Abstract
Background
Vasoactive medications are commonly used in the treatment of critically ill patients, but their impact on the development of ICU-acquired weakness is not well described. The objective of this study is to evaluate the relationship between vasoactive medication use and the outcome of ICU-acquired weakness.
Methods
This is a secondary analysis of mechanically ventilated patients (N = 172) enrolled in a randomized clinical trial of early occupational and physical therapy vs conventional therapy, which evaluated the end point of ICU-acquired weakness on hospital discharge. Patients underwent bedside muscle strength testing by a therapist blinded to study allocation to evaluate for ICU-acquired weakness. The effects of vasoactive medication use on the incidence of ICU-acquired weakness in this population were assessed.
Results
On logistic regression analysis, the use of vasoactive medications increased the odds of developing ICU-acquired weakness (odds ratio [OR], 3.2; P = .01) independent of all other established risk factors for weakness. Duration of vasoactive medication use (in days) (OR, 1.35; P = .004) and cumulative norepinephrine dose (μg/kg/d) (OR, 1.01; P = .02) (but not vasopressin or phenylephrine) were also independently associated with the outcome of ICU-acquired weakness.
Conclusions
In mechanically ventilated patients enrolled in a randomized clinical trial of early mobilization, the use of vasoactive medications was independently associated with the development of ICU-acquired weakness. Prospective trials to further evaluate this relationship are merited.
Trial Registry
ClinicalTrials.gov; No.: NCT01777035; URL: www.clinicaltrials.gov
Key Words: critical illness, critical care outcomes, humans, ICUs, muscle weakness, vasoconstrictor agents
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU-AW, ICU-acquired weakness; OR, odds ratio
Generalized neuromuscular weakness is a common complication of critical illness. It is estimated that at least 25% of patients who require prolonged mechanical ventilation develop ICU-acquired weakness (ICU-AW).1, 2, 3 ICU-AW can lengthen the duration of mechanical ventilation and is associated with increased mortality.4, 5, 6 The functional impairments resulting from ICU-AW can persist for years after discharge.7
Many risk factors for the development of ICU-AW have been described, including pharmacologic interventions used in the treatment of critically ill patients, such as glucocorticoids and neuromuscular blocking agents.8 It is unclear what role other pharmacologic agents used in the ICU, such as vasoactive medications, have in the development of ICU-AW.
Vasoactive medications are used commonly in the treatment of critically ill patients with shock, a life-threatening condition of circulatory failure. Their use allows sustained perfusion to vital organs while the underlying cause of the shock is treated. A portion of patients who receive vasoactive medications will experience adverse effects related to their use. It is well recognized that increased adrenergic stimulation associated with vasoactive medication use can lead to cardiac consequences such as increased rates of arrhythmias and myocardial ischemia.9, 10
Clinically, an association between the use of vasoactive medications and critical illness polyneuropathy has been described, but little remains known about the impact of this class of medications on the development of clinically apparent weakness.11, 12 In addition, a limited number of studies show that in animal models, stimulation of β-adrenergic receptors at high doses in vivo can lead to apoptosis and necrosis in skeletal muscles, similar to what is seen in cardiac myocytes.13, 14, 15 This work suggests biologic plausibility for a link between the use of vasoactive medications in the ICU and skeletal muscle injury that may increase the risk of developing ICU-AW. To further investigate this, we performed a secondary analysis of the association between the use of vasoactive medications and the occurrence of ICU-AW in mechanically ventilated patients enrolled in a clinical trial of early mobilization.
