Abstract
The detrimental effects of synthetic fungicides have increased the emphasis for biological control as an effective and safe sustainable alternative method. In the present work, a potent rhizospheric actinobacterium MR14 showed broad spectrum antifungal and plant growth promoting activities indicating the potential to fulfill the need. Phylogenetic analysis confirmed that the isolate could be assigned as new species of the Streptomyces, coded as Streptomyces sp. MR14. It formed clade with Streptomyces daghestanicus but with very low bootstrap value (14%). The MR14 supernatant showed potent antagonistic activity against 13 different tested fungal phytopathogens. The most and least sensitive fungal phytopathogens were found to be Pyricularia oryzae and Fusarium oxysporum with inhibition zones of 31 mm and 11 mm, respectively. The antifungal metabolites produced by strain MR14 were thermostable, photostable, and remained active at extreme acidic and neutral pH. In pot experiments, the Streptomyces sp. MR14 cells, supernatant and extract significantly suppressed Fusarium wilt caused by Fusarium moniliforme in tomato plants. Various growth parameters such as shoot and root lengths, and plant fresh and dry weights were significantly enhanced by 19.65 to 321.62% over the pathogen infested plants only. The treatment with culture cells/supernatant/extract in the rhizosphere soil also reduced the microbial count as compared to control. In addition, the strain also possessed plant growth promoting potential which was indicated by the increase in various agronomic traits from 3.64 to 116.88%. This study provided a scientific validation that the new rhizobacterium Streptomyces sp. MR14 could be further developed as bioformulation, exhibiting biocontrol and plant growth promoting capabilities.
Keywords: Fungal phytopathogens, Biocontrol, Plant growth promotion, Rhizosphere, Streptomyces, Fusarium wilt
Introduction
Worldwide rapidly increasing population is one of the greatest challenges for the agriculture field. This global struggle becomes more disastrous with the loss of crop yield due to the phytopathogens (bacterial, fungal, pests, nematodes etc.) especially, various fungal phytopathogens (Oerke 2006). The current strategies employed to control plant fungal diseases are mainly the application of synthetic fungicides including triazoles and acylalanines, and the development of resistant varieties (Emmert and Handelsman 1999). However, the arbitrary use of synthetic fungicides has resulted in the development of fungicide resistance in the pathogens, environmental pollution, ecological imbalance in the soil and adverse effects on human health and beneficial microflora (Fox et al. 2007; Thind 2008; Ntalli and Menkissoglu-Spiroudi 2011). So, their use is being restricted in several countries which further necessitate the quest for biological control as an effective and long lasting alternative to control various soil-borne fungal diseases. The specificity to the host plant, adaptiveness of most biocontrol agents (BCAs) to the environment, the involvement of several mechanisms of disease suppression by a single microorganism and the complex organismal interactions contribute to the belief that the biological control is more effective and durable than chemical fungicides (Sharma and Sharma 2008; Manhas and Kaur 2016).
Among natural sources, microorganisms always have been accounted as the solution to every problem and now become the center of intensive research globally. Thus far, various microbial antagonists of rhizospheric origin including species of Bacillus, Pseudomonas, Streptomyces, Trichoderma and nonpathogenic Fusarium have been effectively used as BCAs (Heydaria and Pessarakli 2010; Faheem et al. 2015). They are also used for promotion of the plants by enhancing the nutrient availability to the plants (Gopalakrishnan et al. 2011).
Such novel microbial species having biocontrol and plant growth promoting capabilities mainly reside at interface between roots of higher plants and soil i.e. rhizosphere and benefit the host through a number of mechanisms (Heydaria and Pessarakli 2010; Shobha and Kumudini 2012). In well-studied rhizosphere of a healthy plant, availability of nutrients and organic materials derived from the root exudates become the driving force for the abundance of diverse microbial community (Raaijmakers et al. 2009). The complex plant–microbe interactions such as biogeochemical cycling of nutrients, protection from phytopathogens and production of various growth promoting hormones affect the plant health and function which in turn improve the global productivity (Berendsen et al. 2012; Philippot et al. 2013).
Among the rhizospheric microbial community, the immense importance is given to the genus Streptomyces for the production of vast array of compounds with pharmaceutical and agricultural importance (Prabavathy et al. 2006; Palaniyandi et al. 2013). Spore formation by these filamentous and Gram-positive rhizobacteria also offers an additional advantage to be developed as plant growth improving agents through formulations (Emmert and Handelsman 1999; Shobha and Kumudini 2012). Some species of Streptomyces have already been successfully developed into formulations to control fungal phytopathogens on various crops. Commercially available wettable formulation Mycostop consisting of spores and mycelium of Streptomyces griseoviridis has been extensively used in Europe and North America for the protection of ornamental and vegetable crops (Tahvonen and Avikainen 1987). Another non commercial wettable formulation prepared from Streptomyces sp. Di-944 prevents damping off disease of tomato caused by Rhizoctonia solani (Sabaratnam and Traquair 2002). However, more novel species of Streptomyces are still waiting to be identified and developed as biocontrol and plant growth promoting agents.
Keeping in view the importance of Streptomyces as biocontrol agents, a potent streptomycete isolate, designated as MR14, exhibiting antifungal and plant growth promoting activities was isolated from the rhizosphere soil of mustard (Brassica nigra), collected from Amritsar (India). The objective of this study was to characterize the isolate MR14 using polyphasic approach and to assess the in vivo effect of the isolate to control Fusarium wilt caused by F. moniliforme on the tomato plants. Further, the in vivo potential of strain on plant growth was evaluated by observing its effect on various agronomic traits in tomato plant.
Materials and methods
Sample collection and isolation of Streptomyces sp. MR14
The strain MR14 (MTCC 12924) was isolated from soil sample collected from rhizosphere of mustard (B. nigra) plant, from the designated site MR-ASR (31.63°N 74.87°E), situated in Amritsar, Punjab (India) (Kaur et al. 2013). The isolate exhibited antagonistic activity against different fungal phytopathogens and also various plant growth promoting activities such as production of indole acetic acid, siderophore and ammonia. The isolate MR14 was maintained on Starch Casein Nitrate Agar medium (SCNA; g/L): starch 10.0, casein 0.3, KNO3 2.0, NaCl 2.0, K2HPO4 2.0, MgSO4·7H2O 0.05, CaCO3 0.02, FeSO4·7H2O 0.01 and agar 20.0) slants at 4 °C in the refrigerator and as spore suspensions in 20% (v/v) glycerol at − 70 °C in an ultra-low temperature freezer.
Test microorganisms
Various phytopathogenic fungi used in this study viz. Alternaria brassicicola (MTCC 2102), Colletotrichum acutatum (MTCC 1037), Cladosporium herbarum (MTCC 351), Fusarium oxysporum (MTCC 284), Alternaria solani (MTCC 2101), Pyricularia oryzae (MTCC 1477) and Fusarium oxysporum f.sp. dianthi (MTCC 6659) were obtained from Microbial Type Culture Collection (MTCC) and Gene Bank, Institute of Microbial Technology (IMTECH), Chandigarh, India. Cercospora beticola (KJ461435), Exserohilum sp., Fusarium moniliforme, Colletotrichum gloeosporioides, Alternaria mali and Alternaria alternata were isolated in the laboratory. All the fungal cultures were maintained on Potato Dextrose Agar (PDA) slants at 4 °C.
