(s):
Gaps in our knowledge were identified, and hypotheses coupled with suggestions for future experiments were presented.
Results:
A total of 64 papers were identified as relevant, of which 53 were original research articles and 11 were book chapters and reviews covering morphogenesis and clinical issues. Investigators utilized multiple species including, human, mouse, chick, Xenopus, bovine, and sheep.
Conclusion:
Fundamental understanding of the morphogenesis of the male reproductive tract is limited, especially the morphogenesis of the rete testis and efferent ducts. Therefore, it is not surprising that we do not understand how each part unites to form a whole. Only one mechanism of joining of one part of the tract to another was identified: the joining of the Wolffian duct to the cloaca via controlled apoptosis.
Keywords: efferent ducts, epididymis, mesonephric tubules, morphogenesis, rete testis, Wolffian duct
INTRODUCTION
In viewing morphogenesis of the male reproductive tract from afar, it is quite clear that it is the sum of the parts that makes the whole. Initially, it appears that each piece of the tract, the seminiferous tubules, rete testis, efferent ducts, and Wolffian duct develops in isolation, and yet somehow, they come together to make a continuous duct system that is so vital for spermatozoa to develop and mature. It is all about the connection. So much is devoted to focusing on the individual parts that it is easily forgotten that each is joined and function as one. In this brief overview, we will outline what is known and what is not known and offer a multitude of ideas (i.e., hypotheses) as to what we think might be happening during the morphogenesis of the rete testis, efferent ducts, and Wolffian duct, and how they connect. The focus will be on the mouse and humans, although other species will be highlighted where appropriate. In addition, we will address the clinical importance of these connections, primarily focusing on epididymal disjunction, the failure of the efferent ducts to connect to the testis.
‘BEFORE I CAN CONNECT I NEED TO UNDERGO TUBULOGENESIS, ELONGATE AND MIGRATE TOWARDS, AND JOIN THE CLOACA,’ SAYS THE WOLFFIAN DUCT
The renal (Wolffian/mesonephric/nephric duct) anlagen is derived from the intermediate mesoderm, and in human embryos at Carnegie Stage (CS) 11, the Wolffian ducts undergo mesenchymal–epithelial transitions to form two ducts (Jacob et al., 2012). Studies using chick and Xenopus have shown that the signals that define the fate of those mesenchymal cells in the intermediate mesoderm have been suggested to originate from the trunk paraxial mesoderm, lateral plate mesoderm, roof plate mesoderm, and the surface ectoderm. In addition, there appears to be coordinated growth between the development of the somites and Wolffian duct elongation (Obara-Ishihara et al., 1999; Atsuta & Takahashi, 2015; see also Takahashi et al., 2018). The cells that respond to those signals form a cord and loose contact with the surrounding intermediate mesoderm, coalesce, and migrate toward the cloaca. Studies have shown that retinoic acid from the surface ectoderm, bone morphogenic proteins (BMPs) from the lateral plate mesoderm, and activin from the roof plate mesoderm are signaling molecules that define the renal anlagen. They act through a number of genes forming a complex gene regulatory network, for example, forkhead box1/2 (Foxc1/2), Nodal, homeobox DNA-binding protein (Hox), HoxB4, HoxA6, paired-Box (Pax) Pax2/8, and LIM (LIM domain is a cysteine-rich zinc-binding domain) homeobox 1 (Lim1) (see Stewart & Bouchard, 2014; Marcotte et al., 2014; McMahon, 2016 for more detailed reviews). Similarly, gene networks have been defined for Wolffian duct tubulogenesis and its migration toward the cloaca (see Marcotte et al., 2014) and includes genes such as Pax2/8, Lim1, GATA-binding factor 3 (Gata3), empty spiracles homeobox 2 (Emx2), ectopic virus integration site 1 (Evi1), inhibitor of DNA-binding 4 (Id4), and placenta-specific 8 (Plac8). Examples of gene knockouts that result in the failure of the Wolffian duct to form and/or migrate are Pax2/8 and Gata3, respectively (see Dressler et al., 1993; Torres et al., 1995; Boualia et al., 2013).
