Abstract
Purpose
Parkinson’s disease (PD) is associated with non-motor symptoms (NMS) that can cause progressive disability and impact quality of life of people with PD (PwP) and increase burden on care partners. This survey was designed to evaluate the prevalence, impact, and educational preferences regarding NMS on PwP and their care partners.
Patients and methods
A 17-question survey was sent to the total membership of PMDAlliance, a nonprofit organization reaching 3,685 households of PwP. Care partners and other interested individuals could also respond. The survey was conducted using Survey Monkey, an online survey platform, and included distinct questions for respondents with and without NMS.
Results
A total of 700 individuals responded to the survey. Of the respondents, 378 (54%) were care partners and 287 (41%) were PwP. About 90% of the respondents reported having experience with NMS in PwP, including sleep problems (84%), cognitive symptoms (76%), anxiety (65%), depression (56%), hallucinations (40%), and delusions (23%). NMS in PwP were reported by more care partners (97%) than PwP (80%). NMS had at least some impact on quality of life for 84% of the respondents; 48% indicated that NMS represented a greater challenge than motor symptoms. Care partners were more likely than PwP to report that NMS were more challenging than motor symptoms (58% vs 32%). Respondents with and without NMS indicated a desire for NMS education.
Conclusion
This survey underscores the significant impact of NMS on the quality of life of PwP and highlights the need for improved recognition and education about its effects.
Keywords: sleep, cognition, anxiety, depression, hallucinations, delusions, treatment
Introduction
Parkinson’s disease (PD) is a common and complex neurodegenerative disease traditionally characterized by progressive motor symptoms, including bradykinesia, rigidity, and tremor.1 However, PD is also associated with numerous non-motor symptoms (NMS), such as cognitive impairment, neuropsychiatric symptoms, sleep disorders, and olfactory dysfunction, some of which may precede the development of motor dysfunction.1,2 Moreover, the NMS of PD may have a greater impact on quality of life than motor symptoms and are significantly associated with reduced well-being.2–5 In fact, in advanced PD, NMS are an important determinant of loss of independence and residential placement of people with PD (PwP) outside their homes.3,6 Consequently, NMS contribute substantially to the burden of PwP and their care partners.4 Studies have reported that the care partner burden associated with PD is primarily dependent on PwP’s NMS and is correlated with NMS severity.7,8
The incidence and impact of NMS of PD have been extensively documented in observational studies of PwP3,5,9 and more recently in innovative studies that utilized a clinimetric approach for evaluating the severity of NMS affecting PwP.10–15 However, few reports of the burden of NMS from the perspectives of PwP or their care partners are available. Furthermore, evidence of the burden of NMS in the real world, outside the context of a clinical trial or a physician’s office, is even rarer.
The objective of this study was to evaluate the impact of NMS on PwP and care partners using an online survey addressed directly to potential respondents and not mediated by health care professionals. In this survey, responses provided by PwP and care partners were evaluated as a single respondent data set and as separate subgroups.
Methods
Design
This was a survey among members of the Parkinson and Movement Disorders Alliance (PMDAlliance), a nonprofit organization that promotes the daily health and well-being of PwP and their care partners. The objective of the survey was to better understand the perceptions, experiences, and educational needs of patients with NMS of PD and their care partners. The survey was conducted using Survey Monkey, an online survey platform, between March 19, 2018 and March 31, 2018. Potential respondents were solicited via e-mail 3 times in the course of 10 days. In order to ensure unique responses, respondents were only allowed to submit one questionnaire entry.
Study population
The target study population and potential respondents were the members of PMDAlliance, which includes PwP and their care partners. At the time of the survey, there were 3,685 names in the membership list of the PMDAlliance; 48% were PwP, 48% were care partners, and 4% were “other” (including health care professionals and non–care partners’ adult children); approximately 75% of this population is >65 years of age. Care partners are defined as unpaid individuals (usually family members or close friends) who have assumed the primary responsibility for assisting PwP in managing their health. PwP and care partners were included in the study population regardless of their experience of NMS. In addition, the care partners who responded to the survey were not necessarily the care partners of the PwP who responded to the survey.
