Abstract
In this study, we report a metallogel developed based on metal-phenolic coordination of natural low-cost polyphenolic molecule and metal ions. Gelation occurs by mixing tannic acid (TA) and group (IV) titanium ions (TiIV) to form TA-TiIV gel. The TA-TiIV gel exhibits good capability to incorporate diverse metal ions by in situ co-gelation. Herein, five antimicrobial metal ions, i.e. ferric (FeIII), copper (CuII), zinc (ZnII), cobalt (CoII) and nickel (NiII) ions, were employed to include in TA-TiIV gels for developing intelligent dressings for infected wounds. The chemical and coordinative structures of TA-TiIV metallogels were characterized by UV-Vis and Fourier-transform infrared (FT-IR) spectroscopies. Cytotoxicity of antimicrobial metallogels was explored by MTT assay with NIH 3T3 fibroblasts. The release of metal ions was evaluated by inductively coupled plasma mass spectrometry (ICP-MS), indicating the different releasing profiles upon the coordinative interactions of metal ions with TA. The formation and disassembly of metallogels are sensitive to the presence of acid and an oxidizer, H2O2, which are substances spontaneously generated in infected wounds due to the metabolic activity of bacteria and the intrinsic immune response. The CuII releasing rates of TA-TiIV-CuII metallogels at different pH values of 5.5, 7.4 and 8.5 have been studied. In addition, addition of H2O2 trigger fast release of CuII as a result of oxidation of galloyl groups in TA. Consequently, the antimicrobial potency of TA-TiIV-CuII metallogels can be simultaneously activated while the wounds are infected and healing. The antimicrobial property of metallogels against Gram-negative Escherichia coli, and Gram-positive Methicillin-Resistant Staphylococcus aureus (USA300) and Staphylococcus epidermidis has been investigated by agar diffusion test. In an animal model, the TA-TiIV-CuII metallogels were applied as dressings for infected wounds, indicating faster recovery in the wound area and extremely lower amount of bacteria around the wounds, compared to TA-TiIV gels and gauze. Accordingly, the intelligent nature derived metallogels is a promising and potential materials for medical applications.
Subject terms: Bioinspired materials, Chemical engineering
Introduction
Infections occur when virulence factors expressed by one or more microorganisms in a wound outcompete the host immune system. Dissemination of microorganisms in tissue provokes a series of local and systemic host responses1. Complications of infected wounds depend on the pathogenicity of the microorganisms and on the immune competence of the host, manifesting as clinical signs, such as pain, heat, erythema, edema, tenderness, cellulitis and abscess. The infection in a wound can lead to an acute responses and delay the wound healing process2. Bacteria such as methicillin-resistant Staphylococcus aureus (USA300), Escherichia coli and Staphylococcus epidermidis have been frequently isolated from infected wounds, and become common wound pathogens3.
It has been proven that the pH of a wound environment plays an important role in wound healing because it relates to antimicrobial activity and control infection, angiogenesis, oxygen release, and protease activity4. Bacterial infection leads to the pH fluctuation because of the complex process of healing, immune responses and bacterial metabolism. The pH has been found to fall to the value as low as 5.5 at the infection sites due to low-oxygen fermentation, triggering the production of lactic acid and acetic acid5–7. In addition, the production of hydrogen peroxide (H2O2) in the infecting wounds is the first response of the immune system during inflammation to exert antimicrobial activity8,9. H2O2 is known as a reactive oxygen species (ROS), which accounts for their versatility in mediating host defense against a broad range of pathogens. In addition, studies revealed that H2O2 supports the healing process at a level of µM concentration by serving as a signaling molecule and stimulating an effector cells response10,11. The endogenous substances allow to serve as an environmental stimulus to spontaneously trigger the antimicrobial reactions of medical devices. Therefore, pH- and H2O2-responsive antibiotic delivery systems have received considerable attention due to their great potential and easy applicability in the clinical treatment of infections5,12,13.
Antimicrobial properties of metal ions have been known and explored for many years14. Antibacterial metal ions, such as silver (AgI), zinc (ZnII), cobalt (CoII), nickel (NiII), iron (FeIII) and copper (CuII), have been extensively used in artificial medical implants and devices due to their broad-spectrum antibacterial activity14–16. The toxicity of metals to bacteria is primarily related to strong affinities to biomolecules and production of ROS. Recent studies indicate that different metals cause discrete and distinct types of injuries to microbial cells as a result of protein dysfunction, oxidative stress or membrane damage17. Generally, metals appear to target multiple cellular processes, leading to pleiotropic effects on bacteria17.
Polyphenolic derivatives exist ubiquitously in nature. Their coordinative complexation with metal ions displays a remarkable degree of physico-chemical versatility that has already inspired an ever-growing number of applications as functional materials18–21. Harrington et al. indicated that catecholic amino acid-iron chelate complexes form the peculiar metallopolymeric structures, exhibiting a combination of high hardness and high extensibility21. Recently, Caruso’s group demonstrated metal-phenolic complexation between different metals and natural polyphenolic tannic acid (TA) for assembly of conformal functional coatings20. Moreover, they reported a formation of metal-phenolic supramolecular metallogels by direct complexation of multitopic galloyl groups of TA and group (IV) transition metal of TiIV 14. The metallogels represent excellent versatility to incorporate diverse metal ions, nanomaterials, and polymers via in situ co-gelation without affecting the fundamental gelation process. Additionally, the metallogels display pH-modulated mechanical property, shape persistence, adhesiveness, optical transparency, tunable mechanical properties and self-healing capability14,20.
