Abstract
Infectious bursal disease (IBD), also known as Gumboro disease, is a highly contagious, immunosuppressive disease of young chickens. Although first observed about 60 years ago, to date, the disease is responsible for major economic losses in the poultry industry worldwide. IBD virus (IBDV), a double-stranded RNA virus, exists as two serotypes with only serotype 1 causing the disease in young chickens. The virus infects the bursa of Fabricius of particularly the actively dividing and differentiating lymphocytes of the B-cells lineage of immature chickens, resulting in morbidity, mortality, and immunosuppression. Immunosuppression enhances the susceptibility of chickens to other infections and interferes with vaccination against other diseases. Immunization is the most important measure to control IBD; however, rampant usage of live vaccines has resulted in the evolution of new strains. Although the immunosuppression caused by IBDV is more directed toward the B lymphocytes, the protective immunity in birds depends on inducement of both humoral and cell-mediated immune responses. The interference with the inactivated vaccine induced maternally derived antibodies in young chicks has become a hurdle in controlling the disease, thus necessitating the development of newer vaccines with improved efficacy. The present review illustrates the overall dynamics of the virus and the disease, and the recent developments in the field of virus diagnosis and vaccine research.
Keywords: infectious bursal disease, epidemiology, pathogenesis, diagnosis, vaccines
Introduction
Infectious bursal disease (IBD), also known as Gumboro disease is a highly contagious and immunosuppressive disease of young chickens caused by IBD virus (IBDV) which is responsible for major economic losses in the poultry industry worldwide. IBDV is a double-stranded RNA virus belongs to the genus Avibirnavirus of the family Birnaviridae.1 The genome of the IBDV is bi-segmented and divided into segment A and B. The larger open reading frame 1 (ORF1) of segment A encodes for a 110 kDa polyprotein which auto-catalytically splices into viral proteins VP2 (48 kDa), VP3 (33–35 kDa) and VP4 (24 kDa). IBDV has two recognized serotypes, namely serotypes 1 and 2 and both serotypes of the virus can naturally infect chicken, turkey, duck, guinea fowl, ostriches; the pathogenicity being reported only in chicken by serotype 1. The “re-emergence” of IBDV as antigenic variants and very virulent strains (vvIBDV) had been the reason for significant losses and high mortality in chickens, and the virus is continuously evolving in the field with changes in antigenicity and virulence. VP2 is the major host-protective capsid protein of the virus that carries the immunogenic determinants and is able to elicit neutralizing antibodies. Recently, IBDV has been classified into seven genogroups based on the marked changes in the amino acids in the hypervariable region of the capsid protein VP2 (hVP2) among the different groups, wherein genogroup 1 is distributed globally. Currently, the disease is controlled by live attenuated or inactivated IBDV vaccine, but the live vaccines can revert back to virulence and the traditional vaccines may not give full protection against vvIBDV strain. The inactivated or killed vaccines are usually given to birds in pre-laying stage to induce higher levels of antibody production for at least 2 weeks. However, with the emergence of antigenic variants and very virulent IBDV (vvIBDV), chickens are not fully protected by conventional IBD vaccines. Thus, new generation vaccines are produced with the advantage of their overcoming the interference with maternally derived antibodies (MDA), besides safety, ease of production and stability.
IBDV genome organization
The IBDV genome consists of two segments of double-stranded RNA which are packaged in a non-enveloped icosahedral shell having 32 capsomers, 60 nm in diameter. The structure of the virus is based on a T=13 lattice and the capsid subunits are predominantly trimer clustered.2 The larger segment A encodes for a polyprotein (pVP2–VP4–VP3) which is 3,261 nucleotides long and encodes a 110 kDa precursor protein in a single large ORF. In the VP2 protein, a precursor–product relationship exists as only the larger protein (pVP2, 48 kDa) can be demonstrated in infected cells, and further proteolytic cleavage transforms the precursor into the VP2 protein, present in the complete virus particle.3 This polyprotein can be digested to pVP2 (1–512 amino acids), VP4 (513–791 amino acids) and VP3 (792–1,012 amino acids) proteins by autoproteolysis of VP4 to yield mature VP2–VP4 proteins prior to viral assembly. VP2 being the major host-protective capsid antigen contains at least two epitopes for neutralizing antibodies that protect the susceptible host from vIBDV and is the determinant for cell tropism, tissue culture adaptation and pathogenic phenotype of IBDV.4 VP2 protein has three major domains, namely the base, shell and projection domains. The conserved amino acids form the base and the shell domains whereas the projection domain is formed by a hypervariable region of VP2 spanning amino acids 206–350.5 VP3 (32 kDa) is a group-specific immunogenic protein of IBDV, has cross-reactivity with both serotypes 1 and 2 and following infection the earliest appearing antibodies are directed toward VP3.6,7 Segment A also encodes a 17 kDa non-structural protein VP5, with a partially overlapping ORF.8 VP5 is a class 2 membrane protein with a cytoplasmic N-terminal and extracellular C-terminal domain and plays an important role in pathogenesis. This protein is highly basic, cysteine-rich and semi-conserved among all the serotypes of IBDV.8 VP5 accumulates in the cell membrane resulting in cell disruption and virion release. The smaller segment B encodes VP1 (97 kDa), an RNA-dependent RNA polymerase and it exists both as a free polypeptide and as a genome-linked protein. It plays a key role in the encapsidation of the viral particles and interacts with VP3 to form the VP1-VP3 complex that gives the structural integrity for the viral particles.9
Prevalence
Infectious bursal disease (IBD) has been a serious threat to the poultry industry. The “re-emergence” of IBDV as antigenic variants and hypervirulent strains had been the reason for significant losses and high mortality. However, factors like the dose and virulence of the strain, age and breed of the birds and the presence or absence of passive immunity may be linked with mortality. The disease leads to immunosuppression and the infected birds become susceptible to other viruses, bacteria or parasites. Moreover, the increased usage of antibiotics against secondary infections may also lead to a growing public health concern.
Distribution of IBDV
IBDV termed as avian nephrosis or “classic IBDV” was first reported from Gumboro in Delaware, USA in the year 196210 and hence the name of the disease was originated besides “IBD” or “infectious bursitis.” The disease has spread to most parts of the USA between 1960 and 1964, and affected Europe in between 1962 and 1971.11 IBDV strains based on virus neutralization assay are classified into serotype 1 and serotype 2, While serotype 1 viruses are pathogenic to chickens and based upon the mortality and bursal lesions, the virus can be categorized into attenuated (atIBDV), classical virulent (cvIBDV), antigenic variant (avIBDV) and very virulent (vvIBDV) subtypes.12 Serotype 2 viruses are isolated from turkeys and are avirulent to chickens. The vvIBDV strains that were more virulent than classical strains causing mortality rates of 90% were detected in Holland in 1986. The virus strain DV86 then had spread to the UK in 1988 and was detected in Japan and Belgium.13 Since then the acute form of the disease has spread to most of the countries including all of Asia, Central Europe and Russia, the Middle East, South America14 with only Australia, New Zealand, Canada and the USA remaining free till 2008. However, there are significant differences between the African, European and Asian vvIBDV strains, suggestive of an independent evolution.14 However, the variant strain of serotype 1 virus that emerged in the USA in 1985 causes no mortality but induces rapid and severe bursal atrophy. The live-attenuated vaccines developed against classical strain fail to respond against the variant strains. Moreover, in the USA, the monoclonal antibodies developed against VP2 fail to react with one-third of the 300 IBDV field isolates that proves the rapidity with which the virus had evolved. Based on a recent study, it is proposed that, worldwide, about 60–76% of IBDV isolates are of vvIBDV genotype.15 Rampant vaccination without genotype matching resulted in genetic diversification of circulating viruses through reassortment that acquired segment A from very virulent IBDV and segment B from classical atIBDV D78 strain in Poland.16 The chimeric virus caused 80% mortality in Specific Pathogen Free chickens along with acute form of bursal lesions.
