Abstract
Background.
Motoneuron loss is a severe medical problem that can result in loss of motor control and eventually death. We have previously demonstrated that partial motoneuron loss can result in dendritic atrophy and functional deficits in nearby surviving motoneurons, and that treatment with androgens can be neuroprotective against this dendritic atrophy. Exercise has been also been shown to be protective following a variety of neural injury models and, in some cases, is dependent on androgen action.
Objective.
In this study, we explored whether exercise shows the same neuroprotective effect on induced dendritic atrophy as that seen with androgen treatment.
Methods.
Motoneurons innervating the vastus medialis muscles of adult male rats were selectively killed by intramuscular injection of cholera toxin-conjugated saporin. Following saporin injections, some animals were allowed free access to a running wheel attached to their home cages. Four weeks later, motoneurons innervating the ipsilateral vastus lateralis muscle were labeled with cholera toxin-conjugated horseradish peroxidase, and dendritic arbors were reconstructed in three dimensions.
Results.
Dendritic arbor lengths of animals allowed to exercise were significantly longer than those not allowed to exercise.
Conclusions.
These findings indicate that exercise following neural injury exerts a protective effect on motoneuron dendrites comparable to that seen with exogenous androgen treatment.
Keywords: steroids, neuroprotection, morphology, dendrites
Introduction
Neurodegenerative disease or injury often results in the loss of spinal motoneurons. For example, motor neuron diseases (e.g., amyotrophic lateral sclerosis, ALS13) are characterized by progressive loss of motoneurons. The loss of spinal motoneurons results in muscle weakness, loss of muscular control, and can eventually cause complete paralysis.13 Alternatively, spinal cord injury33 or damage to spinal nerves resulting in laceration and avulsion of spinal roots (e.g., cauda equina injury35) can lead to the death of motoneurons and motor dysfunction.
Importantly, after such insults surviving motoneurons also show a variety of morphological and functional changes. For example, motoneurons undergo dendritic retraction and atrophy after injury (e.g., after spinal cord injury).10 Similarly, after peripheral axotomy, motoneurons show functional changes as well as dendritic atrophy.37,51
We have begun to examine the effects of motoneuron loss on the structure and function of surviving motoneurons using a rat model of motoneuron death. Our previous studies have demonstrated that surviving motoneurons respond to the loss of their neighbors with marked dendritic atrophy.11,12,32 This induced atrophy is responsible for at least some of the movement deficits that accompany degenerative movement disorders and spinal cord trauma, as it results in reduced excitability of the remaining motoneurons.32 Given that we currently lack the technology to replace dead motoneurons, developing the ability to protect surviving motoneurons from injury-induced atrophy is an important goal.
Treatment with gonadal steroid hormones promotes a wide array of neuroprotective and neurotherapeutic effects.20 In our model of induced motoneuron loss, treatment with exogenous testosterone attenuates dendritic atrophy, as well as the attenuated excitability, in motoneurons.12,32 This effect of androgens is mediated via classical receptor activation, and blockade of androgen receptors with flutamide completely prevents the neuroprotective effects from induced dendritic atrophy.11
Exercise has also been demonstrated to be neurotherapeutic in multiple models of neuronal injury and disease. For example, exercise is moderately effective in treating some symptoms of ALS. When compared to matched patients who did not exercise, patients assigned to a low-intensity, twice-daily exercise program designed to improve muscular endurance had reduced muscle spasticity and increased scores on the ALS functional rating scale after three months.16 It should be noted that reports of the clinical outcomes of exercise over time on ALS are mixed. This is partially due to the relatively small number of subjects and high dropout rates as the disease progresses, both of which contribute to difficulty conducting randomized clinical trials and reaching statistical significance.14
The therapeutic effects of exercise include indirect effects, such as reduction of neuroinflammation following spinal cord injury,43 and also more direct protective effects on the preservation of motoneuron dendritic structure.21 In addition, exercise can also induce upregulation of neurotrophic factors that promote neuroplasticity [e.g., brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF)].24,34,53 Following axotomy, exercise increases both the number of regenerating axons sprouting from the proximal stump of the axotomized fibular nerve and axon elongation in the injured peripheral nerve when compared to non-exercised animals.41 Androgens have been directly implicated in the positive effects of exercise after injury. For example, treadmill training results in both increases in serum testosterone levels and enhances axon regeneration after axotomy in male rats; castration prevents treadmill training effects on axon regeneration.59 In the same axotomy model, treatment of exercised animals with the androgen receptor antagonist flutamide reduces the median length of regenerating axons to lengths comparable with animals who did not exercise,50 suggesting that androgen receptor action is a necessary driver of the neuroprotective benefits of exercise following injury. It is thought that the exercise-induced increases in testosterone in turn upregulates BDNF and its receptor, trkB,18,50 both of which are regulated by androgens in motoneurons and their target muscles.38,55 This androgen-dependency of exercise’s neurotherapeutic effects following axotomy leads us to believe that exercise may be a viable substitute for exogenous androgen therapy following partial motoneuron loss. Thus, in the present study, we tested whether exercise was neuroprotective against induced dendritic atrophy following partial motoneuron depletion.
Methods
In rats, the quadriceps muscles of the thigh comprise the vastus lateralis, vastus medialis, vastus intermedialis, and the rectus femoris. These muscles are innervated by motoneurons located in column 3 of the lateral motor column in the L2 spinal segment36 and project via the femoral nerve to the four muscles of the quadriceps. The distribution of somata and dendritic arbors of the motoneurons innervating the individual muscles of the ipsilateral quadriceps complex overlap extensively in the lateral motor column,36 making it possible to partially and selectively deplete this motor population and study the effects of that depletion on the surviving motoneurons.
To examine whether exercise is neuroprotective on motoneuron dendrites following partial motoneuron depletion, motoneurons were selectively killed by intramuscular injection of the toxin saporin to the vastus medialis. Motoneurons innervating the adjacent vastus lateralis were later visualized via retrograde labeling and dendritic arbors were reconstructed in three dimensions.