Methods
Study Design and Patients
This study is a secondary analysis of a randomized controlled trial (N = 172) of patients in the medical ICU randomized to receive early physical and occupational therapy within 72 h of mechanical ventilation (early mobilization) or standard care with therapy as ordered by the primary team.16 Patients included were those enrolled in a completed trial of short-term outcomes of an early mobility intervention (n = 104) and patients enrolled in an ongoing trial (ClinicalTrials.gov: NCT01777035) with the same protocol examining the long-term outcomes of an early mobility intervention (n = 68).16 Adult patients greater than 18 years old and admitted to the medical ICU were eligible. Inclusion criteria for early mobility were mechanical ventilation for greater than 24 h but less than 72 h at the time of enrollment. The baseline functional status of all patients was assessed using the Barthel Index, with a score greater than 70 required for study inclusion.17, 18 Exclusion criteria included rapidly changing neurologic conditions, cardiac arrest, elevated intracranial pressure, more than one absent limb, pregnancy, terminal condition (life expectancy less than 6 months), traumatic brain injury, multiple limb fractures or open wounds, or severe chronic pain syndrome on admission. The institutional review board for human studies approved the protocols (11-0218), which were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, and written consent was obtained from the subjects or their surrogates.
All enrolled patients who were mechanically ventilated received daily interruption of sedatives,19 protocol-based weaning from mechanical ventilation,20 and enteral feeding. Severity of illness was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II score on admission and change in Sequential Organ Failure Assessment (SOFA) score from admission to 48 h.21, 22, 23, 24, 25 All patients received daily assessment for the presence of sepsis.26 The initiation and choice of specific vasoactive medications were determined by the primary medical service. The type and dose of vasoactive medication received were recorded daily for all enrolled patients.
All patients had an assessment by physical and occupational therapists blinded to randomization assignment on hospital discharge. The strength of three muscle groups in each upper and lower extremity was measured by Medical Research Council (MRC) score, using a scale from 0 to 5.27, 28 ICU-AW was diagnosed at the time of this assessment when an awake and attentive patient had a muscle strength sum score < 48 out of a maximal score of 60.1
Statistical Analysis
Data were analyzed with Stata 14.1 (StataCorp LP) software. Baseline and outcomes variables were depicted as medians (interquartile ranges). We used the Wilcoxon-Mann-Whitney two-sample rank-sum test to compare continuous variables and the χ2 test or Fisher exact test where appropriate to compare categorical variables. A univariable analysis of the outcome of interest, occurrence of ICU-AW on hospital discharge, was performed, evaluating the effect of early mobilization, currently established risk factors for ICU-AW, and the use of vasoactive medications. To assess the effect of vasoactive medication use on the occurrence of ICU-AW, logistic regression analysis was performed, correcting for risk factors that showed a trend toward significance (P ≤ .1) on univariable analysis and others that were linked to the outcome on a biologically plausible basis. Hierarchical entry of each variable was performed. Goodness of fit was assessed by the Hosmer-Lemeshow test. Using this model, additional logistic regression analysis was performed to assess the effect of vasoactive medication duration of use (days) and dose (normalized by weight) on the occurrence of ICU-AW. Assessment of a dose-dependent response to norepinephrine was performed by grouping patients according to low, medium, or high total dose requirement, with analysis using χ2 for trend.
As a sensitivity analysis, a Fine-Gray competing risk regression was performed using the time of discharge with weakness as the outcome and time of death as the competing risk, with the same confounding variables as the logistic regression. Further evaluation was done with a subgroup analysis of patients who survived to hospital discharge. Logistic regression was performed to assess the effect of vasoactive medication use on ICU-AW in the subgroup of survivors, using the variables selected in original model.
Results
Univariable Analysis
A total of 80 of the 172 patients demonstrated ICU-AW on hospital discharge. Baseline characteristics of patients in the control and early mobilization treatment groups were comparable. Patients with ICU-AW were older, had higher APACHE II scores, a higher incidence of sepsis, longer hospital length of stay, and longer duration of mechanical ventilation (Table 1). Importantly, the use of steroids and neuromuscular blockers did not differ between the groups.
Table 1.