Characterization and identification of isolate MR14 using polyphasic approach
Morphological, biochemical and physiological characterization
The isolate MR14 was characterized on the basis of cultural characteristics (sporulation, color of the aerial and substrate mycelia and soluble pigment production in the medium) as per methods prescribed in International Streptomyces Project (Shirling and Gotllieb 1966). The isolate was inoculated on SCNA and different ISP media (ISP-1, ISP-2, ISP-3, ISP-4, ISP-5, ISP-6 and ISP-7) for 7 days at 28 °C. Morphological properties of the isolate were observed by the light microscope (Olympus) at 100X, and micro-morphological properties such as spore chain and spore surface morphologies were determined by scanning electron microscopy (Carl Zeiss model EVOLS 10). Assimilation of variety of sugars as carbon sources was studied according to Shirling and Gotllieb (1966). d-glucose, xylose, lactose, maltose, cellulose, glycerol, starch, sucrose, fructose and l-inositol (1%) (HiMedia, India) were added to the basal medium after filter sterilization. The capability of the isolate to produce industrially important enzymes (amylase, protease, lipase, cellulase, chitinase, pectinase, gelatinase, urease, catalase and oxidase), and H2S, and utilization of citrate were determined according to Cowan and Steel (1965). Indole production and Methyl Red and Voges Proskauer (MR––VP) tests were performed as recommended by Holding and Collee (1971). Physiological tests were performed by growing the strain on SCNA at different temperatures (20–50 °C) and different NaCl concentrations (0–20% w/v). Analysis of isomers of diaminopimelic acid (DAP) in the cell wall and sugars in the whole-cell hydrolysate was done according to the method given by Lechevalier and Lechevalier (1970).
Genomic characterization based on 16S rRNA gene sequencing
For 16S rRNA gene sequencing, the genomic DNA of the isolate was extracted following the method given by Marmur (1961), and then amplification of the 16S rRNA gene sequence was performed by polymerase chain reaction (PCR) using primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-AGAAAGGAGGTGATCCAGGC-3′). The obtained PCR product was purified using QIA quick gel extraction kit (Qiagen, Germany). The purified PCR product got sequenced from Institute of Microbial Technology (IMTECH), Chandigarh, India. Using the EzTaxon server (http://www.ezbiocloud.net/), identification of phylogenetic neighbors and calculation of pair wise 16S rRNA gene sequence similarities were achieved (Chun et al. 2007). The almost complete sequence (1434 bp) of isolate MR14 and the sequences of phylogenetic neighbors were aligned using Clustal W Program. Phylogenetic trees were constructed according to the neighbor joining and maximum-parsimony algorithms using bootstrap values based on 1000 replications with the MEGA 6.0 software (Felsenstein 1985; Tamura et al. 2013).
In vitro antifungal activity profile of Streptomyces sp. MR14 against fungal phytopathogens
For the inoculum preparation, the growth of 7 days old streptomycete MR14 was transferred aseptically into the seed medium (SCN broth) and incubated for 48 h at 28 °C on an orbitek rotary shaker at 180 rpm. Fermentation was carried out by inoculating the production medium (SCN broth) with seed culture (2%) and incubated at 28 °C for 1 week under agitation at 180 rpm. After every 24 h, the flasks were harvested and the culture broth was centrifuged at 10,000 rpm for 20 min at 4 °C to separate the biomass. The biomass was dried at 60 °C for 2 days, weighed and expressed in mg on dry weight basis.
The remaining cell free culture supernatant was used to check the activity against different fungal phytopathogens by agar well diffusion method (Bauer et al. 1966). The PDA (Potato Dextrose Agar; Hi-Media) plates, inoculated with test fungi (100 μL; 105 spores/mL), were punctured with sterile cork borer to make wells (9 mm), and 200 μL of culture supernatant was added to each well under aseptic conditions. Prior to incubation, the plates were kept at 4 °C for 30 min for diffusion of the antifungal compounds. Then the PDA plates were incubated at 28 °C for 4–5 days. The antifungal activity of the isolate was detected as clear zones of inhibition around wells, measured in millimeters.
Stability of antifungal compounds in culture supernatant of Streptomyces sp. MR14
To check the thermostability of the antifungal metabolites produced by Streptomyces sp. MR14, culture supernatant was kept at different temperatures (− 20 °C, 37 °C, 50 °C, 70 °C, 100 °C, autoclaving) for one hour. Photostability was tested by exposing the supernatant separately to UV and sunlight for 1 h. To investigate the enzyme stability, culture supernatant was treated with 0.1 mg/mL proteinase K and trypsin at 37 °C for 60 min. The effect of pH on antifungal activity was investigated by adjusting the pH of culture supernatant at 2.0 and 14.0, followed by incubation for 1 h at 28 °C. All the treated samples were then checked for the residual activity using F. moniliforme. The shelf life of the antifungal metabolites at 4 °C was determined by storing the culture supernatant in refrigerator and checking the antifungal activity at regular intervals of time for 6 months.
Extraction of bioactive metabolites
The extraction of antifungal metabolites was done using solvent–solvent extraction technique. The culture supernatant of Streptomyces sp. MR14 was extracted twice in the ratio of 1:1 (culture supernatant: solvent) with six different solvents of a wide range of polarity, namely, n-butanol, methanol, ethyl acetate, chloroform, diethyl ether and hexane. The organic phase was concentrated to dryness using rotavapor (BUCHI). The obtained crude extracts were redissolved in respective solvents and tested for their antifungal activity using agar disc diffusion assay (Bauer et al. 1966). As the maximum recovery of antifungal metabolites was obtained in ethyl acetate further work was done using ethyl acetate extract of MR14. For in vivo biocontrol assay, the ethyl acetate extract of MR14 (250 µg/mL) was dissolved in 0.5% DMSO.
In vivo biocontrol of F. moniliforme causal organism of Fusarium wilt by Streptomyces sp. MR14 and its effect on plant growth promotion in tomato plants
In vivo pot experiment was conducted in the month of 10th Oct–25th Nov, 2017 at Guru Nanak Dev University, Amritsar using soil drenching method. The aim was to investigate the potentiality of the culture cells, culture supernatant as well as culture extract of Streptomyces sp. MR14 to control the F. moniliforme (causal fungal phytopathogen of Fusarium wilt) and promote various plant growth traits. Seeds of tomato (Solanum lycopersicum Mill., variety Pusa Ruby, susceptible to F. moniliforme) were sown in sterilized soil at 28 ± 2 °C for 1 month. The pots (8 cm diameter) containing 100 g of autoclaved soil were divided into 8 groups and each group was given different treatment. Group 1 (C) control: soil was treated with 10 mL water only, Group 2 (P) fungal pathogen: soil was infested with 10 mL of fungal F. moniliforme spore suspension (1 × 106 spores/mL); Group 3 (CC + P) culture cells and pathogen: soil was inoculated with 10 mL of pathogen spore suspension and 10 mL of Streptomyces sp. MR14 culture cell suspension (1 × 106 cells/mL) prepared in autoclaved water; Group 4 (CS + P) culture supernatant and pathogen: soil was inoculated with 10 mL of F. moniliforme suspension and 10 mL of culture supernatant obtained from 4 days old fermentation broth of Streptomyces sp. MR14; Group 5 (CE + P) culture extract and pathogen: soil was inoculated with 10 mL of fungal spore suspension and 10 mL of culture extract of Streptomyces sp. MR14 dissolved in water (250 µg/mL); Group 6 (CC) culture cells only: soil was treated with 10 mL of cell suspension (1 × 106 cells/mL) of Streptomyces sp. MR14. Group 7 (CS) culture supernatant only: soil was treated with 10 mL of culture supernatant; Group 8 (CE) culture extract only: treated with culture extract of Streptomyces sp. MR14 (250 µg/mL). Along with each treatment, the tomato seedlings with true stage leaves were then transplanted singly into pots. Each treatment group was replicated three times and the pots were kept under natural conditions. Plants were watered daily and the wilting of tomato plant at pre-emergence growth stage was recorded after 45 days of treatments. The plants were uprooted and fresh and dry weights of tomato seedlings were recorded.