Animals
Adult male Sprague-Dawley rats (approximately 100 days old; Envigo, Indianapolis) were maintained on a 12:12-hour light/dark cycle with unlimited access to food and water. We used the toxin saporin, conjugated to the cholera toxin B subunit, to kill motoneurons selectively. Saporin is a ribosome-inactivating protein; it kills cells by irreversibly inactivating ribosomes thereby halting protein synthesis.46 Cholera toxin-conjugated saporin is retrogradely transported from the site of injection and kills motoneurons innervating the injected musculature within 3–6 days.19
Rats were anesthetized with isoflurane, and motoneurons innervating the left vastus medialis muscle were selectively killed by intramuscular injection of cholera toxin-conjugated saporin (2 µl, 0.1%; Advanced Targeting Systems, Inc., San Diego, CA). Some rats were not treated further (n = 6), whereas others were allowed free access to exercise wheels (width = 4.375 in; diameter = 14.5 in; circumference = 45.5 in) attached to their home cages (n = 11) immediately following saporin injections. Wheel revolutions were tracked daily to ensure that rats were engaging in exercise for the duration of the recovery period. To control for potential effects of exercise, a group of intact animals (n = 4) was included, as well as a group of untreated animals who received no exercise (n = 5). Because some of the animals in the study (overall n = 26) were not included in all analyses due to histological or histochemical compromise, group sizes for each analysis are reported individually below.
Histochemical and Histological Processing
Four weeks after saporin injection, animals were reanesthetized, and the left vastus lateralis muscle (ipsilateral to the saporin-injected vastus medialis muscle in saporin animals) was exposed and injected with horseradish peroxidase (HRP) conjugated to the cholera toxin B subunit (BHRP; 2µL, 0.2%; Invitrogen, Carlsbad, CA). BHRP labeling permits population-level quantitative analysis of motoneuron somal and dendritic morphologies.31 Forty-eight hours after BHRP injection, a period that ensures optimal labeling of motoneurons,31 animals were weighed, given an overdose of urethane (approximately 0.25 g/100 g body weight), and perfused intracardially with saline followed by cold fixative (1% paraformaldehyde/1.25% glutaraldehyde). To confirm the specificity of the saporin injections, the vastus medialis and the vastus lateralis muscles were removed bilaterally immediately after perfusion and weighed. The lumbar portion of the spinal cord of each animal was removed, postfixed in the same fixative for 5 hours, and then transferred to sucrose phosphate buffer (10% w/v, pH 7.4) overnight for cryoprotection. Spinal cords were then embedded in gelatin, frozen, and sectioned transversely at 40 µm; all sections were collected into four alternate series. One series was stained with thionin for use in cell counts. For visualization of BHRP, the three remaining series were immediately reacted using a modified tetramethyl benzidine protocol, mounted on gelatin-coated slides, and counterstained with thionin.
Motoneuron Number and Morphology
Motoneuron Counts.
To identify the appropriate area within the lateral motor column for motoneuron counts in the unreacted series, we used a method similar to that of Little et al.32 Briefly, for each animal, the range of sections in which motoneurons labeled with BHRP after injection into the vastus lateralis muscle were present in the reacted series was identified. Motoneurons were then counted in the appropriate matching sections in the unreacted series. For each animal, estimates of the total number of motoneurons in the left and right lateral motor columns were obtained using the optical disector method using Stereo Investigator (MBF Bioscience, Williston, VT) as previously described.32 Counts were made at x937.5 under brightfield illumination. Quadriceps motoneurons are easily recognizable as large, darkly staining, multipolar cells.
A counting frame (110 µm × 80 µm) was moved systematically throughout an area of each ventral horn (~500 µm × 500 µm, defined by the actual distribution of BHRP-labeled somata) in each section within the identified range. Only motoneurons in which there was a clear nucleus and nucleolus were counted, provided they did not contact the forbidden lines of the counting frame; motoneuron nucleoli were counted as they appeared while focusing through the z-axis, and nucleoli in the first focal plane (i.e., “tops”) were excluded to avoid double counting. The length of the disector was approximately 16 µm, which was adequate for visualizing nucleoli in multiple focal planes. Motoneuron counts were derived from a mean of 11.08 sections spaced 480 µm apart and distributed uniformly through the entire rostrocaudal extent of the quadriceps motoneuron pool range. This sampling scheme produced an average estimated coefficient of error (CE) of .053. Cell counts for each animal were corrected for the proportion of sections sampled, and then expressed as a ratio (motoneuron number on the saporin-injected side relative to that on the untreated side) to quantify the magnitude of motoneuron depletion examined (untreated, n = 5; SAP, n = 6; SAP+EXERCISE, n = 10; intact+EXERCISE, n = 4).
Using similar methods, the number of BHRP-filled motoneurons was assessed in all sections of the reacted series through the entire rostrocaudal extent of their distribution for all animals. Counts of labeled quadriceps motoneurons were made under brightfield illumination, where somata could be visualized and cytoplasmic inclusion of BHRP reaction product confirmed (untreated, n = 5; SAP, n = 6; SAP+EXERCISE, n = 6; intact+EXERCISE, n = 4).
Dendritic Length.
For each animal, dendritic lengths in a single representative set of alternate series were measured under darkfield illumination (untreated, n = 5; SAP, n = 6; SAP+EXERCISE, n = 6; intact+EXERCISE, n = 4). Beginning with the first section in which BHRP-labeled fibers were present, labeling through the entire rostrocaudal extent of the quadriceps motoneuron dendritic arbor was assessed; BHRP labeling spanned a rostrocaudal distance of an average of over 3200 µm. BHRP-labeled fibers were traced in every third section (480 µm apart) in three dimensions using a computer-based morphometry system (Neurolucida, MBF Bioscience, Williston, VT) at a final magnification of x250. Average dendritic length per labeled motoneuron was estimated by summing the measured dendritic lengths of the series of sections, multiplying by three to correct for sampling, then dividing by the total number of labeled motoneurons in that series. This method does not attempt to assess the actual total dendritic length of labeled motoneurons,30 but it has been shown to be a sensitive and reliable indicator of changes in dendritic morphology after a variety of manipulations, including after the death of neighboring motoneurons.11,12,19,32
Dendritic Distribution.
To assess potential redistributions of dendrites across treatment groups, for each animal the composite dendritic arbor created in the length analysis was divided using a set of axes oriented radially around the center of the collective labeled somata. These axes divided the spinal cord into twelve bins of 30° each. The portion of each animal’s dendritic arbor per labeled motoneuron contained within each location was then determined. This method provides a sensitive measure of dendritic redistribution after injury.10
Dendritic Extent.