Endpoints
Two separate sets of questions were developed: one set for respondents who answered affirmatively to a question gauging whether they or their care recipient with PD currently experienced NMS and a second set of questions for respondents who indicated no current experience with NMS. Both sets of questions are included in Online Supplementary Table 1.
Statistical analyses
Responses were tabulated, analyzed, and reported as percentages. The Student t-test was used to compare responses across groups of respondents and evaluate statistical significance of the differences. There was no adjustment for multiple comparisons.
Results
Respondents
The survey was addressed to the total membership of the PMDAlliance (approximately N=3,685). A total of 700 respondents completed the survey, either totally or partially. Sample sizes vary from question to question because not all respondents completed all questions included in the survey. Of the 700 respondents, 54% were care partners and 41% were PwP. The remaining 5% were non–care partners/family members of PwP or “others.” Given that the vast majority of respondents who were not PwP were care partners, we refer to the nonpatient group as “care partners” in this report.
Among the 700 respondents, 90% reported that the PwP experienced NMS. Notably, a greater percentage of care partners reported NMS than did PwP (97% vs 80%).
Reported NMS
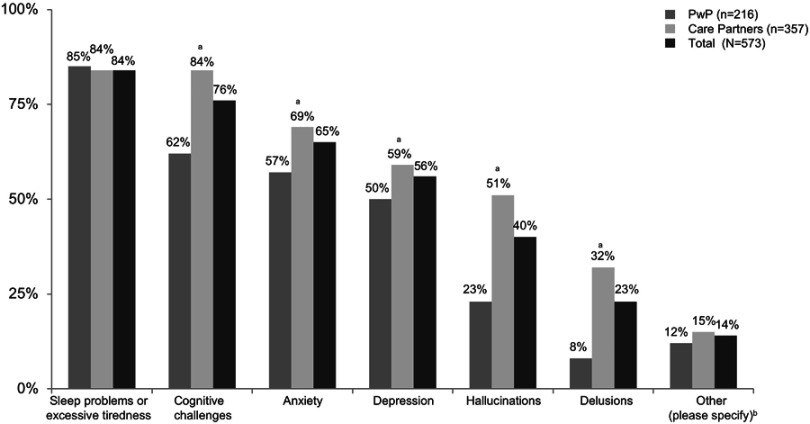
Overall, more than half of respondents (responses pooled for 573 PwP and care partners) reported sleep problems or excessive tiredness (84%), cognitive challenges (76%), anxiety (65%), or depression (56%) among PwP (ie, reported by PwP themselves or their care partners). Respondents also reported a high prevalence of hallucinations (40%) and delusions (23%) among PwP (ie, reported by PwP themselves or their care partners) (Figure 1).
Figure 1.
Reported prevalence of specific non-motor symptoms among 573 survey respondents (March 19, 2018–March 31, 2018). aDifferences in groups (PwP vs care partners) are statistically significant at the 95% confidence level. bOther specified responses: constipation, apathy, nightmares, anger, and mood swings.
Abbreviation: PWP, people with Parkinson’s disease.
There were differences in reported NMS prevalence in PwP between care partners and PwP, with care partners reporting higher prevalence across all symptoms except sleep problems or excessive tiredness (reported by 84% of the care partners vs 85% of the PwP). The differences in reported NMS prevalence between care partners (357 respondents) and PwP (216 respondents) were statistically significant for cognitive challenges (84% vs 62%), anxiety (69% vs 57%), depression (59% vs 50%), hallucinations (51% vs 23%), and delusions (32% vs 8%) (Figure 1).
Among respondents with NMS experience, as PwP or care partners (579 respondents), the onset of NMS occurred within the first 3 years after PD diagnosis for more than half (53%), and within the first 5 years after diagnosis for 72%. The reported onset of NMS appeared to be largely similar for affected PwP, whether reported by them or by their care partners.
Management
Responses indicated that most PwP routinely visited a movement disorder specialist for PD management (70% of 656 respondents, PwP or care partners). The responses of PwP and care partners regarding the health care providers responsible for managing PD were largely similar.