Univariable Analysis of Baseline and Outcome Characteristics
| Variable | ICU-AW (N = 80) | No ICU-AW (N = 92) | P Value |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 61 (49-72) | 50 (31-64) | .0002 |
| Female, No. (%) | 41 (51.3%) | 45 (48.9%) | .76 |
| BMI, kg/m2 | 28.1 (23.7-34.4) | 27.5 (24.4-33.5) | .96 |
| APACHE II | 24 (20-30) | 17 (13-22) | < .0001 |
| Sepsis | 66 (82.5%) | 60 (65.2%) | .01 |
| Diabetes | 24 (30%) | 26 (28.3%) | .8 |
| Outcome characteristics | |||
| Early mobility | 33 (41.3%) | 50 (54.4%) | .09 |
| Ventilator use, d | 5 (2.8-8.3) | 2.9 (1.7-4.6) | < .0001 |
| Hospital length of stay, d | 17.1 (9-27.9) | 10.6 (6.7-15.9) | .0001 |
| ΔSOFA (0-48 h) | 0 (−2 to 2) | 0 (−2 to 1) | .24 |
| MRC score on hospital discharge | 34.5 (0-43.5) | 56 (51-59) | < .0001 |
| Mean arterial pressurea | 79 (73-87) | 86 (79-93) | .0008 |
| Median glucoseb | 135 (116-152) | 125 (113-142) | .11 |
| Medications received | |||
| No. (%) receiving corticosteroids in ICU | 55 (68.8%) | 62 (67.4%) | .85 |
| No. (%) receiving neuromuscular blocker | 5 (6.25%) | 3 (3.3%) | .35 |
| No. (%) receiving vasopressors | 59 (73.8%) | 31 (33.7%) | < .0001 |
| No. (%) receiving multiple vasopressors | 45 (56.3%) | 17 (18.5%) | < .0001 |
Data represent No. (%) or median (IQR). APACHE = Acute Physiology and Chronic Health Evaluation; ICU-AW = ICU-acquired weakness; IQR = interquartile range; MRC = Medical Research Council; SOFA = Sequential Organ Failure Assessment.
Median area under the curve of mean arterial pressure (mm Hg) during ICU stay.
Median glucose during ICU stay (mg/dL).
Patients with ICU-AW had lower mean arterial pressure by area under the curve analysis during the ICU admission [79 (73-87) vs 86 (79-93) mm Hg; P = .0008]. Correspondingly, more patients with ICU-AW received vasoactive medications during their hospitalization compared with patients who did not demonstrate ICU-AW (73.8% vs 33.7%; P = < .0001). Norepinephrine was the most commonly used medication. Sixty percent of patients with ICU-AW received norepinephrine compared with 24% of patients without ICU-AW (P < .0001). Patients with ICU-AW also received vasopressin (48% vs 22%; P = .0002) and phenylephrine (31% vs 5%; P < .0001) more frequently than patients without ICU-AW. Dobutamine, dopamine, and epinephrine were used less frequently and were not significantly different between the groups (e-Table 1).
Multivariable Analysis
Based on the univariable analysis and biologic plausibility, the following independent variables were included in the multivariable analysis for the outcome of ICU-AW: early mobilization, age, severity of illness as defined by APACHE II score, sepsis, duration of mechanical ventilation, hospital length of stay, mean arterial pressure, and vasoactive medication use. Since patients included in the analysis were enrolled in two different studies, this was accounted for in the model as well. There was no multicollinearity among the predictors. The model was well calibrated by the Hosmer-Lemeshow test (P = .77), and the area under the receiver operating characteristic curve for ICU-AW on hospital discharge was 0.86.
In the multivariable analysis, use of vasoactive medications was associated with a more than threefold increase in the odds of developing ICU-AW on hospital discharge (odds ratio [OR], 3.2; 95% CI, 1.29-7.95; P = .01), independent of other established risk factors for ICU-AW (Table 2). APACHE II score, hospital length of stay, and age were also independently associated with increased odds of developing ICU-AW. Early mobility intervention independently decreased the odds of developing ICU-AW. Further, for every day that a patient received a vasoactive medication the odds of developing ICU-AW increased 35% (OR, 1.35; 95% CI, 1.1-1.65; P = .004).
Table 2.