Economic impact
The economic impact of IBD on the poultry industry is difficult to assess due to the complex nature of losses associated with the disease. IBDV infection-induced immunodeficiency in chickens makes the flock susceptible to other viral, bacterial, and parasitic infections, thus resulting in indirect losses. Being resistant to most of the disinfectants and environmental factors, the poultry house remains contaminated with IBDV that persists on the premises and tends to reappear in subsequent flocks.
The acute or chronic IBD in the flock reduces the efficiency of production and net profitability in terms of feed conversion ratio. The avIBDV strains prevalent in Saskatchewan farms probably attributed to substantial economic losses of about 3.9 million kg per year to broiler meat industry.17
Molecular epidemiology and phylogeny analysis of IBDV
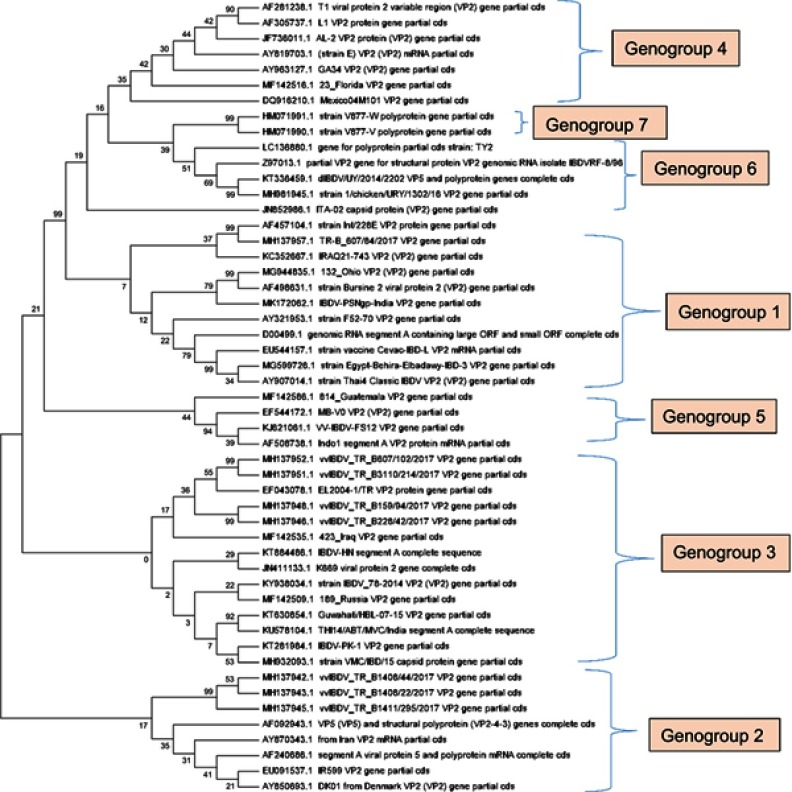
Vaccination is the most important measure to control IBD; however, extensive usage of live vaccines has resulted in the evolution of new strains. Despite the regular vaccination schedule, there are still reports of IBDV outbreaks around the globe. By closely studying the evolution of IBDV, it is possible to find the cause of these outbreaks. The major capsid protein VP2 plays a pivotal role in antigenic variation by which the virus can escape neutralizing antibodies. Most of the amino acid (aa) changes among the antigenically different IBDVs are clustered in the hypervariable region of VP2 (hVP2). Thus, this hypervariable region of VP2 is the obvious target for IBDV detection, evolution and pathogenic variation. The hVP2 region has two major hydrophilic domains, namely major hydrophilic peak A (aa 212–224) and peak B (aa 314–325) that form hairpin loops PBC (aa 219–224) and PHI (aa 316–324), respectively. The minor hydrophilic peak 1 (aa 248–254) and peak 2 (aa 279–290) of hVP2 form loops PDE (aa 249–254) and PFG (aa 279–284), respectively.18 Either single or combined mutations in hVP2 region affect the virulence pattern of the virus. Recent IBDV field outbreaks from various geographic locations revealed amino acid exchanges at minor hydrophilic peak domain of hVP2.19 Mutations at the variable domains between amino acids position 206 and 350, where the neutralizing antibody binds, results in immune evasion of the virus. The substitution of the amino acids at positions 253 (Q253H), 279 (D279N) and 284 (A284T) on the VP2 domain of vvIBDV resulted in loss of virulence of the virus. But a single point mutation in the 253 (H253Q/N) or 249 (R249Q), however, drastically increased the virulence of an attenuated IBDV strain. The mutation at the amino acid position 254 with serine in place of glycine (loop PDE)20 results in vaccination failure. Although sequencing of the whole IBDV genome is useful for phylogenetic characterization, it is impractical to replicate in large number of isolates. Recently, the virus has been classified into seven genogroups. Genogroup 1 generally comprises the classical IBDV and is present worldwide, genogroup 2 primarily comprises the antigenic variants predominant in the USA and genogroup 3 comprises predominantly of the vvIBDV pathotype or few are vvIBDV reassortants, distributed worldwide. An antigenic drift at aa position 222, (A222T) the genetic hallmark, has been observed in the population of genogroup 3 due to selective antigenic pressure with vaccination. Moreover, the genogroup 3 reassortants have a different segment B and do not have the typical amino acids found in the vvIBDV. Most of the virulent viruses of Indian origin also belong to genogroup 3 (Figure 1). However, the viral isolates that did not clearly fit into any of the three major genogroups were classified separately. The genogroup classification method classified these viruses into four new genogroups 4–7. A genogroup 4 virus is characterized by 222S, 272T, 289P, 290I and 296F and is distributed worldwide but was most commonly isolated from Latin America. Genogroup 5 viral strains included Mexican recombinant classical and variant viruses with amino acid changes in both PDE and PHI. These viruses have variant-type amino acid sequences in the PBC loop, whereas the PFG loop is more similar to the classical viruses. The PDE loop differed from reference variant and classical strains with the presence of 251N and 254N, and the PHI loop of genogroup 5 revealed two unique substitutions: S317K and A321P.21 Genogroup 6 consisted of samples from Saudi Arabia with 92.26–93.64% identity to the Italian genotype that is characterized by 220H, 222Q, 253E, 254S and 321V and has 94.02–95.40% identity to Russian isolate. Genogroup 7 is composed of viruses from Australia and has two distinct groups of IBDV; the classical strains that are similar to V877 and 002-73 and antigenic variant strains that are similar to 05-5 and 08/95 viruses.22
Figure 1.
Phylogenetic analysis of the nucleotide sequences of hVP2 infectious bursal disease virus (IBDV). The evolutionary history was inferred using the Neighbour-Joining method. The bootstrap consensus tree inferred from 1,000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in <50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. The analysis involved 51 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + noncoding. All positions containing gaps and missing data were eliminated. There were a total of 336 positions in the final dataset. Evolutionary analyses were conducted in MEGA7.90
Pathogenesis of IBDV
The disease strikes young chickens at 3–6 weeks of age, while sub-clinical form of infection is established in older birds. Layer type chickens are more susceptible to vvIBDV than broiler type23 and higher mortality rates are observed in light than heavy breeds. As mentioned earlier, IBDV has two serotypes, serotype 1 and serotype 2 however serotype 1 develops IBD in chicks. Apparent pathological lesions are observed with experimental infection on pigeon and guinea fowl.24 The primary organ of predilection is bursa of Fabricius (BF) where majority of the B cells are in actively dividing stage in young chicks. Faeco-oral route and inhalation are the major routes of entry of the virus that replicates in gut-associate macrophages and lymphoid cells and results in primary viremia through portal circulation. Following primary viremia, the virus reaches BF by 11-hr post inoculation and after active replication in bursal follicles and B cells, the virus enters the bloodstream to cause secondary viremia. This leads to spread of the virus in other organs like kidneys and muscle tissue that leads to pathognomonic clinical signs and death. Following infection of the BF, degeneration and necrosed B-cell follicles especially IgM+ cells are detected immediately, with associated infiltration of inflammatory cells such as heterophils. As the inflammation reduces, there is necrosis and phagocytosis of heterophils and plasma cells, fibroplasia in the interfollicular connective tissue, and ultimately bursal atrophy.25 With concurrent histological changes, molecular variations like upregulation of antiviral genes that are involved in the type I interferon (IFN) response, pro-apoptotic genes, and pro-inflammatory cytokines and chemokines, presumably from the infected B cell population appears.26 VP2 and VP5 proteins of the virus induce apoptosis in B cells and other lymphoid cells thereby causing cell depletion.27
Other organs show pathological signs like splenomegaly, petechial hemorrhages on the mucosa at the juncture of the proventriculus and diffused hemorrhages in the thigh and breast muscles. Lesions in the caecal tonsils, thymus, spleen and bone marrow confirm infection with vvIBDV, with the harderian gland being severely affected following infection in the day-old chicks.28 Repopulation of B cells in the BF happen in the recovered birds. The cells arrange in two different types of follicles either the long follicles which are the repopulated endogenous bursal stem cells in the survived birds, and can mount their own immunity or the small, poorly developed follicles lacking a distinct medulla and cortex due to the damage following infection.29
Immune response towards IBDV infection
IBDV causes immunosuppression in chickens, and BF is the target organ for viral replication. The stage of B cell differentiation in the BF plays an important role in viral replication as the stem cells and peripheral B cells do not support viral replication. The acute phase of the disease lasts for 7–10 days, during which the B cells are depleted in the bursal cortex and medulla, peripheral blood and thymic medulla, and the BF becomes atrophic. The viral antigen can also be detected in the peripheral lymphoid organs like caecal tonsils, spleen and thymus besides BF. The disease when not fatal leads to immunosuppression with reduced antibody response. The chickens fail to produce antibodies against other viral diseases and lead to subsequent outbreaks with the surviving birds tend to show high anti-IBDV antibody titers.
