Abstract
Patients with common variable immunodeficiency (CVID) can develop granulomatous-lymphocytic interstitial lung disease (GLILD), which is associated with increased morbidity and mortality. Treating GLILD is a significant challenge because it is rare and can be pathologically heterogeneous. Here we describe two cases of patients with CVID-associated GLILD with biopsies demonstrating loosely organized tertiary lymphoid structures (TLSs). Based on the pivotal role that B cells play in TLS initiation and maintenance, we hypothesized that using rituximab monotherapy for B-cell depletion alone would be sufficient for the disruption of the pathologic process underlying GLILD. These two cases demonstrate that adapting a strategy of B cell depletion monotherapy may be effective in TLS-associated conditions such as GLILD.
Key Words: B cells, common variable immunodeficiency, granulomatous lymphocytic interstitial lung disease, rituximab, tertiary lymphoid tissue
Abbreviations: CVID, common variable immunodeficiency; GLILD, granulomatous-lymphocytic interstitial lung disease; ILD, interstitial lung disease; TLS, tertiary lymphoid structure
Common variable immunodeficiency (CVID) is a heterogeneous group of disorders encompassing a clinical syndrome of recurrent sinopulmonary infections in the setting of hypogammaglobulinemia and ineffective antigen-specific antibody production.1 Granulomatous-lymphocytic interstitial lung disease (GLILD) is a unique form of interstitial lung disease (ILD) seen mainly in patients with CVID, characterized on pathology by the presence of varying degrees of granulomas and differing patterns of lymphoproliferation (ie, lymphocytic interstitial pneumonia, follicular bronchiolitis, and so on).2 The occurrence of GLILD in CVID is significant because of its association with an increased rate of mortality, likely driven by their progressive ILD. Patients who have GLILD have a median survival of 13.7 years, compared with 28.8 years in patients with CVID without ILD.
No established guidelines exist for the treatment of GLILD. Systemic steroid therapy has been used with minimal therapeutic benefit,3 and steroid-sparing agents have been described at a case report level.1, 4 In the largest retrospective review to date, seven patients with GLILD and CVID were treated with combination azathioprine and rituximab. Azathioprine was chosen to target T cells, and rituximab for B cells, as both were found in patient lung biopsies.3 Rituximab is an anti-CD20 antibody that is highly effective at specifically eliminating B cells from the body, and azathioprine is a general purine synthesis inhibitor that results in the inhibition of all dividing cells. While all patients had improvement in their pulmonary function and radiographic abnormalities, it is not clear if dual therapy for both T and B lymphocytes is necessary. In this context, both medications can have treatment-limiting side effects, particularly the less specific azathioprine. Consistent with this, two of the seven patients with CVID-GLILD from the above-cited study were unable to tolerate oral azathioprine because of significant gastrointestinal toxicity.3
T and B lymphocytes in GLILD lung biopsies form tertiary lymphoid structures (TLSs). Recent developments in the understanding of fundamental lymphocyte biology have elucidated a joint requirement for B and T cells to initiate and maintain inflammatory (ie, secondary and tertiary) lymphoid structures. This information suggests that B-cell targeting alone, with the highly specific rituximab, would be sufficient for TLS resolution. Here we report that treatment with rituximab monotherapy in two patients with biopsies showing TLSs may be as effective as combination chemotherapy, and less toxic.
Case 1
A 36-year-old man with known CVID and receiving monthly intravenous immunoglobulin therapy originally presented to the pulmonary clinic for evaluation of an incidentally noted 2-cm nodule in the right lower lobe. He underwent a right lower lobe wedge resection of the nodule with pathology demonstrating B cell-predominant interstitial lymphoid hyperplasia with frequent germinal center formation and rare nonnecrotizing granulomas, consistent with GLILD. Several months after diagnosis, he developed dyspnea at work while lifting heavy objects, and a repeat CT chest scan demonstrated an increasing number of lower lobe-predominant nodules. Pulmonary function testing demonstrated a normal diffusion capacity of the lungs for carbon monoxide, corrected for hemoglobin (Dlco[Hb]). He was started on rituximab and azathioprine but developed significant nausea and vomiting with an associated 20-pound weight loss thought to be secondary to the azathioprine, and therefore both medications were discontinued after 6 weeks of therapy. He initially noted improvement in his dyspnea, but subsequently developed worsening cough and shortness of breath. He was treated with antibiotics and steroids with some improvement in his symptoms. However, 1 year later, he re-presented to the clinic with some worsening of his shortness of breath, and a repeat CT chest scan was consistent with advancing GLILD due to an increased number of small nodules (Fig 1A). Rituximab monotherapy was provided with a target frequency of every 2 weeks, and this resulted in subsequent resolution of his symptoms and radiographic abnormalities over an approximate 1-year period (Fig 1B).