Multivariable Analysis of ICU-Acquired Weakness
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Vasoactive medication | 3.2 | 1.29-7.95 | .01 |
| APACHE II | 1.08 | 1.01-1.15 | .02 |
| Sepsis | 0.91 | 0.32-2.62 | .85 |
| Hospital length of stay, d | 1.05 | 1.01-1.08 | .009 |
| Age, y | 1.03 | 1.0-1.05 | .03 |
| Ventilator use, d | 1.07 | 0.98-1.16 | .15 |
| Early mobilization | 0.38 | 0.17-0.85 | .02 |
| Mean arterial pressurea | 0.97 | 0.92-1.02 | .21 |
| Study group | 0.34 | 0.14-0.8 | .01 |
APACHE II = Acute Physiology and Chronic Health Evaluation II; OR = odds ratio for developing ICU-acquired weakness.
Median area under the curve of mean arterial pressure (mm Hg) during ICU stay.
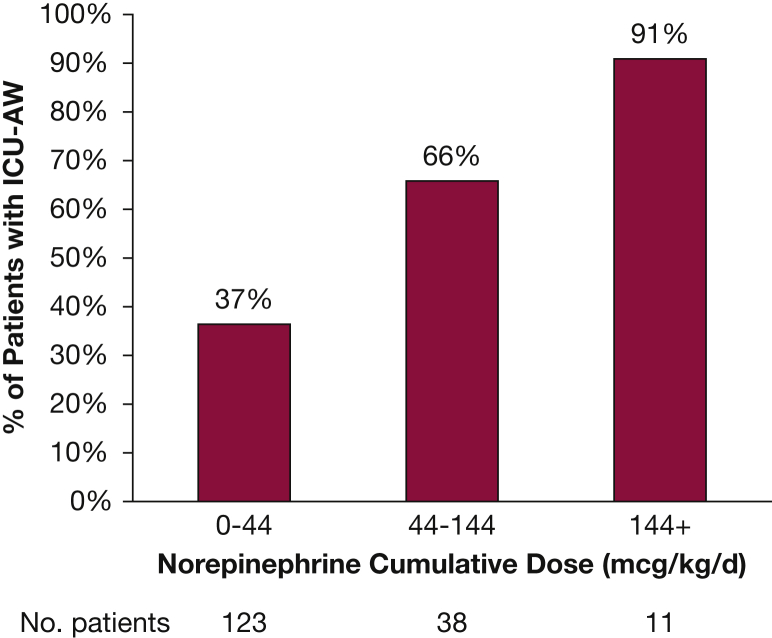
Given the hypothesis that the type of vasoactive medication used may have a differential effect on skeletal muscle via the β-adrenergic receptor, a multivariable analysis was performed with vasoactive medications separated into two variables: those that stimulate the β-adrenergic receptor (norepinephrine, epinephrine, dopamine, and dobutamine) and those that do not (phenylephrine and vasopressin). In this multivariable analysis only the β-adrenergic group was significantly associated with the outcome of ICU-AW (OR, 3.67; 95% CI, 1.44-9.36; P = .006). In order to further assess this, the cumulative doses of norepinephrine (μg/kg/d), phenylephrine (μg/kg/d), and vasopressin (units/d) were analyzed. For every 1-μg/kg/d dose of norepinephrine a patient received, the odds of developing ICU-AW increased 1% (OR, 1.01; 95% CI, 1.001-1.02; P = .04). A dose-dependent response was evident, with increasing incidence of ICU-AW seen with increasing cumulative norepinephrine dose (Fig 1). This relationship was not seen with vasopressin (OR, 1.01; 95% CI, 0.99-1.04; P = .28) or phenylephrine (OR, 1.97; 95% CI, 0.52-7.44; P = .32). The numbers of patients in groups receiving cumulative doses of dopamine, dobutamine, and epinephrine were small and not significantly different when comparing patients with and without ICU-AW, and thus were not included in the analysis.
Figure 1.
Incidence of ICU-acquired weakness (ICU-AW) with increasing cumulative dose of norepinephrine. *The proportion of patients with ICU-AW significantly increases with increasing cumulative dose of norepinephrine (χ2 for trend P < .0001).