Estimation of bacterial and fungal counts in the rhizosphere of the tomato plants
The rhizosphere soils of the treated plants were carefully sampled after 45 days. One gram of the soil was suspended in 9 mL of sterile distilled water and vortexed at high speed. The serial dilutions were prepared and aliquots of 0.1 mL from 10−3 dilution were spread on to Nutrient Agar (NA) amended with cycloheximide (50 µg/mL) and Potato Dextrose Agar (PDA) medium for bacterial and fungal counts, respectively. The NA and PDA plates were incubated at 37 °C and 28 °C, respectively. The CFUs (colony forming units) were counted after 24 h for bacteria and after 72 h for fungi.
Statistical analysis
Data collected from the above experiments were subjected to statistical analysis where values were represented as their mean ± SD. To compare difference in means, one way analysis of variance (ANOVA) with Tukey’s post hoc test was performed using SPSS statistical analysis software (Version 20.0; IBM SPSS). Statistically significant difference was considered at p ≤ 0.05. Correlation analysis was also done using SPSS to determine the relationship between antifungal activity and biomass obtained.
Results
Characterization and identification of isolate MR14 using polyphasic approach
Morphological, biochemical and physiological characterization
The actinobacterium MR14 displayed different colony morphological characteristics on various ISP media as shown in Table 1. Soluble pigment was not produced by the isolate on any of the media except ISP-7 medium which is the characteristic of melanin pigment production. The isolate showed cream sporulation and aerial mycelium and light yellow color substrate mycelium on the SCNA medium. Morphological characteristics such as spore chains of the isolate MR14 in the light microscope (100X) were observed as flexuous sporophores and placed in Rectus-Flexibilis (RF) group of Streptomyces (Fig. 1a). Micromorphological studies by SEM revealed chains of smooth surface spores on aerial mycelium, bearing 20–30 cylindrical spores (1.5–2.0 µm length and 1.5 µm width) (Fig. 1b).
Table 1.
Cultural characteristics of Streptomyces sp. MR14 on ISP media and SCNA medium
| Medium | Growth | Aerial mycelium | Substrate mycelium | Diffusible pigment |
|---|---|---|---|---|
| ISP-1 | Very good | Cream | Brown | – |
| ISP-2 | Very good | Creamish yellow | Light brown | – |
| ISP-3 | – | – | – | – |
| ISP-4 | Fair | Cream | Light brown | – |
| ISP-5 | Very good | Cream | Grey | – |
| ISP-6 | Good | Creamish yellow | Yellow | – |
| ISP-7 | Very good | Light brown | Brown | Brown |
| SCNA | Good | Cream | Creamish yellow | – |
Fig. 1.
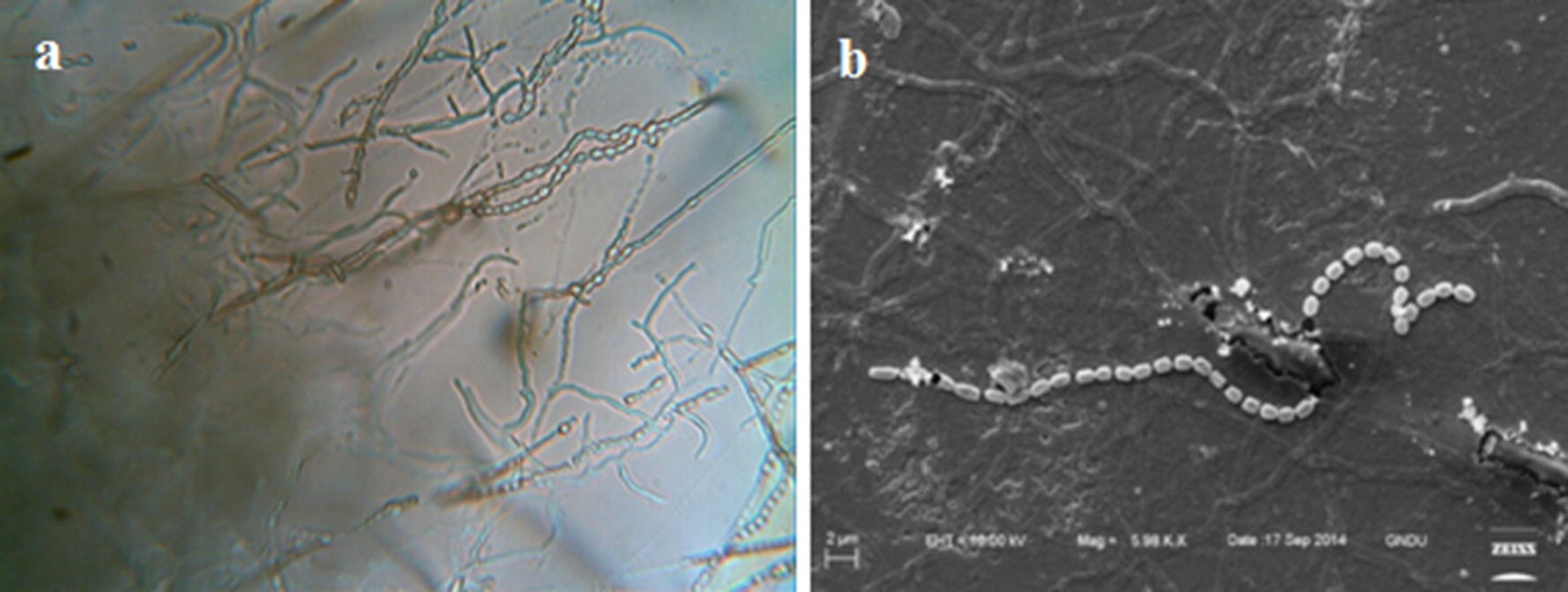
a Light micrograph at 100X showing rectus-flexibilis spore chains on aerial mycelium of Streptomyces sp. MR14 grown on Starch Casein Nitrate Agar for 5 days at 28 °C. b Electron microscopic view of aerial mycelium showing smooth surface of spores of Streptomyces sp. MR14 at 6000X
The isolate grew well between 20 and 40 °C (optimum at 30 °C). It tolerated NaCl concentration up to 5.0%. It produced industrially important extracellular enzymes such as amylase, lipase, protease and urease by degrading their respective substrates starch, lipid, casein and urea. However, response to pectin, cellulose, citrate, chitin and gelatin degradation was negative. The strain neither reduced nitrate nor produced H2S. It gave positive results for both indole production and VP test. The strain was able to utilize most of the tested sugars as sole carbon source except xylose, arabinose and fructose. The maximum growth of MR14 was observed on medium containing starch and glycerol. Chemotaxonomic analysis of the isolate showed the presence of ll-diaminopimelic acid (ll-DAP) as the diagnostic amino acid in cell wall lysate and absence of characteristic sugars in whole cell hydrolysates (Table 2). Based on morphological, cultural and chemotaxonomical studies, strain MR14 belonged to genus Streptomyces which was further supported by 16S rRNA sequencing.