Studies examining Wolffian duct migration in the chick have shown the importance of FGF signaling (Atsuta & Takahashi, 2015; Takahashi et al., 2018). The leader cells are characteristically mesenchymal, and during their migration, they stay that way as long as they are exposed to a high concentration of fibroblast growth factor 8 (FGF8), which is produced by the mesenchyme within the tail bud region. In addition, these cells are also attracted to FGF8, thereby allowing them to migrate in a defined direction. As the axis of the animal elongates, the most anteriorly positioned Wolffian duct cells move further away from the tail bud and are therefore exposed to lower concentrations of FGF8. These cells now undergo a mesenchymal-epithelial transition and form a lumen. The mechanisms of lumen formation are not known, but presumably it involves either cavitation and/or cord hollowing (see reviews by Lubarsky & Krasnow, 2003; Andrew & Ewald, 2010; Jewett & Prekeris, 2018).
The final stage of Wolffian duct elongation is its joining with the cloaca, and a recent study by Hoshi et al. (2018) has shown very nicely how controlled apoptosis regulates the joining of these two tissues. As the mouse Wolffian duct touches the cloaca, it induces a subset of cells within itself and in the cloaca to undergo apoptosis in a rearranged-during-transfection (Ret) and yes-associated protein (Yap)-signaling-dependent manner.
During the remaining embryonic period, the Wolffian duct undergoes extensive elongation and coiling (see reviews by Tomaszewski et al., 2007; Hinton et al., 2011; Hirashima, 2014; Murashima et al., 2015; Hinton & Avellar, 2018; Avellar & Hinton, 2018), which will not be discussed further because it is beyond the scope of this overview.
‘CONNECT WITH ME,’ SAYS THE RETE TESTIS TO THE EFFERENT DUCTS
As the mesonephric duct (Wolffian duct) migrates toward the cloaca, it induces a series of aggregates of mesenchyme that form renal vesicles, which then form S- or J-shaped tubules. One end of the tubule attaches to the Wolffian duct, and the other end forms a corpuscle with a Bowman’s capsule and glomerulus; this complete structure is very similar to the renal proximal tubule. Later, the most cranial and the most caudal mesonephric tubules undergo regression leaving a few in close proximity to the testis. The survival signals that allow those tubules opposite the testis to survive are unclear, but presumably, they originate from the testis itself. Some evidence to support this hypothesis is that in the humans, mesonephric tubular cells opposite the testis express the anti-apoptotic protein B-cell lymphoma 2 (BCL-2) whereas those tubules that underwent regression expressed the pro-apoptotic protein, tumor protein 53 (p53) (Carev et al., 2006). In addition, those tubules/cells that survive highly express the proliferation marker Ki-67, leading to the idea that the signals/morphogens originating from the testis are pro-proliferative and anti-apoptotic. Epidermal growth factor (EGF) is a putative candidate signal/morphogen given that it prevents cell death in the developing kidney. Alternatively, a gradient of FGF8, as observed in the chick, may be responsible for not only guiding the cells to the testis but may also act similar to that observed during the migration of the Wolffian duct to the cloaca, and then to form a lumen (see above; Atsuta & Takahashi, 2015). Therefore, the driving force for mesonephric tubule elongation may be a combination of migrating and dividing tip cells distally and dividing cells in the more proximal regions. Although Bcl-2 null mutant mice develop abnormal renal growth and polycystic kidney disease (Veis et al., 1993; Hammerman, 1998), studies have not been performed as to whether changes in the efferent ducts are observed. The human homolog of avian virus myelocytomatosis oncogene (N-myc) has also been implicated in the survival of mesonephric tubules (Malynn et al., 2000), and mesonephric tubule number has been shown to be dependent upon sonic hedgehog (Shh; Murashima et al., 2014). Table 1 outlines several gene mutations in which a mesonephric tubule phenotype is observed. Interestingly, the mesonephric tubules at mouse E10.5-12.5 are capable of transporting a number of organic ions and cations using both influx and efflux transporters (Lawrence et al., 2018). This study supports the hypothesis that the mesonephric tubules provide an early secretory role for the embryo. However, fluid secretion into the mesonephric tubules may also have a role during morphogenesis of the efferent ducts as observed in other tissues (Navis & Bagnat, 2015). It is certainly well-known that the ability of the efferent ducts to transport ions and water across its epithelium continues into and throughout adulthood.
Table 1.