Subgroup Analysis
In order to assess for death as a competing risk for the detection of ICU-AW we performed a competing risk regression that showed use of vasoactive medications remained independently associated with ICU-AW (subdistribution hazard ratio, 2.45; P = .006). Additional analysis of patients who survived to hospital discharge with ICU-AW (N = 46) and without ICU-AW (N = 92) was also performed. In this multivariable analysis, the use of vasoactive medications was associated with significantly increased odds of developing ICU-AW (OR, 5.37; 95% CI, 1.65-17.5; P = .005) (e-Table 2).
Discussion
As survivorship of critical illness continues to improve, there is an increasing emphasis on improving the outcomes of these survivors. ICU-AW is known to have detrimental effects on both short- and long-term outcomes, and therefore understanding the pathogenesis and identifying those at highest risk of developing neuromuscular weakness are critical. The role vasoactive medications may play has been unclear to this point.
We show in a population of critically ill, mechanically ventilated patients that the use of vasoactive medications is independently associated with the diagnosis of ICU-AW at hospital discharge. In fact, each day a patient received a vasoactive medication significantly increased the odds of developing neuromuscular weakness. In addition to the duration of vasopressor support, the cumulative dose of the β-agonist norepinephrine significantly increased the odds of developing ICU-AW. Importantly, this effect is independent of other known risk factors for ICU-AW, which include sepsis and markers of severity of illness, such as APACHE II score, and duration of mechanical ventilation. Other risk factors such as steroid use and neuromuscular blockade did not differ significantly between the groups and did not significantly impact outcomes in our analysis. The effect of vasoactive medication use remained significant when evaluating the subgroup of patients that survived to hospital discharge. This suggests that vasoactive medications, above and beyond being a marker for other risk factors associated with ICU-AW, may have a direct and independent effect on the development of neuromuscular weakness.
The role adrenergic stimulation may play in the outcomes, neuromuscular and otherwise, of critically ill patients is important to consider. An increase in circulating catecholamines is the natural and necessary response to shock, but in some critically ill patients, excess adrenergic stimulation from both endogenous and exogenous catecholamines can have detrimental effects. Many of the adverse effects of elevated circulating catecholamines appear to be mediated through the β-adrenergic receptor. The effect of β-adrenergic stimulation on the heart can lead to elevated heart rate, increased rates of arrhythmias, myocardial ischemia, and direct toxic effects on cardiac myocytes leading to apoptosis and fibrosis.9, 10, 29, 30, 31 While these are the most well-known adverse effects of adrenergic stimulation, there is significant evidence supporting effects on other organ systems as well. There is a growing body of evidence supporting the potentially detrimental immunomodulatory effects of norepinephrine use, which is believed to be mediated through activation of the β-adrenergic receptor.32 In the coagulation system, induction of a hypercoagulable state occurs in a dose-dependent response to epinephrine.29, 33 The metabolic system is also affected, with evidence supporting numerous metabolic effects of adrenergic stimulation including catecholamine-induced hyperglycemia.29, 34, 35
Given the presence of adrenergic receptors on skeletal muscle (predominantly β-adrenergic receptors), it is not surprising that skeletal muscle may be susceptible to adverse effects of excess adrenergic stimulation as well. The results of our study show an association between the cumulative dose of norepinephrine (but not vasopressin or phenylephrine) and the development of ICU-AW. This finding supports the theory introduced in animal models that stimulation of β-adrenergic receptors by vasoactive medications may have a toxic effect on skeletal myocytes.13, 14, 15, 36, 37 Although these studies provide biologic plausibility for an independent detrimental effect of excessive β-adrenergic stimulation on skeletal muscle, further studies are clearly needed in this area to confirm the association between specific vasoactive medication use and the development of neuromuscular weakness in humans and to elucidate the mechanism underlying this association. Given that the Surviving Sepsis Campaign guidelines recommend norepinephrine as the initial vasoactive of choice, future trials that aim to illuminate a potential causal role of β-adrenergic stimulation in the development of neuromuscular weakness may have important implications.38
Limitations
This is a retrospective study, and therefore carries the limitations of this type of analysis. The analysis included the characteristics known to affect the development of ICU-AW, but there can be no guarantee that it was not confounded by inherent differences between the patients with and without ICU-AW and/or those requiring vs not requiring vasoactive drugs that were not accounted for in the model. As an exploratory analysis, unmeasured confounding related to clinician’s choice to initiate specific vasoactive medications may also affect these findings. A retrospective independent association does not prove causation. Therefore, these findings need to be evaluated in prospective manner.