The comparability of BHRP labeling across groups was assessed by quantifying both the rostrocaudal and the radial extent of quadriceps motoneuron dendritic arbors. The rostrocaudal extent of the dendritic arbor was determined by recording the rostrocaudal distance spanned by labeled dendrites for each animal. The maximal radial extent of the arbor in the transverse plane was also measured for each animal, using the same radial axes and resultant 30° bins used for the dendritic distribution analysis: for each bin, the linear distance between the center of the quadriceps motor pool and the most distal BHRP-filled process was measured. Radial dendritic extent is independent of overall dendritic length and reflects the maximal linear distance (in the transverse plane) of BHRP transport to the most distal dendritic processes.
All procedures were performed in accordance with the Indiana University Animal Care and Use Guidelines. All data were analyzed by t-tests or analyses of variance followed by post hoc analyses using Fisher’s least significant difference (LSD). Digital light micrographs were obtained using an MDS 290 digital camera system (Eastman Kodak Company, Rochester, NY). Brightness and contrast of these images were adjusted in Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
Results
Running Wheel Revolutions
Animals ran consistently over the four weeks they were allowed access to running wheels, averaging 3920 ± 366 (mean ± SEM) revolutions per day (2.82 ± 0.26 miles). Injection of saporin had no effect on exercise, and average daily running wheel revolutions did not differ from those of intact animals [F(1,13) = 1.22, ns]. Overall, animals ran an average cumulative total of 83.97 ± 7.88 miles over the four weeks of ad lib exercise.
Muscle Weights
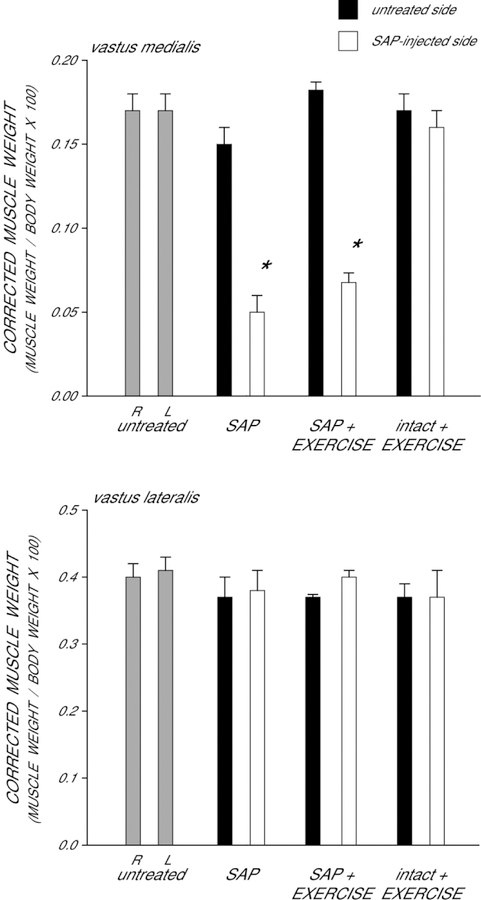
Differences in body weight were present across groups [F(3,22) = 12.15, p < 0.0001], and thus raw muscle weights were corrected for body mass to assess potential effects of saporin and/or exercise on muscle weight (Fig. 1). In untreated animals, the corrected weights of the right (0.17 ± 0.01) and left (0.17 ± 0.01) vastus medialis muscles were similar [t(4) = 2.14, ns]. Although the weights of the uninjected (right) vastus medialis muscles were not affected [F(3,22) = 2.73, ns], injection of saporin into the left vastus medialis resulted in notable muscle atrophy across the saporin groups [overall average of 68% reduction in weight; F(3,22) = 69.34, p < 0.0001]. Compared to those of untreated animals, saporin-injected animals had vastus medialis weights that were 74% lighter (LSD, p < 0.0001). Exercise did not prevent muscle weight loss; compared to those of untreated animals, saporin-injected rats allowed to exercise has vastus medialis weights that were 62% lighter (LSD, p < 0.0001). Muscle weights across saporin groups did not differ from each other (LSD, ns).
Figure 1.
Weights of the vastus medialis muscles corrected by body weight in untreated animals, saporin-injected animals that either received no further treatment (SAP) or were given ad lib exercise (SAP+EXERCISE), and intact animals given ad lib exercise (intact+EXERCISE) at four weeks after saporin injection. Gray bars represent weights from the right (R) and left (L) sides in untreated animals. Black bars represent weights from the untreated contralateral (right) side of the quadriceps muscle, and white bars represent weights from the saporin injected (left) side of the quadriceps muscle of saporin-injected animals. Saporin injection reduced the weight of the vastus medialis muscle; exercise had no effect on muscle weight. Bar heights represent means ± SEM. * indicates significantly different from untreated animals.
The effect of saporin injection on quadriceps weight was specific to the injected muscle. In untreated animals, the corrected weights of the right (0.40 ± .02) and left (0.41 ± .02) vastus lateralis muscles were similar [paired t-test, t(4) = .43, ns]. The weights of the vastus lateralis muscles on the untreated (right) side did not differ across groups [F(3,22) = .74, ns]. Most importantly, the weights of the vastus lateralis muscles adjacent of the saporin-injected vastus medialis muscles also did not differ across groups [F(3,22) = .41, ns]. Exercise had no effect on vastus lateralis muscle weight (LSDs, ns).