Mouse models which show defects in mesonephric tubules (MT)
| Genes | Type of mutation |
Phenotype of the mutant |
References |
|---|---|---|---|
| Pax2 | KO | Absence of MT | Torres et al. (1995) |
| Pax2/8 | dKO | Absence of MT | Bouchard et al. (2002) |
| Gata3 | KO | Absence of MT | Grote et al. (2006) |
| Osr1 | KO | Absence of MT | Wang et al. (2005) |
| Wnt9b | KO | Absence of MT | Carroll et al. (2005) |
| Wt-1 | KO | Absence of caudal MT | Sainio et al. (1997) |
| Six-1 | KO | Absence of caudal MT | Kobayashi et al. (2007) |
| FGFR8 | T-Cre | Regression of cranial mesonephros | Kitagaki et al. (2011) |
| FGFR1/2 | T-Cre | Dysgenesis of MT | Kitagaki et al. (2011) |
| Pax3-Cre | Absence of MT | Poladia et al., (2016) | |
| Shh | KO | Numerous ectopic MT | Murashima et al. (2014) |
| c-ret | ret-k | Reduced number of MT | Schuchardt et al. (1996) |
| Fox1/2 | Foxc1/Mf1 ch, KO | Numerous ectopic MT | Kume et al. (2000) |
The formation of the efferent ducts in the chick, sheep, and bovine is different than that observed in rodents in that it appears to involve de novo formation of the mesonephric tubules from the Bowman’s capsule in the chick, and the mesonephric giant corpuscle in the sheep and bovine (Budras & Sauer, 1975; Zamboni & Upadhyay, 1982; Wrobel, 2001; Wrobel & Schimmel, 2001). In the case of the bovine, the mesonephric tubules develop as a secondary set of tubules, not from the primary set of mesonephric tubules as is the case for rodents (Wrobel & Schimmel, 2001). There are very few studies focusing on the development of the human mesonephric ducts/efferent ducts given the availability of material. However, using standard histological approaches, Jacob et al. (2012) showed very nicely the arrangement of the mesonephric tubules and their association with the Wolffian duct at their distal ends and their association with mesonephric corpuscles at their proximal ends in human embryonic mesonephros at CS14/15. It is suggested that the mesonephric tubules comprise a secretory and collecting part, of which the collecting part is more distal, closest to the testis and survives. The more distal region regresses, and the proximal region elongates eventually joining the testis (Jacob et al., 2012).
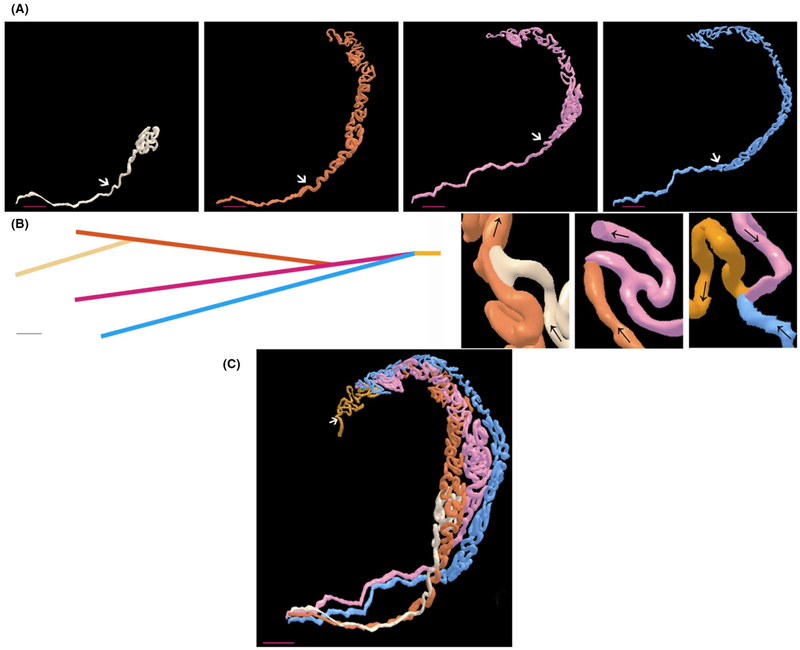
Three-dimensional imaging of the adult rodent efferent ducts has proven very helpful in understanding their morphogenesis, but they do not provide a clear understanding as to whether the individual tubules fuse with each other and/or undergo branching, which is characteristically observed during the morphogenesis of the prostate and kidney. The blind-ended tubules that are seen in the adult may provide clues. If the tubules fuse with one another but one or two fail to fuse at one end, then a blind-ended tubule will result. Possible evidence that the tubules have fused is shown in the study by Vazquez et al. (2003) who described the tubules as being ‘linked’ during mouse embryonic mesonephric tubule development. Interestingly, in their 3D reconstruction study, they also described the tubules as having either a downward or upward rotation, although reason for the rotation is unclear. If the tubules do undergo branching morphogenesis, then it is a little more difficult to reconcile how blind-ended tubules form. Lambot and colleagues (Lambot et al., 2009) performed 3D reconstruction of the efferent ducts and showed their characteristic branching/fusion pattern. Somewhat surprisingly, the efferent ducts do not appear to coil around each other but lie in parallel/closely aligned to each other. The ducts are highly coiled within themselves, although they are quite straight for a short distance, about 10 mm of their length, before they attach to the testis (Fig. 1).