Protection against the disease does not depend solely on the humoral immunity; cell-mediated immunity through T cell involvement is also important. IgM+ B cells serve as targets for IBDV30 and CD4+ and CD8+ T cells, along with the macrophages accumulate in the BF even as early as 1 dpi29 with upregulation of IFN-γ, IL-8 and IL-6 transcription and apoptotic mediators like nitric oxide or TNF-α.
The role of cell-mediated immunity following IBDV infection is well established31,32 with the localization of bursal mRNA transcription of the pro-inflammatory cytokines IL-1β, IL-6, CXCLi2 and IFN-γ together with downregulation of transcription growth factor-β4. In vivo challenge with vvIBDV UK661 strain upregulated the transcription levels of types I, II and III IFNs as well as IL-18, IL-4 and IL-13 cytokines.33
Diagnosis
Clinical signs and differential diagnosis
Clinical manifestation is dependent on various factors like age, strain of the virus, maternal antibody titer, type of vaccine used, breed of the bird, etc. The incubation period is 2–3 days, after which the infected birds show distress, depression, ruffled feathers, anorexia, diarrhea and soiled vent; classical strains of virus can cause 10–50% mortality rates in infected flocks, whereas vvIBDV strains can cause 50–100% mortality. The clinical disease lasts for 3–4 days, followed by rapid recovery of the surviving birds. Few diseases, namely avian coccidiosis, Newcastle disease, chicken infectious anemia, stunting syndrome, mycotoxicosis and nephropathogenic forms of infectious bronchitis are differentially diagnosed with IBD. The BF is the principal target organ to differentiate between these diseases on postmortem examination of the dead birds. In acute form, the bursa is turgid, oedematous, and sometimes hemorrhagic and turns atrophic within 7–10 days. The bursa is atrophic in sub-clinical IBD and can be mistaken with Marek’s disease or infectious anemia.
Virological diagnosis
In the acute phase of the infection, IBDV could be detected within the first 3 days post infection in the bursa. Confirmation of clinical disease or detection of subclinical form is best done by immunological assays as it is difficult to isolate the virus.
Virus isolation
During the acute phase of the disease, the bursa is collected from the susceptible chicks and a 20% bursal homogenate in PBS solution is prepared from the pooled bursa. The isolation of IBDV strains is done by infecting the embryonated chicken eggs following inoculation onto the chorio-allantoic membrane (CAM). IBDV isolation in chicken primary bursal cells from the BF has also been reported.34 However, isolation of very virulent form of the virus is not recommended in cell culture. Death due to IBDV infection begins 3 days post-inoculation (dpi) with typical signs of hemorrhages and edema in the embryos. The best method for virus isolation is done by inoculating bursal homogenate to SPF chickens and the virus is then isolated from the bursal tissue 3 dpi. The virus is then titrated and the endpoint titers are calculated based on specific deaths by inoculating 10-day-old chicken embryos through CAM route.
Detection of viral antigens
Agar gel immunodiffusion (AGID) test detects the antigen in the bursa by placing the minced bursa from susceptible chicks in the wells of the AGID plate against known positive serum. Freeze-thaw cycles of the minced tissue release the IBDV antigens from the tissue and the freeze-thaw exudate is used to fill the wells.35 Antigen-capture-ELISA was described for the detection of serotype 1 IBDV in which the ELISA plates were coated with mouse anti-IBDV monoclonal antibodies (Mabs) or chicken anti-IBDV polyclonal sera.36 A panel of seven Mabs, namely 1, 3, 4, 6, 7, 8 and 9 were used in which Mabs 2 and 3 bound with classical strain but not vvIBDV strain.37
Molecular diagnostic tests
Sequencing the hVP2 gene together with pathogenicity testing in chickens is the most accurate and accepted method for identifying the IBDV strains. The amplification of hVP2 gene by reverse transcription PCR (RT-PCR) followed by sequencing and phylogenetic analysis represents the only valuable tool for the classification of IBDV strains.14
Real-time RT-PCR
Real-time RT-PCR allows IBDV differentiation based upon time and number of samples that can be tested simultaneously. The RT-PCR SYBR green technology is robust and may serve as a useful tool with high capacity for diagnostics as well as in viral pathogenesis studies. Different types of infectious IBDV strains including, virulent strain DK01, classic strain F52/70 and vaccine strain D78 were quantified and detected in infected BF and cloacal swabs by real-time RT-PCR with SYBR green dye.38
Loop-mediated isothermal amplification (LAMP)
LAMP compared to RT-PCR is 10 times more sensitive, rapid and specific assay and is the method of choice for field virus detection with no cross-reactivity.39 The test is specific as 2–3 pairs of primers are used to amplify the more conserved-targeted regions.
One-step strip test
One-step strip tests based on colloidal gold labeled monoclonal antibodies were developed to detect the IBDV antigen. The test was found to be highly sensitive, specific and rapid for diagnosis of the infection in the field when compared with AGID test.40
Serological tests
The commonly used tests for serological diagnosis are AGID,41 virus neutralization test (VNT),42 or ELISA.43 The simplest of all is AGID assay, however, its sensitivity and specificity may vary among laboratories and is time consuming. VNT has the highest specificity and at the same time, it correlates with protection. But the test is labor intensive, and moreover requires the facilities of a virology laboratory, thus impractical for routine use. For IBDV serology, an ELISA kit based on the whole virus as an alternative to AGID test involves the process of growing and purifying IBDV and again is restricted by safety considerations. ELISA kits or latex agglutination assay44 based on the recombinant VP2/VP3 protein could be simpler and safer to produce with higher sensitivity and specificity as the recombinant proteins used in the assay is immunodominant and devoid of any nonspecific moieties as present in whole cell preparations.7,43 As VP3 is a group-specific major immunogenic protein of IBDV, following infection with live or inactivated IBDV, the earliest appearing antibodies are directed toward VP3.6
Vaccination and management strategies
Strict hygiene measures, vaccination with conventional live attenuated and inactivated viral vaccines have been used to prevent IBD. However, eliminating the sturdy and the persistent IBDV particles from the farm is a difficult task as the virus remains infectious for 122 days in a chicken house and for 52 days in feed and water.45 Therefore, routine sanitary measures must rigorously be followed to control IBDV. Disinfection may reduce the virus load and thus reduce the risk of transmission. The mechanical vectors such as mosquitoes, mealworms, and smaller rodents must be eradicated. On the farms where IBDV outbreaks have occurred, the virus may be considered endemic. Improper cleaning of the hoods will expose the young birds to the virus at an early age. Early subclinical infection is the main cause of economic loss as the disease can cause severe, long-lasting suppression of the immune system and the immunocompromised birds do not respond well to vaccination and are more susceptible to other infections. Culling the infected chickens and controlling other flocks from being infected is costly. Thus, vaccination remains the method of choice to control IBD. However, vaccine failures do occur due to the evolution of the virus even with strict vaccination practices.