Table 2.
Morphological, physiological and biochemical characteristics of Streptomyces sp. MR14
| Characteristic | MR14 |
|---|---|
| Spore mass | Cream |
| Spore chain | Recti-flexibilis |
| Spores shape | Cylindrical |
| Substrate mycelium | Creamish yellow |
| Aerial mycelium | Cream |
| Diffusible pigment | – |
| Sugar pattern | No characteristic sugar |
| Diaminopimelic acid | ll-DAP |
| Production of melanoid pigment on | |
| Peptone yeast extract agar (ISP-7) medium | + (Brown) |
| Biochemical characteristics | |
| Amylase | + |
| Protease | + |
| Chitinase | – |
| Cellulase | – |
| Lipase | + |
| Gelatinase | – |
| Urease | + |
| Pectinase | – |
| H2S production | – |
| Nitrate reduction | – |
| Catalase | + |
| Oxidase | + |
| MR | – |
| VP | + |
| Citrate utilization | – |
| Indole production | + |
| Tolerance to NaCl | 5% |
| Growth temperature | 30 °C (20 to 35 °C) |
| Utilization of sugar | |
| Maltose | + |
| d-Glucose | + |
| Sucrose | + |
| Lactose | + |
| Inositol | + |
| d-Xylose | – |
| d-Fructose | – |
| Starch | ++ |
| Glycerol | ++ |
| Arabinose | – |
| Rhamnose | + |
| Raffinose | + |
Phylogenetic and genomic analyses based on 16S rRNA Sequencing
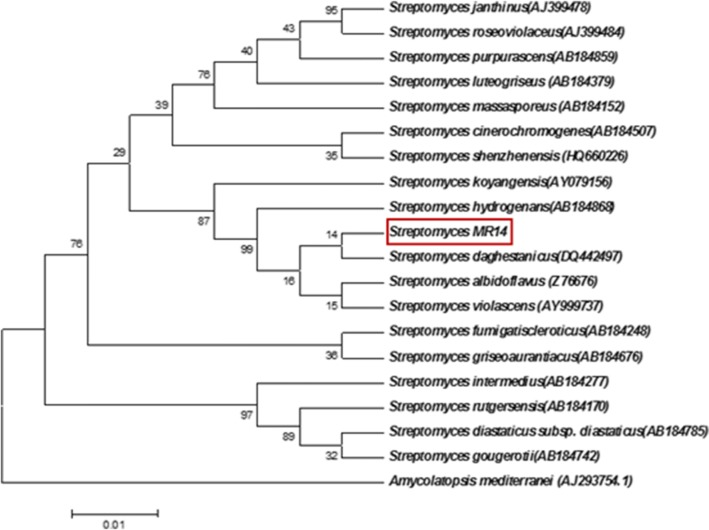
16S rRNA gene sequence (1434 bp) of strain MR14 was compared with all available nucleotide sequences of other closely related Streptomyces species using the EzTaxon database. The data showed 100% sequence similarity with four Streptomyces spp. i.e. Streptomyces violascens ISP 5183 (T) AY999737, Streptomyces hydrogenans (AB184868), Streptomyces daghestanicus (DQ442497) and Streptomyces albidoflavus DSM 40455 (T) (Z76676). Further confirmation was done by constructing phylogenetic trees using neighbor-joining (Fig. 2) and maximum-parsimony algorithms (Fig. 3). In the phylogenetic tree constructed using the neighbor-joining method, MR14 formed clade with S. daghestanicus (DQ442497). However, the lower bootstrap value of 14 (< 50%) excludes the possibility of specific relatedness between the two species. This relationship was also supported by maximum parsimony tree where MR14 formed a phyletic branch different from all closely related species, showing low bootstrap value (34%). Thus on the basis of this, MR14 can be assigned as a new sp. of Streptomyces, designated as Streptomyces sp. MR14. The 16S rRNA gene sequence of the isolate MR14 has been deposited in the GenBank database under the accession number KY522669. The culture has been deposited in Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh (India), an International Depository Authority, and the accorded accession number is MTCC-12924.
Fig. 2.
Neighbor-joining tree based on nearly complete 16S rRNA gene sequences showing the position of isolate Streptomyces sp. MR14 amongst its phylogenetic neighbors. Bootstrap values (expressed as percentages of 1000 replications) are shown at the nodes. Amycolatopsis mediterranei (AJ293754.1) was used as an outgroup. GenBank accession numbers are given in parentheses
Fig. 3.
Maximum-parsimony phylogenetic tree based on 16S rRNA gene sequences of Streptomyces strains showing the position of isolate Streptomyces sp. MR14. Bootstrap values (expressed as percentages of 1000 replications) are shown at the nodes
In vitro antifungal activity profile of Streptomyces sp. MR14 against fungal phytopathogens
In vitro activity profile demonstrated that the production of antifungal metabolites (in terms of inhibition zone) in culture broth by strain MR14 commenced on 1st day of incubation, reached the maximum after 4 days and then declined as the incubation was further extended. The maximum biomass production was also observed after 4 days of incubation which indicated positive correlation between the antifungal activity and growth (Fig. 4).
Fig. 4.
Growth and antifungal activity of Streptomyces sp. MR14 against different phytopathogens; **correlation is significant at 0.01% level; *correlation is significant at 0.05% level
As the maximum antifungal activity was achieved at the 4th day of incubation, the spectrum of the antagonistic potential of the Streptomyces sp. MR14 was assessed using 4 days old culture supernatant against a variety of fungal phytopathogens. The strain displayed antifungal activity against all the tested fungal phytopathogens with inhibition zones ranging from 31 ± 0.0 to 11 ± 0.5 mm (Fig. 5). The highest activity, in terms of inhibition zone, was observed against P. oryzae (31 mm) closely followed by Exserohilum sp. and C. gloeosporioides (29 mm). Moderate activity was observed against C. acutatum (28 mm), A. brassicicola (27 mm), A. alternata (26 mm), A. solani (26 mm), A. mali (25 mm) and C. herbarum (21 mm), and weak activity was detected against F. monilifome (16 mm), C. beticola (16 mm) and F. oxysporum (11 mm).
Fig. 5.
A Antifungal activity of Streptomyces sp. MR14 against different fungal phytopathogens; bars with different letters are statistically different (Tukey’s HSD, p ≤ 0.05). B The clear zones around the wells showing antifungal activity of Streptomyces sp. MR14 by well diffusion method against (i) A. brassicicola (ii) A. mali (iii) A. alternata (iv) A. solani (v) C. acutatum (vi) C. gloeosporioides (vii) Exserohilum sp. (viii) F. moniliforme
Stability of antifungal compounds in culture supernatant of Streptomyces sp. MR14
The stability of antifungal compounds present in the culture supernatant was checked for various physical stresses (Table 3). In terms of inhibition zone, the active metabolites responsible for the antifungal activity of Streptomyces sp. MR14 were found to be completely stable up to 50 °C for one hour, and loss of 22.86% in activity was observed at 70 °C. More than 50% activity retained after boiling for one hour and no activity was observed after autoclaving. The metabolites were also found to be photo stable because 97.14% of activity retained after exposure to UV light for 1 h. Antifungal compounds were also stable to enzyme proteinase K, showing 97.14% residual activity after treatment. More than 50% of residual antifungal activity was observed even at extreme acidic and basic pH of 2.0 and 14.0. Only 20% of loss in activity was observed even after 6 months storage of culture supernatant of Streptomyces sp. MR14 at 4 °C which indicated long shelf life of antifungal components at refrigeration temperature.
