Abstract
Background
Cough is common in pulmonary TB and other chronic respiratory infections. Identifying features that predict whether pulmonary TB is the cause would help target appropriate individuals for rapid and cost-effective screening, potentially limiting disease progression and preventing transmission to others.
Methods
A systematic literature search for individual studies to answer eight key questions (KQs) was conducted according to established Chest Organization methods by using the following databases: MEDLINE via PubMed, Embase, Scopus, and the Cochrane Database of Systematic Reviews from January 1, 1984, to April 2014. Searches for KQ 1 and KQ 3 were updated in February 2016. An updated KQ 2 search was undertaken in March 2017.
Results
Even where TB prevalence is greatest, most individuals with cough do not have pulmonary TB. There was no evidence that 1, 3, or 4 weeks’ duration were better predictors than cough lasting ≥ 2 weeks to screen for pulmonary TB. In people living with HIV (PLWHIV), screening for fever, night sweats, hemoptysis, and/or weight loss in addition to cough (any World Health Organization [WHO]-endorsed symptom) increases the diagnostic sensitivity for TB. Although the diagnostic accuracy of symptom-based screening remains low, the negative predictive value of the WHO-endorsed symptom screen in PLWHIV may help to risk-stratify individuals who are not close TB contacts and who do not require further testing for pulmonary TB in resource-limited settings. However, pregnant PLWHIV are more likely to be asymptomatic, and the WHO-endorsed symptom screen is not sensitive enough to be reliable. Combined with passive case finding (PCF), active case finding (ACF) identifies pulmonary TB cases earlier and possibly when less advanced. Whether outcomes are improved or transmission is reduced is unclear. Screening asymptomatic patients is cost-effective only in populations with a very high TB prevalence. The Xpert MTB/RIF assay on sputum is more cost-effective than clinical diagnosis. To our knowledge, no published comparative studies addressed whether the rate of cough resolution is a reliable determinant of the response to treatment or whether the rate of cough resolution was faster in the absence of cavitary lung disease. All studies on cough prevalence in Mycobacterium avium complex (MAC) lung disease, other nontuberculous mycobacterial infections, fungal lung disease, and paragonimiasis were of poor quality and were excluded from the evidence review.
Conclusions
On the basis of relatively few studies of fair to good quality, we conclude that most individuals at high risk and household contacts with cough ≥ 2 weeks do not have pulmonary TB, but we suggest screening them regardless of cough duration. In PLWHIV, the addition of the other WHO-endorsed symptoms increases the diagnostic sensitivity of cough. Earlier screening of patients with cough will help diagnose pulmonary TB sooner but will increase the cost of screening. The addition of ACF to PCF will increase the number of pulmonary TB cases identified. Screening asymptomatic individuals is cost-effective only in groups with a very high TB prevalence. Data are insufficient to determine whether cough resolution is delayed in individuals with cavitary lung disease or in those for whom treatment fails because of drug resistance, poor adherence, and/or drug malabsorption compared with results in other individuals with pulmonary TB. Cough is common in patients with lung infections due to MAC, other nontuberculous mycobacteria, fungal diseases, and paragonimiasis.
Key Words: cough, evidence-based medicine, fungal infections, Mycobacterium avium complex, nontuberculous mycobacterial, paragonimiasis, TB
Abbreviations: ACF, active case finding; ART, antiretroviral therapy; CD4, CD4-positive T lymphocytes; CHEST, American College of Chest Physicians; DOTS, Directly Observed Treatment, Short Course; GRADE, Grading of Recommendations Assessment, Development and Evaluation; KQ, key question; MAC, Mycobacterium avium complex; MDR-TB, multidrug-resistant TB; PCF, passive case finding; PICO, population, intervention, comparison, outcome; PLWHIV, people living with HIV; QUADAS, Quality Assessment of Diagnostic Accuracy Studies; WHO, World Health Organization; Xpert MTB/RIF, automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampin resistance
Summary of Recommendations and Suggestions
1. For patients with cough in high TB prevalence countries, particularly in high risk groups (eg, inmates, people living with HIV [PLWHIV], or close contacts of pulmonary TB), we suggest that individuals with cough be evaluated for pulmonary TB because of the implications of active pulmonary TB both to the individual and to public health are of great importance (Ungraded Consensus-Based Statement).
Remarks: Evaluation for pulmonary TB should be undertaken even though most individuals with cough will not have pulmonary TB.
2. For patients with cough in high TB prevalence countries or settings, we suggest that they be screened for TB regardless of cough duration (Grade 2C).
Remarks: We found low-quality evidence that the prevalence of pulmonary TB was similar whether patients in such settings were screened after cough durations of ≥ 1, 2, 3, or 4 weeks.
3. For patients with cough and at risk of pulmonary TB but at low risk of drug-resistant TB living in high TB prevalence countries, we suggest that XpertMTB/RIF testing, when available, replace sputum microscopy for initial diagnostic testing, but chest x-rays should also be done on pulmonary TB suspects when feasible and where resources allow (Ungraded Consensus-Based Statement).
4. For patients with cough suspected to have pulmonary TB and at high risk of drug-resistant TB (eg, those with a prior history of treatment for pulmonary TB, contacts of drug-resistant TB cases and/or living in countries with a high drug-resistant TB prevalence), we suggest that XpertMTB/RIF assay, where available, replace sputum microscopy but sputum mycobacterial cultures, drug susceptibility testing and chest x-rays should be performed when feasible and where resources allow (Ungraded Consensus-Based Statement).
5. For patients with cough with or without fever, night sweats, hemoptysis, and/or weight loss, and who are at risk of pulmonary TB in high TB prevalence countries, we suggest that they should have a chest x-ray if resources allow (Ungraded Consensus-Based Statement).
6. For PLWHIV with cough who also complain of fever, night sweats, hemoptysis, and/or weight loss (WHO-endorsed symptoms) and are at risk for TB, we suggest screening for pulmonary TB because the presence of these symptoms increases the likelihood that the affected individual has pulmonary TB (Grade 2C).
Remarks: All of the included studies were limited to PLWHIV.
7. For patients with cough in high TB prevalence populations, we suggest the addition of active case finding (ACF) to passive case finding (PCF) because it may improve outcomes in patients with pulmonary TB (Ungraded Consensus-Based Statement).
8. For patients with cough in high TB prevalence populations, we suggest the addition of ACF to PCF because it may reduce transmission (Ungraded Consensus-Based Statement).
9. For patients with cough suspected to have pulmonary TB, we suggest that available financial modeling algorithms be used to estimate costs associated with different screening strategies because cost-effectiveness studies have not yet been performed (Ungraded Consensus-Based Statement).
