Dear Editor
Accumulating evidence indicates that sleep-dependent memory consolidation of both procedural and declarative memories is impaired in schizophrenia (SZ) (Manoach et al., 2004; Manoach et al., 2016). However, it is unclear if these impairments are specific to SZ, or are also present in other disorders with psychosis. Bipolar disorder (BD) frequently presents with psychotic symptoms and displays cognitive deficits similar to those seen in SZ (Lewandowski et al., 2011). Moreover, SZ and BD are characterized by overlapping brain abnormalities (Yuksel et al., 2012), including in hippocampal-prefrontal cortex connectivity (Benson et al., 2014), which has recently been shown to be associated with procedural memory consolidation deficit in SZ (Genzel et al., 2015). We therefore investigated overnight procedural memory consolidation in patients with psychotic BD using a motor sequence task (MST), and compared their performance to healthy controls (HC) and patients with a SZ diagnosis.
Patients were inpatients at the McLean Hospital Schizophrenia and Bipolar Disorder Unit diagnosed with BD with psychotic features (n=29) or SZ (n=21). All but four patients were taking antipsychotic medications. Data for HC are taken from previous studies (n=15 from Djonlagic et al., 2012; n=17 from Wamsley et al., 2012). To better match the ages of HCs to the BD and SZ, we created two control subgroups. For BD controls (HCBD) we omitted the 10 oldest male HCs (n = 22), and for SZ controls (HCSZ) we omitted the 10 oldest HCs, regardless of sex (n = 22).
Exclusion criteria for patients and HC, methods used for diagnosis and symptom assessment, demographic comparisons and clinical characteristics between groups can be found in Supplementary Materials.
We administered the motor sequence task (MST)as previously described (Wamsley et al., 2012). In brief, participants were instructed to press “4–1-3–2-4” on the keyboard “as quickly and accurately as possible” with their left hand for each of twelve 30-sec trials, separated by 30-sec rest periods (see Supplementary Materials for details). Participants performed the MST twice: first during a training session, then during a test session the following day. Patients’ sleep was monitored by wrist actigraphy (Mini-Mitter Actiwatch-64).
The primary outcome measures were: (1) practice-dependent learning (PDL) during training and (2) overnight improvement (OI) from training to the next day’s test session. PDL was calculated as the percent increase in the number of correctly typed sequences from the first trial to the average of the last three trials in the training session. OI was calculated as the percent increase in the number of correct sequences from the last three training trials to the first three test trials. Details of statistical analyses are provided in Supplementary Materials.
PDL was seen during training for all groups (BD: 56.9 ± 66%, t = 3.9, p = 0.001; HCBD: 71.8 ± 80%, t = 4.5, p < 0.001; HCSZ: SZ: 62.4 ± 66%, t = 4.1, p < 0.001; HCSZ: 51.9 ± 60%, t = 3.8, p = 0.001), with no significant differences between groups (BD vs. HCBD: t = 0.64, p = 0.52, BD vs. SZ: t = 0.26, p = 0.80; SZ vs. HCSZ: t = 0.51, p = 0.61; Figure 1A).
Figure 1.