In this study, we attempted to develop antibacterial metallogels with pH- and H2O2-responsive properties as dressings for infected wounds. The metallogels were synthesized via co-gelation of TA, high-oxidation state TiIV and antimicrobial metal ions for formation of three-dimensional metal-phenolic networks (MPNs). The antimicrobial metal ions of ZnII, CoII, NiII, FeIII and CuII were included in the metallogels for comparative study in terms of their release profiles, cytotoxicity, and antimicrobial potency. Moreover, the antibacterial effect of metallogels can be triggered by acids originated from the metabolic activity of bacteria, and endogenously produced H2O2 after skin wound occurs upon disassembly of the MPNs. UV-vis spectroscopy and Fourier-transform infrared spectroscopy (FTIR) were employed for analysis of coordinative structures of metallogels. The metal ion release profiles and responsiveness of the metallogels to pH and H2O2 were determined by inductively coupled plasma mass spectrometry (ICP-MS). The antimicrobial properties and cytotoxicity of all metal ions were accessed by minimum inhibitory concentration (MIC) test and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The antimicrobial effect of metallogels against Gram-negative E. coli, and Gram-positive S. aureus and S. epidermidis was investigated by agar diffusion test. In an animal model, biocompatible TA-TiIV-CuII metallogels were developed as dressings for infected wounds on mice in comparison with TA-TiIV gels and gauze. Ultimately, the versatility and robustness, combined with the low cost, biocompatibility, ease, and scalability of the coordination-driven assembly make the metallogels excellent candidates for medical applications.
Experimental Section
Materials
Tannic acid (TA, ACS reagent), titanium (IV) bis (ammonium lactato) dihydroxide (Ti-BALDH) solutions 50 wt% in H2O, iron (III) hexahydrate (FeCl3.6H2O), copper (II) chloride (CuCl2), cobalt (II) bromide (CoBr2), nickel (II) chloride (NiCl2), zinc (II) chloride (ZnCl2), 2,6-dichlorophenolindophenol, trizma hydrochloride (Tris), copper assay kit and peroxidase from horseradish (HRP) lyophilized powder were purchased from Sigma-Aldrich. Phosphate buffer saline (PBS) was obtained from Acros Organics. Luria-Bertani (LB) and tryptic soy broth (TSB) were purchased from Gibco. MTT assay kit and sodium hydroxide (NaOH) were acquired from Thermo Fisher Scientific. Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco. NIH 3T3 fibroblasts were obtained from Bioresource Collections and Research Center (BCRC), Taiwan. S. epidermidis ATCC12228, methicillin-resistant S. aureus (USA300, ATCC BAA-1718) and E. coli ATCC12435 were acquired from Bioresource Collections and Research Center in Taiwan. All the water used was purified to 18.2 mΩ using a Millipore water purifications system, and filtered using a 0.22 µm filter.
Preparation of antimicrobial metallogels
All solutions were prepared under a nitrogen atmosphere using standard Schlenk techniques. The preparation of TA-TiIV metallogels was referred to previous study14. Briefly, 5 wt% of TA solution was prepared in deionized water. The pH of the TA solution was adjusted to approximately 7 by adding 1 M NaOH solution. 50 wt.% of Ti-BALDH stock solutions was then mixed with the TA solutions to afford a TA:Ti molar ratio of 1:5. The mixture was thoroughly shaken for 10 s to make sure all the components were well mixed and homogeneous. The mixed solution was left to stand for 20 min and the color of the solution turned from orange to red. The state of the solution was turned from sol to gel. The hydrogel was removed from the tube and denoted as TA-TiIV.
The antimicrobial metal ions of FeIII, CuII, CoII, NiII and ZnII were introduced individually for preparation of the metallogels by co-gelation. The TiIV and antimicrobial metal ions were added into the TA solution, prepared as above mentioned. The mixed solution had a molar ratio of TA:TiIV:M to be 1:4:1, where M is referred to the antimicrobial metal ions. The mixture was shaken with vortex for 10 s and then stood on a bench for 20 min.
Spectroscopic characterizations of metallogels
UV-vis spectroscopy was used to examine the coordination reaction of TA and TiIV in an aqueous solution. UV–vis spectra were obtained on a spectrophotometer (V-600, JASCO, MD) at room temperature. Three solutions were prepared prior to the measurements. Control samples of TA and TiIV solutions were prepared in oxygen-free deionized water at concentrations of 0.1 and 0.3455 wt%, respectively. The pH of the TA solution was controlled at 7. The TA-TiIV solution was prepared as above mentioned.
Fourier-transform infrared spectroscopy (FTIR, Jasco FT/IR-410) was employed to analyze chemical and coordination structures of metallogels. TA-TiIV metallogels were dried in liquid nitrogen under reduced pressure for 4 h. The dried TA-TiIV was grounded by a pestle and blended with KBr. The mixed powder was compressed into a pellet. FTIR was operated in a wavenumber range of 400 to 4000 cm−1.
Release profiles of metal ions
Metal ion release profile was measured and recorded for 15 h using inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500ce, Japan). The metal-contained metallogels were cut into round discs with a diameter of 15 mm and thickness of 1.5 mm. The metallogel disc was immersed into 5 mL of PBS buffer at pH of 7.4 at 37 °C with agitation at 30 rpm. At each time point, the solution was collected for measuring the accumulative release of metal ions. 5 mL of fresh PBS buffer has added to compensate for replacing solutions.
pH- and H2O2-responsiveness of metallogels
The pH- and H2O2-responsiveness of TA-TiIV-CuII metallogels were characterized by measuring the amount of released CuII. The released CuII was quantified at incubation time points of 12, 24 and 36 h by ICP-MASS. The TA-TiIV-CuII metallogels were cut into round discs with a diameter of 15 mm and thickness of 1.5 mm. For the pH-responsiveness, the metallogels, buffered solutions at pH values of 5.5, 7.4 and 8.4 were prepared. The TA-TiIV-CuII metallogels were immersed in 5 mL of buffered solutions at 37 °C with agitation at 30 rpm. At every 12-h period of time, the concentration of CuII in the solution was measured using the assay kit. Afterward, fresh 5 mL of the buffered solution was added for the next measurement.
