Abstract
Background
Many respiratory conditions have been attributed to toxic dust and fume exposure in World Trade Center (WTC) rescue and recovery workers, who frequently report symptoms of OSA. We examined the prevalence of new-onset OSA and tested if the prevalence and severity of OSA are related to the presence of chronic rhinosinusitis (CRS).
Methods
A total of 601 subjects (83% men; age, 33-87 years; BMI, 29.9 ± 5.5 kg/m2) enrolled in the WTC Health Program, excluding those with significant pre-September 11, 2001, snoring or prior CRS, underwent two nights of home sleep testing. OSA was defined as Apnea Hypopnea Index 4% ≥ 5 events/h or respiratory disturbance index of ≥ 15 events/h. CRS was assessed using nasal symptom questionnaires.
Results
The prevalence of OSA was 75% (25% no OSA, 46% mild OSA, 19% moderate OSA, and 10% severe OSA), and the prevalence of CRS was 43.5%. Compared with no CRS, new and worsening CRS was a significant risk factor for OSA with an OR of 1.80 (95% CI, 1.18-2.73; P = .006) unadjusted and 1.76 (95% CI, 1.08-2.88; P = .02) after adjustment for age, BMI, sex, gastroesophageal reflux disorder, and alcohol use.
Conclusions
The high prevalence of OSA in WTC responders was not explained fully by obesity and sex. Possible mechanisms for the elevated risk of OSA in subjects with CRS include increased upper airway inflammation and/or elevated nasal/upper airway resistance, but these need confirmation.
Key Words: chronic rhinosinusitis, nasal symptoms, OSA, WTC exposure
Abbreviations: 9/11, September 11, 2001; AHI, Apnea Hypopnea Index; ARES, Apnea Risk Evaluation System; CRS, chronic rhinosinusitis; EPOS, 2012 European Position Paper on Rhinosinusitis and Nasal Polyps; GERD, gastroesophageal reflux disorder; RDI, respiratory disturbance index; WTC, World Trade Center; WTCHP, WTC Health Program
After the World Trade Center (WTC) disaster on September 11, 2001 (9/11), an estimated 40,000 individuals were exposed to significant amounts of dust while working in rescue, recovery, and debris removal.1 A significant number of these subjects reported new-onset or worsening nasal and upper airway2, 3 symptoms and sleep complaints suggestive of OSA.4 In addition, we have previously shown OSA was present in 96% of WTC responders with chronic rhinosinusitis (CRS) and/or gastroesophageal reflux disorder (GERD) who were referred to our sleep center and who reported their snoring began immediately after their WTC exposure. This unusually high prevalence of presumed new-onset OSA was not related to obesity, the usual major risk factor for OSA,5 implying other mechanisms must be important in the pathogenesis of OSA in these subjects.
OSA is a chronic condition with recurrent episodes of partial or complete upper airway collapse during sleep. The main risk factors for OSA are obesity, aging, and male sex. OSA is highly prevalent in the general population, with estimates ranging from 34% to 50% in men and 17% to 23% in women.6, 7 Upper airway inflammation from exposure to toxic dust/fumes resulting in mucosal congestion could provide an alternate mechanism for development of compromised upper airway patency during sleep. There is evidence in the literature for chronic nasal obstruction/congestion as a risk factor for snoring8, 9; however, evidence relating this to OSA is limited and inconsistent.9, 10
The WTC responder cohort provides an opportunity to explore the relationship between CRS and OSA because both conditions were prevalent. OSA risk in WTC subjects, estimated by questionnaire,4 has been related to dust exposure and, in symptomatic subjects referred to a sleep clinic for OSA evaluation, appears to increase with comorbid CRS and GERD.11
In the present study (WTCSNORE [Obstructive Sleep Apnea in WTC Responders: Role of Nasal Pathology]), we recruited subjects without evidence of pre-9/11 OSA to determine the prevalence of new-onset OSA and examine the association of post-9/11 OSA and CRS.
Methods
Subjects for this study were drawn from those enrolled in the WTC Health Program (WTCHP) Clinical Centers of Excellence at the Environmental and Occupational Health Sciences Institute of Rutgers Biomedical and Health Sciences, the New York University School of Medicine at Bellevue Hospital, and the Icahn School of Medicine at Mount Sinai Hospital. Preliminary findings have been presented as posters/abstracts at American Thoracic Society international conferences.
The study protocol was approved by the institutional review boards of Rutgers Biomedical and Health Sciences (Pro2012002164), New York University School of Medicine (I12-02578) and the Icahn School of Medicine at Mount Sinai (HS#16-00511), and all subjects signed informed consent.
Study Population and Clinical Evaluation
There were 634 subjects with documentation of no history of snoring prior to 9/11 from a multidimensional interviewer-administered exposure and snoring symptom questionnaire administered at the time of first enrollment to the WTC responder clinic.
Inclusion Criteria
Inclusion criteria included all patients seen in WTC responder clinics, or those previously seen and listed in the database.
Exclusion Criteria
Exclusion criteria included the following: (1) gross skeletal alterations affecting the upper airway (eg, micrognathia), (2) unstable chronic medical conditions known to affect OSA (eg, congestive heart failure, stroke), (3) pregnancy or intent to become pregnant within the period of the protocol, (4) inability to sign informed consent form, and (5) habitual snorer or diagnosis of OSA prior to 9/11.