Figure 1.
Three-dimensional models of the adult mouse efferent ducts. (A) Models of individual efferent ducts. The proximal region is the lower left side and the region that connects to the testis. The upper right side is the distal region and connects to the common duct/epididymis. The white arrows indicate the limit established arbitrarily between the convoluted region and the non-convoluted/straight region. Scale bar is 400 μm. (B) Diagram of the connections between individual efferent ducts. Colors are assigned according to the order in which they connect. First connection is between tan and orange ducts, second connection is between orange and pink ducts, and the third connection is between pink and blue ducts. The common duct, which results once all the connections are established, is shown in yellow. Scale bar is 2 mm. (C) Three-dimensional model of the whole efferent duct tree. The beginning of the epididymis is shown by the white arrow. Scale bar is 400 μm. Images are reproduced by kind permission of Dr. Gilbert Vassart (Lambot et al., 2009) and Wiley Press. The legend is modified from the original figure legend.
It should be pointed out that in humans, the majority of the caput region of the epididymis is primarily efferent ducts and not epididymal duct. The branching pattern of the human efferent ducts is quite complex with multiple efferent ducts connecting to the main epididymal duct (Yeung et al., 1991; Ilio & Hess, 1994).
The mechanism(s) by which tubules join the rete testis is/are also unclear, but may involve a similar mechanism observed when the Wolffian duct fuses with the cloaca (Hoshi et al., 2018; see above), or a virus type of fusion event. If we postulate the former event to occur, then cells at the tip of the mesonephric tubules and the cells of the rete testis undergo apoptosis as the mesonephric tubules approach the testis. The openings on each structure will then allow for fusion/joining. There are other potential mechanisms by which tubes connect as has been shown in Xenopus laevis, Drosophila melanogaster, Danio rerio, and Caenorhabditis elegans (see review by Kao, 2013). For example, as the tubes approach each other, there is considerable modification of the tight junctions within the epithelial cells resulting in a transitional mesenchymal type of cell that allows for invasion between the most distal ends of the tubes. Once the cells have invaded, they presumably undergo mesenchymal–epithelial transition, become polarized, and form a lumen. This type of invasive behavior has been observed during kidney development whereby the nephron precursor cells invade into the collecting duct epithelium (Georgas et al., 2009; Kao et al., 2012). It is unclear at this time as to the identification of the invasion cues, but Lhx1 (Lim1) and Bmp2 have been suggested to be involved in the fusion/invasion process (Georgas et al., 2009).
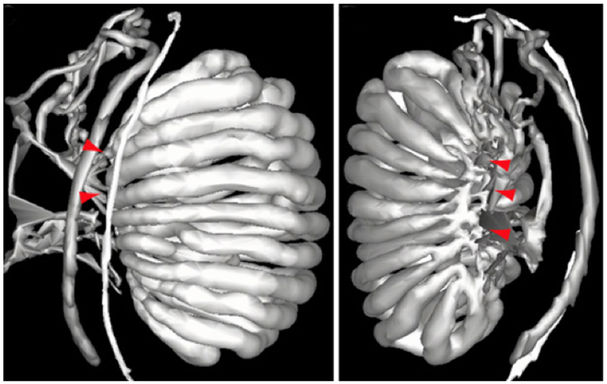
Perhaps the least studied tissue of the male reproductive system is the embryonic development of the rete testis. Combes et al. (2009) provided evidence that the rete testis initially forms as a flattened perforated interconnection between the mesonephric tubules and the testicular cords at approximately E15.5 in the mouse (see Fig. 2). Therefore, the subheading should be ‘Connect with me says the testis cords to the mesonephric tubules.’ In early post-natal development, the rete testis cells express Wilms’ tumor 1 (Wt1), which is a Sertoli cell marker, but the expression is lost between P25-38. However, rete testis cells do not express another Sertoli cell marker, double-sex and mab-3-related transcription factor 1 (Dmrt1) during early post-natal development (Malolina & Kulibin, 2017). As Sertoli cells are derived from the coelomic epithelium, then the data obtained from this study would suggest that rete testis cells are also derived from the coelomic epithelium and not the mesonephros. However, rete testis cells do not express Drmt1, so it is not entirely clear as to the origin of rete testis cells. Further, this was only one Sertoli cell marker that was used, and further markers are needed to draw a more definitive conclusion as to the origin of the rete testis cells.
