Abstract
Background
The relationship of poor sleep patterns to the increased risk of obesity has been reported, but the results are variable. This study evaluated the association between objectively measured sleep patterns and obesity in a representative adult population of Hispanic/Latino subjects living in the United States.
Methods
This cross-sectional study was an analysis of a multicenter, community-based cohort of 2,156 participants aged 18 to 64 years from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Recruitment was conducted in San Diego, California; Chicago, Illinois; Bronx, New York; and Miami, Florida. Models were controlled for age, sex, ethnic background, site, income, education, and apnea-hypopnea index. Seven days of wrist actigraphy data were collected. Obesity was defined as BMI ≥ 30 kg/m2, and abdominal obesity was defined as waist circumference ≥ 88 cm in women and ≥ 102 cm in men. Napping was defined as more than one 15-min nap per week.
Results
An inverse linear relationship was found between sleep duration and prevalence of obesity (P linear trend ≤ 0.01). A reduction of 1 h sleep increased obesity prevalence by 4.1% (95% CI, 1.6-6.6; P = .002) and abdominal obesity prevalence by 3.6% (95% CI, 1.1-6.1; P = .007). Daytime napping increased obesity prevalence by 10.4% (95% CI, 3.5-17.3; P = .004) and abdominal obesity prevalence by 7.1% (95% CI, 1.0-13.2; P = .02).
Conclusions
In a population of young to older adult Hispanic/Latino subjects, we found an inverse linear association between sleep duration and the prevalence of obesity. Daytime napping was strongly associated with greater adiposity. Interventional and longitudinal studies are needed to better understand how abnormal sleep patterns contribute to the obesity epidemic.
Key Words: actigraphy, adiposity, habitual short sleep duration, Hispanic subjects, napping and obesity
Abbreviation: AHI, apnea-hypopnea index
Sleep duration is an important health risk factor. Long sleep may be a marker for disease and inflammation,1 whereas short sleep has been linked to cardiovascular disease,2 hypertension,3, 4 obesity,5 diabetes,6 increased pain,7 and all-cause mortality.8
Self-reported short sleep has been associated with greater obesity prevalence in adults and children.9 Others described greater obesity with long sleep.9, 10, 11 Some studies described a U-shaped relationship between sleep duration and obesity,9, 12 although others found no association,13 and still others found an association only in men.14 Such variability led some to question the validity of self-reported sleep duration to assess obesity risk.9
Using actigraphy to assess the association between sleep duration and obesity has also yielded conflicting results. In the Coronary Artery Risk Development in Young Adults (CARDIA) study, short sleep and greater sleep fragmentation were associated with higher BMI.15 The Osteoporotic Fractures in Men Study (MrOS) reported a U-shaped relationship between sleep duration and obesity in older men.16 In the Rotterdam Study, both short and long sleepers were more likely to be obese than those sleeping 7 to 8 h.13 More recently, the Multi-Ethnic Study of Atherosclerosis (MESA) reported an inverse linear relationship between sleep duration and obesity.17 Methodologic differences and differences in the age of the study populations may account for the variability in results.13, 15, 16, 17
It is unclear which aspect of sleep is most relevant to the obesity risk. Most have focused on sleep duration. However, in the MrOS and the Study of Osteoporotic Fractures (SOF), sleep duration variability and daytime napping were associated with obesity, suggesting that other sleep patterns may be important.18
Hispanic/Latino individuals are the largest minority in the United States that is at high risk for obesity and related diseases.19, 20, 21, 22 Compared with white subjects, Hispanic/Latino individuals have been understudied.23, 24, 25 The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a partial attempt to remedy this disparity. We evaluated the health of > 16,000 adult US Hispanic subjects. The Sueño ancillary study used actigraphy to objectively evaluate the association between sleep patterns and obesity. We controlled for relevant confounders to overcome methodological limitations in the literature and hypothesized that poor sleep patterns, including short sleep, would be associated with greater risk of obesity.
Subjects and Methods
The HCHS/SOL Study
The HCHS/SOL is a longitudinal, multicenter, community-based cohort examining the prevalence and risk factors of chronic disease among US Hispanic/Latino subjects. The protocol has been described elsewhere.26, 27 Briefly, we recruited a representative sample population using a two-stage probability-based sampling scheme to randomly select households based on census block groups in the following: Bronx, New York; Miami, Florida; Chicago, Illinois; and San Diego, California.