Motoneuron Counts
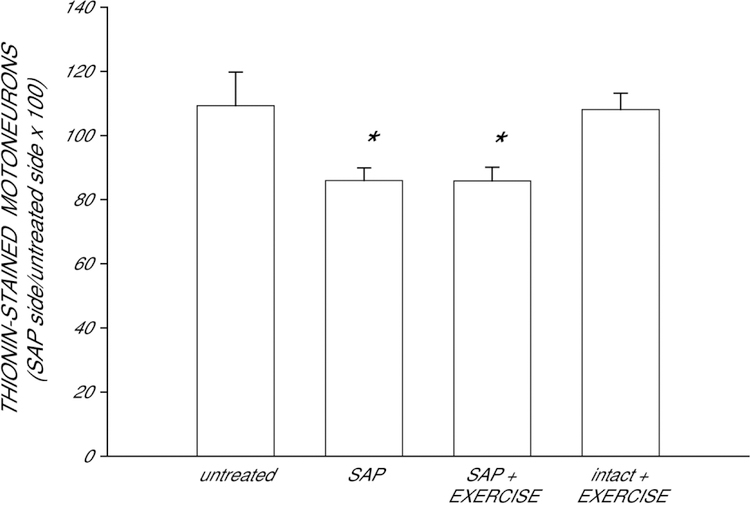
In untreated animals, the number of motoneurons within the identified quadriceps range did not differ between the left (251.2 ± 14.28) and right (237.6 ± 24.97) motor column [paired t-test, t(4) = 0.63, ns]. Motoneuron counts indicated that saporin was effective in inducing partial motoneuron depletion from the quadriceps motor pool (Fig. 2). Injection of saporin into the left vastus medialis muscle resulted in the death of ipsilateral quadriceps motoneurons, significantly reducing the number of motoneurons in the left motor column relative to that of the right [F(3,21) = 4.59, p < 0.02]. Unilateral injection of saporin into the left vastus medialis resulted in a 21% reduction in the relative number of motoneurons compared with that of untreated animals (LSD, p < 0.009). Exercise did not prevent this reduction (overall average of 21% reduced; LSDs, p < .009 compared to untreated animals). As expected, exercise had no effect on motoneuron number in intact animals (LSD, ns).
Figure 2.
Numbers of quadriceps motoneurons in untreated animals, saporin-injected animals that either received no further treatment (SAP) or were given ad lib exercise (SAP+EXERCISE), and intact animals given ad lib exercise (intact+EXERCISE) at four weeks after saporin injection, expressed as a ratio of motoneuron number ipsilateral to the saporin-injected muscle relative to that on the untreated side. Saporin killed approximately 21% of the ipsilateral quadriceps motoneurons, regardless of subsequent treatment. Bar heights represent means ± SEM. * indicates significantly different from untreated animals.
Motoneuron Morphometry
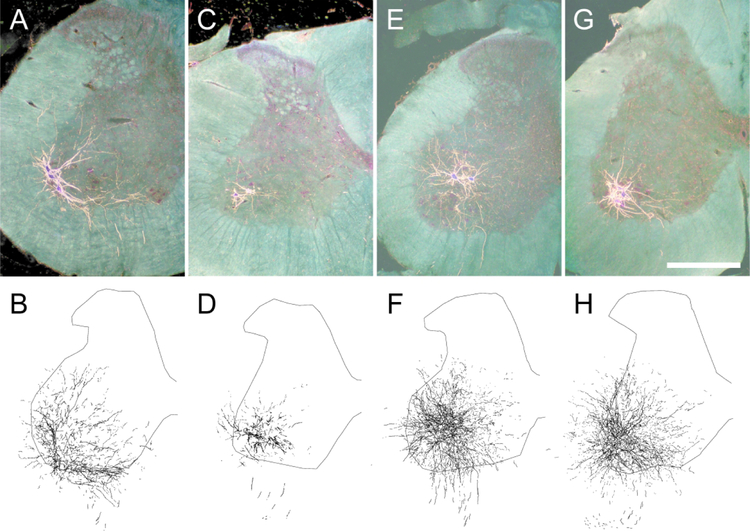
Injection of BHRP into the left vastus lateralis successfully labeled ipsilateral quadriceps motoneurons in all groups (Fig. 3). The dendritic arbor of quadriceps motoneurons was strictly unilateral, with extensive ramification along the ventrolateral margins of the gray matter and in the lateral funiculus, as well as throughout the ventral horn. An average of 36.95 (± 3.83) motoneurons per animal was labeled with BHRP, and this did not vary across groups [F(3,17) = 1.03, ns].
Figure 3.
Darkfield digital micrographs of transverse hemisections through the lumbar spinal cords and computer-generated reconstructions BHRP-labeled somata and processes of an untreated animal (A,B), and saporin-injected animals with either no further treatment (C,D) or given ad lib exercise (E,F), and an intact animal given ad lib exercise (G,H) after BHRP injection into the left vastus lateralis muscle. Computer-generated composites of BHRP labeling were drawn at 480 μm intervals through the entire rostrocaudal extent of the quadriceps motor pool; these composites were selected because they are representative of their respective group average dendritic lengths. Scale bar = 500 µm.
Dendritic Length.
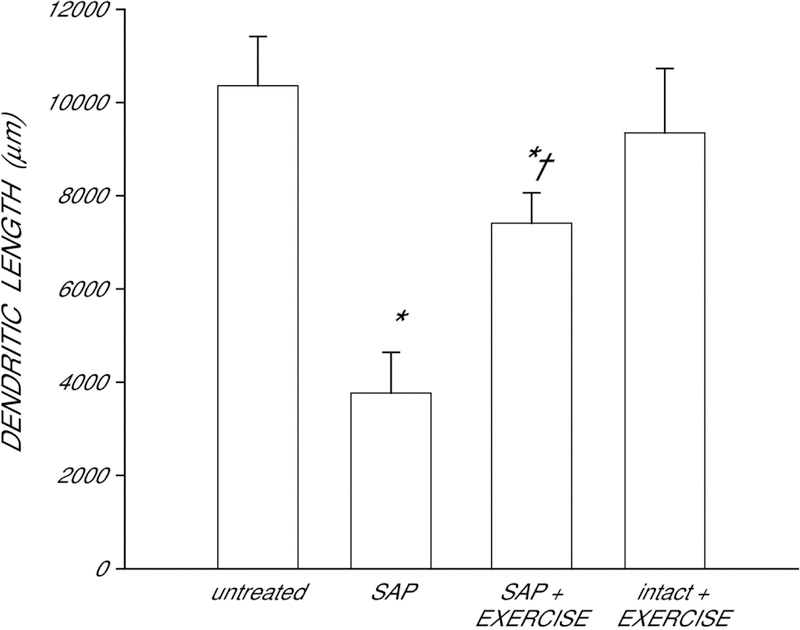
After saporin-induced motoneuron death, surviving neighboring quadriceps motoneurons underwent marked dendritic atrophy (Fig. 4). Dendritic length was decreased by 64% in saporin-injected animals compared to that of untreated animals [LSD, p < .0001; overall test for the effect of group on dendritic length F(3,17) = 9.67, p < .0006]. However, whereas dendritic length in saporin-injected animals who exercised were also shorter than that of untreated animals (LSD, p < 0.04), exercise attenuated dendritic atrophy, with dendritic length being reduced on average by only 28%. Compared with saporin animals who received no further treatment, saporin-injected animals who exercised had dendritic lengths were 97% longer (LSD, p < 0.02). Exercise in intact animals had no effect on dendritic length (LSD, ns).