For the H2O2 responsiveness test, the concentration of CuII in ppm, released from TA-TiIV-CuII metallogels, was determined after incubation with H2O2 in a buffer at pH 5.5 at 37 °C for 36 h. To further confirm the H2O2 responsiveness of metallogels, HRP, that catalyzes the oxidation of various organic substrates by H2O2, was included in the metallogels to observe the release of CuII. For the HRP encapsulation, HRP in PBS was introduced to mix with the TA solution to achieve the final concentration in each gel is 62.5 U/gel and 31.5 U/gel. The size of the metallogels was described as above, which is a round disc with a diameter of 15 mm and thickness of 1.5 mm. After mixture, TiIV and CuII were added and vortexed for 10 s. The metallogels containing HRP (TA-TiIV-CuII-HRP) were formed after 20 min. The test for the release of CuII in the presence of H2O2 was determined at pH 5.5 at 37 °C for 36 h.
Minimum inhibitory concentrations (MICs) of metal ions
MIC is the concentrations at which an antibacterial agent experiences the complete inhibitions of the growth of microorganisms22. The MICs of metal ions were determined using the microbroth dilution method in a 96-well plate for the three bacteria (E. coli, S. aureus and S. epidermidis). E. coli was grown overnight in LB media, and S. aureus and S. epidermidis were grown overnight in TSB media. The bacterial solutions were diluted to an optical density of 0.1 at 670 nm (OD670) in corresponding medium. Consequently, 100 µL of the bacterial solutions were distributed to each well of the 96-well plate. The concentrations of metal ions were prepared, ranging from 0.125 µM to 32 µM. The bacterial solutions in wells were incubated with metal ions for 10 h, followed by measuring the OD670 values of the testing solutions by microplate reader (Synergy 2 Multi-Mode, BioTek, USA). The growth curves of bacteria in metal ion solutions were shown in Supplementary Information (Figs S1–S3).
Cytotoxicity of metal ions
The cytotoxicity of metal ions for NIH 3T3 fibroblast cells was evaluated by MTT assay. The concentrations of metal ion solutions were prepared ranging from 31.25 µM to 250 µM. 3T3 fibroblasts cells were cultured in a 24-well plate at a seeding concentration of 1 × 104 cell/well in DMEM containing 10% of FBS in a humidified 5% CO2 incubator at 37 °C for 24 h. Afterward, the culture medium was replaced by FBS-free DMEM, which contained metal ions of interest at various concentrations. After incubation for 24 h, the MTT solution was introduced to the culture medium to a final concentration of 0.5 mg/mL. MTT, a yellow tetrazole, is reduced to purple formazan in living cells. Therefore, after incubations for 3 h, the medium was replaced by DMSO in each well to dissolve the purple formazan crystals. The absorbance of solutions was quantified by a spectroscopic reader at 550 nm. The cell culture without treatment was set as a control. The resulting value was averaged from triplicate samples.
Tests for zone of inhibition
Antibacterial activity of metallogels was accessed by disc diffusion test by measurement of zone of inhibition. E. coli was cultured in sterile LB liquid culture medium; S. epidermidis and USA300 were incubated in sterile TSB at 37 °C for 24 h. Then, the bacterial solutions were diluted to 0.1 of OD670 using the corresponding medium, followed by dropping 10 µL of diluted bacterial solutions onto sterile agar plates and spreading homogeneously. Six samples of metallogels, TA-TiIV, TA-TiIV-FeIII, TA-TiIV-CuII, TA-TiIV-CoII, TA-TiIV-NiII, and TA-TiIV-ZnII were cut into a round shape with a diameter of 8 mm and thickness of 1.5 mm. The samples were placed on the agar plates in an incubator for 24 h. The zone of inhibition is measured using a pair of calipers. Its size is rounded off to the closest millimeter, and the diameter of the disk is also included.
Animal tests
This study was carried out in strict accordance with an approved Institutional Animal Care and Use Committee (IACUC) protocol at National Central University (NCU), Taiwan. The experimental animals and were purchased from BioLASCO Taiwan Co., Ltd. The animal experiments were conducted referring to previous work23. Six female mice weighed 250–300 g were used in this study. Animals were anesthetized via injection of 2,2,2-tribromoethanol. Dorsal skin was shaved and sanitized. A circular full-thickness wound was created by a punch with a 6 mm diameter. 10 µL of pathogenic S. aureus bacterial solution at a concentration of 107 CFU/mL in PBS was dropped on the wounds. Three dressings, including gauze, TA-TiIV and TA-TiIV-CuII metallogels were placed on the wounds, followed by covering Tegaderm (3 M, USA) to immobilize the dressings. Dressings were changed on day 1, day 4, and day 6. The appearance of wounds was observed and photographed. The analytical equations of the percentage of the wound area were based on the ratio of recovered wound area and created wound area on day 0. The animal experiments completed when wounds with one type of dressing were apparently healed. Animals were euthanized at the endpoint of day 8. In addition, the concentrations of S. aureus on infected wounds with treatment of TA-TiIV, and TA-TiIV-CuII were determined on 8 day. The cotton stick was used to collect bacteria on wounds and put into 1 mL PBS solution, followed by bacterial culture on TSB agar supplemented with 1% methicillin and incubation at 35 °C for 24 h. Afterward, the colony forming unit (CFU) on agar was calculated.
Statistical analysis
Values are expressed as means ± standard deviation (S.D.) unless otherwise indicated. Student’s t-test was used for comparisons of data to assess significant differences.