Between May 2008 and June 2011, a total of 16,145 adults aged 18 to 74 years were recruited. Participants self-identified as Hispanic/Latino. Information on demographic characteristics, socioeconomic status, lifestyle habits, and medical history was obtained through English or Spanish questionnaires per the participant's preference.26 Baseline examination included height, weight, waist circumference, and percent body fat.28 The apnea-hypopnea index (AHI) was determined according to results of home sleep apnea testing using the ARES Unicorder 5.2 (B-Alert).29, 30
Sueño Ancillary Study
From December 2010 to December 2013, a total of 2,189 HCHS/SOL participants aged 18 to 64 years were recruited; this group included Hispanic/Latino subjects from Central American, Cuban, Dominican, Puerto Rican, Mexican, and South American backgrounds. Participants with physician-diagnosed narcolepsy, sleep apnea, using CPAP, or AHI ≥ 50 events/hour (HCHS/SOL threshold for therapy) on home sleep testing were excluded. Details of the Sueño study protocol have been described elsewhere.31, 32
Participants completed questionnaires about employment, work schedule, use of caffeine, and hypnotic agents. Height, weight, and bioelectric impedance percent body fat were measured. Waist circumference from the HCHS/SOL baseline examination was used. BMI was calculated as kilograms divided by meters squared. Obesity was defined as a BMI ≥ 30 kg/m2 and abdominal obesity as a waist circumference ≥ 102 cm for men and ≥ 88 cm for women. Participants wore an Actiwatch Spectrum (Philips Respironics) on the nondominant wrist continuously for 7 days and completed daily sleep diaries.
The study protocols for the HCHS/SOL and Sueño ancillary studies were approved by the institutional review boards at each participating site, and all participants provided written informed consent. The institutional review board committee names and approval numbers are given in e-Appendix 1.
Actigraphy
Actigraphy and ambient light data were collected in 30-s epochs. Upon awakening, participants recorded bed and wake-up times. Records were scored at the reading center in Boston, Massachusetts. Rest periods and daytime napping were determined by following a standardized protocol previously published using the Actiwatch event marker, sleep diaries, light data, and actigraphy activity.31 Sleep-wake status for each epoch was computed by using the Actiware 5.59 scoring algorithm. Epochs were scored as awake if activity counts exceeded a 40-count threshold. Sleep onset was defined by using a threshold of 5 immobile minutes and sleep offset by using a threshold of 0 immobile minutes. This scoring algorithm was previously validated.33, 34, 35 A day was not included if there were > 4 h of missing data or > 2 min missing during a sleep period. Only participants with at least 5 days of valid actigraphy data were included.
Actigraphy Measurements
Actigraphy measurements were reported as the mean of valid days; sleep duration was defined as the time scored as sleep during the main rest period. For shift workers, the main rest period may have occurred partially during the day. Sleep duration was categorized into < 5, 5 to < 6, 6 to < 7, 7 to < 8, and ≥ 8 h of sleep.
Sleep quality was assessed by using the sleep fragmentation index and sleep efficiency. The sleep fragmentation index was calculated as the percentage of mobile epochs (ie, activity count ≥ 2) plus the percentage of immobile epochs (activity count < 2) that were ≤ 1 min long during the main rest period.31, 36 Sleep efficiency was calculated as time scored as sleep from sleep onset to sleep offset divided by the duration of the main rest period.
Naps were defined as self-reported periods of rest based on event markers or sleep diary, outside the main rest period, containing at least 15 min of actigraphically scored sleep. Napping was defined as more than one nap during the 7 days of recording.
Covariates
We included age (modeled continuously), sex, education, and household income as covariates known to affect obesity and sleep.31, 37, 38, 39, 40 Education was dichotomized as less than high school or high school and higher. Annual household income was categorized as less than $20,000, $20,000 and higher, or missing. Study site, ethnicity, and AHI (continuous variable) were also included.
Statistical Analyses
Dependent variables in all models included BMI, waist circumference, percent body fat, obesity, and abdominal obesity. Predictors of obesity included sleep duration, sleep efficiency, sleep fragmentation index, and napping. Because of previous reports of a U-shaped association between sleep duration and obesity, we categorized sleep duration and assessed the relationship by using a test of linear trend. We also modeled sleep duration continuously with both linear and quadratic terms to assess for a nonlinear relationship. Preliminary analyses suggested a linear relationship in models predicting obesity and abdominal obesity prevalence. As a result, all subsequent models used survey linear regression to assess the relationship between sleep measures and obesity accounting for the complex study design, including clustered sampling and sampling weights. For continuous obesity measures, this approach resulted in estimation of a slope (change in obesity per unit change in sleep). For dichotomous obesity measures, this approach resulted in estimation of prevalence difference per unit change in sleep measure.41 Initial analyses were age- and sex-standardized to distributions of the 2010 US Census. Subsequent analyses additionally adjusted for study site, ethnic background, educational level, household income, and AHI.42
Sensitivity analyses were performed excluding all participants who reported shift work (n = 447) and those with moderate to severe sleep apnea (AHI ≥ 15 events/hour; n = 188). All analyses were completed by using complex survey procedures in SAS version 9.3 (SAS Institute, Inc.).
Results
The Sueño ancillary study included 2,189 participants. Of these, 2,156 had ≥ 5 days of valid actigraphy data. Only participants with complete covariate data were included in analyses. The sample varied from 2,040 for percent body fat, 2,099 for obesity, and 2,113 for abdominal obesity.
Tables 1 and 2 present baseline characteristics standardized to age and sex distribution (2010 US Census) for obesity (BMI) and abdominal obesity (waist circumference), respectively. According to the BMI criteria, 43% were obese, and 62% were abdominally obese. Obesity was associated with higher AHI (P < .0001) and higher unemployment (P ≤ .01).