Figure 1.
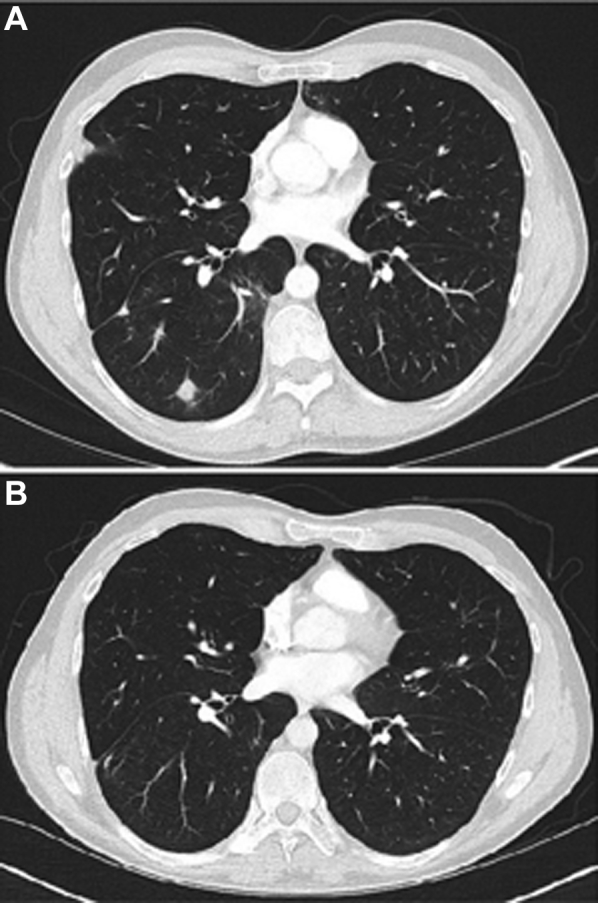
A and B, CT scans of the chest from a patient (case 1) with granulomatous-lymphocytic interstitial lung disease associated with common variable immunodeficiency before (A) and 1 year after (B) treatment with rituximab monotherapy.
Case 2
A 33-year-old woman originally presented to the pulmonary clinic with progressive dyspnea with exertion. A CT scan of the chest demonstrated multiple peribronchovascular lower lobe-predominant nodules of varying density. A video-assisted thorascopic surgical lung biopsy of the right lower lobe was performed, with pathology demonstrating peribronchiolar and interstitial lymphocytic infiltrate with tertiary lymphoid follicles, scattered giant cells, and nonnecrotizing granulomas (Fig 2). Because of her history of recurrent sinusitis and shingles, a workup for immune deficiency was initiated and she was eventually found to have hypogammaglobulinemia consistent with CVID. She was started on monthly intravenous immunoglobulin therapy. Despite this, she developed recurrent lower respiratory tract infections over the subsequent 4 years, requiring prolonged courses of antibiotics and intermittent steroids. She also had progressively deteriorating exercise capacity. Serial CT chest scans showed worsening, widespread multifocal ground-glass and partially solid nodules, interlobular septal thickening, mild bronchiectasis, and bulky mediastinal and hilar lymphadenopathy (Fig 3A). Spirometry showed preserved total lung capacity, FEV1, and FVC, but a decline in Dlco[Hb] from 26.97 mL/min/mm Hg (100% predicted) to 18.03 mL/min/mm Hg (69% predicted). Because of progression of her underlying lung disease, the decision was made to start rituximab (375 mg/m2 once per week) for 4 weeks, repeated every 4 to 6 months for a total of 16 infusions as monotherapy. After the completion of 12 infusions, a repeat CT chest scan 1 year after initiation of rituximab monotherapy showed dramatic improvement in her parenchymal abnormalities and decreased lymphadenopathy (Fig 3B). Her Dlco[Hb] improved to 22.38 mL/min/mm Hg (86% predicted), and she has noted improvement in her exercise capacity.