Results and Discussion
Formation of metallogels
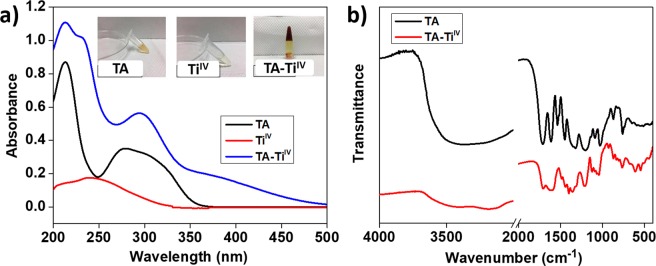
TA and TiIV were mixed at pH 7 for the formation of TA-TiIV metallogels. UV-vis and FTIR spectroscopies were employed to characterize coordinative interactions of the metal ions and phenolic groups. In Fig. 1a, the UV-Vis spectrum of the TA solution presents two absorbance peaks at 213 and 279 nm. The TiIV solution shows a broad band in the range 225–275 nm with a maximum at 239 nm. After adding the TiIV solution in the TA solution, an increasing in absorbance in a range of 375–450 nm, corresponding to coordinative interaction. The results are consistent with the previous literature14, indicating the formation of bis/tris-type chelating structures24. TA provides multiple galloyl chelating sites, which can coordinate with various transition metals. The stable coordination structure between TA and TiIV can be explained by high oxidation state and formal charge of TiIV, which plays a significant role in the solvent-trapping process of the gelation. Moreover, the inserted photographs in Fig. 1a display appearance of TA, TiIV solutions and the TA-TiIV metallogel. The gel was formed after mixing for 15 min and the color of solutions turned to red, reflecting the absorbance at high wavelength from the UV-vis spectroscopic measurements.
Figure 1.
Characterizations of the formation of TA-TiIV metallogels using UV-vis (a) and FTIR (b) spectroscopies. The inserted photographs in (a) indicate the solutions of TA, TiIV, and TA-TiIV in tubes.
Additionally, the interactions between TA and TiIV in metallogels were investigated by FTIR spectroscopy (Fig. 1b). The FTIR spectrum of TA shows a characteristic peak at 3369 cm−1 for stretching vibration of H-bonded –OH25, and a peak at 1711 cm−1 for stretching vibration of C=O (carboxylic ester) groups26, and a peak at 1612 cm−1 for aromatic C=C in free phenolic groups27. Moreover, peaks at 1535 and 1447 cm−1 were attributed to vibrations of aromatic C-C stretching25. Peaks at 1320 and 1202 cm−1 correspond to O-H deformation of phenolic, and a peak at 758 cm−1 was represented for vibration of aromatic C-H4,16. The shifts of the characteristic peaks for TA-TiIV metallogels with respect to the TA alone have been proven by the disappearance of the H-bonded –OH and manifested by a broadened peak at 3182 cm−1 due to the formation the coordinative bonding between –OH and TiIV. Moreover, the peaks for the stretching of aromatic C–C in TA shifted to 1484 and 1439 cm−1 due to deprotonations of the hydroxyl groups and complexation with metal ions. In addition, the coordinative interaction attributed to the shifts of the vibrations of O–H bonds to 1400, 1216, and 764 cm−1 4,25. Consequently, the formation of metallogels through the chelation of phenolic groups in TA and TiIV was demonstrated by spectroscopic analysis.
Release profiles of antimicrobial metal ions from metallogels
Antimicrobial metal ions of FeIII, CuII, CoII, NiII, and ZnII were incorporated into the metallogels. The release profiles of the metal ions were recorded by using ICP-MASS to determine the difference in the coordination strength with phenolic groups of TA (Fig. 2). The metallogels of TA-TiIV-FeIII, TA-TiIV-CuII, TA-TiIV-CoII, TA-TiIV-NiII, and TA-TiIV-ZnII were immersed into PBS at pH 7.4 and physiological temperature of 37 °C. As shown in Fig. 2, NiII was released from TA-TiIV-NiII fastest, followed by CoII, ZnII, CuII, and FeIII. The release profiles of metals from metallogels were fitted using Higuchi model28:
where Q is cumulative release amount, t is the release time in hour, and KH is the Higuchi dissolution constant. In this work, the KH values for FeIII, CuII, CoII, NiII, and ZnII were 0.03, 0.18, 1.81, 2.20, and 0.41, respectively. Therefore, the KH values reflect the release order. The different release profiles of metal ions can be explained by the strength of coordinative interaction with phenolic groups. According to empirical hard-soft acid-base theory (HSAB)17, acidic phenolic components, such as TA, have a pKa value in the range of 7–9, thus they are easily deprotonated at or below physiological pH. Deprotonated polyphenol ligands behave as hard Lewis bases, giving rise to large metal-binding constants with hard Lewis acids, such as TiIV and FeIII. In contrast, CuII, ZnII, NiII and CoII are moderate Lewis acids and do not bind as strongly as hard Lewis acid to hard oxygen atoms of phenolic groups. Moreover, previous study indicated CuII has a higher binding constant with phenolic groups than ZnII 29, which is consistent with higher ZnII release rate than CuII from TA-TiIV metallogels. It is worthwhile to note that undetectable release of TiIV was found from the ICP-MASS detection, thereby the structure of the metallogels was maintained throughout the testing period.
Figure 2.
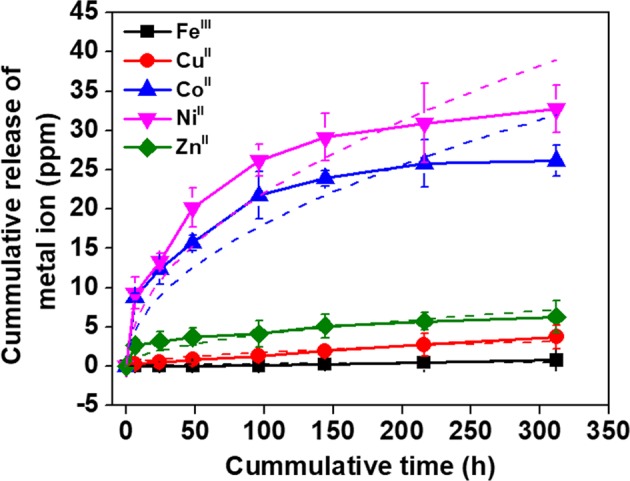
The release profiles of metal ions from metallogels in PBS at pH 7.4 and 37 °C. The cumulative release concentrations of metal ions were recorded using ICP-MASS for 13 days. The data was fitted by using the Higuchi model (dash lines).