10. For patients with chronic cough in low income countries, we suggest that strategies for pulmonary TB diagnosis should focus on improved case detection rather than diagnostic testing (Ungraded Consensus-Based Statement).
Approximately 9.6 million people developed active TB worldwide in 2014, and 1.5 million died as a result.1 The consequences of TB are greatest in the developing world where the prevalence is greatest and resources to diagnose and treat it are most limited. Cough, often productive of sputum, is the most common symptom in individuals with pulmonary TB. According to the World Health Organization (WHO), cough lasting ≥ 2 weeks warrants investigation in resource-limited countries where the prevalence of TB is high, defined as ≥ 100 in 100,000, because of the risks associated with delayed diagnosis to infected individuals and the risk of continued transmission to their contacts.2, 3 Resource-limited countries include those defined by the World Bank as having low income (ie, gross national income per capita ≤ $1,025 and lower middle income with a gross national income between $1,026 and $4,035).4
The prevalence of pulmonary TB in individuals with cough depends on the diagnostic criteria and the population surveyed. If the diagnosis is based on sputum microscopy, many active pulmonary TB cases will be missed because microscopy has a sensitivity of approximately 60%.5 The prevalence will be greater if sputum also is submitted for culture and/or if clinical or chest imaging evaluations are included.6 An important development was the introduction and increased use of rapid point-of-care diagnostics such as a nucleic acid amplification test to diagnose Mycobacterium tuberculosis infection and rifampin resistance rapidly (Xpert MTB/RIF; Cepheid) in resource-limited settings.6 It demonstrated a stronger diagnostic performance than sputum microscopy in a multicenter study and in a meta-analysis and an excellent diagnostic accuracy to detect rifampin-resistant Mycobacterium tuberculosis strains, a surrogate marker for multidrug-resistant TB (MDR-TB).6, 7 Rapid and accurate identification of MDR-TB cases is critical in many areas in the world where MDR-TB is highly prevalent.6, 8 Conversely, the prevalence of cough is greatest in those with smear-positive sputum. However, even in areas with a high TB prevalence, the majority of coughs are due to another cause.9, 10 Common causes include acute viral bronchitis, chronic bronchitis, respiratory complications due to cigarette smoking, and outdoor or indoor air pollution including biomass smoke inhalation, as well as asthma, gastroesophageal reflux disease, upper airway disease, and angiotensin-converting-enzyme-inhibitor-induced cough.
This report updates the 2006 American College of Chest Physicians (CHEST) guideline chapter on cough due to TB and other chronic infections.11 It aims to determine the optimal time to test patients with cough for pulmonary TB; whether screening for the other WHO-endorsed symptoms (fever, night sweats, hemoptysis, or weight loss), in addition to cough, increases the likelihood that the individual has pulmonary TB; and the impact of active case finding (ACF) on diagnostic yield, treatment start times, clinical outcomes, and transmission rates.10, 11
Other aims were to determine the most cost-effective diagnostic strategy to ascertain whether cough is due to TB, the rate of cough resolution with successful treatment of pulmonary TB, and the expected rate of cough resolution in cavitary compared with noncavitary pulmonary TB. Whether there are characteristic features that distinguish cough due to nontuberculous mycobacteria, fungal disease, or paragonimiasis from other conditions was also examined.
Methods
The methodology of the CHEST Guideline Oversight Committee was used to select the Expert Cough Panel chair and the International Panel of Experts to perform the systematic review, synthesis of the evidence, and development of the recommendations and suggestions.12
Key Question Development
Clinical key questions (KQs) were developed using the population, intervention, comparison, outcome (PICO) format (Table 1). The following questions were addressed:
Table 1.
List of the Eight PICO Questions, Inclusion and Exclusion Criteria for the Study Populations and Comparator Groups, Study Designs, Interventions, and Primary Outcomes
| Study Characteristic | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| KQ 1: when to suspect cough is due to TB | ||
| Populations | Coughing patients in high-prevalence areas | None |
| Interventions | Sputum microscopy, culture, Xpert MTB/RIF, CXR | None |
| Comparators | Cough ≥ 2 wk vs other durations | None |
| Outcome | TB diagnostic rate | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 2: diagnostic sensitivity of any WHO-endorsed symptom vs cough in PLWHIV | ||
| Populations | PLWHIV in high-prevalence countries | None |
| Intervention | Addition of any WHO-endorsed symptom | None |
| Comparator | Mainly current cough | None |
| Outcome | Diagnostic sensitivity of active PTB | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 3: ACF vs PCF | ||
| Populations | Coughing patients in high-prevalence areas | None |
| Interventions | ACF or adding ACF to PCF | None |
| Comparator | PCF | None |
| Outcome | Diagnostic yield of PTB | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 4: cost-effectiveness | ||
| Populations | People in high-prevalence areas | None |
| Interventions | Sputum microscopy, culture, Xpert MTB/RIF, CXR | None |
| Comparators | Patients identified passively | None |
| Outcomes | TB diagnosis rates and successful treatment rates | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 5: how quickly should cough resolve with effective treatment | ||
| Populations | People with PTB | None |
| Interventions | Sputum microscopy, culture, Xpert MTB/RIF, CXR | None |
| Comparators | Patients in whom treatment failed | None |
| Outcome | Cough resolution rate in patients treated successfully | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 6: differences in cough duration in DS cavitary vs DS noncavitary lung disease | ||
| Populations | Patients with DS cavitary TB | None |
| Intervention | WHO-approved drug treatment for TB | None |
| Comparators | Patients with DS noncavitary TB | None |
| Outcome | Differences in cough duration | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 7: are there distinctive cough features in patients with Mycobacterium avium lung disease | ||
| Populations | Patients with M avium lung disease | None |
| Interventions | Diagnostic testing: symptoms, sputum, CXR | None |
| Comparators | Healthy individuals, patients with other lung diseases | None |
| Outcomes | Discriminating features of cough in M avium lung disease | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
| KQ 8: are there distinctive cough features in patients with fungal lung diseases | ||
| Populations | Patients with fungal lung diseases | Pneumocystis jirovecii |
| Interventions | Diagnostic testing: symptoms, sputum analysis, CXR | None |
| Comparators | Healthy individuals, patients with other lung diseases | None |
| Outcomes | Discriminating features in patients with fungal lung diseases | None |
| Study design | RCT, cohort, case-control, prospective, retrospective | Series with < 10 patients |
ACF = active case finding; CXR = chest radiograph; DS = drug sensitive; KQ = key question; PCF = passive case finding; PICO = population, intervention, comparison, outcome; PLWHIV = people living with HIV; PTB = pulmonary TB; RCT = randomized control trial; Xpert MTB/RIF = a nucleic acid amplification test to diagnose Mycobacterium tuberculosis infection and rifampin resistance rapidly; WHO = World Health Organization.