NMS impact
NMS had some, quite a bit, or very much of a negative impact on quality of life for PwP according to 84% of the respondents (570 respondents, PwP or care partners). About 20% of the respondents indicated that NMS impacted or disrupted quality of life “very much.” However, more care partners (352 respondents) than PwP (210 respondents) indicated that NMS had either “very much” of an impact (28% vs 7%) or “quite a bit” of an impact (38% vs 26%) on quality of life while more PwP than care partners (42% vs 24%) stated that NMS had “some” impact on quality of life.
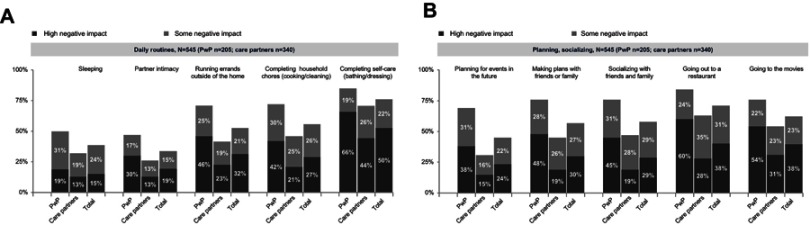
When asked to qualify the specific impact of NMS on daily life (545 respondents, PwP or care partners), more than half of respondents indicated that NMS had some negative impact or a high negative impact on running errands outside of the home (53%), completing household chores (53%), completing self-care (72%), making plans with friends and family (57%), socializing with friends and family (58%), going out to restaurants (69%), and going out to movies (61%), as shown in Figure 2A and B. A comparison of responses revealed that more PwP than care partners indicated that NMS had a “high negative impact” or “some negative impact” on daily routines and planning/socializing.
Figure 2.
Impact of non-motor symptoms on specific aspects of daily living as reported by 205 PwP and 340 care partners (March 19, 2018–March 31, 2018) (A and B).
Abbreviation: PWP, people with Parkinson’s disease.
Perceptions of NMS
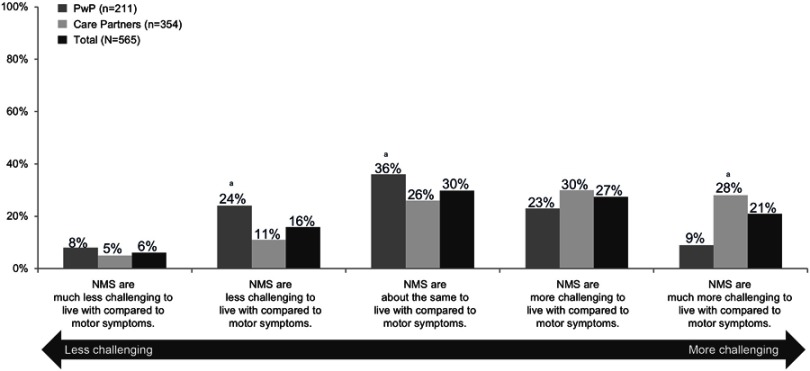
Overall (565 respondents, PwP or care partners) nearly half of respondents (48%) indicated that NMS represent a greater challenge than motor symptoms (Figure 3). However, a greater percentage of care partners (354 respondents) reported that NMS were more or much more challenging than motor symptoms compared with responses from PwP (211 respondents), 58% vs 32%. Despite the perceived impact of NMS, 50% of the respondents (37% of the PwP and 48% of the care partners) indicated that their friends and family had very little or no understanding of the impact of PD on daily living.
Figure 3.
Relative impact of non-motor symptoms compared with motor symptoms as reported by 211 PwP and 354 care partners (March 19, 2018–March 31, 2018). aDifferences in groups (PwP vs care partners) are statistically significant at the 95% confidence level.
Abbreviations: NMS, non-motor symptoms; PwP, people with Parkinson’s disease.