Table 1.
Age- and Sex-standardized Mean and Prevalence of Key Measures According to Obesity (BMI ≥ 30 kg/m2) Status, HCHS/SOL Sueño Ancillary Study (N = 2,156)
| Variable | Not Obese | Obese | P Value |
|---|---|---|---|
| AHI, events/h sleep | 2.9 ± 0.2 | 5.7 ± 0.4 | < .0001 |
| Percent body fat | 28.1 ± 0.2 | 39.5 ± 0.3 | < .0001 |
| Average sleep duration, min/d | 406.8 ± 2.5 | 394.6 ± 2.9 | .001 |
| Average sleep efficiency, % | 88.5 ± 0.2 | 87.9 ± 0.3 | .074 |
| Average sleep fragmentation index, % | 21.2± 0.3 | 22.4 ± 0.4 | .025 |
| Napping prevalence (≥ 2 d, > 15-min nap) | 0.22 ± 0.02 | 0.30 ± 0.02 | .002 |
| Education: less than high school (prevalence) | 0.29 ± 0.02 | 0.31 ± 0.02 | .447 |
| Household income: < $20,000 (prevalence) | 0.45 ± 0.02 | 0.51 ± 0.03 | .096 |
| Shift work (prevalence) | 0.23 ± 0.02 | 0.24 ± 0.02 | .516 |
| Not currently employed (prevalence) | 0.38 ± 0.02 | 0.49 ± 0.02 | .001 |
Survey linear regression was performed to estimate means and prevalence with sampling weight and standardized to the age and sex distribution of the 2010 US Census. Mean ± SEM are estimated for continuous variables, prevalence ± SEM for dichotomized variables. AHI = apnea-hypopnea index; HCHS/SOL = Hispanic Community Health Study/Study of Latinos.
Table 2.
Age- and Sex-standardized Mean and Prevalence of Key Measures According to Abdominal Obesity Status, HCHS/SOL Sueño Ancillary Study (N = 2,156)
| Variable | Not Obese | Obese | P Value |
|---|---|---|---|
| AHI, events/h sleep | 2.4 ± 0.2 | 5.5 ± 0.3 | < .0001 |
| Percent body fat | 26.8 ± 0.3 | 38.0 ± 0.4 | < .0001 |
| Average sleep duration, min/d | 407.8 ± 2.9 | 396.6 ± 2.8 | .007 |
| Average sleep efficiency, % | 88.4 ± 0.2 | 88.1 ± 0.2 | .438 |
| Average sleep fragmentation index, % | 21.3 ± 0.4 | 22.1 ± 0.4 | .193 |
| Napping prevalence (≥ 2 d, > 15-min nap) | 0.22 ± 0.02 | 0.28 ± 0.02 | .025 |
| Education: less than high school (prevalence) | 0.26 ± 0.02 | 0.33 ± 0.02 | .036 |
| Household income: < $20,000 (prevalence) | 0.44 ± 0.03 | 0.50 ± 0.02 | .056 |
| Shift work (prevalence) | 0.24 ± 0.02 | 0.22 ± 0.02 | .542 |
| Not currently employed (prevalence) | 0.37 ± 0.02 | 0.46 ± 0.02 | .01 |
Survey linear regression were performed to estimate means and prevalence with sampling weight and standardized to the age and sex distribution of the 2010 US Census. Mean ± SEM are estimated for continuous variables, prevalence ± SEM for dichotomized variables. Abdominal obesity was defined as waist circumference in male subjects ≥ 102 cm and in female subjects ≥ 88 cm. See Table 1 legend for expansion of abbreviations.
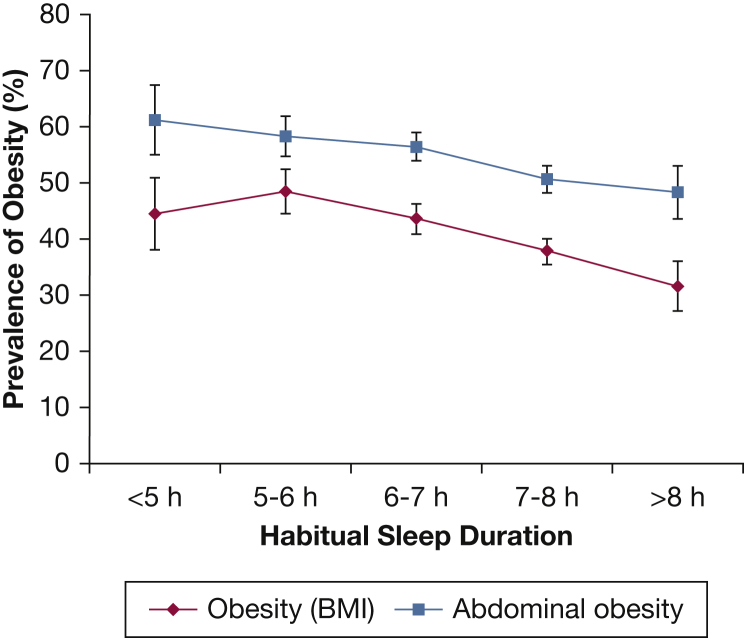
Figure 1 displays the age- and sex-adjusted prevalence of obesity and abdominal obesity according to sleep duration categories. Sleeping > 8 h had the lowest prevalence of adiposity, with an inverse linear relationship (P = .002 and P = .01 test of linear trend for obesity and abdominal obesity, respectively). The test of a quadratic sleep duration effect was nonsignificant.