An early method of prevention is exposing the young chicks to IBDV46 and this technique although reduces mortality but often results in immunosuppression and further dissemination of the field virus. Live-attenuated vaccines based on mild field isolates after passaging in specific-pathogen-free eggs were developed. They are still widely used today in parent stock as a primary vaccine for controlling the very virulent IBD in many countries. Until the 1980s, mortality caused by IBD was effectively controlled by vaccination. With the emergence of Delaware variants in the USA in the mid-1980s and the emergence of very virulent forms of the virus in Europe and Asia in 1989 resulted in vaccination failures.14,25,46 It is essential to prevent the infection at an early age, so that the immunosuppressive effect of IBDV could be controlled. This can be achieved by immunization of the parent stock. Inactivated vaccines along with oil-adjuvants boost the immune response and the maternal immunity may be extended to 3–5 weeks. When young chickens are to be vaccinated with attenuated vaccines, timing of vaccination is important as too early vaccination may lead to neutralization of the vaccine by MDA, and on the contrary, the birds may remain unprotective if vaccinating too late due to the low level of MDA. Monitoring the antibody level in a breeder flock or its progeny can aid in determining the right time to vaccination.25 The MDA level can be determined by serological monitoring and the right time of vaccination could also be determined. Vaccines may be administered by intramuscular injection, by spray or by mixing in drinking water. Chickens vaccinated with IBDV in early life before 7 days of age and revaccinating with an inactivated, oil-adjuvant IBD vaccine at 18 weeks of age can produce and maintain high levels of virus-neutralizing antibody through 10 months of lay.47 Moreover, due to early vaccination, the vaccine virus will spread in the poultry farm and indirectly could provide immune response to the other susceptible chicks.48
Live-attenuated vaccines are referred to as mild, intermediate, or “intermediate plus” (hot) vaccines based on the ability to cause varying degree of histopathological lesions and are suitable for mass vaccination preferably through drinking water to induce robust cellular and humoral immunity. The mild vaccines do not cause bursal damage in chicks but have poor efficacy in the presence of MDA or vvIBDV infection. Vaccines of higher pathogenicity (intermediate or “intermediate plus”) may break through the high levels of maternal immunity but may produce bursal lesions, with subsequent immunosuppression leading to a secondary infection. In addition, they may not protect against infection with vvIBDV49 or antigenic variants. Inactivated vaccines, mostly formulated as water-in-oil emulsions, are usually administered in the breeder hens for vertical transmission of high, uniform, and persistent antibody titers to the progeny.50 Intermediate and “hot” vaccines are mostly used to overcome the MDA in young broilers. The possibility of reversion to virulence, generation of reassortment strain and vaccine reactions resulting in disease or production loss may be the few undesirable side effects. Due to these limitations, the new generation vaccines have been developed to control IBDV (Table 1).
Table 1.
Recent development in IBDV vaccines research
| Platform | Gene targeted | Expression system | Efficacy |
|---|---|---|---|
| Subunit vaccine | Mimotope | Escherichia coli | 100% protection against mortality82 |
| Hypervariable region of VP2 | Pichia pastoris | 100% protection upon challenge53 | |
| Live viral vectored | VP2/PP | Fowl pox virus | Low protection with VP2 and no protection with PP54 |
| VP2 | Herpesvirus of turkey | 100% protection in day-old vaccination75 | |
| VP2 | MDV | 55% protection7 | |
| VP2 | NDV | 90% protection77 | |
| SVP | VP2 | Pichia pastoris | 500 µg SVP with and without adjuvant conferred 100% protection but with moderate bursal damage60 |
| VP2 | Saccharomyces cerevisiae | SVP alone and as a DNA prime-protein boost, 100% protection, Th1 and Th2 mixed response31,44 | |
| Adjuvant with IBD vaccine | Recombinant IFNs and IL-1β in combination | Escherichia coli | Increased humoral responses in immunologically mature but not day-old chicken83 |
| Porcine lactoferrin | Pichia pastoris | Increased CMI response84 | |
| Chicken beta-defensin-1 genetically fused with VP2 | as DNA vaccine | 100% protection, Increased CD3+, CD4+ and CD8+ T-cell subtypes64 | |
| Salmonella typhimurium fliC + VP2 | as DNA vaccine | 80% protection, increased humoral and cell mediated immunity65 | |
| cHSP70 + VP2 |
as DNA vaccine | 100% protection, increased expression of IFN-γ and IL-12, IL-10, increased ELISA Ab31 | |
| VP2-4-3 and chicken IL-18 | as DNA vaccine | 93% protection, increased induction of IFN-γ and IL-485 |
Abbreviations: MDV, Marek’s disease virus; NDV, Newcastle disease virus; PP, polyprotein VP2-4-3; VP2, Virus protein 2; SVP, subviral particle; IFN, interferon; IL, interleukin; cHSP70, C-terminal domain of heat shock protein 70 of Mycobacterium tuberculosis; fliC, flagellin; ELISA, Enzyme linked immunosorbent assay; Ab, antibody; IBDV, infectious bursal disease virus SVP, subviral particle.
Subunit vaccines
The major capsid protein VP2 has proved to be an important target for generating cellular and humoral immune responses against IBDV infection.31 The VP2/3/4 polyprotein or VP2 (rVP2) alone has been expressed in different expression systems like Escherichia coli,51 Lactococcus lactis,52 Saccharomyces cerevisiae,31,43 Pichia pastoris,53 fowlpox virus,54 baculovirus,55 Semliki Forest virus,56 and even plant expression systems.57 In experimental studies, rVP2 protein can induce partial to 100% protection. The recombinant-based vaccines could allow the development of differentiation of infected from vaccinated animals (DIVA) strategy to differentiate the vaccinated from infected ones. However, this new generation vaccine needs to be administered along with adjuvants parenterally and repeated booster immunizations increase its manufacturing cost. Till date, recombinant vaccines based on VP2 expressed in E. coli, P. pastoris and baculovirus have been licensed for commercial use.
Virus-like particle (VLP) based vaccine
VLPs are robust protein cages in the nanometer range that mimic the overall structure of the native virions but lack the viral genome. The trimmed VP2 and VP3 genes of IBDV generated a VLP in baculovirus expression system.58 Attenuated pathogens are commonly excellent inducers of T cell as well as B cell responses, but as discussed earlier have chances of reversion to a more virulent phenotype. Non-infectious subunits of pathogens such as recombinant proteins, peptides or sugars are poorly immunogenic and have to be formulated with immune-stimulating adjuvants. The immunogenicity produced by a VLP-based vaccine is much better than pVP2 subunits and IBDV polyprotein expression products.59 The VLP scaffold is produced by the electrostatic interaction between VP2 and VP3 proteins. In another strategy, 23 nm subviral particles (SVPs) in yeast (P. pastoris) expression system was produced that provided partial protection upon IBDV oral challenge and complete protection by intramuscular challenge.60 IBDV SVPs expressed in S. cerevisiae are based on the assembly of a single protein (VP2) into 20 trimeric clusters of VP2 with T1 symmetry having a diameter of approximately 22 nm43 (Figure 2). The SVPs-based IBD vaccine could completely protect the birds upon vvIBDV challenge and induced both humoral and cell-mediated immune responses.31 A single shot of SVPs IBD vaccine in the hatchery could eliminate the costly, time-consuming vaccination in the field even in the presence of MDA antibodies (data not shown).