Table 3.
Effect of temperature, pH, light and enzymatic treatments on the antifungal activity of culture supernatant of Streptomyces sp. MR14
| Treatment | Streptomyces sp. MR14 | |
|---|---|---|
| Zone of inhibition (mm) against F. moniliforme | Residual activity (%) | |
| Control (untreated) | 35.0 ± 0.0 | 100.0 |
| Heat treatment | ||
| 37 °C, 1 h | 35.0 ± 0.1 | 100.0 |
| 50 °C, 1 h | 34.0 ± 0.2 | 97.14 |
| 70 °C, 1 h | 27.0 ± 1.0 | 77.14 |
| 100 °C, 1 h | 18.0 ± 0.5 | 51.42 |
| 121 °C, 45 min | 0.0 ± 0.0 | 0.0 |
| Low temperature treatment | ||
| − 20 °C, 1 h | 35.0 ± 0.1 | 100.0 |
| Enzymatic treatment | ||
| Proteinase K | 34.0 ± 0.0 | 97.14 |
| Photostability | ||
| Sunlight, 1 h | 15.0 ± 0.5 | 42.85 |
| UV light, I h | 34.0 ± 0.1 | 97.14 |
| pH tolerance | ||
| pH 2.0 | 33.0 ± 0.5 | 94.28 |
| pH 14.0 | 20.0 ± 1.0 | 57.14 |
Values represented as mean ± S.D (n = 3)
Extraction of bioactive compound/s
For the recovery of bioactive compounds present in the culture supernatant of Streptomyces sp. MR14, solvents of different polarity were used. Among all the tested solvents, complete extraction of the bioactive metabolites was obtained using ethyl acetate at pH 5.0. Approximately 60 mg of solvent extract was obtained from 1 L of fermentation broth which was redissolved in 0.5% DMSO prior to the biocontrol assay.
In vivo biocontrol of F. moniliforme causal organism of Fusarium wilt by Streptomyces sp. MR14 and its effect on plant growth promotion in tomato plants
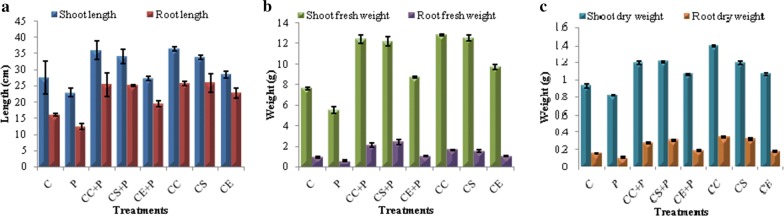
The pot experiments were carried out to evaluate in vivo biocontrol efficiency of strain MR14 against F. moniliforme. The data collected in terms of various agronomic traits of plant which are indicators of plant health and condition, such as shoot length, root length, shoot and root fresh and dry weights are shown in Table 4, Figs. 6, 7. The pots containing soil drenched with culture cells/culture supernatant/culture extract of strain MR14 and fungal pathogen significantly enhanced the root and shoot lengths, and fresh and dry weights of the tomato plants over the plants which were treated with pathogen only. Among the three groups, the soil treated with culture cells/supernatant and pathogen showed higher biocontrol efficiency than the culture extract and pathogen.
Table 4.
Biocontrol and plant growth promoting effect of Streptomyces sp. MR14 on various growth traits of tomato seedlings infested with fungal phytopathogen F. moniliforme
| Group | Shoot length (cm) | Root length (cm) | Shoot fresh weight (g) | Root fresh weight (g) | Shoot dry weight (g) | Root dry weight (g) |
|---|---|---|---|---|---|---|
| C | 27.5 ± 5.1a | 16.2 ± 0.4a | 7.648 ± 0.1a | 0.949 ± 0.07a | 0.936 ± 0.02a | 0.154 ± 0.007a |
| P | 22.9 ± 1.4b | 12.4 ± 0.9b | 5.53 ± 0.3b | 0.569 ± 0.08b | 0.826 ± 0.01b | 0.107 ± 0.005b |
| CC + P | 35.9 ± 2.8ab (56.77)1 | 25.4 ± 3.7ab (104.84)1 | 12.421 ± 0.4ab (124.61)1 | 2.13 ± 0.2ab (274.34)1 | 1.194 ± 0.02ab (44.55)1 | 0.276 ± 0.009ab (157.94)1 |
| CS + P | 34.1 ± 2.2ab (48.91)1 | 25.2 ± 0.3ab (103.23)1 | 12.218 ± 0.4ab (120.94)1 | 2.399 ± 0.3ab (321.62)1 | 1.208 ± 0.01ab (46.25)1 | 0.299 ± 0.008ab (179.44)1 |
| CE + P | 27.4 ± 0.6 (19.65)1 | 19.5 ± 0.8b (57.26)1 | 8.744 ± 0.1ab (58.12)1 | 1.059 ± 0.02b (86.12)1 | 1.068 ± 0.009ab (29.3)1 | 0.185 ± 0.007ab (72.9)1 |
| CC | 36.4 ± 0.6ab (32.36)2 | 25.8 ± 0.7ab (59.26)2 | 12.822 ± 0.08ab (67.65)2 | 1.651 ± 0.02ab (73.97)2 | 1.404 ± 0.01ab (50.0)2 | 0.334 ± 0.007ab (116.88)2 |
| CS | 33.9 ± 0.6ab (23.27)2 | 25.9 ± 2.8ab (59.88)2 | 12.552 ± 0.3ab (64.12)2 | 1.557 ± 0.1ab (64.07)2 | 1.191 ± 0.02ab (27.24)2 | 0.315 ± 0.011ab (104.55)2 |
| CE | 28.5 ± 0.95 (3.64)2 | 22.8 ± 1.6ab (40.74)2 | 9.743 ± 0.2ab (27.39)2 | 1.053 ± 0.02b (10.96)2 | 1.072 ± 0.02ab (14.53)2 | 0.173 ± 0.011b (12.34)2 |
All data are presented as mean ± SD (n = 3). The same letters with in a column are significantly different (Tukey’s Test p ≤ 0.05). “a” indicates the statistical difference between the control and treated plants; “b” indicates the statistical difference between the pathogen infested and treated plants
C control, P pathogen, CC + P culture cells and pathogen, CS + P culture supernatant and pathogen, CE + P culture extract and pathogen, CC culture cells, CS culture supernatant, CE culture extract
1Values indicate percentage increase over pathogen infested plants
2Values indicate percentage increase over control plants
Fig. 6.
Effect of Streptomyces sp. MR14 and its metabolites as root treatments on S. lycopersicum (tomato) plants to control F. moniliforme causing Fusarium wilt disease a shoot and root lengths of plants, b fresh weights of shoots and roots, c dry weights of shoots and roots. Values were expressed in mean ± standard deviation; C, control (Water only); P, pathogen only; CC + P, Streptomyces sp. MR14 cells + pathogen; CS + P, MR14 supernatant + pathogen; CE + P, Streptomyces sp. MR14 solvent extract + pathogen; CC, MR14 cells only; CS, MR14 supernatant only; CE, solvent extract of strain MR14 only
Fig. 7.