Figure 1.
Age- and sex-standardized prevalence of obesity and abdominal obesity according to sleep duration. There is a linear rise in the prevalence of both obesity and abdominal obesity as average nightly hours of sleep decreases (test of linear trend P = .002 for obesity and P = .01 for abdominal obesity). Prevalence standardized to 2010 US census data. Obesity was defined as BMI ≥ 30 kg/m2. Abdominal obesity defined as waist circumference ≥ 102 cm for male subjects and ≥ 88 cm for female subjects. Mean ± SEM.
Table 3 presents the association between sleep measures and the prevalence of obesity and abdominal obesity adjusted for age, sex, ethnic background, site, education, income, and AHI. A 1-h reduction in sleep increased the prevalence of obesity by 4.1% (95% CI, 1.6 to 6.6; P = .002) and abdominal obesity by 3.6% (95% CI, 1.1 to 6.1; P = .007). Napping increased the prevalence of obesity by 10.4% (95% CI, 3.5 to 17.3; P = .004) and abdominal obesity by 7.1% (95% CI, 1.0 to 13.2; P = .02). After adjusting for sleep duration, napping increased the prevalence of obesity by 9.4% (95% CI, 2.2 to 16.5; P = .01) and abdominal obesity by 6.2% (95% CI, –0.1 to 12.6; P = .05). Sleep efficiency and sleep fragmentation index were not associated with adiposity measures.
Table 3.
Association of Actigraphic Sleep Measures and the Prevalence of Obesity and Abdominal Obesity, HCHS/SOL Sueño Ancillary Study (N = 2,156)
| Actigraphy Variable | Obesity |
Abdominal Obesity |
||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | P Value | Beta | 95% CI | P Value | |
| Sleep duration (per hour) | –4.1 | –6.6 to –1.6 | .002 | –3.6 | –6.1 to –1.1 | .007 |
| Sleep efficiency (%) | –0.1 | –0.7 to 0.5 | .68 | 0.1 | –0.5 to 0.7 | .85 |
| Sleep fragmentation index (%) | 0.1 | –0.3 to 0.5 | .58 | 0.0 | –0.4 to 0.4 | .95 |
| Napping vs no napping | 10.4 | 3.5 to 17.3 | .004 | 7.1 | 1.0 to 13.2 | .02 |
All models account for sampling weights and were adjusted for age, sex, ethnic background, site, income, education, and AHI. Obesity was defined as BMI ≥ 30 kg/m2. Abdominal obesity was defined as waist circumference ≥102 cm for male subjects and ≥ 88 cm for female subjects. Napping was defined as more than one nap of at least 15 min over 1 week. See Table 1 legend for expansion of abbreviations.
Table 4 presents the association between sleep measurements and continuous adiposity measurements. A 1-h reduction in sleep increased BMI by 0.67 kg/m2 (95% CI, 0.30-1.10; P = .001) and waist circumference by 1.5 cm (95% CI, 0.5-2.5; P = .002). Napping increased BMI by 1.36 kg/m2 (95% CI, 0.40-2.30; P = .005) and waist circumference by 3.9 cm (95% CI, 1.7-6.1; P = .0004). After adjusting for sleep duration, napping increase in BMI by 1.20 kg/m2 (95% CI, 0.23-2.16; P = .02) and waist circumference by 3.5 cm (95% CI, 1.3-5.6; P = .002). None of the sleep measures was associated with percent body fat. No relationship was found with sleep efficiency or sleep fragmentation index.
Table 4.
Association of Actigraphic Sleep Measures With Continuous Adiposity Measures, HCHS/SOL Sueño Ancillary Study (N = 2,156)
| Actigraphy Variable | BMI (kg/m2) |
% Body Fat |
Waist Circumference (cm) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P Value | Beta | 95% CI | P Value | Beta | 95% CI | P Value | |
| Sleep duration, h | –0.67 | –1.10 to –0.30 | .001 | –0.34 | –0.90 to 0.20 | .24 | –1.5 | –2.5 to –0.5 | .002 |
| Sleep efficiency, % | 0.04 | –0.04 to 0.12 | .36 | 0.07 | –0.05 to 0.20 | .25 | 0.0 | –0.2 to 0.2 | .99 |
| Sleep fragmentation index, % | –0.01 | –0.07 to 0.05 | .66 | 0.00 | –0.08 to 0.08 | .90 | 0.0 | –0.2 to 0.2 | .73 |
| Napping vs no napping | 1.36 | 0.40 to 2.30 | .005 | 1.26 | –0.01 to 3.20 | .05 | 3.9 | 1.7 to 6.1 | .0004 |
All models account for sampling weights and were adjusted for age, sex, ethnic background, site, income, education, and AHI. Sleep duration, sleep efficiency, and sleep fragmentation index were modeled continuously. Napping was defined as more than one nap of at least 15 min over 1 week. See Table 1 legend for expansion of abbreviations.