Educational preferences
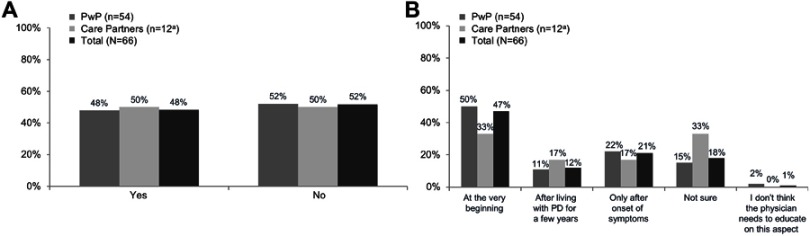
Among the subgroup of respondents who had not experienced NMS or who had not seen it in the PwP they cared for (66 respondents, PwP or care partners), approximately half (48%) indicated that they had received some education on NMS by their health care provider (Figure 4A). When questioned about their preferred timing for receiving information about NMS, nearly half (47%) indicated “at the very beginning” (eg, soon after diagnosis). However, approximately one-third (33%) stated that they did not wish to receive education until the onset of NMS symptoms or after living with PD for a few years. Care partners seemed to favor receiving education later in the course of PD than did PwP themselves (Figure 4B).
Figure 4.
Proportion of 66 respondents without non-motor symptoms (NMS) experience who reported having received NMS education (A) and preferred timing for NMS education (B) (March 19, 2018–March 31, 2018). aLow base size; please consider directional.
Abbreviations: PD, Parkinson’s disease; PwP, people with Parkinson’s disease.
Overall, the majority of respondents (585 respondents, PwP or care partners, regardless of NMS experience) wanted to receive education about cognitive symptoms (72%) and sleep problems (67%). Respondents also indicated a desire for education about anxiety (48%), depression (42%), hallucinations (36%), or delusions (28%). More respondents in the care partner group than in the PwP group indicated a desire for education about NMS, and the between-group differences were statistically significant for cognitive challenges, hallucinations, and delusions.
Discussion
The results of this survey highlight the significant impact of NMS on the quality of life and daily living of PwP, as reported by them and/or care partners. Because the survey was conducted online without the supervision of health care providers, included a relatively large number of respondents, and solicited distinct responses from PwP and care partners, the results provide a unique perspective on the burden of NMS in PD. These findings are particularly relevant given recent evidence indicating that NMS are key contributors to the burden of PD among PwP and care partners.7,16,17 Recognizing and alleviating the burden of care partners could improve their quality of life and enable PwP to remain in the community for longer periods of time.8,16,17
Interestingly, the results of this survey reveal that care partners reported NMS more frequently than did PwP themselves. Differences in the frequency of NMS reports were greatest for cognitive and neuropsychiatric symptoms, such as hallucinations and delusions. Care partners are often in a unique position to detect subtle psychiatric and cognitive changes in their care recipients and might observe these changes before the PwP themselves.8,16 Their accounts could also be useful to clinicians treating PD, whose attention might be more focused on the typical motor symptoms than the behavioral and cognitive NMS of PD.8,18 Although the higher rates of reported NMS by care partners could be attributable, in part, to their greater motivation to respond to surveys regarding NMS than care partners of patients without NMS, these findings are consistent with those of other studies and surveys investigating care partners as proxy symptom reporters, which indicate that care partners tend to report a greater severity of symptoms than the patients themselves.19–21 Nonetheless, PwP recognized the substantial impact of NMS on their daily routines and social activities. In fact, a greater proportion of respondents in the PwP group reported that NMS had a negative impact on their daily routines and social activities than respondents in the care partner group, suggesting that there might be discordance in perceptions of the impact of NMS on daily life in PD between PwP and care partners.
Another noteworthy finding is that a greater proportion of PwP report substantial impact of NMS on concrete activities of daily living or social interaction than care partners. This could be reflective of discordance between perception of general symptoms vs tangible effects on actual activities, which –in turn– may be a relevant distinction to keep in mind when physicians try to gain a full perspective on the overall experience of PwP, whether described by themselves or their care partners.