Triggered release of metal ions from metallogels
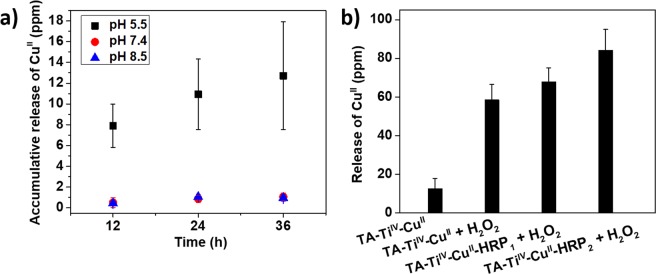
The susceptibility of the coordinative structures between TA and metal ions to pH and H2O2 was investigated to explore responsiveness of the metallogels. In Fig. 3a, three different pH values have been used to test the release profiles of CuII from metallogels of TA-TiIV-CuII. As a result, the release of CuII at pH 5.5 was much faster than that at higher pH, indicating the disassembly of the phenol-metal coordinative structure14. At a low pH value, the galloyl groups of TA are predominately protonated and relatively few phenolate binding sites are available for complexations with CuII, facilitating the high accumulative concentration. On the contrary, at the high pH, the number of deprotonated phenolate groups increases for complexation with CuII.
Figure 3.
The release of CuII from metallogels in responses to the changes in pH (a) and the presence of H2O2 at pH 5.5 (b).
The release of CuII from TA-TiIV-CuII in the presence of oxidizer of H2O2 was measured in Fig. 3b. The concentration of released CuII from metallogels in H2O2 was 4.1-fold higher than that without H2O2. Moreover, the TA-TiIV-CuII metallogels were incorporated with HRP enzymes with a concentration of 31 and 62 U/gel, corresponding to TA-TiIV-CuII-HRP1 and TA-TiIV-CuII-HRP2, respectively. HRP catalyzes the oxidation of various organic substrates by H2O2. Thus, we suspect that the release of CuII should be accelerated in the presence of HRP and H2O2 in the metallogels. The faster release of CuII from the metallogels associated with the concentration of HRP was observed, and, however, the changes were not dramatic. Therefore, in consideration of cost effectiveness and ease of preparation, the metallgels without HRP were employed for biological tests and applications.
The increased release of CuII from the metallogels triggered by H2O2 is ascribed to two mechanisms: 1. the oxidation of phenols to quinones in TA, leading to loss of coordinative capability with CuII 30; 2. the formation of CuI in the presence of H2O2 due to the Fenton-like reaction31. For the antibacterial property, the redox of CuII is majorly responsible for killing bacteria through damaging the outer membrane and inhibiting cell respiration by producing a high level of ROS32. CuII was also found to interfere with the replication of 16 S rRNA genes33. In addition, CuI can directly react with certain metabolic enzymes in bacteria and deactivate them34. Taken together, the results have further strengthened hypothesis of the enhanced antimicrobial property of TA-TiIV-CuII in the presence of H+ and H2O2, which endogenously generate in the infected wounds.
Cytotoxicity test of metallogels
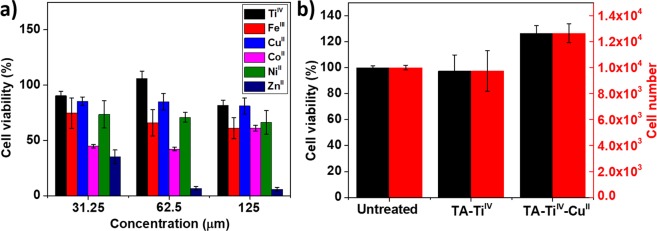
The cytotoxicity of metal ions in solutions was investigated with NIH-3T3 fibroblasts. As shown in Fig. 4a, the concentrations of metal ions were prepared in a range from 31.25 to 125 µM and the solutions were incubated with fibroblasts in a serum-free DMEM for 24 h, followed by the MTT assay. As a result, CoII and ZnII displayed high toxicity to fibroblasts, and TiIV and CuII exhibited negligible toxicity in the range of the concentrations. Moreover, cytotoxicity of the metallogels of TA-TiIV and TA-TiIV-CuII were accessed in Fig. 4b. Obviously, the growth of fibroblasts was not affected by the metallogels, likely due to low cytotoxicity of natural product of TA and slow release of CuII and TiIV. The high cell viability for all samples reveals the low cytotoxicity of the metallogels for medical applications.
Figure 4.
MTT assays for metal ions in solutions (a) and metallogels (b). NIH-3T3 fibroblasts were used for the MTT assays.
Antimicrobial properties of metallogels
MIC tests were conducted for evaluating the antimicrobial capability of all metal ions in solutions. Three bacteria, Gram-negative E. coli, Gram-positive S. aureus, and S. epidermidis, which are common cause of infections involving indwelling foreign devices and surgical wound infections, were employed. In Table 1, E. coli seems susceptible to metal ions, likely reflecting their different degrees of interaction with cell wall structures. Generally, different metals cause distinct types of damages to microbial cells as a result of oxidative stress, protein dysfunction, membrane damage and genotoxicity. CoII was more toxic to bacteria than others. The antimicrobial capabilities of FeIII and CuII metal ions were comparable and moderate.
Table 1.