Demographic Information: WTC Occupational Exposure Assessment and Comorbidities
Information collected after recruitment to the study included age, sex, BMI, weight 3 years prior, current and past smoking, and current regular or intermittent alcohol use. Current medication use, including nasal and oral steroid use or use of nasal or oral decongestants, were also documented. WTC exposure level was obtained from the General Responder Date Center and classified as very high, high, intermediate, or low.3
Diagnosis of comorbid conditions including GERD, posttraumatic stress disorder, depression, anxiety, hypertension, diabetes, and obstructive airways disease was obtained either through questionnaires at time of recruitment (self-reported) or from the General Responder Date Center as a WTC-certified condition.
Snoring
Snoring was assessed by questionnaire at two time points. The questionnaire was adapted from standardized instruments for specific use after exposure to WTC dust to describe symptoms by severity, relation to 9/11, persistence, and recent occurrence. On entry into the WTCHP (mostly 2003-2004), a snoring questionnaire (WTC Visit 1 Snoring Questionnaire) completed by all WTC responders, assessed frequency of snoring in the year before 9/11 and in the month prior based on what others had told them. An ascending score of 0 to 4 was used for frequency of snoring. Questions included frequency of snoring (0 = never or no more than a few times in the year, 1 = sometimes, a few nights per month, 2 = at least once a week, 3 = 3-5 nights/wk, and 4 = 6-7 nights/wk). On entry into WTCSNORE, current snoring was ascertained using responses to the following questions, developed for use in the Sleep Heart Health Study: Have you ever been told you snored? and How often do you snore? Snoring was considered to be present (positive) if it was reported to occur > 3 nights/wk and was used to compare with previous snoring. Responders with habitual snoring prior to 9/11 were defined as those with a score of 3 to 4 in the year prior to 9/11. Responders with new-onset habitual snoring were those with a score of ≤ 2 in the year prior to 9/11 and who then went on to manifest a score of 3 to 4 after 9/11 in the month prior to the initial screening evaluation. Nonsnorers were those with a snoring score of ≤ 2 both prior to and after 9/11.
Subjective Assessment of Nasal Symptoms
A nasal symptoms score was assigned to each subject based on the following current symptoms. The symptoms must have been present for > 8 weeks and be unrelated to an infection, with or without associated symptoms of sinusitis, pharyngitis, or laryngitis. Questions addressed nasal congestion or stuffy nose, sneezing, blocked nose, loss of smell, facial pain or sinus pressure, sore throat or hoarseness, and postnasal drip. Presence of three of more symptoms was considered positive for CRS.12 We also separately redefined CRS based on the 2012 European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) for epidemiologic studies, which defined CRS as the presence of two or more of the following symptoms: (1) nasal blockage/obstruction/congestion, (2) nasal discharge (anterior/posterior nasal drip), (3) facial pain or pressure, and (4) reduction or loss of smell. At least one symptom had to be nasal blockage or nasal discharge.13 For the purpose of analysis regarding a relationship to OSA, nasal symptoms with onset (less than three prior to more than three current) or worsening (more than three prior to a value higher that noted prior) after 9/11 were defined as new or worsening CRS. We performed analysis using both the WTC definition and the EPOS definition of CRS. Current medication use, including nasal and oral steroid use or use of nasal or oral decongestants, was documented.
Subjective Assessment for Sleep Disorders
After recruitment to the study, we used a sleep questionnaire to evaluate for sleep disorders (insomnia, RLS, circadian rhythm disorders) and the Epworth Sleep Scale, a well-validated questionnaire that asks the subject to rate the likelihood of falling asleep in eight commonly encountered situations.
Home Monitoring for OSA
Subjects were given an Apnea Risk Evaluation System (ARES Unicorder; SleepMed Inc) to take home and wear for 2 nights, with a preaddressed mailer to return the device to the sleep laboratory. The ARES Unicorder is worn on the forehead and does not require additional wires to external devices. It measures oxygen saturation and pulse rate from reflectance oximetry, airflow from a nasal cannula/pressure transducer, snoring via an acoustic microphone, head movement from actigraphy, and head position from accelerometers. The device also provides audible alerts during the study if poor quality airflow or oxygen saturation are detected so the subject can reposition the device. The ARES Unicorder has been validated previously by us and shown to provide accurate estimates of sleep-disordered breathing indices when compared with in-laboratory polysomnography.14 We have also assessed night to night variability of the sleep-disordered breathing indices measured with the ARES Unicorder at a median of 3.0 events/h (range, 1-6 events/h) of sleep.15
Analysis of Respiratory Data From ARES
Data from the monitor were autoscored and then manually reviewed by a single trained sleep technician at New York University. Apneas were scored when there was a reduction in airflow to < 10% of baseline for > 10 seconds. Hypopneas 4% were scored for a > 30% reduction in airflow associated with a ≥ 4% decrease in oxygen saturation. HypopneasArsl was scored for a > 30% reduction in airflow associated with only surrogates of arousal (head movement, changes in snoring, or changes in pulse rate; these were then edited for a disappearance of flow limitation and a marked [> 150%] increase in flow amplitude at the end of the event). We have validated these rules for hypopnea previously for the diagnosis of OSA against standard polysomnography.16 Apnea Hypopnea Index (AHI) 4% was calculated as apneas + hypopneas 4% divided by total valid recording time. The respiratory disturbance index (RDI) was calculated as apneas + hypopneas 4% + hypopneasArsl divided by the total valid recording time. AHI 4% and RDI for each participant was recorded as the weighted average of each index for the two nights combined based on the duration of recording.