KQ 1: When should we suspect cough is due to TB in high-prevalence settings?
KQ 2: What is the impact of including the other WHO-endorsed symptoms (fever, night sweats, hemoptysis, and weight loss) with cough compared with cough alone on the detection of TB in patients at high risk?
KQ 3: Is actively screening more effective than passively screening for TB?
KQ 4: What is the most cost-effective strategy to diagnose TB in individuals with cough in high-prevalence countries?
KQ 5: How quickly should cough resolve with effective antimicrobial therapy in patients with pulmonary TB?
KQ 6: Is there a difference in cough duration between patients treated with drug-sensitive cavitary disease vs drug-sensitive noncavitary pulmonary TB?
KQ 7: In coughing patients with lung disease, are there features that suggest Mycobacterium avium complex lung disease?
KQ 8: In coughing patients with lung disease, are there features to suggest fungal infection or paragonimiasis?
Systematic Literature Search
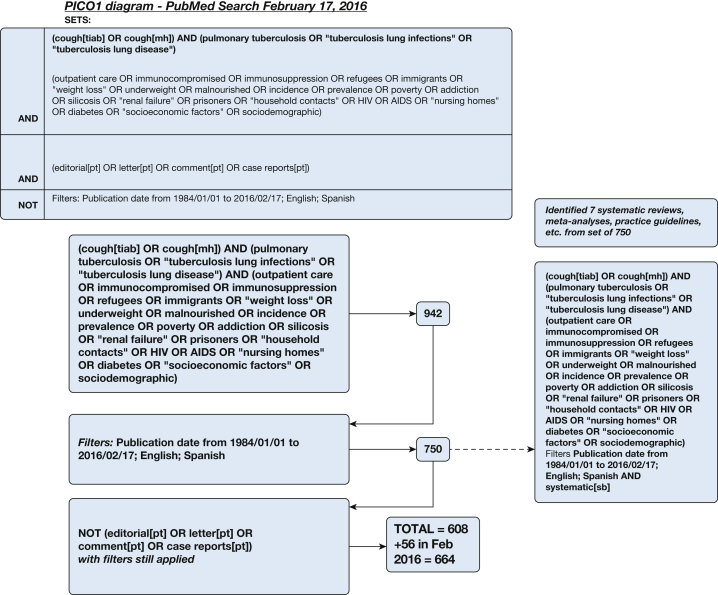
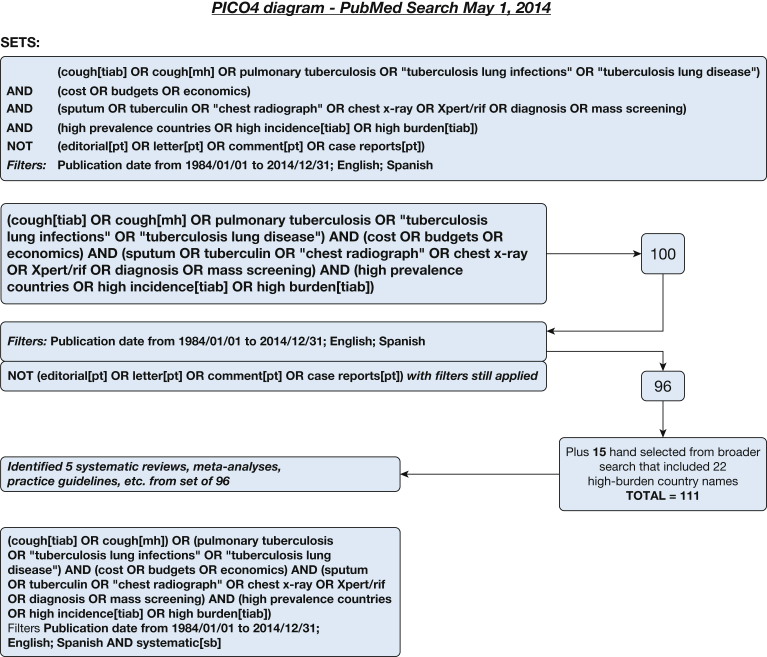
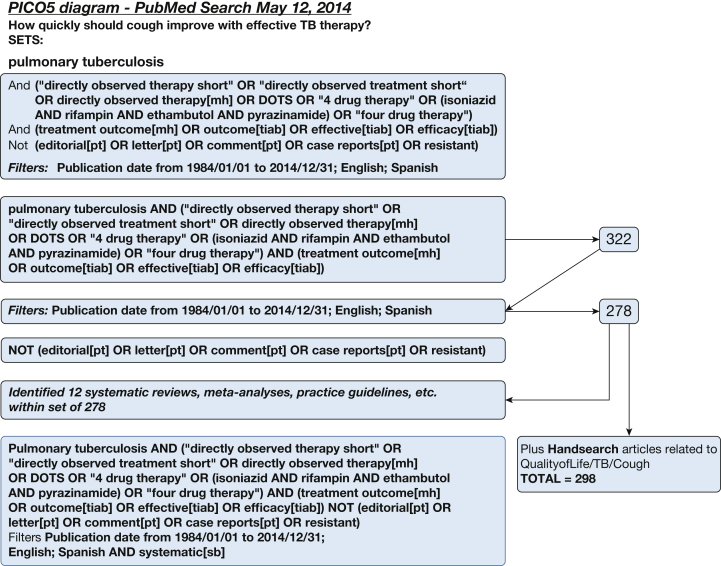
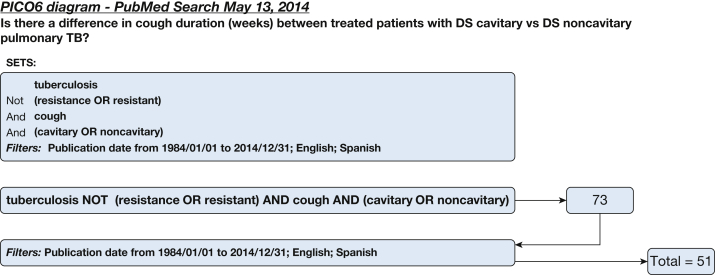
A systematic literature search for individual studies for each of the KQs was conducted using the following databases: MEDLINE via PubMed, Embase, Scopus, and the Cochrane Database of Systematic Reviews with date limitations from January 1, 1984, to April 2014. Searches for KQs 1 and 3 were updated in February 2016, and the search for KQ 2 was undertaken in March 2017. A combination of keywords specific to the topic was used to identify studies. All searches were limited to the English and Spanish languages. The search strategies are shown in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8.