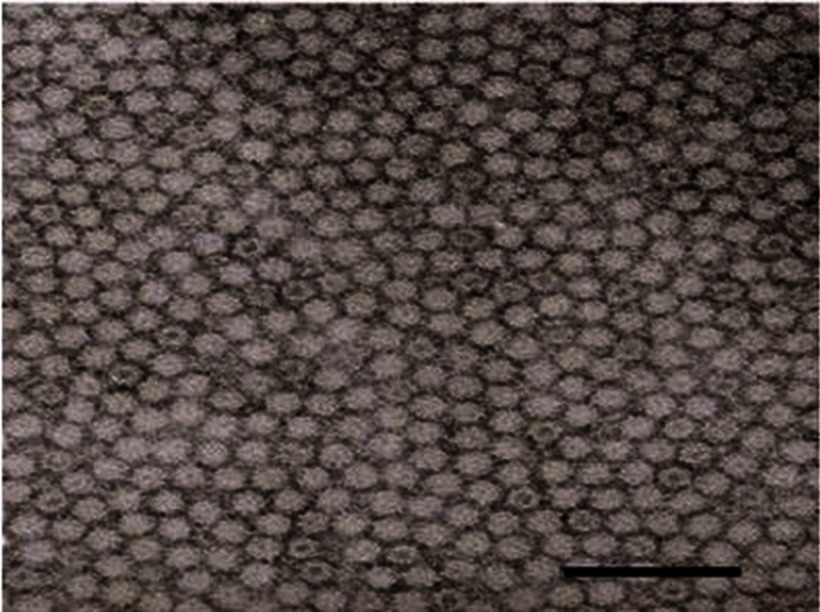
Figure 2.
Transmission electron micrograph of cesium chloride gradient purified Infectious bursal disease virus (IBDV) virus protein 2 (VP2) subviral particles (SVPs), negatively stained with sodium phosphotungstate. Reprinted from.43 Copyright 2009, with permission from Elsevier. Bar = 100 nm.
Notes: Dey S, Upadhyay C, Mohan CM, Kataria JM, Vakharia VN. Formation of subviral particles of the capsid protein VP2 of infectious bursal disease virus and its application in serological diagnosis. J Virol Methods. 2009;157(1):84–89
DNA vaccines
A DNA vaccine expressing VP2 can induce both humoral and cell-mediated immune responses, but the protective efficacy varies from 40% to 80% and often has resulted in bursal lesions resulting in immunosuppression. IBDV polyprotein encoding cDNA is a better candidate than VP2 encoding cDNA.61 For better results, priming in ovo or at 1-day-old followed by boosting with inactivated vaccine or fowlpox vectored vaccine is performed.62 In ovo delivery alone without a boost vaccine, however, did not induce sufficient protective immunity. However, the level of protection depends on the quantity of DNA used in the priming vaccine, the challenge strain of viruses used, age of the bird and route of vaccination. The failure to achieve complete protection with VP2 gene-based DNA vaccine led to improved strategies based on the incorporation of cytokines genes or cytosine-phosphate-guanine (CpG) motifs.20 A DNA vaccine carrying VP234 gene of IBDV and IL-18 enhanced the immune response and protection efficacy to 93% against vvIBDV.63 In a study, complete protection with a DNA vaccine encoding the IBDV VP2 protein fused with defensin (AvBD1) gene was reported with the DNA vaccine encoding VP2 protein alone providing 80% protection.64 A chimeric DNA vaccine encoding C-terminal HSP70 of Mycobacterium tuberculosis fused with full-length IBDV VP2 gene induced mixed Th1 and Th2 responses31 and completely protected the birds when boosted with SVP-based vaccine in which VP2 has been expressed in S. cerevisiae.43 A TLR-5 ligand flagellin (fliC) antigen of Salmonella typhimurium when fused with an N-terminal of VP2 and injected as a DNA vaccine produced 80% protection but could stimulate both the arms of immunity.65 For achieving a successful immunization with IBDV-DNA vaccine, age of bird, route of vaccine administration and virulence of challenge virus are crucial.
Immune complex vaccine
Immune complex vaccine (Icx) is a cocktail of live pathogenic IBDV strains mixed with anti-IBDV antibodies derived from hyperimmunized chickens sera or recombinant neutralizing antibody and is available commercially.66,67 It can be administered subcutaneously to day-old chicks even in the presence of MDA, resulting in generation of active immune response without causing any vaccine-induced immunosuppression.68,69 Icx vaccines are also used to vaccinate in ovo at day 18 of incubation using automated technology to achieve very precise vaccination. By this route of inoculation, the vaccine induces the formation of more germinal centers in the spleen, thus resulting in localization of IBDV in dendritic and bursal follicles. Post-challenge, IBDV-Icx vaccine efficacy was found to be equal to or better than that of conventional live vaccines. The principle behind this technology is that IBDV antibody in Icx vaccine forms a complex with the virus and thus causes delay in virus detection (5 days) and also a remarkable B cell depletion in bursa and spleen.70
Reverse genetics and live viral-vectored vaccines
Understanding the genome of the viral pathogen helps in targeting the specific antigenic protein, host–pathogen interaction and also reverse engineer the virus to generate a live vaccine candidate. The whole genome sequencing of the IBDV71 paved the way toward the development of a reverse genetic vaccine system.72 The hypervariable region of VP2 gene was changed to an attenuated mutant IBDV from vvIBDV by site-directed mutagenesis72,73 and then rescued by reverse genetics. The mutant virus was able to protect against both classical and antigenic variant IBDV strains.74 There are several reports of using recombinant live viral vectors including herpesvirus of turkey,75,76 NDV,77 fowlpox virus,54 Marek’s disease virus78 and avian adenovirus79 incorporating VP2 gene with the aim of protecting the birds against IBD with a DIVA strategy. Humoral immunity plays an important role in the clearance of IBD following vaccination, with rapid infiltration of T cells into the bursa and upregulation of CMI related genes following infection with very virulent IBD.80 The HVT-VP2 vaccine has been licensed in several countries and is found to be safe as the virus is poorly sensitive to MDA, does not produce bursal lesions and has been extensively validated in field efficacy studies.81 This vaccine can either be used as an in ovo application or given by subcutaneous route in day-old chicks.
Conclusion
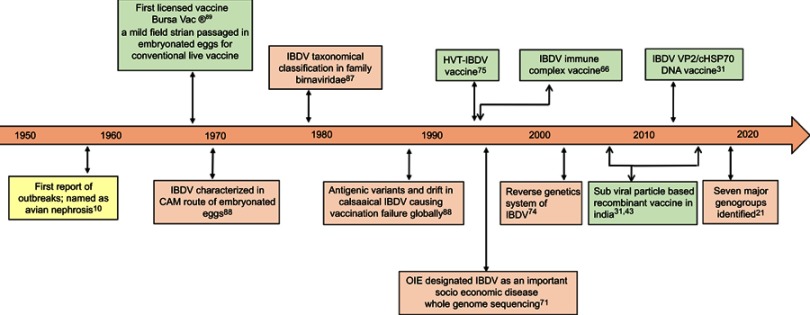
Poultry farming has grown phenomenally since the times of backyard farming to a proliferating and profitable business in many countries across the globe. Intensive poultry production system has led to the occurrence of frequent disease outbreaks with IBD figuring prominently in many countries. Although emerged 60 years back, the disease still poses an economical threat to the poultry industry worldwide. The emergence of antigenic variants and very virulent strains of the virus further complicate the field scenario that contributes to high mortality in birds. The milestone encompassing the IBD research over the years is presented in Figure 3. Recent and future developments in molecular epidemiology, development of new generation diagnosis and vaccines could greatly aid in containment of this scourge in the near future.
Figure 3.
An overview of 60 years of infectious bursal disease (IBD) research.Abbreviations: IBDV, Infectious bursal disease virus; HVT, Herpesvirus of Turkey; VP2, virus protein 2; cHSP70, C-terminal domain of heat shock protein 70 of Mycobacterium tuberculosis; CAM, Chorioallantoic membrane; OIE, Office International des Épizooties [World Organisation for Animal Health].
Disclosure
The authors report no conflicts of interest in regard to this review.