Definition of OSA
OSA was defined as present when AHI 4% was ≥ 5 events/h or when the RDI was ≥ 15 events/h. Mild OSA was defined as AHI 4% < 15 events/h when OSA was present, moderate OSA was defined as AHI 4% ≥ 15 events/h and < 30 events/h, and severe OSA was defined as AHI 4% ≥ 30 events/h.
Statistical Analysis
OSA and CRS were defined as dichotomous variables. We cross-tabulated the distributions of OSA and CRS to calculate frequencies and proportions. Logistic regression analysis, with OSA as the dependent variable and CRS as the independent variable, was used to evaluate the association of OSA and CRS without and after controlling for age, sex, BMI, alcohol use, and comorbid GERD. To evaluate the association of CRS and OSA with exposure, we performed logistic regression analysis with each of CRS (yes/no) and OSA (yes/no) as the dependent variable and exposure levels (very high and high vs low) as the independent variables. Analysis was also without and with controlling for age, sex, BMI, AHI, smoking status, alcohol use, and use of nasal steroids. Because a strong association has previously been found to OSA, we also examined the association of GERD to OSA11 using a similar logistic regression analysis. Interaction of GERD and CRS was also added into the logistic model to compare the odds of OSA between subjects with both GERD and CRS vs those with neither, using linear contrast. Statistical significance was defined by P < .05 (two-sided). To assess for the possibility of recruitment bias because of snoring, we performed a subanalysis examining demographics and answers to the snoring questionnaire in 210 consecutive subjects screened at New York University but not recruited to WTCSNORE and compared their demographics and answers with the snoring questionnaire in the first 209 subjects recruited to WTCSNORE. All statistical analyses were performed using SAS v9.4 (SAS Institute).
Results
Of the 634 subjects without preexisting snoring enrolled, valid data were available on CRS in 617 subjects, for OSA in 601 subjects, and for CRS and OSA in 586 subjects. Patient demographics are shown in Table 1, and details of the respiratory events of the subjects with OSA are shown in Table 2. The study population reflects the proportions that are in the WTCHP for men and women.1 The mean age is 53 years, with a mean BMI of 29.9 kg/m2. A very high prevalence of new-onset OSA was observed in this population: prevalence was 75% with a predominance of mild OSA. However, the mean AHI 4% was in the moderate range (Table 2). Subjects with OSA were more obese and more likely men and older than those without OSA. They had on average gained about 5 lb more weight in the previous 3 years than those without OSA. Subjects with OSA were more likely to use regular alcohol than those without OSA, and as expected, a significantly greater number of subjects with OSA had comorbid hypertension and diabetes (Table 1). Use of nasal or oral steroids, stimulants, and sedative medications was not different between subjects with and without OSA. Prevalence of comorbid depression, anxiety/panic disorder, posttraumatic stress disorder, GERD, and obstructive airways disease was not significantly different between those with and without OSA.
Table 2.
Data From Home Sleep Tests
| All Subjects (N = 601) | No. (%) |
|---|---|
| Without OSA | 150 (25) |
| With OSAa | 451 (75) |
| Mild OSA | 275 (46) |
| Moderate OSA | 116 (19) |
| Severe OSA | 60 (10) |
| Subjects With OSA (n = 451) | Mean ± SD |
|---|---|
| Total recorded time, h (total of 1-3 nights) | 11.4 ± 3.7 |
| Apnea Index (events/h) | 8.2 ± 10.4 |
| AHI 4% (events/h) | 15.5 ± 13.8 |
| RDI (events/h) | 30.6 ± 15.6 |
| Hypopnea (with 4% desaturation) Index (events/h) | 7.4 ± 5.8 |
| Hypopnea (with arousal alone) Index (events/h) | 15.0 ± 6.4 |
| Supine AHI 4% (events/h) | 22.5 ± 19.2 |
| Supine RDI (events/h) | 39.6 ± 20.8 |
| Mean oxygen saturation, % | 95 ± 1.25 |
| Time oxygen saturation < 90%, % | 3.3 ± 6 |
| Time snoring > 30 dB, % | 30 ±14 |
RDI = respiratory disturbance index.
OSA is defined as AHI 4% ≥ 5 events/h or when RDI ≥ 15 events/h. Mild OSA was defined as AHI 4% < 15 events/h when OSA was present, moderate OSA was defined as AHI 4% ≥ 15 events/h and < 30 events/h, and severe OSA was defined as AHI 4% ≥ 30 events/h.
Table 1.