Figure 4.
Dendritic lengths of quadriceps motoneurons in untreated animals, saporin-injected animals that either received no further treatment (SAP) or were given ad lib exercise (SAP+EXERCISE), and intact animals given ad lib exercise. Following saporin-induced motoneuron death, surviving neighboring motoneurons lost almost 64% of their dendritic length. Exercise attenuated this dendritic atrophy, but had no effect in intact animals. Bar heights represent means ± SEM. * indicates significantly different from untreated animals. † indicates significantly different from untreated saporin-injected animals.
Dendritic Distribution.
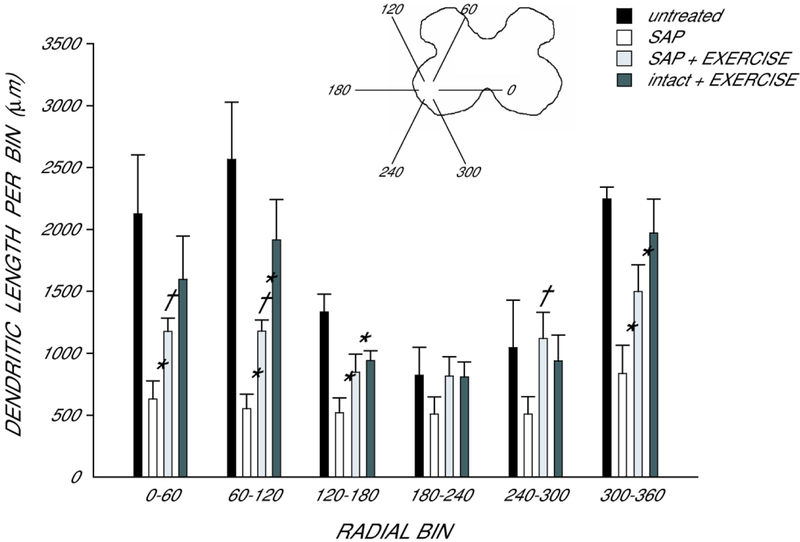
Dendritic length was non-uniform across radial bins, and a repeated measures ANOVA revealed a significant effect of radial location [F(11,187) = 13.46, p < 0.0001; Fig. 5]. Consistent with the results seen in total dendritic length analysis, there was also a significant effect of group [F(3,187) = 10.52, p < 0.0005]. There were reductions in dendritic length throughout the radial distribution, ranging from 38% (180° to 240°) to 79% (60° to 120°) in saporin-injected animals compared with untreated animals [F(1,99) = 26.19, p < .0007]. Saporin-injected animals allowed to exercise showed an attenuation of these reductions, with reductions in dendritic length ranging from no change (180°−300°) to 54% (60° to 120°) compared to untreated animals [F(1,99) = 9.44, p < .02]. Dendritic lengths per bin in exercised saporin-injected animals were longer than those of saporin-injected animals who received no further treatment [F(1,110) = 9.70, p < 0.02] throughout most of the radial distribution, with increases ranging from 61% (180°−240°) to 120% (240°−300°).
Figure 5.
Inset: Drawing of spinal gray matter divided into radial sectors for measure of quadriceps motoneuron dendritic distribution. Length per radial bin of quadriceps dendrites in untreated animals (black bars), saporin-injected animals that either received no further treatment (SAP, white bars) or were given ad lib exercise (SAP+EXERCISE, light gray bars), and intact animals given ad lib exercise (intact+EXERCISE, dark gray bars). For graphic purposes, dendritic length measures have been collapsed into 6 bins of 60° each. Quadriceps motoneuron dendritic arbors display a non-uniform distribution, with the majority of the arbor located between 300° and 120°. Following saporin-induced motoneuron death, surviving neighboring motoneurons had reduced dendritic length throughout the radial distribution. Exercise attenuated this reduction, but had no effect in intact animals. Bar heights represent means ± SEM. * indicates significantly different from untreated animals. † indicates significantly different from untreated saporin-injected animals.
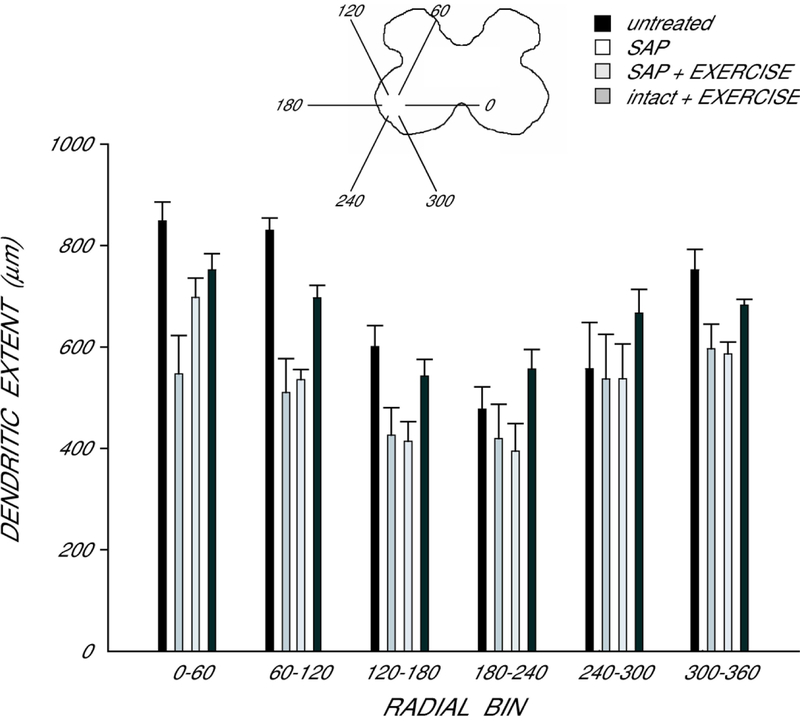
Dendritic Extent.