Figure 2.
Two three-dimensional models from an E15.5 mouse embryo showing the connectivity between the testis cords and the mesonephric tubules. Connections are labeled with red arrowheads. It is at these points that a plexus forms with perforations, which then contracts and narrows to form the rete testis on the dorsomedial aspect of the testis. The images were kindly provided by Dr. Peter Koopman, with the left image (lateral view) being published (Combes et al., 2009) and reproduced by kind permission of Wiley Press.
‘CONNECT WITH ME,’ SAYS THE WOLFFIAN DUCT TO THE EFFERENT DUCTS
During the morphogenesis of the mesonephric tubules/efferent ducts, it is clear that the efferent ducts unite to form a single duct referred to as the common duct (Ilio & Hess, 1994), which joins the main epididymal duct. The mechanism(s) by which the cells of mesonephric tubules migrate toward the Wolffian duct and then fuse with the duct is/are not known, although Sprouty 2 (Spry2) may be a regulator (Chi et al., 2006). A histological study by Vetter & Gibley (1966) using embryonic mice showed very clearly the contact between the mesonephric tubules and the Wolffian duct. However, it did not provide clues as to how that joining occurred. Once the joining/fusion is complete, the renal vesicle then forms the characteristic J-/S-shaped tubules. Therefore, one can hypothesize that a signal promotes the migration of the cells from the renal vesicle toward the Wolffian duct, and elongation may occur via cell proliferation and cell intercalation similar to that observed during Wolffian duct elongation (Xu et al., 2016). Interestingly, the epithelium of the short distal region of the mesonephric tubule is similar to that seen in the Wolffian duct (see review by Sainio, 2003). This has led to the possibility that the efferent ducts may be derived from the mesonephric duct (Sainio et al., 1997).
‘IF WE FAIL TO CONNECT, WE HAVE A PROBLEM’
There are several clinical implications should there be failure of any part to connect. In particular, epididymal disjunction, the failure of the efferent ducts to join the rete testis, is found predominantly in men who were discovered to be cryptorchid at birth. A number of studies have shown that fusion anomalies associated with cryptorchidism are 32–79% of cases. However, this percentage includes minor as well as major fusion anomalies. For example, a minor fusion anomaly would be a disconnection between the caput and cauda from the testis with an intact connection between the testis and efferent ducts. Major fusion anomalies include complete separation between the testis and epididymis, that is, a failure of the efferent ducts to connect, and leading to complete separation of the testis and epididymis with epididymal atrophy (see Caterino et al., 2014). Fusion anomalies can be either unilateral or bilateral, presumably meaning that patients with a unilateral fusion anomaly would be fertile. Interestingly, it is only when an adult male patient undergoes explorative surgery, for example, for varicocoele repair, hydrocele, and/or infertility that the anomaly is found. Further, some studies have shown that epididymal fusion anomalies were observed more frequently in boys when the undescended testis was at a higher level (Kim et al., 2015; Favorito et al., 2017). The reason for this is unclear.
There are several case reports showing various degrees of severity of epididymal disjunction and other epididymal abnormalities. For example, in one study, a 3 year old who underwent orchidopexy had a minor fusion anomaly with separation of the caput and cauda from the testis. The connection between the testis and epididymis was intact but was discovered to have a long looping vas deferens, with both limbs of the vas deferens enclosed in a common sheath of connective tissue (Cundy & Goh, 2017). The mechanism by which this occurs is intriguing; long looping vas deferens occurs in approximately 20% of patients in which their undescended testes were found close to or within the inguinal canal (Miliaras et al., 1997). From these studies, it is apparent that epididymal anomalies associated with cryptorchidism are more complex than previously thought. The positioning of the testis and Wolffian duct within the abdomen has a surprising effect on the type of epididymal anomaly observed in some young patients.
There is now considerable evidence in laboratory animals and in humans exposed to environmental toxicants that an increase in the incidence of cryptorchidism is observed (see reviews by Guerrero-Bosagne & Skinner, 2014; Barthold et al., 2016 and Foster et al., 2017). One study using the rat showed a transgenerational affect when rats were exposed to di(2-ethylhexyl) phthalate (DHEP; Chen et al., 2015). An increase in the incidence of cryptorchidism in male offspring has also been observed in mothers that smoke (Yu et al., 2018). Although these studies have not shown a link between epididymal disjunction and cryptorchidism following exposure to environmental toxicants, we predict that link will be established in future studies.