Recently, a clinimetric approach was utilized to evaluate not only prevalence but also severity of psychiatric symptoms in PD.11 Results indicated that the most severe psychiatric symptoms were somatization, anxiety, phobic anxiety, psychoticism, and neurasthenia (apathy). Somatization was particularly prominent and thought to reflect “distress related to the subjective perception of bodily dysfunctions”.10,11 Phobic anxiety is a marker of impairment in a number of day-to-day functions of patients with PD: being afraid to travel or to go out of home alone. These symptoms are likely to negatively affect the daily life of patients with PD.10–13
Finally, another interesting but rarely investigated topic explored by our survey is the educational preferences of patients and care partners regarding NMS. Previous studies have identified disease education as an overall gap in PD care, a gap that could affect adherence to treatment.22,23 It has been traditionally thought that early conversations with patients and their care partners about PD symptoms could help avoid uncertainty and assist in long-term planning.8 However, among respondents without NMS or experience in caring for PwP with NMS, only 46% preferred to receive NMS education “at the very beginning” of the illness or soon after PD was diagnosed, whereas one-third preferred to receive education only after the onset of symptoms or after living with PD for a few years. These preferences could be particularly relevant to health care practitioners with regard to how to frame conversations about sensitive topics and how to set priorities for discussions with PwP and care partners. It may be generally valuable for PwP and their care partners to be aware of the possibility of NMS early on in their disease course to avoid misattribution of symptoms, but the results of this survey show that, for some patients, deeper education may be preferable later in the disease course.
Our survey had several important limitations. Not all relevant NMS, such as gastrointestinal and urinary disturbances, were specifically addressed in the questionnaire. Additionally, the questionnaire captured limited demographic information other than specifics related to NMS. The age, sex, duration of PD, concomitant illnesses, and medication use of the surveyed or cared-for PwP were not assessed and are not known. The overall frame of reference is that of the PMDAlliance membership, which includes a largely elderly population. As self-selected members of the PMDAlliance, respondents of the survey may not have been fully representative of PwP and their care partners in the community at large (eg, the proportion of PwP cared for by movement disorders specialists was relatively high compared with prior reports)24,25 We also cannot be certain that the PwP group and the patient group reported by caregivers were equivalent. The survey compared answers between PwP and care partners of different family units; given that the clinical details of the PwP who responded and of the PwP for which the care partners responded are not known, it is possible that differences in perception of NMS between PwP and care partners may be due to differences in disease severity or other characteristics. In addition, our study was limited by the fact that respondents with and without NMS answered different questions, particularly regarding their educational preferences. Understanding the educational preferences and their potential differences regarding NMS in PwP with and without NMS and their care partners would have provided an interesting perspective across the PD spectrum, regardless of the type of NMS. Lastly, only 700 of the 3,685 potential respondents answered the survey. Despite its limitations, our survey provides valuable insights into understanding the needs, perspectives, and preferences of PwP and their care partners concerning NMS in PD. Given the substantial impact of NMS on PwP’s quality of life and care partner burden, additional studies of PwP and care partner perspectives on the impact of NMS and unmet needs regarding NMS management are warranted.
There remains a need to more clearly assess both the psychological distress and functional impact caused by NMS in PD, as perceived by both PwP and their caregivers. Investigations are underway to identify potential risk factors and predictors of specific NMS, and how various NMS cluster, as well as their underlying pathophysiology. Future studies could potentially seek to identify patient and caregiver characteristics that influence their perception of impact of NMS and look for modifiable factors. The effects of non-pharmacologic interventions, such as individual and couples psychotherapy, exercise, and meditation need to be assessed. In addition, a better understanding of the impact of various NMS can also help direct development of future pharmacologic treatments.
Conclusions
The outcomes of this survey provide a valuable contribution to a better understanding of the needs, perspectives, and preferences of PwP and care partners related to NMS in PD. Given their substantial impact on patients’ quality of life and care partner burden, addressing NMS should be a regular priority in discussions between health care providers, patients, and care partners.
Acknowledgments
Medical writing assistance for this manuscript was provided by Nicole Cooper and Julia Saiz for Syneos Health and supported by ACADIA Pharmaceuticals Inc. The study was conducted by the PMDAlliance and sponsored by ACADIA Pharmaceuticals Inc.