Characteristics of the Participants With and Without OSA
| Characteristics | All (N= 601) | OSA (n = 451) | No OSA (n = 150) | P Value |
|---|---|---|---|---|
| Age, y | 52.8 ± 8.5 | 53.6 ± 8.3 | 50.2 ± 8.7 | < .0001 |
| Sex | < .0001 | |||
| Male | 498 (83) | 392 (87) | 106 (71) | |
| Female | 103 (17) | 59 (13) | 44 (29) | |
| BMI, kg/m2 | 29.9 ± 5.5 | 30.7 ± 5.5 | 27.4 ± 4.7 | < .0001 |
| Exposure (n = 525) | ns | |||
| Very high and high | 94 (17.9) | 72 (18.4) | 22 (16.4) | |
| Intermediate | 346 (65.9) | 259 (66.2) | 87 (64.9) | |
| Low | 85 (16.2) | 60 (15.3) | 25 (18.7) | |
| Nasal symptom score (n = 586) | 2.4 ± 2.2 | 2.5 ± 2.3 | 2.0 ± 2.1 | .02 |
| CRS+ (≥ 3) WTC (n = 586) | 255 (43.5) | 204 (46.5) | 51 (34.7) | .01 |
| CRS+ (new/worsening group, n = 547) | 216 (39.5) | 176 (42.8) | 40 (29.4) | .006 |
| CRS+ (≥ 2) EPOS (n = 586) | 275 (46.9) | 218 (49.7) | 57 (38.8) | .03 |
| CRS+ (new/worsening group, n = 548) | 237 (43.2) | 191 (46.4) | 46 (33.8) | .012 |
| Snoring (n = 598) | 302 (50.5) | 254 (56.7) | 48 (32.0) | < .0001 |
| ESS (n = 596) | 8.2 ± 4.8 | 8.3 ± 4.9 | 7.9 ± 4.5 | ns |
| FOSQ (n = 545) | 17.5 ± 2.6 | 17.4 ± 2.7 | 17.7 ± 2.4 | ns |
| Weight change in last 3 y, lb (n = 550) | 3.8 ± 17.6 | 4.8 ± 18.3 | 0.7 ± 15.2 | .02 |
| Current smoking status (n = 597) | ns | |||
| Yes | 58 (9.7) | 45 (10.0) | 13 (8.8) | |
| No | 539 (90.3) | 405 (90.0) | 134 (91.2) | |
| Alcohol | < .05 | |||
| Regular | 191 (32.4) | 154 (34.5) | 37 (25.9) | |
| Intermittent | 307 (52.1) | 228 (51.1) | 79 (55.2) | |
| Never | 91 (15.4) | 64 (14.3) | 27 (18.9) | |
| Sedative medications | 127 (21.5) | 96 (21.8) | 31 (20.7) | ns |
| Stimulant medications | 85 (14.4) | 70 (15.9) | 15 (10.0) | ns |
| Oral steroids | 36 (7.5) | 27 (7.6) | 9 (7.0) | ns |
| Nasal steroids | 61 (10.1) | 49 (10.9) | 12 (8.0) | ns |
| OAD | 126 (21.0) | 100 (22.2) | 26 (17.3) | ns |
| Hypertension | 154 (25.9) | 133 (29.8) | 21 (14.2) | < .0001 |
| Diabetes | 51 (8.6) | 46 (10.3) | 5 (3.4) | .007 |
| Anxiety and panic disorder | 136 (25.4) | 107 (26.4) | 29 (22.1) | ns |
| Depression | 110 (20.0) | 88 (21.3) | 22 (16.1) | ns |
| PTSD (n = 573) | 133 (23.2) | 106 (24.7) | 27 (18.9) | ns |
| Insomniaa (n = 589) | 309 (52.5) | 232 (52.6) | 77 (52.0) | ns |
| GERD (n = 544) | 278 (51.1) | 217 (53.3) | 61 (44.5) | ns |
Values are mean ± SD, No. (%), or as otherwise indicated. CRS+ EPOS = ≥2 specific nasal symptoms defined in text under subjective assessment of nasal symptoms; CRS+ WTC = ≥3 nasal symptoms for >8 weeks; EPOS = 2012 European Position Paper on Rhinosinusitis and Nasal Polyps; ESS = Epworth Sleepiness Scale score; FOSQ = Functional Outcomes of Sleep Questionnaire; GERD = gastroesophageal reflux disorder; ns = not significant; OAD = obstructive airway disease; PTSD = posttraumatic stress disorder; WTC = World Trade Center.
Onset and maintenance insomnia.
Although the expected high prevalence of OSA was observed in men with BMI > 30 kg/m2, a high prevalence was also observed in both men and women with a BMI < 30 kg/m2, suggesting mechanisms other than the traditional risk factors for OSA play a role in OSA pathogenesis in this cohort (Table 3).
Table 3.
Prevalence of OSA by Sex, Age, and BMI Category
| Sex | Age Group (y) | BMI (kg/m2) | No. of Subjects in Group | % With OSA |
|---|---|---|---|---|
| Women | 30-49 | < 25 | 12 | 25 |
| 25–29.9 | 7 | 43 | ||
| 30–39.9 | 10 | 30 | ||
| ≥ 40 | 2 | 50 | ||
| 50-70 | < 25 | 25 | 60 | |
| 25–29.9 | 21 | 52 | ||
| 30–39.9 | 13 | 85 | ||
| ≥ 40 | 9 | 89 | ||
| Men | 30-49 | < 25 | 20 | 40 |
| 25–29.9 | 85 | 65 | ||
| 30–39.9 | 88 | 88 | ||
| ≥ 40 | 6 | 100 | ||
| 50-70 | < 25 | 42 | 74 | |
| 25–29.9 | 120 | 76 | ||
| 30–39.9 | 116 | 91 | ||
| ≥ 40 | 10 | 100 |
Subjects > 70 y of age were excluded.
Table 4 shows the sex distribution, age, and snoring complaints in our subgroup of subjects who were not enrolled in WTCSNORE compared with subjects recruited to WTCSNORE. Demographics of the groups did not differ. Of the WTCHP cohort, 33% had pre-9/11 snoring and, by design, WTCSNORE did not include these subjects. A total of 75 of 209 subjects (37%) in WTCSNORE reported significant snoring post-9/11. This was significantly lower than the 51% of subjects not recruited to WTCSNORE, arguing against recruitment bias because of snoring as a symptom of OSA.
Table 4.