An additional limitation of this study is that the patient population analyzed is from a single-center randomized controlled trial, which could lead to exclusion of some patients. Both of these factors can introduce selection bias and therefore limit the generalizability of the findings to a broader patient population.
Conclusions
In a population of critically ill, mechanically ventilated patients enrolled in a clinical trial of early mobility, the use of vasoactive medications was independently associated with the development of ICU-AW. This effect is related to both the duration of vasoactive support and cumulative dose of norepinephrine received.
Acknowledgments
Author contributions: J. P. K. takes responsibility for the content of the manuscript, including the data and analysis. Study concept and design: K. S. W., B. K. P., J. B. H., J. P. K.; acquisition, analysis, or interpretation of the data: K. S. W., B. K. P., E. L. MacK., S. P. G., A. S. P., M. M. C., J. P. K.; drafting of the manuscript: K. S. W., B. K. P., J. B. H., J. P. K.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: K. S. W., B. K. P., M. M. C., J. P. K.; study supervision: J. B. H., J. P. K. All authors read and approved the final manuscript.
Financial/nonfinancial disclosure: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Fellow salary support for K. S. W. was funded by the NIH/NHLBI [Grant No. T32 HL007605]. Salary support for B. K. P. was provided by the Parker B. Francis Fellowship [Grant No. FP062541-01-PR].
Supplementary Data
References
- 1.De Jonghe B., Sharshar T., Lefaucheur J.P. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 2.Fan E., Cheek F., Chlan L. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190(12):1437–1446. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 3.Jolley S.E., Bunnell A.E., Hough C.L. ICU-acquired weakness. Chest. 2016;150(5):1129–1140. doi: 10.1016/j.chest.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermans G., Van Mechelen H., Clerckx B. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 5.Brunello A.G., Haenggi M., Wigger O., Porta F., Takala J., Jakob S.M. Usefulness of a clinical diagnosis of ICU-acquired paresis to predict outcome in patients with SIRS and acute respiratory failure. Intensive Care Med. 2010;36(1):66–74. doi: 10.1007/s00134-009-1645-7. [DOI] [PubMed] [Google Scholar]
- 6.Dinglas V.D., Aronson Friedman L., Colantuoni E. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit Care Med. 2017;45(3):446–453. doi: 10.1097/CCM.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan E., Dowdy D.W., Colantuoni E. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kress J.P., Hall J.B. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 9.De Backer D., Biston P., Devriendt J. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 10.Schmittinger C.A., Torgersen C., Luckner G., Schröder D.C.H., Lorenz I., Dünser M.W. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 2012;38(6):950–958. doi: 10.1007/s00134-012-2531-2. [DOI] [PubMed] [Google Scholar]
- 11.Thiele R.I., Jakob H., Hund E. Sepsis and catecholamine support are the major risk factors for critical illness polyneuropathy after open heart surgery. Thorac Cardiovasc Surg. 2000;48(3):145–150. doi: 10.1055/s-2000-9640. [DOI] [PubMed] [Google Scholar]
- 12.Van den Berghe G., Wouters P., Weekers F. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 13.Burniston J.G., Chester N., Clark W.A., Tan L.-B., Goldspink D.F. Dose-dependent apoptotic and necrotic myocyte death induced by the β2-adrenergic receptor agonist, clenbuterol. Muscle Nerve. 2005;32(6):767–774. doi: 10.1002/mus.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burniston J.G., Clark W.A., Tan L.-B., Goldspink D.F. Dose-dependent separation of the hypertrophic and myotoxic effects of the β2-adrenergic receptor agonist clenbuterol in rat striated muscles. Muscle Nerve. 2006;33(5):655–663. doi: 10.1002/mus.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burniston J.G., Ng Y., Clark W.A., Colyer J., Tan L.-B., Goldspink D.F. Myotoxic effects of clenbuterol in the rat heart and soleus muscle. J Appl Physiol. 2002;93(5):1824–1832. doi: 10.1152/japplphysiol.00139.2002. [DOI] [PubMed] [Google Scholar]