MIC tests for metal ions with E. coli, S. aureus and S. epidermidis.
| MIC (mM) | |||
|---|---|---|---|
| E. coli | S. aureus | S. epidermidis | |
| TiIV | — | — | — |
| FeIII | 4 ± 0.5 | 8 ± 0.5 | 8 ± 0.4 |
| CuII | 4 ± 0.3 | 8 ± 0.7 | 8 ± 1.1 |
| CoII | 0.5 ± 0.2 | 1 ± 0.3 | 2 ± 0.2 |
| NiII | 2 ± 0.2 | 4 ± 0.6 | 4 ± 0.2 |
| ZnII | 1 ± 0.3 | 16 ± 2.2 | 16 ± 1.5 |
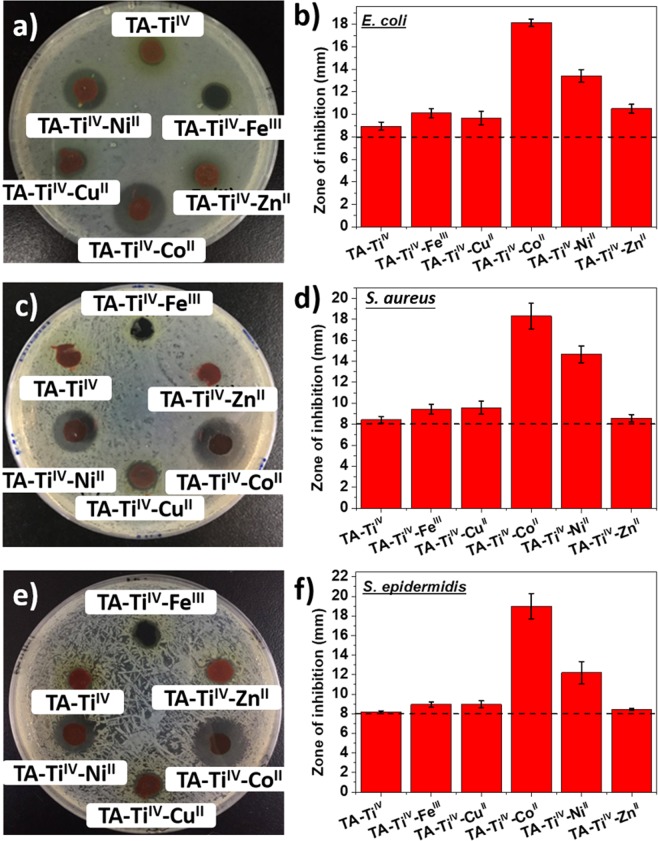
The disc diffusion tests were carried out for the metallogels to compare their antimicrobial properties by measuring the diameter of the inhibition zone in Fig. 5. E. coli (Fig. 5a,b), S. aureus (Fig. 5c,d) and S. epidermidis (Fig. 5e,f) were spread and cultured on agar plates, followed by incubation with metallogels for 24 h. From the diameter measurements of Fig. 5b,d and f, the dashed lines indicate the original diameter of the metallogel discs, which is 8 mm. Therefore, TA-TiIV showed a marginal effectiveness to suppress the growth of bacteria. Moreover, the data from the inhibition zones of TA-TiIV-ZnII are in agreement with that from MIC tests, showing high toxicity to E. coli, but not to S. aureus and S. epidermidis. Moreover, TA-TiIV-CoII exhibited the strongest antimicrobial property against all bacteria, followed by TA-TiIV-NiII. The results can be ascribed to high antimicrobial potency (Table 1) and fast release of CoII and NiII (Fig. 2) from the metallogels. Although the excellent antimicrobial performance with CoII and NiII was observed, the high cytotoxicity and fast release from the metallogels constrain medical applications. On the contrary, TA-TiIV-CuII did not appear to effectively kill bacteria. The good biocompatibility and controlled release of CuII make TA-TiIV-CuII a suitable material for medical uses. Consequently, TA-TiIV-CuII metallogel was employed as a smart dressing for controlled release antimicrobial metal ions in response to the changes in pH and the presence of H2O2. Herein, the responsiveness of TA-TiIV-CuII cannot be performed by the disc diffusion test because acid and H2O2 are bactericidal agents. The test cannot distinguish the antimicrobial roles of metal ions, acid and H2O2. Accordingly, the antimicrobial and responsiveness of TA-TiIV-CuII will be demonstrated on infected wounds in mice.
Figure 5.
The disc diffusion tests for metallogels of TA-TiIV, TA-TiIV-FeIII, TA-TiIV-CuII, TA-TiIV-CoII, TA-TiIV-NiII, and TA-TiIV-ZnII with E. coli (a,b), S. aureus (c,d) and S. epidermidis (e,f). The dashed lines indicate the original diameter of the metallogel discs.
Metallogels as infected wound dressings
The use of TA-TiIV-CuII as a dressing material for infected wounds was conducted to demonstrate the unique characteristics of metallogels. The wounds were created on the back of mice and applied with pathogenic S. aureus. Gauze and TA-TiIV were employed as dressings for comparison. In Fig. 6a, the photographs of wounds with treatment of different dressings were taken periodically to estimate their wound areas. In Fig. 6b, the healing rates of the wounds were determined by reduction in the wound areas. As a result, the wounds with TA-TiIV-CuII displayed faster recovery than that with other dressings. Moreover, the presence of bacteria on wounds was estimated by counting CFU on tryptic soy broth (TSB) agar supplemented with 1% methicillin. The cotton stick was used to collect bacteria on wounds and put into 1 mL PBS solution, followed by bacterial culture on agar plates to calculate colony forming unit (CFU). In Fig. 6c, obviously, the concentration of bacteria on the wounds with TA-TiIV-CuII was extremely lower than that with TA-TiIV, showing good antimicrobial potency of TA-TiIV-CuII.
Figure 6.