Table 5 depicts sensitivity analyses after excluding shift workers (n = 447) and subjects with moderate to severe sleep apnea (n = 188). The relationships between sleep measurements (ie, sleep duration, napping) and adiposity (ie, BMI, waist circumference, percent body fat) were strengthened. Napping increased percent body fat by 1.5% (95% CI, 0.1-2.9; P = .04) only in the model excluding shift workers.
Table 5.
Sensitivity Analyses Evaluating the Association of Sleep Duration and Napping With Continuous Adiposity Measures Excluding Shift Workers (n = 447) and Participants With Moderate to Severe Sleep Apnea (n = 188), HCHS/SOL Sueño Ancillary Study (N = 2,156)
| Actigraphy Variable | BMI (kg/m2) |
Waist Circumference (cm) |
Percent Body Fat |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P Value | Beta | 95% CI | P Value | Beta | 95% CI | P Value | |
| Shift workers excluded | |||||||||
| Sleep duration, h | –0.8 | –1.3 to –0.3 | .001 | –1.8 | –3.0 to –0.6 | .001 | –0.6 | –1.3 to 0.1 | .08 |
| Napping vs no napping | 1.7 | 0.7 to 2.7 | .001 | 4.9 | 2.5 to 7.3 | < .0001 | 1.5 | 0.1 to 2.9 | .04 |
| Moderate to severe OSA excluded | |||||||||
| Sleep duration, h | –0.8 | –1.2 to –0.4 | .0002 | –1.7 | –2.7 to –0.7 | .0006 | –0.5 | –1.1 to 0.1 | .12 |
| Napping vs no napping | 1.3 | 0.3 to 2.3 | .01 | 3.8 | 1.4 to 6.2 | .001 | 1.1 | –0.3 to 2.4 | .12 |
All models account for sampling weights and were adjusted for age, sex, ethnic background, site, income, education, and AHI. Sleep duration was modeled continuously. Napping was defined as more than one nap of at least 15 min over 1 week. Shift worker was defined as those working afternoon, night, split, irregular, or rotating shifts. Moderate to severe sleep apnea was defined as an AHI ≥15 events/hour. See Table 1 legend for expansion of abbreviations.
Discussion
We found an inverse linear relationship between objectively measured sleep duration and the prevalence of obesity (BMI) and abdominal obesity. Lower sleep duration and napping were strongly associated with increased obesity prevalence and a rise in BMI and waist circumference after accounting for important confounders, including sleep apnea. No association was found between sleep quality (ie, sleep efficiency, sleep fragmentation index) and adiposity.
This study may be the most representative evaluation of the association between sleep patterns and obesity using 7 days of actigraphy data concurrently with measures of adiposity. It used a large community-based population of young to older adults in four geographic locations across the United States and controlled for sleep apnea, ethnicity, and other major confounders.
Hispanic/Latino individuals are the largest minority in the United States at high risk for obesity-related diseases.19, 20 However, compared with white and black subjects, Hispanic/Latino subjects have been understudied.23, 24, 25 Chronic insufficient sleep is strongly linked to higher risk of obesity in men, women, and children.10, 43, 44, 45, 46, 47, 48, 49 Only a few studies have focused on the sleep patterns of Hispanic subjects,32, 50, 51, 52, 53 however, and most included only self-reported data.51, 52, 53, 54 Ours is the first study evaluating the association of objectively measured sleep patterns and adiposity in a large representative US Hispanic/Latino population.
Our findings support a strong association between lower sleep duration and obesity, confirming results of studies using self-reported sleep duration9, 10, 12, 13, 14, 43, 44, 45, 46, 47, 48, 49 and objectively measured sleep duration.13, 15, 16, 18, 55 However, instead of a U-shaped relationship described by most,13, 16 or a dichotomous relation showing higher obesity only in the lowest sleep duration category,16, 55 we found an inverse linear relationship (Fig 1). The reason for this variability may be related to methodologic differences among studies.
Studies that evaluated elderly populations mostly report a U-shaped relationship between objective sleep duration and adiposity. The MrOS, SOF,16 and the Rotterdam13 studies evaluated elderly men and women (aged > 68 years) and described a U-shaped relationship with percent body fat, and with BMI in the Rotterdam study. Moraes et al55 described a U-shaped relationship with BMI in subjects aged > 50 years and a dichotomous relationship in younger subjects. The MESA study described an inverse linear relationship between sleep duration and obesity (BMI ≥ 30 kg/m2) in an older population (68.6 ± 9.2 years).17 However, the relationship with abdominal obesity was U-shaped. In contrast, we report an inverse liner relationship with both obesity (BMI ≥ 30 kg/m2) and abdominal obesity in a younger population (average age, 47 years; range, 18-64 years). The CARDIA study evaluated young adults (aged 18-30 years)15 and reported an inverse linear relationship with BMI, similar to our findings. The literature and our findings suggest that the inverse linear relationship between sleep duration and adiposity may weaken into a U-shaped relationship with aging.10 This age hypothesis in the pattern of the association between sleep duration and adiposity needs further investigation.