References
- 1.Hon CC, Lam TT, Yip CW, et al. Phylogenetic evidence for homologous recombination within the family Birnaviridae. J Gen Virol. 2008;89(12):3156–3164. doi: 10.1099/vir.0.2008/004101-0. [DOI] [PubMed] [Google Scholar]
- 2.Böttcher B, Kiselev NA, Stel’Mashchuk VY, Perevozchikova NA, Borisov AV, Crowther RA. Three-dimensional structure of infectious bursal disease virus determined by electron cryomicroscopy. J Virol. 1997;71(1):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller H, Becht HE. Biosynthesis of virus-specific proteins in cells infected with infectious bursal disease virus and their significance as structural elements for infectious virus and incomplete particles. J Virol. 1982;44(1):384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt M, Yao K, Liu M, Heckert RA, Vakharia VN. Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. J Virol. 2001;75(24):11974–11982. doi: 10.1128/JVI.75.24.11974-11982.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayliss CD, Spies U, Shaw K, et al. A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. J Gen Virol. 1990;71(6):1303–1312. doi: 10.1099/0022-1317-71-6-1303 [DOI] [PubMed] [Google Scholar]
- 6.Fahey KJ, O’donnell IJ, Bagust TJ. Antibody to the 32K structural protein of infectious bursal disease virus neutralizes viral infectivity in vitro and confers protection on young chickens. J Gen Virol. 1985;66(12):2693–2702. doi: 10.1099/0022-1317-66-12-2693 [DOI] [PubMed] [Google Scholar]
- 7.Singh NK, Dey S, Mohan CM, Kataria JM, Vakharia VN. Evaluation of four enzyme linked immunosorbent assays for the detection of antibodies to infectious bursal disease in chickens. J Virol Methods. 2010;165(2):277–282. doi: 10.1016/j.jviromet.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Mundt E, Beyer J, Müller H. Identification of a novel viral protein in infectious bursal disease virus-infected cells. J Gen Virol. 1995;76(2):437–443. doi: 10.1099/0022-1317-76-2-437 [DOI] [PubMed] [Google Scholar]
- 9.Tacken MG, Rottier PJ, Gielkens AL, Peeters BP. Interactions in vivo between the proteins of infectious bursal disease virus: capsid protein VP3 interacts with the RNA-dependent RNA polymerase, VP1. J Gen Virol. 2000;81(1):209–218. doi: 10.1099/0022-1317-81-1-209 [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove AS. An apparently new disease of chickens: avian nephrosis. Avian Dis. 1962;6(3):385–389. doi: 10.2307/1587909 [DOI] [Google Scholar]
- 11.Faragher JT. Infectious bursal disease of chickens. Vet Bull. 1972;42:61–369. [Google Scholar]
- 12.Van Den Berg TP, Morales D, Eterradossi N, et al. Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathol. 2004;33(5):470–476. doi: 10.1080/03079450400003650 [DOI] [PubMed] [Google Scholar]
- 13.Van Den Berg TP, Gonze M, Meulemans G. Acute infectious bursal disease in poultry: isolation and characterisation of a highly virulent strain. Avian Pathol. 1991;20(1):133–143. doi: 10.1080/03079459108418748 [DOI] [PubMed] [Google Scholar]
- 14.Berg TP. Acute infectious bursal disease in poultry: a review. Avian Pathol. 2000;29(3):175–194. doi: 10.1080/030794500750047234 [DOI] [PubMed] [Google Scholar]
- 15.Jackwood DJ, Sommer-Wagner S. Genetic characteristics of infectious bursal disease viruses from four continents. Virology. 2007;365(2):369–375. doi: 10.1016/j.virol.2007.03.046 [DOI] [PubMed] [Google Scholar]
- 16.Pikuła A, Lisowska A, Jasik A, Śmietanka K. Identification and assessment of virulence of a natural reassortant of infectious bursal disease virus. Vet Res. 2018;49(1):89. doi: 10.1186/s13567-018-0586-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachar T, Popowich S, Goodhope B, et al. 5-year study of the incidence and economic impact of variant infectious bursal disease viruses on broiler production in Saskatchewan, Canada. Can J Vet Res. 2016;80(4):255–261. [PMC free article] [PubMed] [Google Scholar]
- 18.Coulibaly F, Chevalier C, Delmas B, Rey FA. Crystal structure of an Aquabirnavirus particle: insights into antigenic diversity and virulence determinism. J Virol. 2010;84(4):1792–1799. doi: 10.1128/JVI.01536-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackwood DJ, Sommer-Wagner SE. Amino acids contributing to antigenic drift in the infectious bursal disease Birnavirus (IBDV). Virology. 2011;409(1):33–37. doi: 10.1016/j.virol.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 20.Negash T, Liman M, Rautenschlein S. Mucosal application of cationic poly (D, L-lactide-co-glycolide) microparticles as carriers of DNA vaccine and adjuvants to protect chickens against infectious bursal disease. Vaccine. 2013;31(36):3656–3662. doi: 10.1016/j.vaccine.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 21.Michel LO, Jackwood DJ. Classification of infectious bursal disease virus into genogroups. Arch Virol. 2017;162(12):3661–3670. doi: 10.1007/s00705-017-3383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignjatovic J, Sapats S. Confirmation of the existence of two distinct genetic groups of infectious bursal disease virus in Australia. Aust Vet J. 2002;80(11):689–694. doi: 10.1111/j.1751-0813.2002.tb11299.x [DOI] [PubMed] [Google Scholar]
- 23.Tippenhauer M, Heller DE, Weigend S, Rautenschlein S. The host genotype influences infectious bursal disease virus pathogenesis in chickens by modulation of T cells responses and cytokine gene expression. Dev Comp Immunol. 2013;40(1):1. doi: 10.1016/j.dci.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Kasanga CJ, Yamaguchi T, Wambura PN, Munang’andu HM, Ohya K, Fukushi H. Detection of infectious bursal disease virus (IBDV) genome in free-living pigeon and guinea fowl in Africa suggests involvement of wild birds in the epidemiology of IBDV. Virus Genes. 2008;36(3):521–529. doi: 10.1007/s11262-008-0219-z [DOI] [PubMed] [Google Scholar]
- 25.Eterradossi N, Saif YM. Infectious bursal disease In: Saif YM, Fadly AM, Glisson JR, Mcdougald LR, Nolan LK, Swayne DE, editors. Diseases of Poultry. 12th ed. Ames: Iowa State University Press; 2008:185–208. [Google Scholar]
- 26.Ruby T, Whittaker C, Withers DR, et al. Transcriptional profiling reveals a possible role for the timing of the inflammatory response in determining susceptibility to a viral infection. J Virol. 2006;80(18):9207–9216. doi: 10.1128/JVI.00929-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y, Zheng S. Infectious bursal disease virus–host interactions: multifunctional viral proteins that perform multiple and differing jobs. Int J Mol Sci. 2017;18(1):161. doi: 10.3390/ijms18010161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdul R, Murgia MV, Rodriguez-Palacios A, Lee CW, Saif YM. Persistence and tissue distribution of infectious bursal disease virus in experimentally infected SPF and commercial broiler chickens. Avian Dis. 2013;57(4):759–766. doi: 10.1637/10448-110812-Reg.1 [DOI] [PubMed] [Google Scholar]
- 29.Withers DR, Young JR, Davison TF. Infectious bursal disease virus-induced immunosuppression in the chick is associated with the presence of undifferentiated follicles in the recovering bursa. Viral Immunol. 2005;18(1):127–137. doi: 10.1089/vim.2005.18.127 [DOI] [PubMed] [Google Scholar]
- 30.Sharma JM, Kim IJ, Rautenschlein S, Yeh HY. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev Comp Immunol. 2000;24(2–3):223–235. [DOI] [PubMed] [Google Scholar]