Figure 1.
Key question 1 search strategy: When should we suspect cough is due to TB in high-prevalence settings? mh = mesh heading; PICO = population, intervention, comparison, outcome; pt = publication type; sb = subset; tiab = title and abstract.
Figure 2.
Key question 2 search strategy: What is the impact of including the other World Health Organization-endorsed symptoms (fever, night sweats, hemoptysis, and weight loss) with cough compared with cough alone on the detection of TB in patients at high risk? See Figure 1 legend for expansion of abbreviations.
Figure 3.
Key question 3 search strategy: Is actively screening more effective than passively screening for TB? See Figure 1 legend for expansion of abbreviations.
Figure 4.
Key question 4 search strategy: What is the most cost-effective strategy to diagnose TB in individuals with cough in high-prevalence countries? See Figure 1 legend for expansion of abbreviations.
Figure 5.
Key question 5 search strategy: How quickly should cough resolve with effective antimicrobial therapy in patients with pulmonary TB? DOTS = Directly Observed Treatment, Short Course. See Figure 1 legend for expansion of other abbreviations.
Figure 6.
Key question 6 search strategy: Is there a difference in cough duration between patients treated with drug-sensitive cavitary disease vs drug-sensitive noncavitary pulmonary TB?
Figure 7.
Key question 7 search strategy: In coughing patients with lung disease, are there features that suggest Mycobacterium avium complex lung disease? See Figure 1 legend for expansion of abbreviation.
Figure 8.
Key question 8 search strategy: In coughing patients with lung disease, are there features to suggest fungal infection or paragonimiasis? See Figure 1 legend for expansion of abbreviations.
The titles and abstracts of the search results were reviewed independently in parallel by three members of the writing group (S. K. F., P. E., and D. A. F.) to identify potentially relevant articles on the basis of the inclusion and exclusion criteria. Discrepancies were resolved by discussion. Studies deemed eligible then underwent a second round of full-text screening for final inclusion. Important data from each included study then were extracted into structured evidence tables.
Quality Assessment
Included studies were assessed for quality and risk of bias by using the modified Quality Assessment of Diagnostic Accuracy Studies (QUADAS) form for diagnostic studies13
Grading the Evidence and Development of Recommendations
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profiles were created to grade the overall quality of the body of evidence supporting each outcome on the basis of five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of the evidence for each outcome was rated as high, moderate, or low or very low. Recommendations were drafted by the panel for each KQ that had sufficient evidence. Recommendations were graded using the CHEST grading system that is composed of two parts: the strength of the recommendation (either strong or weak) and a rating of the overall quality of the body of evidence.12. In instances in which there was insufficient evidence, but a recommendation still was warranted, a weak suggestion was developed, and “Ungraded Consensus-Based Statement” replaced the grade.
All drafted recommendations and suggestions were presented to the full Cough Expert Panel in an anonymous voting survey to achieve consensus through a modified Delphi technique. Panelists were requested to indicate their level of agreement on each statement by using a 5-point Likert scale.12 Panelists also had the option to provide open-ended feedback on each statement with suggested edits or general comments.
Guideline Framework
Recommendations were graded according to the GRADE framework adopted by CHEST.12 This framework separates the process of rating the quality of evidence from that of determining the strength of the recommendation. The quality of evidence was based on risk of bias, inconsistency, indirectness, reporting bias, and imprecision.13 The quality of evidence (ie, the confidence in estimates) was rated as high (A), moderate (B), or low or very low (C). The strength of the recommendation was determined according to the quality of the evidence.14 Level 1 represented strong recommendations in which benefits or harms clearly outweighed the other. Level 2 represented a recommendation in which it was not clear that the benefits outweighed the harms (or vice versa), and new research could change the direction or strength of these recommendations.
Developing Recommendations and Suggestions
To be included in this guideline, a recommendation or suggestion had to be voted on by 75% of the eligible members of the entire Cough Expert Panel and achieve ratings of strongly agree or agree by 80% of the voting panelists.
Results
KQ 1: When Should We Suspect Cough Is Due to TB in High-Prevalence Settings?
Figure 1 illustrates the abstract search strategy for KQ 1, and selected abstracts are shown in Table 2. The systematic literature search for individual studies was conducted using MEDLINE via the PubMed database in April 2014 and identified 608 abstracts. Another 56 were identified from the Embase and Scopus databases and an updated PubMed database search in February 2016. There were seven relevant cross-sectional comparative trials among the 664 citations identified by the search strategy. The three listed in Table 2 were rated as fair, and four were rated as poor primarily because of unexplained subject loss and other unclear study reporting. They were assessed using the QUADAS-2 tool, and the four rated as poor were excluded. Deficiencies in the excluded studies included the following: subjects were not necessarily representative of the population of note, inclusion and exclusion criteria were not always stated clearly, large numbers of potential subjects were not included without explanation but presumably because they declined to participate, and diagnostic standards were not always stated clearly. In some cases, individuals who could not provide sputum for assessment were included in the analysis.
Table 2.