Sleep apnea is strongly associated with obesity56 and potentially an important confounder of the association between sleep duration and adiposity. In our analyses, we excluded subjects with AHI > 15 events/h and controlled for AHI to remove the confounding effect of sleep apnea. The CARDIA and Rotterdam studies13, 15 did not control for sleep apnea. However, in the CARDIA study, the association between sleep duration and BMI was strongest in subjects who snored,15 and in the Rotterdam study, the association with BMI was no longer significant after adjusting for sleep fragmentation.13 These findings suggest that sleep disturbances, such as in sleep apnea, are important confounders. In the MESA study, models that controlled for sleep apnea attenuated the associations between sleep duration and obesity,17 again underlining the importance of controlling for sleep apnea when evaluating the association between sleep duration and obesity.
We found a strong association between napping and obesity, suggesting that other sleep patterns may be contributing to adiposity. Our findings confirm the results of the MrOS and SOF studies that reported an association with napping and increased obesity.18 The mechanism for the association of napping and adiposity is unclear. However, napping can be a marker of insufficient sleep or poor sleep quality,57 potentially driving the association. However, in the present study, controlling for sleep apnea and sleep duration minimally attenuated the association, suggesting that napping by itself may be a risk factor for adiposity.
It is unclear why we found no relationship between markers of sleep quality and adiposity as others have reported. The CARDIA study and the MrOS and SOF studies reported an association with sleep fragmentation15 and sleep duration variability18 respectively, and higher BMI. The MESA study reported an association between sleep efficiency and adiposity.17 Differences in ethnicity and age may contribute to the variability in results. We studied a population of younger Hispanic/Latino individuals, whereas others evaluated a mix of racial groups, mostly elderly.13, 17, 18 Racial differences in sleep quality exist,58, 59, 60 potentially confounding the association between sleep quality and adiposity. In older adult subjects, BMI underestimates adiposity,61 yet the elderly are more prone to increased adiposity.62 Also, the circadian pacemaker becomes progressively disturbed with aging, resulting in sleep pattern changes63, 64, 65 and potentially confounding the association between sleep quality and adiposity. More research is needed to understand the effect of aging and ethnicity in the association of sleep quality and adiposity.
The literature evaluating the relationship of actigraphic sleep patterns and adiposity is limited by wide methodologic and population differences that restrict generalization. These include disparate population size,16, 55 wide ethnic mix,17 short duration of actigraphy evaluation,55 and population age extremes ranging from young adults15 to the very elderly.13, 16, 18 A major strength in the present study is the age-sex representative sample population of adult Hispanic/Latino individuals living across the United States and the careful objective evaluation of sleep quality variables derived from actigraphy over 7 days, which were correlated to obesity measurements (ie, BMI, percent body fat) made during the week of data acquisition. However, the home sleep study and waist circumference measurements were obtained from the baseline assessment made in the parent HCHS/SOL study 6 to 24 months prior to the actigraphy recordings. The assumption that abdominal obesity and AHI remained stable during this time represents a significant limitation. Also, the cross-sectional nature of this study precludes inferring causality and represents a limitation. Further research is needed to assess causality that could include interventional studies to modify sleep duration and longitudinal evaluation of sleep duration and napping on adiposity.
Conclusions
In a large representative sample population of adult Hispanic/Latino subjects living across the United States, we found an inverse linear association between objectively measured sleep duration and the prevalence of obesity. We also found an association between daytime napping and adiposity, highlighting the potential contribution of other sleep patterns to obesity. The age distribution of the population under study may be an important determinant of the pattern of the association between sleep duration and adiposity. Interventional and longitudinal studies are needed to better understand how sleep patterns contribute to the obesity epidemic currently affecting the United States, especially among minority populations.20, 21, 22
Acknowledgments
Author contributions: J. S. L. takes responsibility for the content of the manuscript, including the accuracy of the data and analysis, and affirms that the manuscript is an honest, accurate, representation of the study design and aims. J. S. L. contributed to the design of the study, data collection, data analysis, interpretation, and was the primary writer of the manuscript. J. W. performed the statistical analysis and contributed substantially to the study design, analysis interpretation, and writing of the manuscript. S. R. P. was the principal investigator in the Sueño ancillary study and contributed substantially to the study design, data collection, analysis and interpretation, and writing of the manuscript. A. R. R., D. S.-A., G. S., and G. A. T. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. R. P. has received grant funding from the American Sleep Medicine Foundation and the ResMed Foundation and has also served as consultant for Covidien. None declared (J. S. L., J. W., A. R. R., D. S.-A., G. S., G. A. T.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the participants of the Hispanic Community Health Study/Study of Latinos and participants of the Sueño ancillary study for their willingness to take part in this research. They also thank the Hispanic Community Health Study publications committee for the review of this work and the National Heart, Lung, and Blood Institute for its support of this research project.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) [Grant HL098297 and HL127307]. In addition, the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) was conducted as a collaborative study supported by contracts from the NHLBI to the University of North Carolina [Grant N01-HC65233], University of Miami [Grant N01-HC65234], Albert Einstein College of Medicine [Grant N01-HC65235], Northwestern University [Grant N01-HC65236], and San Diego State University [Grant N01-HC65237]. The following institutes/center/offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: the National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements.