- 16.Schweickert W.D., Pohlman M.C., Pohlman A.S. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin C., Wade D.T., Davies S., Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 18.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel Index. Maryland State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 19.Kress J.P., Pohlman A.S., O’Connor M.F., Hall J.B. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 20.Girard T.D., Kress J.P., Fuchs B.D. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 21.Jones A.E., Trzeciak S., Kline J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent J.L., Moreno R., Takala J., Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 23.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 24.Ferreira F.L., Bota D.P., Bross A., Melot C., Vincent J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 25.Vincent J.L., de Mendonca A., Cantraine F., Working Group on “Sepsis-Related Problems” of the European Society of Intensive Care Medicine Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleyweg R.P., van der Meche F.G., Schmitz P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 28.Dyck P.J., Boes C.J., Mulder D. History of standard scoring, notation, and summation of neuromuscular signs: a current survey and recommendation. J Peripher Nerv Syst. 2005;10(2):158–173. doi: 10.1111/j.1085-9489.2005.0010206.x. [DOI] [PubMed] [Google Scholar]
- 29.Dünser M.W., Hasibeder W.R. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24(5):293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 30.Iwai-Kanai E., Hasegawa K., Araki M., Kakita T., Morimoto T., Sasayama S. α- and β-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100(3):305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 31.Todd G.L., Baroldi G., Pieper G.M., Clayton F.C., Eliot R.S. Experimental catecholamine-induced myocardial necrosis. II. Temporal development of isoproterenol-induced contraction band lesions correlated with ECG, hemodynamic and biochemical changes. J Mol Cell Cardiol. 1985;17(7):647–656. doi: 10.1016/s0022-2828(85)80064-x. [DOI] [PubMed] [Google Scholar]
- 32.Stolk R.F., van der Poll T., Angus D.C., van der Hoeven J.G., Pickkers P., Kox M. Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med. 2016;194(5):550–558. doi: 10.1164/rccm.201604-0862CP. [DOI] [PubMed] [Google Scholar]
- 33.de Montmollin E., Aboab J., Mansart A., Annane D. Bench-to-bedside review: β-adrenergic modulation in sepsis. Crit Care. 2009;13(5):230. doi: 10.1186/cc8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trager K., DeBacker D., Radermacher P. Metabolic alterations in sepsis and vasoactive drug-related metabolic effects. Curr Opin Crit Care. 2003;9(4):271–278. doi: 10.1097/00075198-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Trager K., Radermacher P., Debacker D., Vogt J., Jakob S., Ensinger H. Metabolic effects of vasoactive agents. Curr Opin Anaesthesiol. 2001;14(2):157–163. doi: 10.1097/00001503-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Burniston J.G., Tan L.B., Goldspink D.F. Relative myotoxic and haemodynamic effects of the β-agonists fenoterol and clenbuterol measured in conscious unrestrained rats. Exp Physiol. 2006;91(6):1041–1049. doi: 10.1113/expphysiol.2006.035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldspink D.F., Burniston J.G., Ellison G.M., Clark W.A., Tan L.-B. Catecholamine-induced apoptosis and necrosis in cardiac and skeletal myocytes of the rat in vivo: the same or separate death pathways? Exp Physiol. 2004;89(4):407–416. doi: 10.1113/expphysiol.2004.027482. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes A., Evans L.E., Alhazzani W. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.