Descriptions of the Retained Studies for KQ 1
| Study/Year | Inclusions | Exclusions | Study Arms | Method of Assessment | Outcomes | Limitations and Risk of Bias | Key Messages |
|---|---|---|---|---|---|---|---|
| Bastos et al15/2007 | Outpatients in the primary care unit in Rio de Janeiro, Brazil | Age < 15 y previous TB pregnancy | Cough ≥ 1 wk Cough ≥ 2 wk Cough ≥ 3 wk Cough ≥ 4 wk |
Three sputa smears and culture | 765 of 7,174 (10.7%) cough ≥ 1 wk 15 of 542 (2.8%) had pulmonary TB Cough ≥ 1 wk, 1 of 68 (1.5%) Cough ≥ 2 wk, 6 of 130 (4.6%) Cough ≥ 3 wk, 1 of 52 (1.9%) Cough ≥ 4 wk, 6 of 292 (2.1%) |
Cough duration was subjective. Not all eligible patients were screened, and the reasons why were not given, nor were study withdrawals explained. It is not clear how many patients submitted 1, 2, or 3 sputum specimens. Details of indeterminate specimens (eg, those with bacterial overgrowth) were not provided. It is not clear that the study was adequately powered. |
In a population with a high pulmonary TB prevalence, screening patients for cough < 3 wk may improve the proportion of TB cases diagnosed. If pulmonary TB is diagnosed sooner, it might result in earlier treatment, potentially improving outcomes and potentially reducing the risk of transmission. It would result in more individuals being screened and greater screening costs. |
| Ngadaya et al16/2009 | Outpatients at six hospitals in Dar es Salaam, Tanzania | Age < 5 y | Cough < 2 wk Cough ≥ 2 wk |
Three sputum smears, two smears positive for diagnosis |
2,274 of 65,530 (3.5%) had cough 241 of 2,214 (10.9%) cough < 2 wk 21 (8.7%) were smear positive 1,973 of 2,214 (89.1%) cough ≥ 2 wk and 250 (12.7%) were smear positive (P = .074) |
Although the prevalence of pulmonary TB was the same in patients with cough < 2 and ≥ 2 wk, it is not clear that the study was adequately powered. Cough duration was subjective. Details of indeterminate specimens (eg, bacterial overgrowth) were not provided. Withdrawals from the study were not explained. |
The prevalence of smear-positive pulmonary TB was the same in those with cough < 2 wk as it was in those with cough ≥ 2 wk. |
| Santha et al17/2005 | Outpatients in government health units in six districts in India | Age ≤ 15 y Receiving TB treatment Previously seen at facilities |
Cough ≥ 2 wk Cough ≥ 3 wk |
Three sputum smears | 2,210 of 55,561 (4%) had cough 267 of 2,210 (12.1%) were smear positive 1,370 of 55,561 (2.5%) cough ≥ 3 wk 182 of 1,370 (13.3%) were smear positive |
Cough duration was subjective. It was unclear what proportion of patients submitted 1, 2, or 3 specimens. Details of indeterminate specimens (eg, bacterial overgrowth) were not provided. |
The prevalence of smear-positive pulmonary TB was the same in patients with cough ≥ 2 wk as in those with cough ≥ 3 wk. Screening at ≥ 2 wk rather than ≥ 3 wk results in more patients being screened and greater costs. |
See Table 1 legend for expansion of abbreviation.
Among the three retained studies, one compared individuals with cough lasting 1, 2, 3, and 4 weeks in an outpatient primary health unit in Rio de Janeiro, Brazil, to determine the prevalence of culture-positive pulmonary TB.15 The prevalence of pulmonary TB was similar whether patients were screened after 1, 2, 3, or 4 weeks. The evidence profile for KQ 1 is presented in Table 3.
Table 3.
Grading of Recommendations Assessment, Development and Evaluation Evidence Profile for TB KQ 1
| Quality Assessment |
No. of Patients |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | … | … | Relative (95% CI) | Absolute | ||
| Cough duration ≥ 2 wk vs ≥ 3 wk | ||||||||||||
| Two | Cross-sectional | Seriousa | No serious risk | No serious risk | No serious risk | Undetected | 277 of 2,684 cough ≥ 2 wk | 189 of 1,714 cough ≥ 3 wk | 10% vs 11% | … | Very low | … |
| Cough duration < 2 wk vs ≥ 2 wk | ||||||||||||
| Two | Cross-sectional | No serious risk | No serious risk | No serious risk | No serious risk | Undetected | 22 of 309 cough < 2 wk | 263 of 2,447 cough ≥ 2 wk | 7% vs 10% | … | Low | … |
Date: February 2016.
Question: When is cough in high-prevalence settings caused by TB?
Setting: High prevalence of TB.
See Table 1 legend for expansion of abbreviation.
In the study by Santha et al,17 21.5% of eligible subjects did not provide a sputum sample or provided fewer than the required three specimens.
The prevalence of smear-positive pulmonary TB in individuals aged ≥ 5 years, attending six different outpatient clinics in Dar es Salaam, Tanzania, with cough < 2 weeks was compared with the prevalence in those with cough ≥ 2 weeks.16 Among 65,530 clinic attendees, 2,274 (3.5%) reported cough, and 2,214 (97.4%) complaining of cough recalled its duration. Among 1,973 patients with cough ≥ 2 weeks, 250 (12.7%) had smear-positive pulmonary TB, vs 21 of 241 (8.7%) with cough < 2 weeks. The difference between the two groups was not statistically significant.
A study in Indian government health facilities in six districts compared the prevalence of smear-positive pulmonary TB in individuals with cough ≥ 2 weeks vs that in individuals with cough ≥ 3 weeks.17 Individuals were questioned about cough and its duration. All new adult attendees with cough ≥ 2 weeks were referred and were asked to provide three sputum specimens. A total of 2,210 of 55,561 (4%) reported cough ≥ 2 weeks. Among these, 267 (12%) were smear positive. Cough was present for ≥ 3 weeks in 1,370 (2.5%), and 182 (13%; P not significant) were smear positive.
In areas of high TB prevalence, the proportion of individuals with cough due to pulmonary TB is even greater in certain subpopulations such as PLWHIV or close contacts of individuals with pulmonary TB, but even in these subpopulations, the majority of coughs are not due to pulmonary TB.9, 10, 18
The WHO and Practical Approach to Lung Health in South Africa recommend that individuals with cough lasting ≥ 2 weeks in low-income, high-prevalence countries be screened for TB.2, 3 This systematic review aimed to determine whether cough ≥ 2 weeks is the optimal duration to initiate screening for pulmonary TB. Other investigators variously have considered screening for cough of shorter or longer durations, including 1, 3, or 4 weeks. Reducing the time before obtaining sputum for microscopic examination in individuals with cough might increase the number of cases diagnosed and result in pulmonary TB being diagnosed earlier, although that was not evident in the three comparative studies included in the KQ 1 analysis, but the program costs will be increased and whether outcomes are improved is unclear.19 Moreover, sputum testing and/or chest radiographs may not be diagnostic earlier, resulting in a higher false-negative rate.20 Conversely, later testing may result in individuals being more ill at the time of diagnosis, potentially requiring more expensive care and transmitting disease to more people.21
Another important consideration is the diagnostic tools available to screen for TB in patients with cough in resource-limited settings. Sputum smear testing has significant diagnostic performance limitations and has suboptimal sensitivity for TB screening purposes. Diagnostic imaging and microbiologic culture testing are usually more sensitive and specific than symptoms, including cough, for the diagnosis of pulmonary TB, but availability of these tests can be an issue in resource-limited settings. Despite this barrier, these tests should be used routinely for patients suspected of having smear-negative pulmonary TB with cough where the resources are available. The addition of chest radiographs to sputum analysis will increase the number of cases diagnosed.6, 22 Earlier diagnosis does not guarantee that treatment will be started earlier or that treatment completion rates will be increased.23 Thus, screening initiatives for early TB diagnosis should be implemented as part of a comprehensive TB control program.