Ethics
This study only involved the use of anonymous survey responses, and researchers did not have any access to any identifiable information about the survey respondents; thus, it did not require approval by ethical review boards. All authors had access to any data requested, reviewed and approved the manuscript, and vouch for the accuracy and completeness of the data. The corresponding author had full access to all the data and had final responsibility to submit for publication.
Disclosure
NH reports personal fees from AbbVie, ACADIA, Adamas, Eli Lilly, Parkinson Study Group, Sunovion, UCB, and Voyager, outside the submitted work. RAH reports personal fees or other support from AbbVie Inc, Acorda Therapeutics, ACADIA Pharmaceuticals, Adamas Pharmaceuticals, Alphasights, ApoPharma, Back Bay Life Science, Baron Capital, Biotie Therapies, Cerecor Inc., ClearView Healthcare Partners, CNS Ratings, LLC., Cynapsus Therapeutics, DDB Health LLC, Decision Resources Group, eResearch Technology, Extera Partners, GE Healthcare, Health Advances, Guidepoint Global, HealthLogix, Health and Wellness Partners, Hepatares Therapeutics, Impax Laboratories, Impel Neuropharma, Intec Pharma Ltd., IQVIA, Jazz Pharmaceuticals, Kashiv Pharma LLC, Kyowa Kirin Pharmaceutical Development, Ltd., Life Sciences, Lundbeck LLC, The Lockwood Group, MEDACorp, MEDIQ, Medscape, Michael J. Fox Foundation, Mitsubishi Tanabe Pharmaceuticals, Movement Disorder Society, National Institutes of Health, Neurocea LLC, Neurocrine Biosciences, Neuroderm, Neuropore Therapies, Parkinson Study Group, Partner’s Healthcare, Peerview Press, Penn Technology Partnership, Pennside Partners, Perception OpCo, Precision Effect, Pfizer, Inc., Pharma Two B, Phase Five Communications, Putnam Associates, Quintiles, Regenera Pharma, RMEI Medical Education for Better Outcomes, SAI Med Partners LLC, Sarepta Therapeutics, Schlesinger Associates, Scion Neurostim, LLC, Seagrove Partners, LLC, Seelos, Sunovion Pharmaceuticals, Sun Pharma, Teva Pharmaceutical Industries, US WorldMeds, Vista Research, WebMD, Windrose Consulting Group, AstraZeneca, Axovant Sciences, Biogen Inc., Cavion, Enterin Inc., Intec Pharma Ltd, NeuroDerm Ltd., Lundbeck, Dart NeuroScience LLC, Prexton Therapeutics, Revance Therapeutics Inc., Sunovion Pharmaceuticals, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 2.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–450. doi: 10.1038/nrn.2017.62 [DOI] [PubMed] [Google Scholar]
- 3.Duncan GW, Khoo TK, Yarnall AJ, et al. Health-related quality of life in early Parkinson’s disease: the impact of nonmotor symptoms. Mov Disord. 2014;29(2):195–202. doi: 10.1002/mds.25664 [DOI] [PubMed] [Google Scholar]
- 4.Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559–568. doi: 10.1016/S1474-4422(18)30127-3 [DOI] [PubMed] [Google Scholar]
- 5.van Uem JMT, Marinus J, Canning C, et al. Health-related quality of life in patients with Parkinson’s disease—A systematic review based on the ICF model. Neurosci Biobehav Rev. 2016;61:26–34. doi: 10.1016/j.neubiorev.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–942. [DOI] [PubMed] [Google Scholar]
- 7.Grün D, Pieri V, Vaillant M, Diederich NJ. Contributory factors to caregiver burden in Parkinson disease. J Am Med Dir Assoc. 2016;17(7):626–632. doi: 10.1016/j.jamda.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Hiseman JP, Fackrell R. Caregiver burden and the nonmotor symptoms of Parkinson’s disease. Int Rev Neurobiol. 2017;133:479–497. doi: 10.1016/bs.irn.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;21(7):916–923. doi: 10.1002/mds.20844 [DOI] [PubMed] [Google Scholar]