In agreement with the nonuniform dendritic distribution of quadriceps motoneurons apparent in Fig. 3, radial extent differed across bins (Fig. 6), and a repeated measures ANOVA revealed a significant effect of location [F(11,187) = 14.81, p < 0.0001]. However, radial dendritic extent did not differ across groups [F(3,187) = 2.10, ns]. Rostrocaudal dendritic extent also did not differ across groups [F(3,17) = 2.30, ns], ], spanning 3776.0 ± 546.3 µm in untreated animals, 3760.0 ± 402.1 µm in saporin-injected animals who received no further treatment, 2760.0 ± 336.0 µm in exercised saporin-injected animals, and 2700.0 ± 326.8 in exercised intact animals.
Figure 6.
Inset: Drawing of spinal gray matter divided into radial sectors for measure of quadriceps motoneuron radial dendritic extent. Radial extents of quadriceps dendrites in untreated animals (black bars), saporin-injected animals that either received no further treatment (SAP, white bars) or were given ad lib exercise (SAP+EXERCISE, light gray bars), and intact animals given ad lib exercise (intact+EXERCISE, dark gray bars). For graphic purposes, dendritic extent measures have been collapsed into 6 bins of 60° each. Extent measures did not differ across groups. Bar heights represent means ± SEM.
Discussion
Surviving motoneurons respond to the death of neighboring motoneurons with marked dendritic atrophy, and treatment with testosterone is protective to this atrophy.12,19,32 Exercise is neuroprotective following motoneuron injury (e.g., axotomy41,59), and these neurotherapeutic effects have been demonstrated to be both dependent on androgens59 and mediated by androgen receptor action.50 In this study, we explored whether exercise shows a similar neuroprotective effect against induced dendritic atrophy as that seen with androgen treatment. We observed that exercise attenuates induced dendritic atrophy following the death of nearby motoneurons. Furthermore, this attenuation of dendritic atrophy was of a comparable magnitude to that seen with exogenous testosterone treatment. We hypothesize that the similarity in protection from induced dendritic atrophy seen with both androgen treatment and exercise suggests that these treatments are acting through a common mechanism.
Specificity and Effectiveness of Saporin Injections
Consistent with our previous findings,11,12,32 saporin injection into the vastus medialis significantly decreased muscle weight (68%) and reduced the number of motoneurons in the quadriceps pool (21%). Intramuscular injection of saporin had no effect on the adjacent, un-injected vastus lateralis muscle and there were no differences in the number of labeled motoneurons following injection of BHRP into the vastus lateralis. Thus, saporin injections were effective at the targeted vastus medialis muscle and did not spread to the adjacent vastus lateralis muscle, an important consideration for interpreting the effects seen on the morphology of surviving motoneurons.
Exercise had no effect on preventing saporin-induced decreases in weight of the injected vastus medialis muscle, nor did it prevent saporin-induced motoneuron death. This is consistent with our previous findings concerning treatment with testosterone.12,19,32 Thus, the beneficial effects of exercise on the morphology of neighboring surviving motoneurons cannot be attributed to differences resulting from the degree of peripheral damage or an attenuation of the ability of saporin to kill motoneurons.
Although saporin injection was effective at both the targeted muscle and innervating motoneurons, it did not impair the ability of the saporin-treated rats to exercise. Running wheel performance in saporin-treated animals did not differ from that of intact animals, and animals in both groups ran an impressive overall cumulative distance of almost 84 miles over the four weeks of treatment. Thus, the exercise manipulation was successful, providing a neurotherapeutic treatment to the saporin-treated rats.
Causes and Protection from Dendritic Atrophy
Consistent with our previous studies, saporin-induced motoneuron death resulted in a pronounced dendritic atrophy in surviving nearby quadriceps motoneurons.11,12,32 We have previously ruled out that this dendritic atrophy is the result of the loss of afferent fibers from the saporin-injected muscle,11 or the pronounced increase in activated microglia in the quadriceps motor pool following saporin-induced motoneuron death.12 We have speculated that the induced death of motoneurons could result in the release of toxins [e.g., inflammatory cytokines (IL-6, IL-1β, TNF-α), purines (ATP), glutamate, and matrix metalloproteinases (MMPs)] into the extracellular space, in close proximity to the dendrites of the surviving motoneurons resulting in direct damage.12,19 Such local changes in the cellular microenvironment would be consistent with the general atrophy seen throughout the dendritic distribution.
These possible explanations as to the cause of the observed dendritic atrophy also provide possible explanations as to the mechanism of how exercise is conferring neuroprotective effects to the motoneurons. Exercise upregulates both antioxidant enzymatic activity39 and the presence of heat shock proteins in skeletal muscle.29,42 These upregulations are theorized to be adaptive mechanisms in response to the oxidative stress and other biochemical changes experienced during exercise, and thus could contribute to a neuroprotective effect on motoneuron dendrites.
Interestingly, many of these effects of exercise are also regulated by androgens. Testosterone regulates proteins thought to be involved in neuroprotection, including expression of heat shock proteins48,61 and proteins with antioxidant functions (e.g., catalase1). Furthermore, both testosterone27,55 and exercise15,22,53 have also been associated with upregulation of neurotrophic factors that promote neuroplasticity (e.g., BDNF) and the cytoskeletal protein β-tubulin in neurons.
The protection from induced dendritic atrophy in surviving quadriceps motoneurons obtained in the present study through ad lib exercise was remarkably similar to what we have previously reported through treatment with exogenous testosterone.11,12,32 It is tempting to speculate that the similarity in protection from induced dendritic atrophy in surviving motoneurons seen with both exercise and androgen treatment indicates that these treatments are acting through a common mechanism. Such a common mechanism has been hypothesized for the effects of exercise and androgens on axonal regeneration following axotomy (an “in series mechanism”50). As described above, androgens have been directly implicated in the positive effects of exercise after injury.18,50,59 Exercise has commonly been associated with testosterone and its role in anabolic muscle growth.8,9 Exercise training results in elevations in serum testosterone,28,45,59 although intensity, duration, prior conditioning of the subject, time point of measurement (e.g,. immediately after exercise, hours after exercise, at rest, etc.57), or the type of exercise training44,52 all modulate serum testosterone levels.