An important development was the introduction and increased use of rapid point-of-care diagnostics in resource-limited settings. The Xpert MTB/RIF test demonstrated a stronger diagnostic performance than sputum microscopy in a multicenter study.6 A systematic review estimated that it increases pulmonary TB detection among culture-confirmed cases by 23% (95% credible interval, 15%-32%) compared with smear microscopy. As an initial diagnostic test replacing sputum microscopy, the estimated pooled sensitivity of the Xpert MTB/RIF test was 89% (95% credible interval, 85%-92%), and pooled specificity was 99% (95% credible interval, 98%-99%). For PLWHIV, the estimated pooled sensitivity of the Xpert MTB/RIF test was 79% (95% credible interval, 70%-86%).7 This finding suggests that it potentially can replace smear microscopy as initial testing in low- and high-prevalence settings.24 The Xpert MTB/RIF test also has a robust diagnostic performance to detect rifampin-resistant TB as a surrogate marker of MDR-TB.5, 6 However, effective treatment regimens for MDR-TB cases continue to rely on information from mycobacterial cultures and drug susceptibility testing in most settings.25 Submitting sputum for culture and/or Xpert MTB/RIF assay will increase the cost of screening but also will increase the number of cases diagnosed.9, 26
Suggestions
1. For patients with cough in high TB prevalence countries, particularly in high risk groups (eg, inmates, people living with HIV [PLWHIV], or close contacts of pulmonary TB), we suggest that individuals with cough be evaluated for pulmonary TB because of the implications of active pulmonary TB both to the individual and to public health are of great importance (Ungraded Consensus-Based Statement).
Remarks: Evaluation for pulmonary TB should be undertaken even though most individuals with cough will not have pulmonary TB.
2. For patients with cough in high TB prevalence countries or settings, we suggest that they be screened for TB regardless of cough duration (Grade 2C).
Remarks: We found low-quality evidence that the prevalence of pulmonary TB was similar whether patients in such settings were screened after cough durations of ≥ 1, 2, 3, or 4 weeks.
3. For patients with cough and at risk of pulmonary TB but at low risk of drug-resistant TB living in high TB prevalence countries, we suggest that XpertMTB/RIF testing, when available, replace sputum microscopy for initial diagnostic testing, but chest x-rays should also be done on pulmonary TB suspects when feasible and where resources allow (Ungraded Consensus-Based Statement).
4. For patients with cough suspected to have pulmonary TB and at high risk of drug-resistant TB (eg, those with a prior history of treatment for pulmonary TB, contacts of drug-resistant TB cases and/or living in countries with a high drug-resistant TB prevalence), we suggest that XpertMTB/RIF assay, where available, replace sputum microscopy but sputum mycobacterial cultures, drug susceptibility testing and chest x-rays should be performed when feasible and where resources allow (Ungraded Consensus-Based Statement).
5. For patients with cough with or without fever, night sweats, hemoptysis and/or weight loss, and who are at risk of pulmonary TB in high TB prevalence countries, we suggest that they should have a chest x-ray if resources allow (Ungraded Consensus-Based Statement).
KQ 2: What Is the Impact of Including the Other WHO-Endorsed Symptoms (Fever, Night Sweats, Hemoptysis, and Weight Loss) With Cough Compared With Cough Alone on the Detection of TB in Patients at High Risk?
Figure 2 outlines the abstract search strategy for KQ 2. The search was undertaken on March 20, 2017, and six, 127, and 48 abstracts were identified in the Cochrane Database of Systematic Reviews, PubMed, and Scopus, respectively. Among the 181 identified abstracts, 36 were selected for full-text review, and 20 were excluded because they were commentaries or reviews and did not provide data or distinguish cough from the other WHO-endorsed symptoms. Some reports combined other symptoms with WHO-endorsed symptoms (eg, fatigue, dyspnea, and chest pain) and were included for quality assessment. Some studies included physical findings, specific chest radiographic abnormalities (eg, apical disease or clinical or laboratory findings such as BMI, erythrocyte sedimentation rate, C-reactive protein level, Xpert MTB/RIF testing of sputum, tuberculin skin test results, or CD4-positive T lymphocytes [CD4] cell counts).
The 16 selected references underwent quality review with the QUADAS-2 tool. Nine were rated as poor primarily because they lacked a comparison population or the index test formed part of the reference test and were excluded. Five were rated good and two were rated fair and were reviewed. Six of the seven were cross-sectional in design. The included studies focused on PLWHIV in high-risk, low-resource countries in sub-Saharan Africa and Southeast Asia.
Because these studies are observational and are imprecise as reflected in the CIs, the data were rated as low quality according to the GRADE system. In addition, the heterogeneity in the results, partly related to differences in the subpopulations and settings, precluded developing an evidence profile.
A prospective cohort study examined the prevalence of TB in 361 PLWHIV eligible for antiretroviral therapy (ART; WHO stage 4 or CD4 < 200 cells/mm3) in South Africa.27 Patient evaluation included a repeat assessment 3 to 6 months after the initial one. Among 361 trial participants, 64 (18%) had culture-positive sputum, and 114 (32%) fulfilled any TB case definition (ie, positive microbiologic test results or clinical improvement after 2 months of treatment). Of the 114, 99 (87%) had pulmonary TB, five (4%) had extrapulmonary TB, and 10 (9%) had both. The WHO-endorsed symptom screen (ie, the presence of any one WHO-endorsed symptom) was more sensitive but less specific than cough (Table 4).
Table 4.