Figure 2.
A, Hematoxylin and eosin-stained sections of right middle and right lower lobes show a peribronchiolar (*) and interstitial (**) chronic inflammatory infiltrate consisting of nonnecrotizing granulomata and tertiary lymphoid structures (left). At higher magnification (right), the lymphoid structures consist of predominantly small lymphocytes with admixed larger forms and tingible-body macrophages. B, Immunohistochemical studies reveal that the follicles consist predominantly of CD20? B cells with ringing by CD3? T cells. The B cells are positive for CD10, suggestive of follicular center origin, but negative for BCL-2 within the follicular structures (F), which favors a reactive over a neoplastic process. CD21 highlights a preserved, compact underlying follicular dendritic cell meshwork. Additional special studies for fungal (methenamine silver stain), mycobacterial (acid fast), and Epstein-Barr virus infection (in situ hybridization for EBV-encoded RNA) produced negative results (data not shown).
Figure 3.

A and B, CT scans of the chest from a patient (case 2) with granulomatous-lymphocytic interstitial lung disease associated with common variable immunodeficiency before (A) and 1 year after (B) starting rituximab monotherapy.
Discussion
The clinical heterogeneity of disorders that both lead to CVID and contribute to the lymphocytic infiltrates seen in GLILD renders treatment choices difficult. Because of the rarity of GLILD, rigorous immunophenotyping and randomized treatment studies are not feasible, and therapy thus far has been guided primarily by expert opinion and small case series. Here we describe the successful treatment of two patients with CVID-associated GLILD by rituximab monotherapy. This therapeutic strategy was chosen on the basis of the supposition that TLSs are a significant driver of clinical symptoms in GLILD, and emerging evidence of the key role of B cells in TLS initiation and maintenance, in addition to the superior toxicity profile and specificity of B cell-depleting therapy compared with combination chemotherapy.
Despite significant heterogeneity in the proportion of T and B cells comprising the pathology in patients with GLILD, lymphocytic infiltrates can be observed to segregate into distinct B- and T-cell zones.5 In five patient biopsies, regardless of the makeup of the lymphocytic infiltrate, B cells can be found primarily in the context of ectopic follicles, with T cells circumscribing B-cell zones, thereby creating loosely organized TLSs. TLSs develop postnatally in response to environmental antigenic stimulation, and are organized structures, similar to secondary lymphoid organs such as lymph nodes and the spleen.6 TLSs have been found in many chronic pulmonary diseases, including mycobacterial infection, COPD,7 hypersensitivity pneumonitis, asthma, and pulmonary complications from autoimmune diseases such as rheumatoid arthritis8 and Sjögren’s syndrome. Furthermore, not only are TLSs more prevalent in autoimmune-associated interstitial lung disease compared with normal and noninflammatory lung conditions, but the presence of TLSs has also been associated with increased alveolar anti-cyclic citrullinated peptide antibodies and local tissue damage in this context, suggesting that TLSs are not only bystanders in chronic inflammation but may play a role in pathogenesis in the context of autoimmune disease.8
Unlike secondary lymphoid organs, which occur in lymph nodes and spleen, TLSs are not encapsulated and exist in various degrees of organization. In general, TLSs typically contain a B-cell follicle with a surrounding T-cell zone made up of primarily follicular helper T (TFH) cells and are further supported by stromal cells such as follicular dendritic cells that help to organize and maintain the follicle.6 The release of local homeostatic chemokines, and the interactions between the various immune and stromal cells, all play a crucial role in the initiation and maintenance of these TLSs.9 Experimental evidence has shown that B cell-deficient animals fail to form mature TLSs in a cardiac allograft model,10 and in particular, loss of lymphotoxin-expressing B cells results in failure of maturation of mucosal-associated TLSs in the gastrointestinal tract.11 T cells, particularly TFH cells, are essential for the survival and affinity maturation of B cells within the follicle.12 Blocking the inducible costimulatory (ICOS) pathway, which has been shown to impair TFH cell development, reduces the formation of TLSs in a pathologic atherosclerosis model, although it did not completely eliminate the presence of B-cell clusters.13 Within these lymphoid structures, T- and B-cell responses are interdependent on each, and targeting either population would likely disrupt the integrity of the follicle.