Supplementary Data
References
- 1.Prather A.A., Vogelzangs N., Penninx B.W. Sleep duration, insomnia, and markers of systemic inflammation: results from the Netherlands Study of Depression and Anxiety (NESDA) J Psychiatr Res. 2015;60:95–102. doi: 10.1016/j.jpsychires.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayas N.T., White D.P., Manson J.E. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 3.Gangwisch J.E., Heymsfield S.B., Boden-Albala B. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb D.J., Redline S., Nieto F.J. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 5.Itani O., Jike M., Watanabe N., Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Knutson K.L., Ryden A.M., Mander B.A., Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 7.Weingarten J.A., Dubrovsky B., Basner R.C., Redline S., George L., Lederer D.J. Polysomnographic measurement of sleep duration and bodily pain perception in the Sleep Heart Health Study. Sleep. 2016;39(8):1583–1589. doi: 10.5665/sleep.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallicchio L., Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 9.Marshall N.S., Glozier N., Grunstein R.R. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Patel S.R., Hu F.B. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutson K.L. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaput J.P., Després J.P., Bouchard C., Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec Family Study. Obesity (Silver Spring) 2007;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg J.F., Knvistingh Neven A., Tulen J.H. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008;32(7):1083–1090. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 14.Sun W., Huang Y., Wang Z. Sleep duration associated with body mass index among Chinese adults. Sleep Med. 2015;16(5):612–616. doi: 10.1016/j.sleep.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Lauderdale D.S., Knutson K.L., Rathouz P.J., Yan L.L., Hulley S.B., Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170(7):805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S.R., Blackwell T., Redline S. and the Osteoporotic Fractures in Men Research Group; Study of Osteoporotic Fractures Research Group. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32(12):1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogilvie R.P., Redline S., Bertoni A.G. Actigraphy measured sleep indices and adiposity: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2016;39(9):1701–1708. doi: 10.5665/sleep.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel S.R., Hayes A.L., Blackwell T. and the Osteoporotic Fractures in Men (MrOS), Study of Osteoporotic Fractures (SOF) Research Groups. The association between sleep patterns and obesity in older adults. Int J Obes (Lond) 2014;38(9):1159–1164. doi: 10.1038/ijo.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhupathiraju S.N., Hu F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell B., Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Females, Hispanics and older individuals are at greatest risk of developing metabolic syndrome in the U.S. Diabetes Metab Syndr. 2016;10(4):230–233. doi: 10.1016/j.dsx.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Isasi C.R., Ayala G.X., Sotres-Alvarez D. Is acculturation related to obesity in Hispanic/Latino adults? Results from the Hispanic Community Health Study/Study of Latinos. J Obes. 2015;2015:186276. doi: 10.1155/2015/186276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan R.C., Avilés-Santa M.L., Parrinello C.M. Body mass index, sex, and cardiovascular disease risk factors among Hispanic/Latino adults: Hispanic Community Health Study/Study of Latinos. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy V.H., Krumholz H.M., Gross C.P. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 24.Schmotzer G.L. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis. 2012;22(2):226–230. [PubMed] [Google Scholar]
- 25.Wendler D., Kington R., Madans J. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavange L.M., Kalsbeek W.D., Sorlie P.D. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorlie P.D., Aviles-Santa L.M., Wassertheil-Smoller S. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong W.W., Strizich G., Heo M. Relationship between body fat and BMI in a US Hispanic population-based cohort study: results from HCHS/SOL. Obesity (Silver Spring) 2016;24(7):1561–1571. doi: 10.1002/oby.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redline S., Sotres-Alvarez D., Loredo J. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westbrook P.R., Levendowski D.J., Cvetinovic M. Description and validation of the apnea risk evaluation system: a novel method to diagnose sleep apnea-hypopnea in the home. Chest. 2005;128(4):2166–2175. doi: 10.1378/chest.128.4.2166. [DOI] [PubMed] [Google Scholar]
- 31.Patel S.R., Weng J., Rueschman M. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38(9):1497–1503. doi: 10.5665/sleep.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley K.A., Weng J., Sotres-Alvarez D. Actigraphic sleep patterns of U.S. Hispanics: the Hispanic Community Health Study/Study of Latinos. Sleep. 2017;40(2):zsw049. doi: 10.1093/sleep/zsw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushida C.A., Chang A., Gadkary C. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 34.Marino M., Li Y., Rueschman M.N. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oakley N.R. Technical Report to Mini Mitter Co, Inc., Bend, OR;; 1997. Validation with polysomnography of the sleep-watch sleep/wake scoring algorithm used by the actiwatch activity monitoring system. [Google Scholar]