Demographics and Snoring Complaints in Subjects From New York University Enrolled in WTCSNORE and Subjects Attending the WTCHP but Not Enrolled in WTCSNORE
| Demographics and Snoring Complaints | WTCSNORE (n = 209) | WTCHP (n = 210) |
|---|---|---|
| Female sex, % | 20.4 | 20.7 |
| Mean age ± SD, y | 51.74 ± 8.27 | 53.9 ± 13.7 |
| WTC Visit 1 Questionnaire, No. (%) | ||
| Pre-September 11, 2001, snoring | … | 70 (33.3) |
| Post-September 11, 2001, snoring (within last month of administering questionnaire) (n = 201) | 75 (37) | 106 (50.7) |
| Current snoring at enrollment in WTCSNORE, No. (%) | 96 (47.3) | NA |
NA = not applicable; WTC = World Trade Center; WTCHP = WTC Health Program.
WTC Dust Exposure and Relationship to New and Worsening CRS After 9/11 and OSA
Intermediate and high levels of WTC dust exposure were associated with higher CRS scores than low levels of exposure unadjusted and after adjusting for age, sex, BMI, and OSA. The mean CRS scores ± SEM were 1.78 ± 0.25 with low level of exposure, 2.53 ± 0.12 with intermediate exposure, and 2.41 ± 0.24 with high levels of WTC dust exposure (P < .05). However, the level of WTC exposure was not different between subjects with and without OSA (Table 1). Additionally, the level of exposure was not associated with greater severity of OSA (data not shown).
Relationship of New and Worsening CRS After 9/11 to New-Onset OSA
There were 216 subjects identified as having new-onset or worsening CRS. Subjects with OSA had higher nasal symptom scores and a significantly higher prevalence of CRS defined by the WTC criteria and by the newer EPOS definition (Fig 1, Table 1). However, in subjects with OSA, no significant differences were seen in respiratory event characteristics between subjects with and without CRS (Table 5, apnea index, supine and total AHI, supine and total RDI, Hypopnea 4%, HypopneaArsl, time < 90%, or time of loud snoring and in the severity of OSA, P = not significant). Using the WTC definition of CRS, we found that after adjusting for age, sex, and BMI (model 1), and adding GERD (model 2) or GERD and alcohol use (model 3), new and worsening CRS was still independently associated with new-onset OSA with an OR ranging from 1.80 to 1.76. Using the new EPOS definition and worsening CRS were independently associated with new-onset OSA adjusting for age, sex, and BMI (OR, 1.56; 95% CI, 1.01-2.40), but not in models 2 or 3 (Table 6).
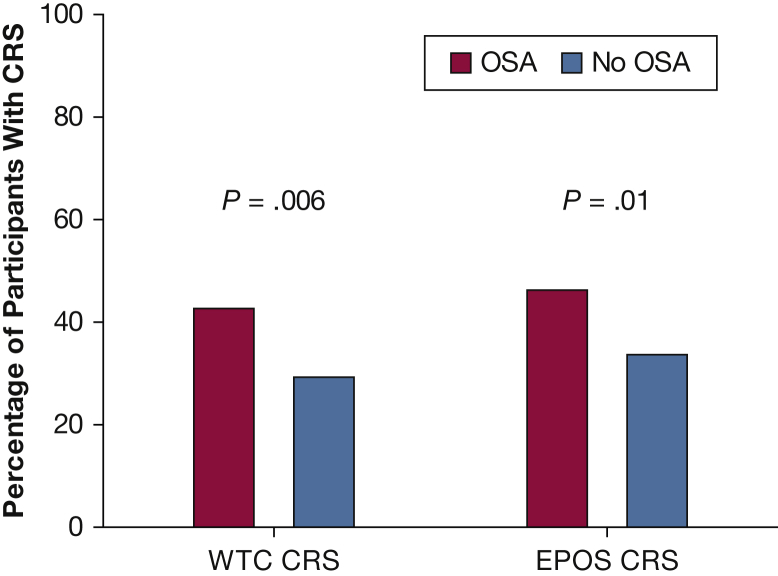
Figure 1.
A significantly higher percentage of participants with OSA (red bars) had new and worsening CRS compared with those without OSA (blue bars) using both the WTC (42.8% vs 29.8%) and EPOS definitions of CRS (46.4% vs 33.8%), respectively. CRS = chronic rhinosinusitis; EPOS = 2012 European Position Paper on Rhinosinusitis and Nasal Polyps; WTC = World Trade Center.
Table 5.
Respiratory Events in Subjects With OSA (CRS Absent Compared With CRS Present)
| Respiratory Events | OSA (n = 411) | OSA With CRS (n = 176)a,b | OSA Without CRS (n = 235)a,b |
|---|---|---|---|
| Mild OSA | 257 (62.6) | 112 (64) | 145 (62) |
| Moderate OSA | 101 (24.6) | 42 (24) | 59 (25) |
| Severe OSA | 53 (12.8) | 22 (13) | 31 (13) |
| Total recording time, h (total of 1-3 nights) | 11.35 ± 3.77 | 11.6 ± 3.8 | 11.2 ± 3.7 |
| Apnea Index, events/h | 8.03 ± 10.40 | 7.9 ± 10.4 | 8.1 ± 10.4 |
| AHI 4%, events/h | 15.3 ± 13.7 | 15.5 ± 13.8 | 15.1 ± 13.6 |
| RDI, events/h | 30.4 ± 15.6 | 30.8 ± 16.0 | 30.2 ± 15.4 |
| Hypopnea (4% desaturation only) Index | 7.2 ± 5.7 | 7.6 ± 6.1 | 7.0 ± 5.4 |
| Hypopnea (arousal only) Index | 15.2 ± 6.6 | 15.4 ± 7.0 | 15.1 ± 6.3 |
| Supine AHI 4% | 22.3 ± 19.3 | 22.2 ± 18.5 | 22.3 ± 19.9 |
| Supine RDI | 39.6 ± 21.0 | 39.9 ± 21.0 | 39.5 ± 21.0 |
| Time oxygen saturation < 90%, % | 3.3 ± 6.2 | 3.4 ± 5.9 | 3.3 ± 6.4 |
| Time snoring > 30 dB, % | 30 ± 14 | 31.0 ± 14.6 | 29.3 ± 14.0 |
Values are No. (%) or mean ± SD. CRS = chronic rhinosinusitis. See Table 2 legend for expansion of other abbreviation.