Descriptions of the Retained Studies for KQ 2
| Study/Year | Inclusions | Exclusions | Study Arms | Method of Assessment | Outcomes | Limitations and Risk of Bias | Key Messages |
|---|---|---|---|---|---|---|---|
| Hanifa et al27/2012 | PLWHIV, ART eligible, age ≥ 17 y, WHO stage 4 or CD4 < 200 mm3 South Africa |
TB treatment within previous 3 mo | Current cough Cough ≥ 2 wk Cough ≥ 3 wk Any WHO-endorsed symptom and/or fatigue, chest pain |
Symptom questionnaire Chest radiograph Sputum smear and culture |
Sensitivity specificity PPV NPV 69% 63% 33% 88% 44% 81% 38% 84% 42% 83% 40% 84% 97% 4% 21% 82% |
It is unclear that the time between the reference standard (symptom screen) and index test was close enough. Sputum samples were collected at two different times. The performance of the index test was not described sufficiently apart from stating that trained nurses administered the symptom questionnaire. |
The prevalence of pulmonary TB is high (32%) among PLWHIV in South Africa who are eligible but have not commenced ART with a median CD4 of 120 mm3. Any WHO-endorsed symptom is more sensitive than cough as a screen for pulmonary TB in this population. |
| Khan et al31/2014 | PLWHIV, ART eligible, age ≥ 18 y South Africa |
Previously investigated for or treated for TB | Not receiving ART Receiving ART |
Symptom questionnaire Chest radiograph Sputum smear and culture |
Any WHO-endorsed symptom, all PLWHIV 72% 50% 12% 95% Any WHO-endorsed symptom, PLWHIV receiving ART 52% 56% 7% 95% Any WHO-endorsed symptom, no ART 91% 33% 20% 95% |
It is not clear whether the standard was sputum smear or culture. Completion of the symptom questionnaire was not described in sufficient detail. The symptom screen was not described adequately to permit replication of the study. Each hospital used different methods to interpret sputum smears. It is not clear that the clinical data would be available in clinical practice. Uninterpretable or intermediate test results were not reported. |
The prevalence of previously undiagnosed pulmonary TB is greater in PLWHIV in South Africa who have not yet started receiving ART. The WHO-endorsed symptom questionnaire was more sensitive in PLWHIV not receiving ART. |
| Kim et al10/2012 | PLWHIV, age > 6 y, with and without ART Southeast Asia Aim was to determine sensitivity of symptoms to identify PLWHIV (ie, those with highly infectious disease) whose results were smear positive |
No chest radiograph or sputum examination in previous 3 mo | Current cough Any WHO-endorsed symptom |
Symptom questionnaire Sputum smear and culture If indicated, other cultures |
Sensitivity of symptoms to identify PLWHIV with smear-positive TB Sensitivity for cough 83% Any WHO-endorsed symptom, sensitivity, specificity, PPV and NPV, respectively 98% 30% 8% 100% |
Index test was not described in adequate detail to permit replication of the test. It is unclear whether uninterpretable or intermediate results were reported. |
PLWHIV attending outpatient clinics in Southeast Asia have a high prevalence of pulmonary TB (14%), and 40% of these were smear positive and considered highly infectious. The median CD4 cell counts were 75 mm3, 125 mm3, and 277 mm3 in smear-positive, smear-negative, and nonpulmonary TB cases, respectively. In PLWHIV with smear-positive pulmonary TB, cough had a sensitivity of 83%, and any WHO-endorsed symptom had a sensitivity of 98%. |
| Rangaka et al32/2012 | PLWHIV who are ART eligible South Africa |
Unwilling to consent | Not receiving ART Receiving ART |
Symptom questionnaire Sputum culture Chest radiography in participants suspected of having TB |
Current cough, all PLWHIV 25% 93% 25% 93% Any WHO-endorsed symptom, all PLWHIV 40% 88% 26% 94% Current cough, PLWHIV not receiving ART 31% 89% 30% 90% Any WHO-endorsed symptom, PLWHIV not receiving ART 48% 80% 26% 91% Current cough, PLWHIV receiving ART 12% 96% 14% 95% Any WHO-endorsed symptom, PLWHIV receiving ART 24% 94% 20% 96% |
The selection criteria were described only as the retrospective selection of subjects with data available for analysis. There could be up to 1 mo between the reference standard and the index test. The index test was described only as the WHO-endorsed symptom screen. |
Among PLWHIV, prevalence of pulmonary TB is less in those receiving ART. Any WHO-endorsed symptom is more sensitive than cough but not sensitive enough to serve as a reliable screen. Both cough and any WHO-endorsed symptom are even less sensitive in those receiving ART. |
| Modi et al30/2016 | PLWHIV, age ≥ 7 y Kenya |
Any HIV treatment in last 2 y Any TB treatment in previous year |
Current cough Any WHO-endorsed symptom |
Symptom questionnaire Physical examination Chest radiograph Sputum smear and culture Xpert MTB/RIF |
Current cough, all PLWHIV 43% 77% 19% 92% Any WHO-endorsed symptom, all PLWHIV 74% 50% 16% 94% Any WHO-endorsed symptom, nonpregnant PLWHIV 78% 42% 16% 93% |
It was unclear whether any positive results from Xpert MTB/RIF or liquid culture were equivalent. The execution of the index case was described only as “the WHO symptom screen was used.” | Among PLWHIV in Kenya, with a median CD4 of 343 mm3, any WHO-endorsed symptom was a more sensitive marker than cough but was not sensitive enough to serve as a screen for TB. Any symptom screen was even less sensitive in pregnant PLWHIV. |
| Pregnant patients | |||||||
| Modi et al30/2016 | PLWHIV, age ≥ 7 y Kenya nonpregnant and pregnant |
Any HIV treatment in last 2 y Any TB treatment in previous year |
Current cough Any WHO-endorsed symptom |
Symptom questionnaire Physical examination Chest radiograph Sputum smear and culture Xpert MTB/RIF |
Any WHO-endorsed symptom, pregnant PLWHIV 28% 75% 6% 95% |
It was unclear whether any positive results from Xpert MTB/RIF or liquid culture were equivalent. The execution of the index case was described only as “the WHO symptom screen was used.” | Any symptom screen was even less sensitive in pregnant PLWHIV. |
| Hoffmann et al28/2013 | Pregnant PLWHIV, age ≥ 18 y South Africa |
< 7 d since diagnosis, already receiving TB treatment | Current cough Any WHO-endorsed symptom |
Symptom questionnaire Sputum smear and culture |
Current cough 23% 92% 7% 98% Any WHO-endorsed symptom 28% 84% 4% 98% |
It is not clear that the reference standard correctly classifies the target condition. Only 16% of the sputum samples were satisfactory. Symptom questions were not described in any detail. It is not clear that the investigators were blinded to the sputum results when they assessed the questionnaire results. |
In pregnant PLWHIV in South Africa, with a median. CD4 of 394 mm3, neither cough nor any WHO-endorsed symptom is sensitive enough to be reliable to screen for pulmonary TB. |
| LaCourse et al29/2016 | Pregnant PLWHIV, age ≥ 16 y Kenya |
TB or latent TB infection, treatment in previous year | Any WHO-endorsed symptom Cough > 2 wk |
Tuberculin skin test Sputum smear and culture Xpert MTB/RIF |
Cough > 2 wk 29% 96% 14% 98% Any WHO-endorsed symptom 43% 81% 5% 98% |
Index test was not described sufficiently to permit replication of the test. It was reported as a structured interview tool that included the WHO-endorsed symptom screen. It is unclear whether the investigator was blinded to the sputum results when interpreting the questionnaire. | Among pregnant PLWHIV in Kenya, 54% receiving. ART and with a median CD4 of 437 mm3, neither cough nor any WHO-endorsed symptom was a sensitive enough indicator of pulmonary TB. |
ART = antiretroviral therapy; CD4 = CD4-positive T lymphocytes; NPV = negative predictive value; PPV = positive predictive value. See Table 1 for expansion of other abbreviations.