CHALLENGES FOR THE FUTURE
It is clear how little we know regarding the mechanisms by which the male reproductive tract connects to make a ductular system capable of transporting spermatozoa from the testis to the vas deferens and to the ejaculatory duct. This is, in part, because we do not fully comprehend the morphogenesis of each part of the system. We have a better understanding of the tubulogenesis and morphogenesis of the Wolffian duct, but very limited understanding of the morphogenesis of the rete testis and mesonephric tubules/efferent ducts. Therefore, once we figure out how each part develops, then we can devote our efforts to identify those mechanisms by which the rete testis joins the mesonephric tubules, which in turn joins the Wolffian duct. The identification of genes, signaling pathways, and the use of live imaging to identify cell behaviors involved in the joining of each part will provide a major contribution as to how the development of the male reproductive system is regulated. In addition, the findings will also contribute to understanding the development of other tubular organs whereby several parts are united, for example, the kidney. It is hoped that from these studies we may finally discern how birth defects such as epididymal disjunction occur especially in those patients with a history of cryptorchidism, and the challenge will be in treating such patients.
ACKNOWLEDGEMENTS
This work was supported by a Eunice Shriver National Institute of Child Health and Human Development, NIH grant HD093703 (BTH) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001—PDSE, process n° 88881.187637/2018-01 (TMS).
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
REFERENCES
- Andrew DJ & Ewald AJ. (2010) Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol 341, 34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsuta Y & Takahashi Y. (2015) FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis. Development 142, 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avellar MCW & Hinton BT. (2018) Epididymis In: Encyclopedia of Endocrine Diseases, 2nd edn, Vol 2 (eds Huhtaniemi I & Martini L), pp. 807–813. Elsevier Inc, Amsterdam, Netherlands. [Google Scholar]
- Barthold JS, Reinhardt S & Thorup J. (2016) Genetic, maternal, and environmental risk factors for cryptorchidism: an update. Eur J Pediatr Surg 26, 399–408. [DOI] [PubMed] [Google Scholar]
- Boualia SK, Gaitan Y, Tremblay M, Sharma R, Cardin J, Kania A & Bouchard M. (2013) A core transcriptional network composed of Pax2/8, Gata3 and Lim1 regulates key players of pro/mesonephros morphogenesis. Dev Biol 382, 555–566. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubüser A & Busslinger M. (2002) Nephric lineage specification by Pax2 and Pax8. Genes Dev 16, 2958–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budras KD & Sauer T. (1975) Morphology of the epididymis of the cock (Gallus domesticus) and its effect upon steroid sex hormone synthesis. I. Ontogenesis, morphology and distribution of the epididymis. Anat Embryol (Berl) 148, 175–196. [DOI] [PubMed] [Google Scholar]
- Carev D, Krnić D, Saraga M, Sapunar D & Saraga-Babić M. (2006) Role of mitotic, pro-mitotic and anti-apoptotic factors in human kidney development. Pediatr Nephrol 21, 627–636. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A & McMahon AP. (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9, 283–292. [DOI] [PubMed] [Google Scholar]
- Caterino S, Lorenzon L, Cavallini M, Cavaniglia D & Ferro F. (2014) Epididymal-testicular fusion anomalies in cryptorchidism are associated with proximal location of the undescended testis and with widely patent process vaginalis. J Anat 225, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, Cao X, Zhou Y, Long C, Lin T, He D, Hua Y & Wei G. (2015) The mechanism of environmental endocrine disruptors (DHEP) induces epigenetic transgenerational inheritance of cryptorchidism. PLoS ONE 10, e0126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Itäranta P, Zhang S & Vainio S. (2006) Sprouty 2 is involved in male sex organogenesis by controlling fibroblast growth factor 9-induced mesonephric cell migration to the developing testis. Endocrinology 147, 3777–3788. [DOI] [PubMed] [Google Scholar]
- Combes AN, Lesieur E, Harley VR, Sinclair AH, Little MH, Wilhelm D & Koopman P. (2009) Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev Dyn 238, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundy TP & Goh DW. (2017) Beware the looping vas deferens on orchidopexy. Urology 104, 194–195. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L & Westphal H. (1993) Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature 362, 65–67. [DOI] [PubMed] [Google Scholar]