Only subjects with new/worsening CRS and no CRS included.
CRS present/absent classification based off World Trade Center CRS definition.
Table. 6.
Unadjusted and Adjusted OR (95% CI) for OSA in Subjects with New-Onset or Worsening CRS using the WTC and the EPOS Definition for CRS
| Nasal Symptom Score | Unadjusted OR (95% CI) | Adjusted for Age, Sex, and BMI (Model 1) | Adjusted for Age, Sex, BMI, and GERD (Model 2) | Adjusted for Age, Sex, BMI, GERD, and Regular Alcohol Use (Model 3) |
|---|---|---|---|---|
| No CRS | 1.0 | 1.0 | 1.0 | 1.0 |
| WTC CRS | 1.8 (1.18-2.73) | 1.69 (1.08-2.65) | 1.76 (1.09-2.85) | 1.76 (1.08-2.88) |
| P value | .006 | .02 | .02 | .02 |
| EPOS CRS | 1.69 (1.12-2.53) | 1.56 (1.01-2.4) | 1.59(1-2.5) | 1.52 (0.95-2.4) |
| P value | .01 | < .05 | .05 | .08 |
GERD alone was not associated with OSA in this population. However, when we compared those subjects who had comorbid GERD and CRS with those with neither, controlling for age, sex, BMI, and alcohol use, a statistically significant association to OSA was found with an OR of 1.86 (95% CI, 1.01-3.39; P < .05).
Discussion
This study has two major findings. First, a very high prevalence of OSA (75%) was observed in a cohort of WTC responders who were prospectively studied. Because we specifically excluded subjects with a diagnosis of OSA or significant snoring prior to September 2001, our prevalence estimate of new-onset OSA is a conservative one for the prevalence of OSA in the entire WTC responder cohort and is much higher than has been described in community cohorts prospectively studied.6, 7 For example, the overall prevalence of OSA was estimated to be 26% among persons 30 to 70 years of age in the US population.7 Similar prevalence rates were reported in community-based studies from Spain (26.2% in men and 28.0% in women)17 and Brazil (46.6% in men and 30.5% in women).18 A much higher prevalence was observed in a population-based study in Switzerland (HypnoLaus) where an AHI of > 5 events was recorded in 83.9% of men and 60.8% of women.6 However, this high prevalence was based on a very low cutoff of this inclusive definition of hypopnea (> 30% drop of airflow lasting at least 10 seconds with either an arousal or ≥ 3% oxygen saturation drop). Using this definition, we would have found a prevalence of OSA of 99% in men and 96% in women in our cohort. Additionally, in our cohort, those subjects with low traditional risk factors for OSA (age 30-49 years and BMI < 30 kg/m2) still had a very high prevalence of OSA (25%-43% in women and 40%-65% in men). In comparison, the expected prevalence in the Wisconsin cohort for this age/BMI was 1.4% to 4.2% in women and 7% to 18% in men.7 Our high prevalence of OSA is therefore not explained solely based on traditional risk factors for OSA in the WTC cohort.
Second, we found a strong association between new and worsening CRS symptoms and new-onset OSA in our study population even after adjusting for well-established risk factors for OSA. Because a high prevalence of GERD is also present, we controlled for the presence of GERD and the association between CRS and OSA persisted. A previous study in a similar group of WTC workers has not found that association, but their sample size was smaller.19 Another study by Glaser et al11 examined the relationship of OSA to GERD and CRS in WTC firefighters. A very high prevalence of OSA (81%) was also observed in this cohort. However, significant differences exist between this study and our study. Most notably, the Glaser et al study examined the prevalence of OSA in participants who were at high risk for OSA with symptoms of snoring or daytime sleepiness and with a BMI of > 30 kg/m2 or if they were referred for polysomnography because of high suspicion for OSA irrespective of BMI. The authors noted an association between OSA and GERD alone as well as between OSA and GERD along with co-existing CRS but not between OSA and CRS alone. This may have been because of a small number of participants with CRS alone in that study.11 Additionally, because participants were a referral population with symptoms of OSA, GERD may have been a result of OSA rather than a cause. Although Glaser et al found an association between arrival at the mound in the immediate aftermath of the WTC towers collapsing and severe OSA, we found an association between level of exposure and increasing CRS scores but not with presence or absence of OSA or to severity of OSA. A direct consequence of WTC exposure may be nasal inflammation leading to CRS symptoms, which would explain the association we found. The lack of association with exposure and OSA suggests that OSA pathogenesis in the responder population is likely multifactorial with CRS playing a significant but not unique role in the development of OSA. The high prevalence of OSA in this population without CRS is evidence for this.