In addition to elevations in serum testosterone, skeletal muscle also contains enzymes that contribute to local steroidogenesis (e.g., 5α-reductase, aromatase2,5), and exercise upregulates the expression of these enzymes.3,4 We have previously speculated that the neuroprotective effects of androgens on motoneuron dendrites could be mediated through androgen receptors at the target musculature.11,12 Support for the target muscle as the site of androgenic action comes from a variety of sources.20 For example, motoneuron dendritic morphology is regulated by androgens acting at the target musculature.25 Androgen action at the target musculature has also been shown to regulate BDNF expression in motoneurons,55 and we have demonstrated that BDNF is critical in maintaining normal dendritic length in motoneurons.54 Thus, it is possible that both exercise and testosterone’s neuroprotective effects following partial motoneuron depletion are due to testosterone binding at the target muscle, signaling the muscle and/or the innervating motoneurons to produce trophic factors which protect motoneurons from dendrite atrophy.
It is also possible that exercise is neuroprotective via a mechanism that does not directly involve androgen signaling. As previously described, BDNF and other neurotrophins can regulate neurite plasticity and accompanying morphological changes. Neurotrophin signaling can be regulated by non-hormonal factors, e.g., electrical stimulation has been observed to increase BDNF expression in motoneurons.6 Thus, it is possible that exercise is neuroprotective due to the increased use and activation of the quadriceps motoneurons.
Exercise also upregulates biochemical substrates that promote vascularization [e.g., vascular endothelial growth factor (VEGF)47]. VEGF has also been implicated in promoting neurite outgrowth in centrally26 and peripherally23 located neurons, and has been shown to be neuroprotective following stroke,17 traumatic brain injury,49 and spinal cord injury58 (but see7). This neuroprotective function of VEGF may be an effect stemming from its role in angiogenesis. As VEGF promotes new vasculature formation, resources are more effectively able to be transported to the tissues undergoing reparative/restorative processes.40,60 Thus, exercise may upregulate VEGF and increased vascularization, which promotes neuroprotection of motoneuron dendrites.
Consequences of Dendritic Morphology on Motoneuron Function
Dendritic morphology has a direct effect on the electrophysiological responses of neurons.56 We have previously demonstrated that after saporin-induced motoneuron death, the resultant dendritic atrophy in remaining quadriceps motoneurons was accompanied by marked reductions in stimulation-evoked activation.32 In saporin-injected animals, stimulation of the dorsal root afferents to the quadriceps motoneurons produced responses in the peripheral nerve whose amplitudes were dramatically reduced compared with those of normal males.32 Both the atrophy in the morphology of quadriceps motoneuron dendrites and functional deficits were attenuated in testosterone-treated saporin animals, providing a functional measure of recovery.32
Comparability of BHRP Labeling
Our measure of dendritic length is dependent on both axonal and dendritic transport of BHRP, and it is possible that confounds arising from saporin injection or exercise could affect retrograde transport. This is an important consideration, as such an artifact could potentially result in artifactual alterations in dendritic morphology. However, no differences in either radial or rostrocaudal extents of quadriceps motoneuron dendrites across groups were observed. Therefore, we believe that the dendritic labeling across groups was comparable and that the shorter dendritic lengths we observed in saporin groups reflect dendritic atrophy.
Conclusion
In this study, we assessed whether exercise is neuroprotective to surviving motoneurons following the induced death of their neighbors. Our findings indicate that exercise is, in fact, neuroprotective to motoneurons following the death of their neighbors, and that the magnitude of this protective effect is comparable to that seen with testosterone treatment. Understanding the cellular and molecular mechanisms that operate in normal and injured neurons is likely to provide important insights for developing therapeutic interventions for nervous system trauma and neurodegenerative diseases. Thus, further research into the mechanism(s) of how exercise confers this neuroprotective effect to motoneurons may lead to the development and optimization of physical therapy regimens.
Acknowledgments
This work was supported by NIH-NINDS NS047264 to D.R.S.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- 1.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res 2001;892:255–262. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa K, Iemitsu M, Maeda S, Jesmin S, Otsuki T, Mowa CN, … Mesaki N. Expression of steroidogenic enzymes and synthesis of sex steroid hormones from DHEA in skeletal muscle of rats. Am J Physiol Endocrinol Metab 2007;292:577–584. [DOI] [PubMed] [Google Scholar]
- 3.Aizawa K, Iemitsu M, Maeda S, Mesaki N, Ushida T, Akimoto T. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med Sci Sports Exerc 2011;43:2072–2080. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa K, Iemitsu M, Maeda S, Otsuki T, Sato K, Ushida T, … Akimoto T. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids 2010;75:219–223. [DOI] [PubMed] [Google Scholar]
- 5.Aizawa K, Iemitsu M, Otsuki T, Maeda S, Miyauchi T, Mesaki N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J Appl Physiol 2008;104:67–74. [DOI] [PubMed] [Google Scholar]
- 6.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB rnRNA in regenerating rat femoral motoneurons. Eur J Neurosci 2000;12:4381–4390. [PubMed] [Google Scholar]
- 7.Benton RL, Whittemore SR. VEGF 165 therapy exacerbates secondary damage following spinal cord injury. Neurochem Res 2003;28:1693–1703. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci 2003;58:1103–1110. [DOI] [PubMed] [Google Scholar]
- 9.Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol 2001;170:27–38. [DOI] [PubMed] [Google Scholar]
- 10.Byers JS, Huguenard A, Kuruppu D, Liu N-K, Xu X-M, Sengelaub DR. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J Comp Neurol 2012;520:2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y, Chew C, Muñoz F, Sengelaub DR. Neuroprotective effects of testosterone metabolites and dependency on receptor action on the morphology of somatic motoneurons following the death of neighboring motoneurons. Dev Neurobiol 2017;77:691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew C, Kiley BJ, Sengelaub DR. Neuroprotective effects on the morphology of somatic motoneurons following the death of neighboring motoneurons: A role for microglia? Dev Neurobiol 2019;79:131–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2001;2:806–819. [DOI] [PubMed] [Google Scholar]