- Favorito LA, Julio-Junior HR & Sampaio FJ. (2017) Relationship between undescended testis position and prevalence of testicular appendices, epididymal anomalies, and patency of processus vaginalis. Biomed Res Int 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WG, Evans JA, Little J, Arbour L, Moore A, Sauve R, Andres Leon J & Luo W. (2017) Human exposure to environmental contaminants and congenital anomalies: a critical review. Crit Rev Toxicol 47, 59–84. [DOI] [PubMed] [Google Scholar]
- Georgas K, Rumballe B, Valerius MT, Chui HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP & Little MH. (2009) Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332, 273–286. [DOI] [PubMed] [Google Scholar]
- Grote D, Souabni A, Busslinger M & Bouchard M. (2006) Pax2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133, 53–61. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagne C & Skinner MK. (2014) Environmental epigenetics and effects on male fertility. Adv Exp Med Biol 791, 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerman MR. (1998) Regulation of cell survival during renal development. Pediatr Nephrol 12, 596–602. [DOI] [PubMed] [Google Scholar]
- Hinton BT & Avellar MCW. (2018) Wolffian duct development In: Encyclopedia of Reproduction, 2nd edn, Vol 1 (ed. Skinner MK), pp. 256–262. Elsevier Inc, Amsterdam, Netherlands. [Google Scholar]
- Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdell-Fattah R & Yang L. (2011) How do you get six meters of epididymis inside a human scrotum? J Androl 32, 558–564. [DOI] [PubMed] [Google Scholar]
- Hirashima T (2014) Pattern formation of an epithelial tube by mechanical instability during epididymal development. Cell Rep 9, 866–873. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Reginensi A, Joens MS, Fritzpatrick JAJ, McNeill H & Jain S. (2018) Reciprocal spatiotemporally controlled apoptosis regulates Wolffian duct cloaca fusion. J Am Soc Nephrol 29, 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilio KY & Hess RA. (1994) Structure and function of the ductuli efferentes: a review. Microsc Res Tech 29, 432–467. [DOI] [PubMed] [Google Scholar]
- Jacob M, Yusuf F& & Jacob HJ. (2012) Development, differentiation and derivatives of the Wolffian and Müllerian ducts In: The Human Embryo (ed. Yamada S), pp. 143–166. InTech, London, UK. [Google Scholar]
- Jewett CE & Prekeris R. (2018) Insane in the apical membrane: trafficking events mediating apicobasal epithelial polarity during tube morphogenesis. Traffic 19, 666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RM. (2013) The luminal connection. Organogenesis 9, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RM, Vasilyev A, Miyawaki A, Drummond IA & McMahon AP. (2012) Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol 23, 1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-O, Na SW, Yu HS & Kwon D. (2015) Epididymal anomalies in boys with undescended testis or hydrocele: significance of testicular location. BMC Urol 15, 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagaki J, Ueda Y, Chi X, Sharma N, Elder CM, Truffer E, Costantini F, Lewandoski M & Perantoni AO. (2011) FGF8 is essential for formation of the ductal system in the male reproductive tract. Development 138, 5369–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Kawakami K, Asashima M & Nishinakamura R. (2007) Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev 124, 290–303. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng K & Hogan BL. (2000) Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127,1387–1395. [DOI] [PubMed] [Google Scholar]
- Lambot MAH, Mendive F, Laurent P, Van Schoore G, Noël JC, Vanderhaeghen P & Vassart G. (2009) Three-dimensional reconstruction of efferent ducts in wild-type and Lgr4 knock-out mice. Anat Rec 292, 595–603. [DOI] [PubMed] [Google Scholar]
- Lawrence ML, Smith JR & Davies JA. (2018) Functional transport of organic ions and cations in the murine mesonephros. Am J Physiol 315, F130–F137. [DOI] [PubMed] [Google Scholar]
- Lubarsky B & Krasnow MA. (2003) Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28. [DOI] [PubMed] [Google Scholar]
- Malolina EA & Kulibin AY. (2017) Rete testis and the adjacent seminiferous tubules during postembryonic development in mice. Russian J Development Biol 48, 385–392. [Google Scholar]
- Malynn BA, de Alboran IM, O’Hagen RC, Bronson R, Davidson L, DePinho RA & Alt FW. (2000) N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev 14, 1390–1399. [PMC free article] [PubMed] [Google Scholar]