Our results for CRS are consistent with the Wisconsin cohort data showing symptoms because of allergic and nonallergic rhinitis as a risk factor for OSA but not related to severity of OSA.9, 20, 21, 22 The prevalence of CRS or nasal symptoms in epidemiologic populations of OSA and nasal symptoms/pathology ranges from 10.4% to 17.4%.10 However, sleep clinic populations report 50% prevalence of nasal and upper airway complaints23, 24 and diagnoses of allergic and nonallergic rhinitis22 are similar to the WTC responder population, suggesting the relevance of our data to these populations.
In our study, 81% of subjects who had CRS had OSA, whereas 71% without CRS had OSA. Data from the literature show that the prevalence of OSA in populations with CRS varies between 15% if it is physician diagnosed25 to 67% when prospectively screened for OSA.26 Therefore, the prevalence of OSA in the WTC cohort, both in those with and without CRS, exceeds the prevalence seen in the general population. Pathogenic mechanisms such as impaired anatomy, low arousal threshold, respiratory instability (loop gain), and impaired upper airway neuromuscular control/muscle compensation need to be further explored in our population.
Although our hypothesis was that new-onset CRS was a risk factor for OSA, the cross-sectional design of our study does not allow us to ascertain which of the conditions occurred first, and there may be reasons for causality in both directions. A large population-based retrospective cohort study comparing the incidence of CRS within 5 years after the index date showed an OR of 3.18 (95% CI, 1.38-5.01) for CRS in subjects with OSA compared with those without OSA.27
Potential mechanisms for an increased risk of OSA related to CRS include the effect of nasal obstruction on collapsibility of the nasopharynx downstream. An increase in nasal resistance is the most likely mechanism and could be caused by inflammation related to an elevated nasal resistance. There is increasing evidence of inflammation in both OSA and CRS with high serum levels of C-reactive protein, IL-1, IL-6, IL-8, and tumor necrosis factor-alpha.28, 29 This inflammation could cause increased upper airway resistance or nasal resistance via local swelling and edema. If CRS is a causative factor for OSA, elimination or reduction of CRS may play a role in preventing OSA. In a randomized double-blind crossover trial of 4 weeks of 100 μg twice daily of intranasal fluticasone, Kiely et al30 showed a significant reduction in AHI in those with OSA (defined as AHI > 10 events/h), with a median within-subject reduction of 6.5 events/h of sleep. However, in our data, we did not see a difference in OSA prevalence in subjects on nasal or oral steroids (77.3%) compared with subjects who were not on steroids (73%), nor did we see a difference in the severity of OSA (mean AHI 4% in subjects with OSA on steroids, 15 events/h vs without steroids, 14.7 events/h).
Alternatively, an abnormal inflammatory response in patients with OSA might contribute to development of CRS.
Finally, as in any cohort study, we may have had a recruitment bias toward greater participation by subjects whose snoring suggested OSA. We tried to limit this by specifically excluding subjects who reported significant snoring prior to 9/11 (ie, we targeted pre-9/11 nonsnorers for inclusion). The percentage of snorers recruited in WTCSNORE is similar to published reports of snoring in the general population.31, 32, 33 If anything, our prevalence estimate of OSA is lower than the actual prevalence because we excluded all pre-9/11 snorers and those already diagnosed with OSA. Another limitation of our study was that although we used two standardized definitions for CRS (WTC and EPOS), we did not perform nasal endoscopy or CT scan demonstrating objective findings of sinusitis.
Conclusions
Our data show that the prevalence of OSA in WTC responders exposed to dust is extremely high and not explained by the usual risk factors of sex and obesity. CRS is a significant independent risk factor for OSA.
Acknowledgments
Author contributions: J. S. and I. A. are guarantors of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. K. B., S. A., I. U., A. T., D. H., H. S., R. E. H., and N. C. recruited patients and collected data. J. S., I. A., D. M. R., S.-E. L., and M. W. analyzed results. J. S., I. A., D. M. R., and S.-E. L. designed the research study. J. S., D. M. R., and I. A. wrote the paper. All authors reviewed the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. S., I. A., K. B., I. U., D. M. R., S.-E. L., N. C., D. H., and R. E. H. report grants from CDC/NIOSH during the conduct of the study. D. M. R. and I. A. report grants, personal fees, nonfinancial support, and other from Fisher Paykel Healthcare outside the submitted work. D. M. R. and I. A. also have patents for modifications of CPAP that result in royalties paid through New York University. None declared (M. W., S. A., A. T., H. S.).
Role of sponsors: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC/NIOSH.
Other contributions: We thank Alan Perez, BS, Jash Vakil, BS, Emma Ducca, BS, Tyler Gumb, BS, Rohan Patel, BS, and Clarimel Cepeda, BS, who participated as research assistants; and the subjects who are enrolled in the WTCHP who participated in the study.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institute for Occupational Safety and Health/Centers for Disease Control and Prevention [Grants U01OH010415, U10OH008232 (DCC), 200-2011-39356 (Mount Sinai CCE), 200-2011-39385 (Rutgers CCE), 200-2011-39384 (NYU CCE)]; and a National Institutes of Health K24 grant [Grant HL109156].