Effect of Streptomyces sp. MR14 and its metabolites on growth of S. lycopersicum (tomato) plants infested with F. moniliforme; C, control (water only); P, pathogen only; CC + P, Streptomyces sp. MR14 cells + pathogen; CS + P, MR14 supernatant + pathogen; CE + P, Streptomyces sp. MR14 solvent extract + pathogen
The strain MR14 not only controlled the disease caused by F. moniliforme but also promoted the growth of the tomato plants. The culture cells, culture supernatant and cell extract significantly increased the shoot (3.64 to 32.36%) and root (40.74 to 59.26%) lengths, fresh weights of shoots (27.39 to 67.65%) and roots (10.96 to 73.97%) and dry weights of shoots (14.53 to 50.0%) and roots (12.34 to 116.88%) over the control plants (Table 4, Figs. 6, 8). These data indicated biocontrol and plant growth promoting potential of Streptomyces sp. MR14 and its metabolites.
Fig. 8.

Plant growth promoting potential of Streptomyces sp. MR14 and its metabolites on growth of S. lycopersicum (tomato) plants; C (untreated plants, water only), CC (plants treated with culture cells only), CS (plants treated with culture supernatant only) and CE (plants treated with culture extract of strain MR14 only)
Estimation of bacterial and fungal counts in the rhizosphere of the tomato plants
After 45 days, bacterial and fungal counts were determined in the rhizosphere of the treated plants (treated with culture cells/supernatant/extract along with pathogen). The results showed that the treatment with culture cells/supernatant/extract and pathogen significantly reduced the other competing bacterial and fungal counts along with the pathogen in the soil. The CFUs in control (water only) soil was very high as compared to the treated soils (Table 5).
Table 5.
Bacterial and fungal counts in rhizosphere soils of control (water only) and different treated plants after 45 days
| Treatment | Microbial count (104 CFU/g) | |
|---|---|---|
| Bacteria | Fungi | |
| Control | 146a | 53a |
| CC + P | 42ab | 15ab |
| CS + P | 34ac | 12ac |
| CE + P | 37ad | 19ad |
The same letters with in a column are significantly different (Tukey’s HSD p ≤ 0.05) and different letters with in a column are statistically insignificant different; Control, tomato plants treated with water only; CC + P, plants treated with Streptomyces sp. MR14 and pathogen; CS + P, plants treated with MR14 supernatant and pathogen; CE + P, plants treated with MR14 solvent extract and pathogen
Discussion
In spite of the remarkable progress attained in plant breeding, and other disease management practices, loss in crop yield due to diseases caused by phytopathogens remains a major limiting factor in the agriculture growth globally. The losses are most likely to affect the tropical and developing countries. The food scarcity due to losses further creates the economical and health problems in community and hinders the development of the country. Much greater emphasis is required to control the phytopathogens by natural means. Biological control using Streptomyces spp. has the potential for the management of these diseases and to be further developed as BCAs and plant growth promoting agents (Hartman et al. 2009). Numerous streptomycete antagonists have been used to promote plant growth, and control soil-borne phytopathogens (Gopalakrishnan et al. 2011; Boukaew and Prasertsan 2014; Faheem et al. 2015).
In the present study, a potent streptomycete strain (MR14) isolated from mustard (B. nigra) rhizosphere was characterized through the polyphasic approach. Based on its cultural properties on different media, the strain belongs to the genus Streptomyces. Furthermore, the strain MR14 was also capable of producing a variety of enzymes such as amylase, lipase, protease and urease and could gain consideration in industrial sector. The bioactive compounds produced by the Streptomyces spp. are mainly secondary metabolites and greatly influenced by the availability of carbon sources given during the growth (Ser et al. 2016; Rani et al. 2018). The results indicated that the strain has the capacity to utilize a variety of substrates such as glucose, sucrose, lactose, inositol, starch, glycerol, rhamnose, raffinose and maltose. Chemotaxonomical markers (ll-DAP in cell wall and no characteristic sugar in whole cell hydrolysate) also supported that the strain MR14 belongs to the genus Streptomyces.
The low bootstrap value (14%) in comparative analysis done by constructing phylogenetic trees suggested the unrelatedness of strain MR14 with the closely related species S. violascens ISP 5183 (T) AY999737, S. hydrogenans (AB184868), S. daghestanicus (DQ442497) and S. albidoflavus DSM 40455 (T) (Z76676). Therefore, it might be assigned as a new sp. of Streptomyces and was supported by Sharma et al. (2014). They observed the low bootstrap value of novel Streptomyces amritsarensis sp. 2A with closely related type strains to which it showed very high i.e. 99.5–99.9% 16S rRNA sequence similarity. The novelty of the sp. was further proved by DNA–DNA hybridization. In contrast, Sahu et al. (2017) isolated a species of the novel genus Allostreptomyces showing the highest (99.0%) 16S rRNA sequence similarity with the type strain Allostreptomyces psammosilenae but with a very high bootstrap value (100%). However, DNA–DNA hybridization showed 54.5% relatedness with the type strain, proving the novelty of the species for which the name Allostreptomyces indica sp. nov. was proposed. Moreover, Streptomyces sp. MR14 is distinguished from the S. daghestanicus (which is phylogenetically more closest to MR14 and in the same clade) in terms of antimicrobial activity as the latter is not reported to possess any antimicrobial activity. S. daghestanicus is able to utilize xylose, fructose, citrate, gelatin and cellulose whereas MR14 does not. The novelty of the strain can be further confirmed by DNA- DNA hybridization.
In vitro antagonistic assay of culture supernatant done by well diffusion method revealed broad spectrum antifungal activity of the compound produced by Streptomyces sp. MR14, inhibiting wide range of fungal phytopathogens with varying degree of inhibition. The variation in antagonistic activity of strain could be related to the test pathogen. The culture supernatant showed pronounced effect against P. oryzae (31.0 ± 0.0), Exserohilum sp. and C. gloeosporioides (29 mm). The results demonstrated that F. monilifome (16 mm), C. beticola (16 mm) and F. oxysporum (11 mm) are more resistant as compared to other tested phytopathogens.
For the development of safe and effective formulation to be used as biocontrol and plant growth promoting agent in fields under varying climatic conditions in different regions, the bioactive compounds must be stable at various temperatures and in soils with different pH. The antifungal metabolites from Streptomyces sp. MR14 were found to be thermo and photo stable and active over extreme acidic and basic pH. This property is also quite useful during isolation, purification and processing of bioactive compounds from commercialization point of view.
In vitro disease suppressive potential of the strains does not ensure their use in the fields because various strains do not acclimatize or the bioactive compounds produced by a strain lose their activity in natural conditions (Li et al. 2011). Bhuiyan et al. (2003) observed that two bacterial isolates Pseudomonas aeruginosa and Burkholderia cepacia completely antagonized the Claviceps africana, the cause of ergot or sugary disease of sorghum (Sorghum bicolor) in vitro but failed to inhibit infection in vivo. Therefore, in vivo experiments were conducted to check the effect of Streptomyces sp. MR14 on disease suppression and promotion of the plant growth under natural conditions.