- 31.Maity HK, Dey S, Mohan CM, et al. Protective efficacy of a DNA vaccine construct encoding the VP2 gene of infectious bursal disease and a truncated HSP70 of Mycobacterium tuberculosis in chickens. Vaccine. 2015;33(8):1033–1039. doi: 10.1016/j.vaccine.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 32.Dey S, Chellappa M, Pathak D, et al. Newcastle disease virus vectored bivalent vaccine against virulent infectious bursal disease and newcastle disease of chickens. Vaccines. 2017;5(4):31. doi: 10.3390/vaccines5040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eldaghayes I, Rothwell L, Williams A, et al. Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 2006;19(1):83–91. doi: 10.1089/vim.2006.19.83 [DOI] [PubMed] [Google Scholar]
- 34.Dulwich KL, Asfor AS, Gray AG, Nair V, Broadbent AJ. An ex vivo chicken primary bursal-cell culture model to study infectious bursal disease virus pathogenesis. J Vis Exp. 2018;4(140):e58489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Organization for Animal Health. Infectious Bursal Disease (Gumboro disease). In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals Chapter 3.3.12; 2016:931–951. [Google Scholar]
- 36.Snyder DB, Marquardt WW, Mallinson ET, Russek E. Rapid serological profiling by enzyme-linked immunosorbent assay. I. Measurement of antibody activity titer against Newcastle disease virus in a single serum dilution. Avian Dis. 1983;27(1):161–170. [PubMed] [Google Scholar]
- 37.Eterradossi N, Arnauld C, Toquin D, Rivallan G. Critical amino acid changes in VP2 variable domain are associated with typical and atypical antigenicity in very virulent infectious bursal disease viruses. Arch Virol. 1998;143(8):1627–1636. [DOI] [PubMed] [Google Scholar]
- 38.Li YP, Handberg KJ, Kabell S, Kusk M, Zhang MF, Jørgensen PH. Relative quantification and detection of different types of infectious bursal disease virus in bursa of Fabricius and cloacal swabs using real time RT-PCR SYBR green technology. Res Vet Sci. 2007;82(1):126–133. doi: 10.1016/j.rvsc.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 39.Dhama K, Karthik K, Chakraborty S, et al. Loop-mediated isothermal amplification of DNA (LAMP): a new diagnostic tool lights the world of diagnosis of animal and human pathogens: a review. Pak J Biol Sci. 2014;17(2):151–166. doi: 10.3923/pjbs.2014.151.166 [DOI] [PubMed] [Google Scholar]
- 40.Zhang GP, Li QM, Yang YY, et al. Development of a one-step strip test for the diagnosis of chicken infectious bursal disease. Avian Dis. 2005;49(2):177–181. doi: 10.1637/7353-030305R.1 [DOI] [PubMed] [Google Scholar]
- 41.Hirai K, Shimakura S, Kawamoto E, et al. The immunodepressive effect of infectious bursal disease virus in chickens. Avian Dis. 1974;18:50–57. doi: 10.2307/1589241 [DOI] [PubMed] [Google Scholar]
- 42.Weisman J, Hitchner SB. Infectious bursal disease virus infection attempts in turkeys and coturnix quail. Avian Dis. 1978;22:604–609. doi: 10.2307/1589635 [DOI] [PubMed] [Google Scholar]
- 43.Dey S, Upadhyay C, Mohan CM, Kataria JM, Vakharia VN. Formation of subviral particles of the capsid protein VP2 of infectious bursal disease virus and its application in serological diagnosis. J Virol Methods. 2009;157(1):84–89. doi: 10.1016/j.jviromet.2008.11.020 [DOI] [PubMed] [Google Scholar]
- 44.Dey S, Mohan CM, Kataria JM inventors; Indian Council of Agricultural Research, patentee. Recombinant antigen based rapid sero siagnosis of infectious bursal disease (IBD). The Patent Office, Govt. of India 277404 2008. March 3.
- 45.Müller H, Mundt E, Eterradossi N, Islam MR. Current status of vaccines against infectious bursal disease. Avian Pathol. 2012;41(2):133–139. doi: 10.1080/03079457.2012.661403 [DOI] [PubMed] [Google Scholar]
- 46.Lasher HN, Box PG. Infectious bursal disease: the past, present situation and future prospects. Zootechnica Int. 1993;16:70–77. [Google Scholar]
- 47.Naqi SA, Marquez B, Sahin N. Maternal antibody and its effect on infectious bursal disease immunization. Avian Dis. 1983;623–631. doi: 10.2307/1590304 [DOI] [PubMed] [Google Scholar]
- 48.van Den Berg TP, Eterradossi N, Toquin D, Meulemans G. Infectious bursal disease (Gumboro disease). Rev Sci Tech. 2000;19(2):509–543. doi: 10.20506/rst.19.2.1227 [DOI] [PubMed] [Google Scholar]
- 49.Rautenschlein S, Kraemer C, Vanmarcke J, Montiel E. Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Dis. 2005;49(2):231–237. doi: 10.1637/7353-030305R.1 [DOI] [PubMed] [Google Scholar]
- 50.Wyeth PJ, Cullen GA. Transmission of immunity from inactivated infectious bursal disease oil-emulsion vaccinated parent chickens to their chicks. Vet Rec. 1978;102(16):362–363. doi: 10.1136/vr.102.16.362 [DOI] [PubMed] [Google Scholar]
- 51.Rong J, Jiang T, Cheng T, et al. Large-scale manufacture and use of recombinant VP2 vaccine against infectious bursal disease in chickens. Vaccine. 2007;25(46):7900–7908. doi: 10.1016/j.vaccine.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 52.Liu L, Zhang W, Song Y, et al. Recombinant Lactococcus lactis co-expressing OmpH of an M cell-targeting ligand and IBDV-VP2 protein provide immunological protection in chickens. Vaccine. 2018;36(5):729–735. [DOI] [PubMed] [Google Scholar]
- 53.Pitcovski J, Gutter B, Gallili G, et al. Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine. 2003;21(32):4736–4743. doi: 10.1016/S0264-410X(03)00525-5 [DOI] [PubMed] [Google Scholar]
- 54.Heine HG, Boyle DB. Infectious bursal disease virus structural protein VP2 expressed by a fowlpox virus recombinant confers protection against disease in chickens. Arch Virol. 1993;131(3–4):277–292. doi: 10.1007/BF01378632 [DOI] [PubMed] [Google Scholar]
- 55.Vakharia VN, Snyder DB, He J, Edwards GH, Savage PK, Mengel-Whereat SA. Infectious bursal disease virus structural proteins expressed in a baculovirus recombinant confer protection in chickens. J Gen Virol. 1993;74:1201–1206. doi: 10.1099/0022-1317-74-6-1201 [DOI] [PubMed] [Google Scholar]
- 56.Phenix KV, Wark K, Luke CJ, et al. Recombinant Semliki Forest virus vector exhibits potential for avian virus vaccine development. Vaccine. 2001;19(23–24):3116–3123. doi: 10.1016/S0264-410X(01)00026-3 [DOI] [PubMed] [Google Scholar]
- 57.Wu H, Singh NK, Locy RD, Scissum-Gunn K, Giambrone JJ. Immunization of chickens with VP2 protein of infectious bursal disease virus expressed in Arabidopsis thaliana. Avian Dis. 2004;48(3):663–668. doi: 10.1637/7104 [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Torrecuadrada JL, Lázaro B, Rodriguez JF, Casal JI. Antigenic properties and diagnostic potential of baculovirus-expressed infectious bursal disease virus proteins VPX and VP3. Clin Diagn Lab Immunol. 2000;7(4):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez-Torrecuadrada JL, Saubi N, Pagès-Manté A, et al. Structure-dependent efficacy of infectious bursal disease virus (IBDV) recombinant vaccines. Vaccine. 2003;21(23):3342–3350. doi: 10.1016/S0264-410X(02)00804-6 [DOI] [PubMed] [Google Scholar]
- 60.Taghavian O, Spiegel H, Hauck R, Hafez HM, Fischer R, Schillberg S. Protective oral vaccination against infectious bursal disease virus using the major viral antigenic protein VP2 produced in Pichia pastoris. PLoS One. 2013;8(12):e83210. doi: 10.1371/journal.pone.0083210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fodor N, Dube SK, Fodor I, et al. Induction of protective immunity in chickens immunised with plasmid DNA encoding infectious bursal disease virus antigens. Acta Vet Hung. 1999;47(4):481–492. [DOI] [PubMed] [Google Scholar]