- 14.Bello‐Haas V Dal, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database of Syst Rev 2013;5:CD005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez‐Pinilla F. Exercise affects energy metabolism and neural plasticity‐related proteins in the hippocampus as revealed by proteomic analysis. Eur J of Neurosci 2006;24:1265–1276. [DOI] [PubMed] [Google Scholar]
- 16.Drory VE, Goltsman E, Reznik JG, Mosek A, Korczyn AD. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci 2001;191:133–137. [DOI] [PubMed] [Google Scholar]
- 17.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res 2013;4:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English AW, Wilhelm JC, Ward PJ. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology 2014;29:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fargo KN, Sengelaub DR. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J Comp Neurol 2004;469:96–106. [DOI] [PubMed] [Google Scholar]
- 20.Foecking EM, Fargo KN, Brown TJ, Sengelaub DR, Jones KJ. Gonadal steroids in regeneration and repair of neuromuscular systems. In: So KF, Xu X-M eds., Neural Regeneration London, England: Elsevier; 2015:129–152. [Google Scholar]
- 21.Gazula V, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol 2004;476:130–145. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 2002;88:2187–2195. [DOI] [PubMed] [Google Scholar]
- 23.Guaiquil VH, Pan Z, Karagianni N, Fukuoka S, Alegre G, Rosenblatt MI. VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. PNAS 2014;111:17272–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyorkos AM, McCullough MJ, Spitsbergen JM. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience 2014;257:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huguenard A, Fernando SM, Monks DA, Sengelaub DR. Overexpression of androgen receptors in target musculature confers androgen sensitivity to motoneuron dendrites. Endocrinol 2011;152:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol 2006;66:236–242. [DOI] [PubMed] [Google Scholar]
- 27.Jones KJ, Oblinger MM. Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J Neurosci 1994;14:3620–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kindermann W, Schnabel A, Schmitt WM, Biro G, Cassens J, Weber F. Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur J Appl Physiol Occup Physio 1982;49:389–399. [DOI] [PubMed] [Google Scholar]
- 29.Kregel KC. Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 2002;92:2177–2186. [DOI] [PubMed] [Google Scholar]
- 30.Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Neurobiol 1991;22:976–988. [DOI] [PubMed] [Google Scholar]
- 31.Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science 1986;232:395–398. [DOI] [PubMed] [Google Scholar]
- 32.Little CM, Coons KD, Sengelaub DR. Neuroprotective effects of testosterone on the morphology and function of somatic motoneurons following the death of neighboring motoneurons. J Comp Neurol 2009;512:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XZ, Xu XM, Hu R, Du C, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 1997;17:5395–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullough MJ, Gyorkos AM, Spitsbergen JM. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 2013;240:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moschilla G, Song S, Chakera T. Post-traumatic lumbar nerve root avulsion. Austral Radiol 2001;45:281–284. [DOI] [PubMed] [Google Scholar]
- 36.Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol 1983;217:75–85. [DOI] [PubMed] [Google Scholar]
- 37.O’Hanlon GM, Lowrie MB. Nerve injury in adult rats causes abnormalities in the motoneuron dendritic field that differ from those seen following neonatal nerve injury. Exp Brain Res 1995;103:243–250. [DOI] [PubMed] [Google Scholar]
- 38.Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB expression in spinal motoneurons. J Neurosci Res 2007;85:303–309. [DOI] [PubMed] [Google Scholar]
- 39.Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol Regul Integr Comp Physio 1994;266:375–380. [DOI] [PubMed] [Google Scholar]
- 40.Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue—beyond blood vessels. Exp Neurol 2004;187:246–253. [DOI] [PubMed] [Google Scholar]
- 41.Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol 2008;211:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med 1991;11:239–246. [DOI] [PubMed] [Google Scholar]
- 43.Sandrow-Feinberg HR, Houlé JD. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res 2015;1619:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato K, Iemitsu M. Exercise and sex steroid hormones in skeletal muscle. J Steroid Biochem Mol Biol 2015;145:200–205. [DOI] [PubMed] [Google Scholar]
- 45.Spiering BA, Kraemer WJ, Vingren JL, Ratamess NA, Anderson JM, Armstrong LE, Niedl BC, Volek JS, Häkkinen K, Maresh CM. Elevated endogenous testosterone concentrations potentiate muscle androgen receptor responses to resistance exercise. J Steroid Biochem Mol Biol 2009;114:195–199. [DOI] [PubMed] [Google Scholar]
- 46.Stirpe F, Barbieri L, Battelli MG, Soria M, Lappi DA. Ribosome-inactivating proteins from plants: present status and future prospects. Bio/Technology 1992;10:405–412. [DOI] [PubMed] [Google Scholar]
- 47.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 2003;117:1037–1046. [DOI] [PubMed] [Google Scholar]
- 48.Tetzlaff J, Tanzer L, Jones K. Exogenous androgen treatment delays the stress response following hamster facial nerve injury. J Neuroendocrinol 2007;19:383–389. [DOI] [PubMed] [Google Scholar]
- 49.Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab 2010;30:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson NJ, Sengelaub DR, English AW. Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Dev Neurobiol 2014;74:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol 1990;35:1–51. [DOI] [PubMed] [Google Scholar]
- 52.Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol 2004;96:531–539. [DOI] [PubMed] [Google Scholar]
- 53.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair 2005;19:283–295. [DOI] [PubMed] [Google Scholar]
- 54.Verhovshek T, Sengelaub DR Trophic effects of BDNF blockade in an androgen-sensitive neuromuscular system. Endocrinol 2010;151:5337–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhovshek T, Sengelaub DR. Androgen action at the target musculature regulates brain-derived neurotrophic factor protein in the spinal nucleus of the bulbocavernosus. Dev Neurobiol 2013;73:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vetter P, Roth A, Häusser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol 2001;85:926–937. [DOI] [PubMed] [Google Scholar]
- 57.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training. Sports Med 2010;40:1037–1053. [DOI] [PubMed] [Google Scholar]
- 58.Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience 2003;120:951–960. [DOI] [PubMed] [Google Scholar]
- 59.Wood K, Wilhelm JC, Sabatier MJ, Liu K, Gu J, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobio 2012;72:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1–42 toxicity through heat shock protein 70. J Neurosci 2004;24:5315–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]