References
- 1.Herbert R., Moline J., Skloot G. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect. 2006;114(12):1853–1858. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Hoz R.E., Shohet M.R., Cohen J.M. Occupational rhinosinusitis and upper airway disease: the world trade center experience. Curr Allergy Asthma Rep. 2010;10(2):77–83. doi: 10.1007/s11882-010-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisnivesky J.P., Teitelbaum S.L., Todd A.C. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: a cohort study. Lancet. 2011;378(9794):888–897. doi: 10.1016/S0140-6736(11)61180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webber M., Lee R., Soo J. Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath. 2011;15(3):283–294. doi: 10.1007/s11325-010-0379-7. [DOI] [PubMed] [Google Scholar]
- 5.Sunderram J., Udasin I., Kelly-McNeil K. Unique features of obstructive sleep apnea in World Trade Center responders with aerodigestive disorders. J Occup Environ Med. 2011;53(9):975–980. doi: 10.1097/JOM.0b013e3182305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinzer R., Vat S., Marques-Vidal P. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T., Finn L., Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med. 2001;161(12):1514–1519. doi: 10.1001/archinte.161.12.1514. [DOI] [PubMed] [Google Scholar]
- 9.Young T., Finn L., Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol. 1997;99(2):S757–S762. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- 10.Stradling J.R., Crosby J.H. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46(2):85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser M.S., Shah N., Webber M.P. Obstructive sleep apnea and World Trade Center exposure. J Occup Environ Med. 2014;56(suppl 10):S30–S34. doi: 10.1097/JOM.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 12.de la Hoz R.E., Shohet M.R., Chasan R. Occupational toxicant inhalation injury: the World Trade Center (WTC) experience. Int Arch Occup Environ Health. 2008;81(4):479–485. doi: 10.1007/s00420-007-0240-x. [DOI] [PubMed] [Google Scholar]
- 13.Bengtsson C., Lindberg E., Jonsson L. Chronic rhinosinusitis impairs sleep quality: results of the GA2LEN Study. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw021. [DOI] [PubMed] [Google Scholar]
- 14.Ayappa I., Norman R.G., Seelall V., Rapoport D.M. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med. 2008;4(1):26–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Gumb T., Twumasi A., Alimokhtari S. Comparison of two home sleep testing devices with different strategies for diagnosis of OSA. Sleep Breath. 2018;22(1):139–147. doi: 10.1007/s11325-017-1547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayappa I., Norman R.G., Suryadevara M., Rapoport D.M. Comparison of limited monitoring using a nasal-cannula flow signal to full polysomnography in sleep-disordered breathing. Sleep. 2004;27(6):1171–1179. doi: 10.1093/sleep/27.6.1171. [DOI] [PubMed] [Google Scholar]
- 17.DurÁN J., Esnaola S., Rubio R., Iztueta Á. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 18.Tufik S., Santos-Silva R., Taddei J.A., Bittencourt L.R. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 19.de la Hoz R.E., Aurora R.N., Landsbergis P., Bienenfeld L.A., Afilaka A.A., Herbert R. Snoring and obstructive sleep apnea among former World Trade Center rescue workers and volunteers. J Occup Environ Med. 2010;52(1):29–32. doi: 10.1097/JOM.0b013e3181c2bb18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNicholas W.T., Tarlo S., Cole P. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126(4):625–628. doi: 10.1164/arrd.1982.126.4.625. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelm C.P., deShazo R.D., Tamanna S., Ullah M.I., Skipworth L.B. The nose, upper airway, and obstructive sleep apnea. Ann Allergy Asthma Immunol. 2015;115(2):96–102. doi: 10.1016/j.anai.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Zheng M., Wang X., Ge S. Allergic and non-allergic rhinitis are common in obstructive sleep apnea but not associated with disease severity. J Clin Sleep Med. 2017;13(8):959–966. doi: 10.5664/jcsm.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreivi H.R., Virkkula P., Lehto J.T., Brander P.E. Upper airway symptoms in primary snoring and in sleep apnea. Acta Otolaryngol. 2012;132(5):510–518. doi: 10.3109/00016489.2011.644803. [DOI] [PubMed] [Google Scholar]
- 24.Lojander J., Brander P.E., Ammala K. Nasopharyngeal symptoms and nasal continuous positive airway pressure therapy in obstructive sleep apnoea syndrome. Acta Otolaryngol. 1999;119(4):497–502. doi: 10.1080/00016489950181062. [DOI] [PubMed] [Google Scholar]
- 25.Alt J.A., DeConde A.S., Mace J.C., Steele T.O., Orlandi R.R., Smith T.L. Quality of life in patients with chronic rhinosinusitis and sleep dysfunction undergoing endoscopic sinus surgery: a pilot investigation of comorbid obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2015;141(10):873–881. doi: 10.1001/jamaoto.2015.1673. [DOI] [PubMed] [Google Scholar]
- 26.Jiang R.S., Liang K.L., Hsin C.H., Su M.C. The impact of chronic rhinosinusitis on sleep-disordered breathing. Rhinology. 2016;54(1):75–79. doi: 10.4193/Rhino15.204. [DOI] [PubMed] [Google Scholar]
- 27.Kao L.T., Hung S.H., Lin H.C., Liu C.K., Huang H.M., Wu C.S. Obstructive sleep apnea and the subsequent risk of chronic rhinosinusitis: a population-based study. Sci Rep. 2016;6:20786. doi: 10.1038/srep20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeem R., Molnar J., Madbouly E.M. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugappan Ramanathan J., Lane A.P. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136(3):348–356. doi: 10.1016/j.otohns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Kiely J.L., Nolan P., McNicholas W.T. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax. 2004;59(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- 31.Yeboah J., Redline S., Johnson C. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population. MESA. Atherosclerosis. 2011;219(2):963–968. doi: 10.1016/j.atherosclerosis.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 33.Young T., Shahar E., Nieto F. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]