- 62.Haygreen EA, Kaiser P, Burgess SC, Davison TF. In ovo DNA immunisation followed by a recombinant fowlpox boost is fully protective to challenge with virulent IBDV. Vaccine. 2006;24(23):4951–4961. doi: 10.1016/j.vaccine.2006.03.060 [DOI] [PubMed] [Google Scholar]
- 63.Li L, Fang W, Li J, Huang Y, Yu L. Oral DNA vaccination with the polyprotein gene of infectious bursal disease virus (IBDV) delivered by the attenuated Salmonella elicits protective immune responses in chickens. Vaccine. 2006;24(33–34):5919–5927. doi: 10.1016/j.vaccine.2006.04.057 [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Yang X, Xie Q, et al. The potent adjuvant effects of chicken beta-defensin-1 when genetically fused with infectious bursal disease virus VP2 gene. Vet Immunol Immunopathol. 2010;136(1–2):92–97. doi: 10.1016/j.vetimm.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 65.Deb R, Dey S, Mohan CM, et al. Development and evaluation of a Salmonella typhimurium flagellin based chimeric DNA vaccine against infectious bursal disease of poultry. Res Vet Sci. 2015;102:7–14. doi: 10.1016/j.rvsc.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 66.Whitfill CE, Haddad EE, Ricks CA, et al. Determination of optimum formulation of a novel infectious bursal disease virus (IBDV) vaccine constructed by mixing bursal disease antibody with IBDV. Avian Dis. 1995;39:687–699. doi: 10.2307/1592404 [DOI] [PubMed] [Google Scholar]
- 67.Ignjatovic J, Gould G, Trinidad L, Sapats S. Chicken recombinant antibodies against infectious bursal disease virus are able to form antibody–virus immune complex. Avian Pathol. 2006;35(4):293–301. doi: 10.1080/03079450600823378 [DOI] [PubMed] [Google Scholar]
- 68.Haddad EE, Whitfill CE, Avakian AP, et al. Efficacy of a novel infectious bursal disease virus immune complex vaccine in broiler chickens. Avian Dis. 1997;41:882–889. doi: 10.2307/1592342 [DOI] [PubMed] [Google Scholar]
- 69.Iván J, Velhner M, Ursu K, et al. Delayed vaccine virus replication in chickens vaccinated subcutaneously with an immune complex infectious bursal disease vaccine: quantification of vaccine virus by real-time polymerase chain reaction. Can J Vet Res. 2005;69(2):135. [PMC free article] [PubMed] [Google Scholar]
- 70.Jeurissen SH, Janse EM, Lehrbach PR, et al. The working mechanism of an immune complex vaccine that protects chickens against infectious bursal disease. Immunology. 1998;95(3):494. doi: 10.1046/j.1365-2567.1998.00617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mundt E, Vakharia VN. Synthetic transcripts of double-stranded Birnavirus genome are infectious. Proc Natl Acad Sci U S A. 1996;93(20):11131–11136. doi: 10.1073/pnas.93.20.11131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Loon AA, De Haas N, Zeyda I, Mundt E. Alteration of amino acids in VP2 of very virulent infectious bursal disease virus results in tissue culture adaptation and attenuation in chickens. J Gen Virol. 2002;83(1):121–129. doi: 10.1099/0022-1317-83-1-121 [DOI] [PubMed] [Google Scholar]
- 73.Raue R, Islam MR, Islam MN, et al. Reversion of molecularly engineered, partially attenuated, very virulent infectious bursal disease virus during infection of commercial chickens. Avian Pathol. 2004;33(2):181–189. doi: 10.1080/03079450310001652112 [DOI] [PubMed] [Google Scholar]
- 74.Mundt E, de Haas N, van Loon AA. Development of a vaccine for immunization against classical as well as variant strains of infectious bursal disease virus using reverse genetics. Vaccine. 2003;21(31):4616–4624. doi: 10.1016/S0264-410X(03)00448-1 [DOI] [PubMed] [Google Scholar]
- 75.Darteil R, Bublot M, Laplace E, et al. Herpesvirus of turkey recombinant viruses expressing infectious bursal disease virus (IBDV) VP2 immunogen induce protection against an IBDV virulent challenge in chickens. Virology. 1995;211(2):481–490. [DOI] [PubMed] [Google Scholar]
- 76.Prandini F, Simon B, Jung A, Pöppel M, Lemiere S, Rautenschlein S. Comparison of infectious bursal disease live vaccines and a HVT-IBD vector vaccine and their effects on the immune system of commercial layer pullets. Avian Pathol. 2016;45(1):114–125. doi: 10.1080/03079457.2015.1127891 [DOI] [PubMed] [Google Scholar]
- 77.Huang Z, Elankumaran S, Yunus AS, Samal SK. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J Virol. 2004;78(18):10054–10063. doi: 10.1128/JVI.78.18.10054-10063.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsukamoto K, Kojima C, Komori Y, Tanimura N, Mase M, Yamaguchi S. Protection of chickens against very virulent infectious bursal disease virus (IBDV) and Marek’s disease virus (MDV) with a recombinant MDV expressing IBDV VP2. Virology. 1999;257(2):352–362. doi: 10.1006/viro.1999.9671 [DOI] [PubMed] [Google Scholar]
- 79.Francois A, Chevalier C, Delmas B, et al. Avian adenovirus CELO recombinants expressing VP2 of infectious bursal disease virus induce protection against bursal disease in chickens. Vaccine. 2004;22(17–18):2351–2360. doi: 10.1016/j.vaccine.2003.10.039 [DOI] [PubMed] [Google Scholar]
- 80.Rautenschlein S, Yeh HY, Sharma JM. The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet Immunol Immunopathol. 2002;89(3–4):159–167. doi: 10.1016/S0165-2427(02)00202-7 [DOI] [PubMed] [Google Scholar]
- 81.Bublot M, Pritchard N, Le Gros FX, Goutebroze S. Use of a vectored vaccine against infectious bursal disease of chickens in the face of high-titred maternally derived antibody. J Comp Pathol. 2007;137(S1):S81–S84. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Fan H, Li Y, et al. Development of a multi-mimotope peptide as a vaccine immunogen for infectious bursal disease virus. Vaccine. 2007;25(22):4447–4455. [DOI] [PubMed] [Google Scholar]
- 83.Schijns VE, Weining KC, Nuijten P, Rijke EO, Staeheli P. Immunoadjuvant activities of E. coli- and plasmid-expressed recombinant chicken IFN-alpha/beta, IFN-gamma and IL-1beta in 1-day- and 3-week-old chickens. Vaccine. 2000;18(20):2147–2154. [DOI] [PubMed] [Google Scholar]
- 84.Hung CM, Yeh CC, Chen HL, et al. Porcine lactoferrin administration enhances peripheral lymphocyte proliferation and assists infectious bursal disease vaccination in native chickens. Vaccine. 2010;28(16):2895–2902. doi: 10.1016/j.vaccine.2010.01.066 [DOI] [PubMed] [Google Scholar]
- 85.Li K, Gao H, Gao L, et al. Adjuvant effects of interleukin-18 in DNA vaccination against infectious bursal disease virus in chickens. Vaccine. 2013;31(14):1799–1805. doi: 10.1016/j.vaccine.2013.01.056 [DOI] [PubMed] [Google Scholar]
- 86.Hitchner SB. Infectivity of infectious bursal disease virus for embryonating eggs. Poult Sci. 1970;49:511–516. doi: 10.3382/ps.0490511 [DOI] [PubMed] [Google Scholar]
- 87.Dobos P, Hill BJ, Hallett R, Kells DT, Becht H, Teninges D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979;32(2):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackwood DH, Saif YM, Hughes JH. Replication of infectious bursal disease virus in continuous cell lines. Avian Dis. 1987;31(2):370–375. doi: 10.2307/1590888 [DOI] [PubMed] [Google Scholar]
- 89.Snedeker C, Wills FK, Moulthrop IM. Some studies on the infectious bursal agent. Avian Dis. 1967;11(4):519–528. doi: 10.2307/1588292 [DOI] [PubMed] [Google Scholar]
- 90.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw005 [DOI] [PMC free article] [PubMed] [Google Scholar]