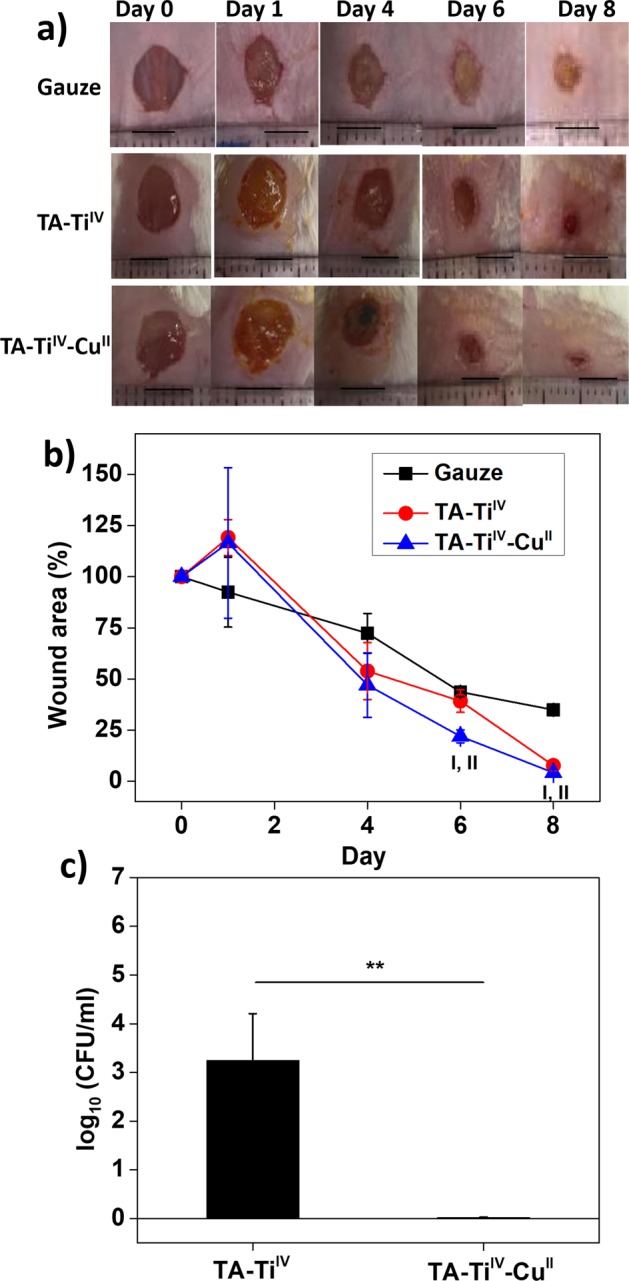
Animal tests for wound dressings for healing of infected wounds. The photographs of wounds with treatment of dressings of gauze, TA-TiIV, and TA-TiIV-CuII (a). Scare bars: 5 mm, n = 3. The wound areas for the wounds with different dressings (b). I: significant difference between gauze and TA-TiIV-CuII (p < 0.05). II: significant difference between TA-TiIV and TA-TiIV-CuII (p < 0.05). Bacterial concentrations of infected wounds with treatment of TA-TiIV, and TA-TiIV-CuII for 8 days (c). **p < 0.01.
Conclusively, TA-TiIV-CuII promotes the healing process of the infected wounds due to multiple functions of high hydration, good biocompatibility, and controlled release of antimicrobial agent. Additionally, the gauze adheres to the newly grown tissues on wounds after 3-day use, which occurs very often with traditional dressings when removal of the hydrogel and scab formation35. TA-TiIV-CuII showed very weak adhesion to the wounds due to the hydrophilic surfaces and low cell attachment36. Moreover, previous research indicates that CuII is well-known as an essential element of angiogenesis37, and interacts with many factors involved in the wound healing process38,39. Therefore, CuII serves not only an antimicrobial agent, but also a promoter for wound recovery. The responsiveness of the coordinative interaction between galloyl groups and CuII to the changes of pH and H2O2 allows spontaneous release of CuII while the wound is infected. The intelligent property and good biocompatibility of the metallogels enable their implementation in the wound management.
Conclusions
A nature derived metallogel was successfully fabricated by simple gelation through coordinative interaction between TA and TiIV. Antimicrobial metal ions were incorporated into the metallogels by co-gelation. Because of unique characteristics of phenol-metal chelating interactions, the release of metal ions can be triggered by acid and H2O2, which are endogenously produced due to the immune response of wounds against bacterial infection. Therefore, the antimicrobial agents spontaneously release while wounds get infected. The cytotoxicity of metal ions and metallogels were carefully evaluated to find a biocompatible dressing material. In this work, TA-TiIV-CuII metallogel possesses characteristics of controlled release, biocompatibility, pH- and H2O2-dependent antimicrobial property, and weak adhesion to wounds. We expect that the further development will establish TA-based metallogels as a smart system for advanced biomedical applications.
Supplementary information
Supplementary Info: Intelligent Metal-Phenolic Metallogels as Dressings for Infected Wounds
Acknowledgements
We acknowledged the financial support for this work provided by the Ministry of Science and Technology (MOST 105-2628-E-008 -007 -MY3).
Author Contributions
H.T.P.A., C.M.H. and C.J.H. have made substantial contribution to conception and design of the study. H.T.P.A. performed the experiments. H.T.P.A. and C.J.H. have made substantial contributions for analysis and interpretation of data. H.T.P.A. and C.J.H. wrote the manuscript and prepared the figures, which are critically reviewed by C.M.H.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47978-9.