Tomato fruit, the product of Solanum lycopersicum L. plants, is one of the most consumed foods worldwide (Borrero et al. 2006). In 2014, India was the second largest country in production, accounting for about 10.95% worldwide (UN Food and Agriculture Organization Report 2016). Fusarium wilt in tomato mainly caused by Fusarium oxysporum f.sp. lycopersici is one of the major soil borne systemic diseases resulting severe reduction in yield (Larkin and Fravel 1998). There are numerous reports of the Streptomyces spp. with potential to control Fusarium wilt caused by F. oxysporum f.sp. lycopersici. In this particular study, the Streptomyces sp. MR14 was evaluated for the first time to control the Fusarium wilt caused by another species of genus i.e. F. moniliforme. F. moniliforme mainly affects the health of the plant by producing various toxins such as fusaric acid, fusarins, gibberellins, moniliformin, and fumonisins (Abbas et al. 1995). Inovejas and Divina (2018) reported the use of methanol extract and nanocomposite of Trichoderma sp. as a potential bio-control against Fusarium moniliforme in tomato plants.
In vivo pot experiments demonstrated that the soil infested with F. moniliforme and Streptomyces sp. MR14 cells/supernatant/solvent extract possessed the significant antagonistic ability to control the Fusarium wilt in tomato plants. The percentage increase in various growth traits over the pathogen infested plants clearly indicated the strong disease suppression by culture cells and culture supernatant and then followed by culture extract. The treatments altered the microbial community in the rhizosphere. Additionally, the strain MR14 produced significant amounts of IAA when grown in broth containing tryptophan, and siderophores and ammonia (Kaur et al. 2013). Further, the pot experiments determined the significant effect of Streptomyces sp. MR14 on various growth parameters of the plant such as root and shoot length, fresh and dry weights of the plants (3.64 to 116.88% increase as compared to the control). Therefore, the data obtained from in vivo experiments suggested that Streptomyces sp. MR14 might be used as a BCA and plant growth promoting agent in two formulations, one containing spores or mycelium and other containing antifungal metabolites produced by it.
The possible mechanism of various BCAs can be the production of antifungal compounds, hydrolytic cell wall degrading enzymes such as chitinase, β-1,3 glucanase and protease, siderophores, hyperparasitism and induced systemic resistance (De-Oliveira et al. 2010; Palaniyandi et al. 2013; Passari et al. 2015). They can further promote the growth of plants directly by enhancing the production of plant growth hormones such as indole-3-acetic acid (IAA) and solubilizing the inorganic phosphate and indirectly by the production of antifungal compounds (Hamdali et al. 2008; El-Tarabily et al. 2009; Goudjal et al. 2016). The results displayed that Streptomyces sp. MR14 cells or supernatant showed more pronounced effect on the biocontrol and plant growth promotion in tomato plant as compared to solvent extract. But, the reduction in microbial community was significantly same when soil was treated with culture cells/supernatant/extract and pathogen. The improvement in different plant growth parameters by culture supernatant or cells might be associated with the ability to produce IAA, ammonia and siderophores, and root colonization. These results suggest that Streptomyces sp. MR14 employ more than one possible mechanisms along with antibiosis to promote plant growth. In addition, Streptomyces sp. MR14 also exhibited antibacterial and nematicidal activities which further increase its effectiveness as biocontrol agent (unpublished observations). Therefore, it might be developed as safe multifunctional biopesticide against wide range of phytopathogens (fungi, bacteria and nematodes) and as bioinoculant to enhance plant growth.
This study concluded with highlighting the true biocontrol and plant growth promoting potential of a new potent rhizospheric actinobacterium Streptomyces sp. MR14. The strain produced stable antifungals as secondary metabolites in culture supernatant possessing broad spectrum activity against different phytopathogens. In vivo studies validated the biocontrol potential of the strain against Fusarium wilt caused by F. moniliforme, and also demonstrated the alteration in the microbial community structure. In addition, the strain significantly improved the growth and yield of tomato plants by reducing the risk of disease. This study opens the way for the development of new BCA and plant growth promoting agent using Streptomyces sp. for agricultural use and thus providing a platform for its usage at commercial level.
Acknowledgements
We duly acknowledge the grant of fellowship under UPE (University with Potential for Excellence) scheme of University Grants Commission, New Delhi, India for providing funds to accomplish this work.
Abbreviations
- BCAs
biocontrol agents
- SCNA
Starch Casein Nitrate Agar
- PDA
Potato Dextrose Agar
- ll-DAP
ll-diaminopimelic acid
- PCR
polymerase chain reaction
- IMTECH
Institute of Microbial Technology
- NA
Nutrient Agar
Authors’ contributions
TK performed and interpreted the data related to isolation and characterization of the strain and drafted linked content of the manuscript. RR was involved in the planning and execution of the research work; analysis and interpretation of the data; manuscript writing. RKM as research supervisor of RR and TK was involved in planning of research work; analysis and interpretation of data; drafting as well as critical editing of the manuscript for intellectual subject matter. All the authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Funding
This work was financially supported by the University Grants Commission (UGC), New Delhi vide letter no. F. No. 43-468/2014(SR) dated 24 September, 2015.
Availability of data and materials
All the data and materials have been provided in main manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Talwinder Kaur, Email: talwinder.kaur83@gmail.com.
Riveka Rani, Email: riveka.microrsh@gndu.ac.in.
Rajesh Kumari Manhas, Phone: +183 2258802-09, Email: rkmanhas@rediffmail.com.