- 10.Carrozzino D, Bech P, Patierno C, et al. Somatization in Parkinson’s disease: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:18–26. doi: 10.1016/j.pnpbp.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Carrozzino D, Morberg BM, Siri C, Pezzoli G, Bech P. Evaluating psychiatric symptoms in Parkinson’s disease by a clinimetric analysis of the Hopkins Symptom Checklist (SCL-90-R). Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:131–137. doi: 10.1016/j.pnpbp.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 12.Carrozzino D, Siri C, Bech P. The prevalence of psychological distress in Parkinson’s disease patients: the brief symptom inventory (BSI-18) versus the Hopkins symptom checklist (SCL-90-R). Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:96–101. doi: 10.1016/j.pnpbp.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 13.Carrozzino D. Clinimetric approach to rating scales for the assessment of apathy in Parkinson’s disease: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94(3):109641. doi: 10.1016/j.pnpbp.2019.109641 [DOI] [PubMed] [Google Scholar]
- 14.Fava GA, Carrozzino D, Lindberg L, Tomba E. The clinimetric approach to psychological assessment: a tribute to Per Bech, MD (1942–2018). Psychother Psychosom. 2018;87(6):321–326. doi: 10.1159/000493746 [DOI] [PubMed] [Google Scholar]
- 15.Onofrj M, Carrozzino D, D’Amico A, et al. Psychosis in parkinsonism: an unorthodox approach. Neuropsychiatr Dis Treat. 2017;13:1313–1330. doi: 10.2147/NDT.S116116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szeto JY, Mowszowski L, Gilat M, Walton CC, Naismith SL, Lewis SJ. Mild cognitive impairment in Parkinson’s disease: impact on caregiver outcomes. J Parkinsons Dis. 2016;6(3):589–596. doi: 10.3233/JPD-160823 [DOI] [PubMed] [Google Scholar]
- 17.Torny F, Videaud H, Chatainier P, Tarrade C, Meissner WG, Couratier P. Factors associated with spousal burden in Parkinson’s disease. Rev Neurol (Paris). 2018;174(10):711–715. doi: 10.1016/j.neurol.2018.01.372 [DOI] [PubMed] [Google Scholar]
- 18.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8(3):193–197. [DOI] [PubMed] [Google Scholar]
- 19.Sebring K, Shattuck J, Berk J, Boersma I, Sillau S, Kluger BM. Assessing the validity of proxy caregiver reporting for potential palliative care outcome measures in Parkinson’s disease. Palliat Med. 2018;32(9):1522–1528. doi: 10.1177/0269216318785830 [DOI] [PubMed] [Google Scholar]
- 20.Schmidt P, Cubillos F, Diaz A, et al., eds. Evaluation of Parkinson’s Disease Psychosis Requires Structured Questioning of Patients and Caregivers. Movement Disorders. 111 River St, Hoboken 07030-5774, NJ: Wiley; 2018. [Google Scholar]
- 21.Schmidt P, Cubillos F, Diaz A, et al., eds. Patient Experience of Parkinson’s Disease Psychosis Management. Movement Disorders. 111 River St, Hoboken 07030-5774, NJ: Wiley; 2018. [Google Scholar]
- 22.Boersma I, Jones J, Carter J, et al. Parkinson disease patients’ perspectives on palliative care needs: what are they telling us? Neurol Clin Pract. 2016;6(3):209–219. doi: 10.1212/CPJ.0000000000000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daley DJ, Myint PK, Gray RJ, Deane KH. Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(10):1053–1061. doi: 10.1016/j.parkreldis.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011;77(9):851–857. doi: 10.1212/WNL.0b013e31822c9123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis AW, Schootman M, Tran R, et al. Neurologist-associated reduction in PD-related hospitalizations and health care expenditures. Neurology. 2012;79(17):1774–1780. doi: 10.1212/WNL.0b013e3182703f92 [DOI] [PMC free article] [PubMed] [Google Scholar]