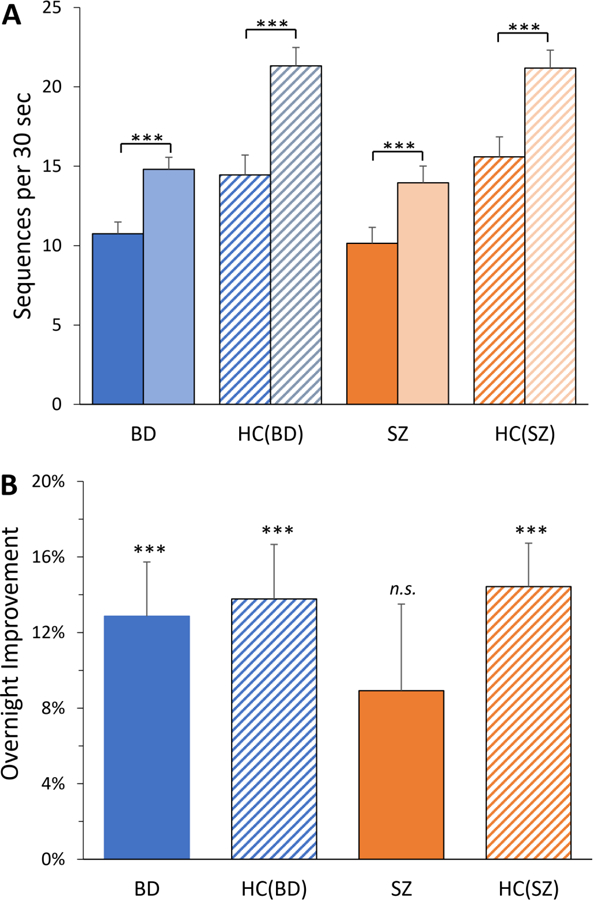
MST performance. (A) Performance at start (first trial; solid and hatched bars) and end (average of last 3 trials; lighter bars) of training session. (B) Overnight improvement in number of correctly typed sequences, from the last 3 trials of evening training to the first 3 trials of the test in the morning, presented as percent increase. Error bars = SEM.
BD and the two control groups (HCBD and HCSZ) showed significant OI of 13–16% (BD: 13.3 ± 16%, t = 4.2, p < 0.001; HCBD: 14.2 ± 14%, t = 4.8, p < 0.0001; HCsz: 15.7 ± 14%, t = 5.1, p < 0.0001), while improvement in SZ failed to reach significance (9.1 ± 21%; t = 1.9, p = 0.07; Figure 1B). OI did not differ significantly between BD and HCBD (F = 0.04, p = 0.85); there were also no significant differences in other comparisons (BD vs. SZ: F = 2.1, p = 0.15; SZ vs. HCSZ: F = 1.3, p = 0.25). Additional exploratory analyses are included in the Supplementary Materials.
This is the first study to investigate overnight memory consolidation in BD. We included HCs and patients with SZ as control groups. Patients with BD exhibited significant overnight improvement in MST performance (p = 0.003), comparable to overnight improvement in HCs (13% vs. 14%, p = 0.83). The overnight improvement in SZ (9%) was larger than that reported in several earlier studies (−1% in Genzel et al., 2011; −3% in Manoach et al., 2004; 5% in Manoach et al., 2010; 2% in Wamsley et al., 2012). The reason for this difference is unclear, although our SZ group was younger than those in prior studies, and younger age is associated with larger improvement in our sample. Our study has several limitations that are described in detail in Supplementary Materials. Notwithstanding these limitations, our findings clearly demonstrate that BD with psychotic features shows normal overnight improvement on the MST, indicating that the deficit in overnight memory consolidation previously reported for SZ is not present across all disorders with psychosis.
Supplementary Material
5. References
- Benson BE, Willis MW, Ketter TA, Speer A, Kimbrell TA, Herscovitch P, George MS, Post RM, 2014. Differential abnormalities of functional connectivity of the amygdala and hippocampus in unipolar and bipolar affective disorders. Journal of affective disorders 168, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A, 2012. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PloS one 7(3), e34106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Ali E, Dresler M, Steiger A, Tesfaye M, 2011. Sleep-dependent memory consolidation of a new task is inhibited in psychiatric patients. Journal of psychiatric research 45(4), 555–560. [DOI] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Cornu M, Jager E, Konrad B, Adamczyk M, Friess E, Steiger A, Czisch M, Goya-Maldonado R, 2015. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biological psychiatry 77(2), 177–186. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Keshavan MS, Ongur D, 2011. Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophrenia research 133(1–3), 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R, 2004. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biological psychiatry 56(12), 951–956. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, Stickgold R, 2016. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol. Psychiat 80(8), 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R, 2010. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. Journal of psychiatric research 44(2), 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS, 2012. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biological psychiatry 71(2), 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel C, McCarthy J, Shinn A, Pfaff DL, Baker JT, Heckers S, Renshaw P, Ongur D, 2012. Gray matter volume in schizophrenia and bipolar disorder with psychotic features. Schizophrenia research 138(2–3), 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