References
- Abbas HK, Tanaka T, Duke SO. Pathogenicity of alternaria alternata and Fusarium moniliforme and phytotoxicity of AAL-toxin and fumonisin B1 on tomato cultivars. J Phytopathol. 1995;143:329–334. doi: 10.1111/j.1439-0434.1995.tb00270.x. [DOI] [Google Scholar]
- Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Patho. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Bhuiyan SA, Ryley MJ, Galea VJ, Tay D. Evaluation of potential biocontrol agents against Claviceps africana in vitro and in vivo. Plant Pathol. 2003;52:60–67. doi: 10.1046/j.1365-3059.2003.00799.x. [DOI] [Google Scholar]
- Borrero C, Ordova´s J, Trillas MI, Avile´s M. Tomato Fusarium wilt suppressiveness. The relationship between the organic plant growth media and their microbial communities as characterised by Biolog®. Soil Biol Biochem. 2006;38:1631–1637. doi: 10.1016/j.soilbio.2005.11.017. [DOI] [Google Scholar]
- Boukaew S, Prasertsan P. Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot. 2014;61:1–10. doi: 10.1016/j.cropro.2014.02.012. [DOI] [Google Scholar]
- Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- Cowan ST, Steel KJ. Manual for the identification of medical bacteria. London: Cambridge University Press; 1965. [Google Scholar]
- De-Oliveira MF, Da Silva MG, Van Der Sand ST. Anti-phytopathogen potential of endophytic actinobacteria isolated from tomato plants (Lycopersicon esculentum) in southern Brazil, and characterization of Streptomyces sp. R18, a potential biocontrol agent. Res Microbiol. 2010;161:565–572. doi: 10.1016/j.resmic.2010.05.008. [DOI] [PubMed] [Google Scholar]
- El-Tarabily KA, Nassar AH, Hardy GESJ, Sivasithamparam K. Plant growth promotion and biological control of Pythium aphanidermatum a pathogen of cucumber, by endophytic actinomycetes. J Appl Microbiol. 2009;106:13–26. doi: 10.1111/j.1365-2672.2008.03926.x. [DOI] [PubMed] [Google Scholar]
- Emmert EAB, Handelsman J. Biocontrol of plant disease: a (Gram-) positive perspective. FEMS Microbiol Lett. 1999;171:1–9. doi: 10.1111/j.1574-6968.1999.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Faheem M, Raza W, Zhong W, Nan Z, Shen Q, Xu Y. Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum f. sp. niveum. Biol Control. 2015;81:101–110. doi: 10.1016/j.biocontrol.2014.11.012. [DOI] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evol. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fox JE, Gulledge J, Engelhaupt E, Burow ME, McLachlan JA. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc Nat Acad Sci USA. 2007;104:10282–10287. doi: 10.1073/pnas.0611710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Pande S, Sharma M, Humayun P, Kiran BK, Sandeep D, Vidya MS, Deepthi K, Rupela O. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011;30:1070–1078. doi: 10.1016/j.cropro.2011.03.006. [DOI] [Google Scholar]
- Goudjal Y, Zamoum M, Sabaou N, Mathieu F, Zitouni A. Potential of endophytic Streptomyces spp. for biocontrol of Fusarium root rot disease and growth promotion of tomato seedlings. Biocont Sci Technol. 2016;26:1691–1705. doi: 10.1080/09583157.2016.1234584. [DOI] [Google Scholar]
- Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y. Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl Soil Ecol. 2008;40:510–517. doi: 10.1016/j.apsoil.2008.08.001. [DOI] [Google Scholar]
- Hartman A, Schmid M, van Tuinen D, Berg G. Plant driven selection of microbes. Plant Soil. 2009;321:235–257. doi: 10.1007/s11104-008-9814-y. [DOI] [Google Scholar]
- Heydaria A, Pessarakli M. A review on biological control of fungal plant pathogens using microbial antagonists. J Biol Sci. 2010;10:273–290. doi: 10.3923/jbs.2010.273.290. [DOI] [Google Scholar]
- Holding AJ, Collee JG. Routine biochemical tests. Methods Microbiol. 1971;6A:1–31. [Google Scholar]
- Inovejas RC, Divina CC. Methanol extract and nanocomposite of Trichoderma sp. as a potential bio-control against Fusarium moniliforme in tomato (Lycopersicon esculentum) Int J Agric Technol. 2018;14:99–108. [Google Scholar]
- Kaur T, Sharma D, Kaur A, Manhas RK. Antagonistic and plant growth promoting activities of endophytic and soil actinomycetes. Arch Phytopathol Plant Protect. 2013 doi: 10.1080/03235408.2013.777169. [DOI] [Google Scholar]
- Larkin RP, Fravel DR. Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 1998;82:1022–1028. doi: 10.1094/PDIS.1998.82.9.1022. [DOI] [PubMed] [Google Scholar]
- Lechevalier HA, Lechevalier MP. A critical evaluation of the genera of aerobic actinomycetes. In: Prauser H, editor. The actinomycetes. Jena: Gustav Fischer; 1970. pp. 393–405. [Google Scholar]
- Li Q, Jiang Y, Ning P, Zheng L, Huang J, Li G, Jiang D, Hsiang T. Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Cont. 2011;58:139–148. doi: 10.1016/j.biocontrol.2011.04.013. [DOI] [Google Scholar]
- Manhas RK, Kaur T. Biocontrol potential of Streptomyces hydrogenans strain DH16 toward Alternaria brassicicola to control damping off and black leaf spot of Raphanus sativus. Front Plant Sci. 2016;7:1869. doi: 10.3389/fpls.2016.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. doi: 10.1016/S0022-2836(61)80047-8. [DOI] [Google Scholar]
- Ntalli NG, Menkissoglu-Spiroudi U, editors. Pesticides of botanical origin: a promising tool in plant protection. Rijeka: InTech; 2011. [Google Scholar]
- Oerke EC. Crop losses to pests. J Agric Sci. 2006;144:31–43. doi: 10.1017/S0021859605005708. [DOI] [Google Scholar]
- Palaniyandi SA, Yang SH, Zhang L, Suh JW. Effects of actinobacteria on plant disease suppression and growth promotion. Appl Microbiol Biotechnol. 2013;97:9621–9636. doi: 10.1007/s00253-013-5206-1. [DOI] [PubMed] [Google Scholar]
- Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP. In vitro and in vivo plant-growth-promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associates with medicinal plants. PLoS ONE. 2015;10:e0139468. doi: 10.1371/journal.pone.0139468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- Prabavathy VR, Mathivanan N, Murugesan K. Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biol Cont. 2006;39:313–319. doi: 10.1016/j.biocontrol.2006.07.011. [DOI] [Google Scholar]
- Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 2009;321:341–361. doi: 10.1007/s11104-008-9568-6. [DOI] [Google Scholar]
- Rani R, Arora S, Kaur J, Manhas RK. Phenolic compounds as antioxidants and chemopreventive drugs from Streptomyces cellulosae strain TES17 isolated from rhizosphere of Camellia sinensis. BMC Complement Altern Med. 2018;18:82. doi: 10.1186/s12906-018-2154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaratnam S, Traquair JA. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplant. Biol Cont. 2002;23:245–253. doi: 10.1006/bcon.2001.1014. [DOI] [Google Scholar]
- Sahu AK, Quadri SR, Agasar D, Al Ruwaili J, Jun-Li W, Dastager SG. Allostreptomyces indica sp. nov., isolated from India. J Antibiot. 2017;70:1000. doi: 10.1038/ja.2017.82. [DOI] [PubMed] [Google Scholar]
- Ser H-L, Law JW-F, Chaiyakunapruk N, Jacob SA, Palanisamy UD, Chan K-G, Goh B-H, Lee L-H. Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: a systematic review. Front Microbiol. 2016;7:522. doi: 10.3389/fmicb.2016.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Sharma S. Control of foliar diseases of mustard by Bacillus from reclaimed soil. Microbiol Res. 2008;163:408–411. doi: 10.1016/j.micres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Sharma D, Mayilraj S, Manhas RK. Streptomyces amritsarensis sp. nov., exhibiting broad-spectrum antimicrobial activity. Antonie Van Leeuwenhoek. 2014;105:943–949. doi: 10.1007/s10482-014-0151-2. [DOI] [PubMed] [Google Scholar]
- Shirling EB, Gotllieb D. Methods for characterization of streptomycetes sp. Int J Syst Bacteriol. 1966;16:313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- Shobha G, Kumudini BS. Antagonistic effect of the newly isolated PGPR Bacillus spp. on Fusarium oxysporum. Int J Appl Sci Eng Res. 2012;1:463–474. doi: 10.6088/ijaser.0020101047. [DOI] [Google Scholar]
- Tahvonen R, Avikainen H. The biological control of seedborne Alternaria brassicicola of cruciferous plants with a powdery preparation of Streptomyces sp. J Agric Sci Finl. 1987;59:199–208. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind TS. Fungicide resistance: a perpetual challenge in disease control. J Mycol Plant Pathol. 2008;38:407–418. [Google Scholar]
- UN Food and Agriculture Organization, Statistics Division (2016) Global tomato production in 2014. FAOSTAT. http://www.fao.org/faostat/en/#home. Accessed 16 Jan 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data and materials have been provided in main manuscript.