Rituximab monotherapy has been shown to be effective in improving clinical symptoms and stabilizing radiographic abnormalities in patients with ILD from Sjögren’s syndrome14 and rheumatoid arthritis,15 two other pulmonary processes in which TLSs have been described. Given the pivotal role of B cells in TLS initiation and propagation, together with the toxicity and superior specificity compared with currently approved T cell-targeted therapies, rituximab monotherapy may be an effective and better tolerated approach than combination chemotherapy in patients with biopsies demonstrating TLSs. On the basis of this scientific rationale and proof of concept demonstrated in the two cases presented here, we propose that rituximab monotherapy may be considered a first-line therapeutic for the treatment of GLILD with biopsies demonstrating the presence of TLSs. Future studies comparing rituximab monotherapy with combination therapy are warranted.
Acknowledgments
Financial/nonfinancial disclosures: G. M. H. is supported by National Institutes of Health grants HL111024, HL135142, and HL130974. D. R. W. is supported by the National Institutes of Health grants AI121394, AI1113217, AI139538, AI137940, and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. None declared (J. N., K. W., M. A.).
References
- 1.Park J.H., Levinson A.I. Granulomatous-lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID) Clin Immunol. 2010;134(2):97–103. doi: 10.1016/j.clim.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Bates C.A., Ellison M.C., Lynch D.A., Cool C.D., Brown K.K., Routes J.M. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114(2):415–421. doi: 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Chase N.M., Verbsky J.W., Hintermeyer M.K. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID) J Clin Immunol. 2013;33(1):30–39. doi: 10.1007/s10875-012-9755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández Pérez E.R. Granulomatous lymphocytic interstitial lung disease. Immunol Allergy Clin North Am. 2012;32(4):621–632. doi: 10.1016/j.iac.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Maglione P.J., Ko H.M., Beasley M.B., Strauchen J.A., Cunningham-Rundles C. Tertiary lymphoid neogenesis is a component of pulmonary lymphoid hyperplasia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;133(2):535–542. doi: 10.1016/j.jaci.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang J.Y., Randall T.D., Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol. 2016;7:258. doi: 10.3389/fimmu.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadava K., Bollyky P., Lawson M.A. The formation and function of tertiary lymphoid follicles in chronic pulmonary inflammation. Immunology. 2016;149(3):262–269. doi: 10.1111/imm.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangel-Moreno J., Hartson L., Navarro C., Gaxiola M., Selman M., Randall T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsughayyir J., Pettigrew G.J., Motallebzadeh R. Spoiling for a fight: B lymphocytes as initiator and effector populations within tertiary lymphoid organs in autoimmunity and transplantation. Front Immunol. 2017;8:1639. doi: 10.3389/fimmu.2017.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motallebzadeh R., Rehakova S., Conlon T.M. Blocking lymphotoxin signaling abrogates the development of ectopic lymphoid tissue within cardiac allografts and inhibits effector antibody responses. FASEB J. 2012;26(1):51–62. doi: 10.1096/fj.11-186973. [DOI] [PubMed] [Google Scholar]
- 11.McDonald K.G., McDonough J.S., Newberry R.D. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174(9):5720–5728. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 12.Sage P.T., Sharpe A.H. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36(7):410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement M., Guedj K., Andreata F. Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015;131(6):560–570. doi: 10.1161/CIRCULATIONAHA.114.010988. [DOI] [PubMed] [Google Scholar]
- 14.Chen M.H., Chen C.K., Chou H.P., Chen M.H., Tsai C.Y., Chang D.M. Rituximab therapy in primary Sjögren’s syndrome with interstitial lung disease: a retrospective cohort study. Clin Exp Rheumatol. 2016;34(6):1077–1084. [PubMed] [Google Scholar]
- 15.Md Yusof MY, Kabia A., Darby M. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology. 2017;56(8):1348–1357. doi: 10.1093/rheumatology/kex072. [DOI] [PMC free article] [PubMed] [Google Scholar]