- Marcotte M, Sharma R & Bouchard M. (2014) Gene regulatory network of renal primordium development. Pediatr Nephrol 29, 637–644. [DOI] [PubMed] [Google Scholar]
- McMahon AP. (2016) Development of the mammalian kidney. Curr Top Dev Biol 117, 31–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliaras D, Vlahakis-Miliaras E, Anagnostopoulos D, Koutsoumis G, Pergamalis G & Miliaras S. (1997) Gross morphologic variations and histologic changes in cryptorchid testes. Pediatr Surg Int 12, 158–162. [PubMed] [Google Scholar]
- Murashima A, Akita H, Okazawa M, Kishigami S, Nakagata N, Nishinakamura R & Yamada G. (2014) Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm. Dev Biol 386, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashima A, Xu B & Hinton BT. (2015) Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl 17, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navis A & Bagnat M. (2015) Developing pressures: fluid forces driving morphogenesis. Curr Opin Genet Dev 32, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara-Ishihara T, Kuhlman J, Niswander L & Herzlinger D. (1999) The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Poladia DP, Kish K, Kutay B, Haines D, Kegg H, Zhao H & Bates CM. (2016) Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291, 325–339. [DOI] [PubMed] [Google Scholar]
- Sainio K (2003) Development of the mesonephric kidney In: The Kidney (eds Vize PD, Woolf AS & Bard JBL) pp. 75–86. Academic Press, Inc, Cambridge, MA. [Google Scholar]
- Sainio K, Hellstedt P, Kreidberg JA, Saxen L & Sariola H. (1997) Differential regulation of two sets of mesonephric tubules by WT-1. Development 124, 1293–1299. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Pachnis V & Costantini F. (1996) Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development 122, 1919–1929. [DOI] [PubMed] [Google Scholar]
- Stewart K & Bouchard M. (2014) Coordinated cell behaviours in early urogenital system morphogenesis. Semin Cell Dev Biol 36, 13–20. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kudo R, Tadokoro R & Atsuta Y. (2018) Coordination between body growth and tissue growth: Wolffian duct elongation and somitogenesis proceed in harmony with axial growth. Int J Dev Biol 62, 79–84. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D & Yao HH. (2007) Essential roles of inhibin Beta a in mouse epididymal coiling. Proc Nat Acad Sci USA 104,11322–11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Gómez-Pardo E, Dressler GR & Gruss P. (1995) Pax-2 controls multiple steps of urogenital development. Development 121, 4057–4065. [DOI] [PubMed] [Google Scholar]
- Vazquez MD, Bouchet P & Vize PD. (2003) Three-dimensional anatomy of mammalian mesonephroi In: The Kidney (eds Vize PD, Woolf AS & Bard JBL) pp. 87–92. Academic Press, Inc, Cambridge, MA. [Google Scholar]
- Veis DJ, Sorensen CM, Shutter JR & Korsmeyer SJ. (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240. [DOI] [PubMed] [Google Scholar]
- Vetter SMR & Gibley CW Jr. (1966) Morphogenesis and histochemistry of the developing mouse kidney. J Morphol 120, 135–156. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lan Y, Cho ES, Maltby KM & Jiang R. (2005) Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol 288, 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel K-H. (2001) Morphogenesis of the bovine rete testis: Extratesticular rete, mesonephros and establishment of the definitive urogenital junction. Anat Embryol (Berl) 203, 292–307. [DOI] [PubMed] [Google Scholar]
- Wrobel K-H & Schimmel M. (2001) Establishment of the urogenital junction in the male bovine embryo: an ultrastructural study. Anat Embryol (Berl) 104, 225–237. [DOI] [PubMed] [Google Scholar]
- Xu B, Washington AM, Domeneniconi RF, Souza ACF, Lu X, Sutherland A & Hinton BT. (2016) Protein tyrosine kinase 7 is essential for tubular morphogenesis of the Wolffian duct. Dev Biol 412, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG, Bergmann M & Schulz H. (1991) Organization of tubules in the human caput epididymidis and the ultrastructure of their epithelia. Am J Anat 191, 261–279. [DOI] [PubMed] [Google Scholar]
- Yu C, Wei Y, Tang X, Liu B, Long C, Lin T, He D, Wu S & Wei G. (2018) Maternal smoking during pregnancy and risk of cryptorchidism: a systematic review and meta-analysis. Eur J Pediatr 178, 287–297. [DOI] [PubMed] [Google Scholar]
- Zamboni L & Upadhyay S. (1982) The contribution of the mesonephros to the development of the sheep fetal testis. Am J Anat 165, 339–356. [DOI] [PubMed] [Google Scholar]