- 36.Kurina L.M., Knutson K.L., Hawkley L.C. Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011;34(11):1519–1526. doi: 10.5665/sleep.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatakis K.A., Kaplan G.A., Roberts R.E. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17(12):948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel S.R., Malhotra A., Gottlieb D.J. Correlates of long sleep duration. Sleep. 2006;29(7):881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorleifsdottir B., Bjornsson J.K., Benediktsdottir B. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53(1):529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 40.Hjorth M.F., Chaput J.P., Michaelsen K. Seasonal variation in objectively measured physical activity, sedentary time, cardio-respiratory fitness and sleep duration among 8-11 year-old Danish children: a repeated-measures study. BMC Public Health. 2013;13:808. doi: 10.1186/1471-2458-13-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellevik O. Linear versus logistic regression when the dependent variable is a dichotomy. Qual Quant. 2009;43:59–74. [Google Scholar]
- 42.Patel S.R., Sotres-Alvarez D., Castaneda S.F. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;38(10):1515–1522. doi: 10.5665/sleep.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theorell-Haglöw J., Berglund L., Berne C., Lindberg E. Both habitual short sleepers and long sleepers are at greater risk of obesity: a population-based 10-year follow-up in women. Sleep Med. 2014;15(10):1204–1211. doi: 10.1016/j.sleep.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Patterson R.E., Emond J.A., Natarajan L. Short sleep duration is associated with higher energy intake and expenditure among African-American and non-Hispanic white adults. J Nutr. 2014;144(4):461–466. doi: 10.3945/jn.113.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buxton O.M., Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 46.Knutson K.L., Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel S.R., Malhotra A., White D.P., Gottlieb D.J., Hu F.B. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Q., Arem H., Moore S.C., Hollenbeck A.R., Matthews C.E. A large prospective investigation of sleep duration, weight change, and obesity in the NIH-AARP diet and health study cohort. Am J Epidemiol. 2013;178:1600–1610. doi: 10.1093/aje/kwt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Cauter E., Knutson K.L. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159:S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakker J.P., Weng J., Wang R., Redline S., Punjabi N.M., Patel S.R. Associations between obstructive sleep apnea, sleep duration, and abnormal fasting glucose. The Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2015;192(6):745–753. doi: 10.1164/rccm.201502-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grandner M.A., Chakravorty S., Perlis M.L., Oliver L., Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seicean S., Neuhauser D., Strohl K., Redline S. An exploration of differences in sleep characteristics between Mexico-born US immigrants and other Americans to address the Hispanic Paradox. Sleep. 2011;34(8):1021–1031. doi: 10.5665/SLEEP.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunningham T.J., Wheaton A.G., Ford E.S., Croft J.B. Racial/ethnic disparities in self-reported short sleep duration among US-born and foreign-born adults. Ethn Health. 2016;21(6):628–638. doi: 10.1080/13557858.2016.1179724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mossavar-Rahmani Y., Jung M., Patel S.R. Eating behavior by sleep duration in the Hispanic Community Health Study/Study of Latinos. Appetite. 2015;95:275–284. doi: 10.1016/j.appet.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moraes W., Poyares D., Zalcman I. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med. 2013;14(4):312–318. doi: 10.1016/j.sleep.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Resta O., Foschino-Barbaro M.P., Legari G. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25(5):669–675. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 57.Ming X., Koransky R., Kang V., Buchman S., Sarris C.E., Wagner G.C. Sleep insufficiency, sleep health problems and performance in high school students. Clin Med Insights Circ Respir Pulm Med. 2011;5:71–79. doi: 10.4137/CCRPM.S7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hale L., Do D.P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–10103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gamaldo A.A., McNeely J.M., Shah M.T., Evans M.K., Zonderman A.B. Racial differences in self-reports of short sleep duration in an urban-dwelling environment. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):568–575. doi: 10.1093/geronb/gbt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X., Wang R., Zee P. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pasco J.A., Nicholson G.C., Brennan S.L., Kotowicz M.A. Prevalence of obesity and the relationship between the body mass index and body fat: cross-sectional, population-based data. PLoS One. 2012;7(1):e29580. doi: 10.1371/journal.pone.0029580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ilich J.Z., Kelly O.J., Inglis J.E. Osteosarcopenic obesity syndrome: what is it and how can it be identified and diagnosed? Curr Gerontol Geriatr Res. 2016;2016:7325973. doi: 10.1155/2016/7325973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youngstedt S.D., Kripke D.F., Elliott J.A., Klauber M.R. Circadian abnormalities in older adults. J Pineal Res. 2001;31(3):264–272. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- 64.Yoon I.Y., Kripke D.F., Elliott J.A., Youngstedt S.D., Rex K.M., Hauger R.L. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51(8):1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 65.Hofman M.A., Swaab D.F. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5(1):33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.