References
- 1.Bessa LJ, Fazii P, Di Giulio M, Cellini L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Int. Wound J. 2015;12:47–52. doi: 10.1111/iwj.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsou TL, et al. Poly(2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release studies. J. Mater. Sci.-Mater. Med. 2005;16:95–100. doi: 10.1007/s10856-005-5954-2. [DOI] [PubMed] [Google Scholar]
- 3.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001;14:244–+. doi: 10.1128/cmr.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 2007;298:413–420. doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 5.Lu ZT, et al. Hydrogel degradation triggered by pH for the smart release of antibiotics to combat bacterial infection. New J. Chem. 2017;41:432–436. doi: 10.1039/c6nj03260e. [DOI] [Google Scholar]
- 6.Handke LD, et al. Staphylococcus epidermidis saeR is an effector of anaerobic growth and a mediator of acute inflammation. Infect. Immun. 2008;76:141–152. doi: 10.1128/iai.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeulen M, et al. Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J. Immunol. 2004;172:3196–3204. doi: 10.4049/jimmunol.172.5.3196. [DOI] [PubMed] [Google Scholar]
- 8.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Vatansever F, et al. Antimicrobial strategies centered around reactive oxygen species - bactericidal antibiotics, photodynamic therapy, and beyond. Fems Microbiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol. Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu GY, Wang Q, Lu SL, Niu YW. Hydrogen Peroxide: A Potential Wound Therapeutic Target? Med. Princ. Pract. 2017;26:301–308. doi: 10.1159/000475501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, et al. Smart H2O2-Responsive Drug Delivery System Made by Halloysite Nanotubes and Carbohydrate Polymers. ACS Appl. Mater. Interfaces. 2017;9:31626–31633. doi: 10.1021/acsami.7b10867. [DOI] [PubMed] [Google Scholar]
- 13.Saravanakumar G, Kim J, Kim WJ. Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges. Adv. Sci. 2017;4:19. doi: 10.1002/advs.201600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahim MA, et al. Metal-Phenolic Supramolecular Gelation. Angew. Chem.-Int. Edit. 2016;55:13803–13807. doi: 10.1002/anie.201608413. [DOI] [PubMed] [Google Scholar]
- 15.Casey AL, et al. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010;74:72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal S, McHale P, Duffy B. Preparation and rapid analysis of antibacterial silver, copper and zinc doped sot-gel surfaces. Colloid Surf. B-Biointerfaces. 2012;94:170–176. doi: 10.1016/j.colsurfb.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 18.Holten-Andersen N, et al. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA. 2011;108:2651–2655. doi: 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedo J, Saiz-Poseu J, Busque F, Ruiz-Molina D. Catechol-Based Biomimetic Functional Materials. Adv. Mater. 2013;25:653–701. doi: 10.1002/adma.201202343. [DOI] [PubMed] [Google Scholar]
- 20.Ejima H, et al. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science. 2013;341:154–157. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 21.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science. 2010;328:216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzola PG, Jozala AF, Novaes LCD, Moriel P, Penna TCV. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009;45:241–248. doi: 10.1590/s1984-82502009000200008. [DOI] [Google Scholar]
- 23.Huang KT, et al. Non-sticky and antimicrobial zwitterionic nanocomposite dressings for infected chronic wounds. Biomater. Sci. 2017;5:1072–1081. doi: 10.1039/c7bm00039a. [DOI] [PubMed] [Google Scholar]
- 24.Sever, M. J. & Wilker, J. J. Visible absorption spectra of metal-catecholate and metal-tironate complexes. Dalton Trans. 1061–1072, 10.1039/b315811j (2004). [DOI] [PubMed]
- 25.Cakar S, Ozacar M. Fe-tannic acid complex dye as photo sensitizer for different morphological ZnO based DSSCs. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2016;163:79–88. doi: 10.1016/j.saa.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Ninan N, Forget A, Shastri VP, Voelcker NH, Blencowe A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces. 2016;8:28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 27.Borgias BA, Cooper SR, Koh YB, Raymond KN. Synthetic, structural, and physical studies of titanium complexes of catechol and 3,5-di-tertbutylcatechol. Inorganic Chemistry. 1984;23:1009–1016. doi: 10.1021/ic00176a005. [DOI] [Google Scholar]
- 28.Lu HW, Zhang LM, Liu JY, Chen RF. Synthesis of an amphiphilic polysaccharide derivative and its micellization for drug release. J. Bioact. Compat. Polym. 2008;23:154–170. doi: 10.1177/0883911507088272. [DOI] [Google Scholar]
- 29.Lee BP, Narkar A, Wilharm R. Effect of metal ion type on the movement of hydrogel actuator based on catechol-metal ion coordination chemistry. Sens. Actuator B-Chem. 2016;227:248–254. doi: 10.1016/j.snb.2015.12.038. [DOI] [Google Scholar]
- 30.Kholdeeva OA, Zalomaeva OV. Recent advances in transition-metal-catalyzed selective oxidation of substituted phenols and methoxyarenes with environmentally benign oxidants. Coord. Chem. Rev. 2016;306:302–330. doi: 10.1016/j.ccr.2015.07.019. [DOI] [Google Scholar]
- 31.Lu Y, et al. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl. Mater. Interfaces. 2018;10:127–138. doi: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battistoni A, et al. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 2000;68:30–37. doi: 10.1128/iai.68.1.30-37.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, et al. Toward a Molecular Understanding of the Antibacterial Mechanism of Copper-Bearing Titanium Alloys against Staphylococcus aureus. Adv. Healthc. Mater. 2016;5:557–566. doi: 10.1002/adhm.201500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho M, Kim J, Kim JY, Yoon J, Kim JH. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010;44:3410–3418. doi: 10.1016/j.watres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Lalani R, Liu LY. Electrospun Zwitterionic Poly(Sulfobetaine Methacrylate) for Nonadherent, Superabsorbent, and Antimicrobial Wound Dressing Applications. Biomacromolecules. 2012;13:1853–1863. doi: 10.1021/bm300345e. [DOI] [PubMed] [Google Scholar]
- 36.Huang CJ, Chu SH, Wang LC, Li CH, Lee TR. Bioinspired Zwitterionic Surface Coatings with Robust Photostability and Fouling Resistance. ACS Appl. Mater. Interfaces. 2015;7:23776–23786. doi: 10.1021/acsami.5b08418. [DOI] [PubMed] [Google Scholar]
- 37.Wang XJ, et al. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016;46:286–298. doi: 10.1016/j.actbio.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Gerard C, Bordeleau LJ, Barralet J, Doillon CJ. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31:824–831. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Sen CK, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol.-Heart Circul. Physiol. 2002;282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info: Intelligent Metal-Phenolic Metallogels as Dressings for Infected Wounds