Another prospective cohort study was designed to determine the dependability of WHO-endorsed symptom screening to diagnose pulmonary TB in pregnant women with HIV infection who were aged ≥ 18 years.28 All clinic patients were eligible for the WHO-endorsed four-symptom screen and sputum smear and culture. Among the 1,415 women who underwent screening, 226 (16%) had a positive symptom screen. Thirty-five (2.5%) had new diagnoses, and 12 were already receiving treatment for pulmonary TB (total prevalence, 3.3%). Median age was 27 years, median gestational age was 24 weeks, and median CD4 cell count was 394 mm3. The sensitivity of cough and/or any WHO-endorsed symptom was poor (Table 4). The investigators concluded that symptom screening had a low sensitivity among pregnant women with HIV infection.28
A survey in eight clinics in Thailand, Cambodia, and Vietnam of PLWHIV, aged ≥ 6 years, collected sputum from 1,980 individuals with or without WHO-endorsed symptoms and found that 272 (14%) had pulmonary TB.10 Among these, 109 (40%) had positive smear and culture results. The investigators surveyed for WHO-endorsed symptoms. In this high-prevalence population in Southeast Asia, the more WHO-endorsed symptoms present the greater the likelihood of pulmonary TB. The investigators analyzed various combinations of symptoms. Individual sensitivities for weight loss, current cough, fever, and fatigue were 84%, 83%, 81%, and 78%, respectively. However, these symptoms were present in 46% to 54% of all participants. The sensitivity of one, two, three, or four WHO-endorsed symptoms (current cough, fever, weight loss, night sweats), was 98%, 90%, 72%, and 51%, respectively, for smear-positive disease. The sensitivity, specificity, negative predictive value, positive predictive value, and prevalence for cough ≥ 2 weeks or any of the four WHO-endorsed symptoms are shown in Table 4.
A cross-sectional study in Kenya identified 288 pregnant women who were HIV positive (age ≥ 16 years, 54% receiving ART, and median CD4 of 437/mm3) who could provide sputum for mycobacterial smear and culture. Median age was 25 years, and median gestational age was 26 weeks. Seven of 288 (2.4%) women had culture-positive pulmonary TB. Among the seven pregnant women with culture positive PTB, two had cough, and three had any WHO-endorsed symptom (Table 4).29
A prospective cohort study of PLWHIV, age ≥ 7 years, in Kenya not previously treated for TB and not receiving ART were screened for pulmonary TB with three sputa for microscopy, culture, and Xpert MTB/RIF testing; chest radiography; and symptom questionnaires. The prevalence of bacteriologically confirmed pulmonary TB was 11.2%. Among those with pulmonary TB, 74.1% had at least one WHO-endorsed symptom. Symptom prevalence was 90.3% in those with smear-positive disease and 63% in those whose results were culture positive but smear negative.30 However, among all PLWHIV, 53.2% had at least one WHO-endorsed symptom. Among PLWHIV without TB, 50.5% had a least one WHO-endorsed symptom. The sensitivity and specificity of current cough or at least one WHO-endorsed symptom are shown in Table 4. The WHO-endorsed symptom screen sensitivity was only 28% in pregnant women with pulmonary TB.30
Chest radiography, WHO-endorsed symptom screening, and sputum testing were planned for 825 PLWHIV in South Africa, and 737 who provided at least one sputum specimen were included.31 Pulmonary TB was diagnosed in 31 of 522 (5.9%) receiving ART and 34 of 215 (15.8%) not receiving ART. The questionnaire performed adequately for those not receiving ART but missed many patients with TB receiving ART (Table 4). Cough was the most common WHO-endorsed symptom in patients with and those without ART, but the report does not show the symptom breakdown in patients with TB and does not provide data to determine whether inclusion of other WHO-endorsed symptoms improved diagnostic sensitivity.
Another South African study in PLWHIV compared symptom screening of those receiving ART with those who did not.32 Symptom screening and sputum culture results were available for 1,429 PLWHIV, 54% receiving ART, and 126 (8.8%) had culture-positive pulmonary TB. Among 654 not receiving ART, culture-positive pulmonary TB was diagnosed in 84 (13%), and among 775 receiving ART, pulmonary TB was diagnosed in 42 (5.4%). For those receiving ART, the WHO-endorsed symptom screen was less sensitive but more specific (Table 4).
6. For PLWHIV with cough who also complain of fever, night sweats, hemoptysis and/or weight loss (WHO-endorsed symptoms) and are at risk for TB, we suggest screening for pulmonary TB because the presence of these symptoms increases the likelihood that the affected individual has pulmonary TB (Grade 2C).
Remarks: All of the included studies were limited to PLWHIV.
KQ 3: Is Actively Screening More Effective Than Passively Screening for TB?
Figure 3 outlines the search strategy used in PubMed, Scopus, and the Cochrane Database of Systematic Reviews and the number of citations identified for the KQ 3 systematic review. The search, undertaken in February 2016, identified 70 titles in PubMed; 111 in Scopus, 51 after removal of duplicates; and five in the Cochrane Database of Systematic Reviews, three after removal of duplicates. Three comparative trials were identified,33, 34, 35 and four others were discovered during the KQ 1 search and bibliographic review36, 37, 38, 39 (Table 5). All seven were cross-sectional studies, but two were based on data from the same region collected 2 years apart, and a third compared results with the previous year’s statistics.33, 37
Table 5.
Descriptions of the Retained Studies for KQ 3
| Study/Year | Inclusions | Exclusions | Study Arms | Method of Assessment | Outcomes | Limitations and Risk of Bias | Key Messages |
|---|---|---|---|---|---|---|---|
| Becerra et al38/2005 | Household contacts, neighbors Lima, Peru, with cough ≥ 2 wk |
Not mentioned | ACF and PCF PCF only |
Number of smear- and culture-positive pulmonary TB cases | Case detection rates: Household cases, ACF and PCF vs PCF only 914 of 100,000 vs 183 of 100,000 (P = .02) Cases among neighbors, ACF and PCF vs PCF only 221 of 100,000 vs 80 of 100,000 (P = .25; not significant) | The ACF and PCF cohort was compared with the previous time period. The study was cross-sectional. Inclusion and exclusion criteria were not stated. Power calculations and demographic characteristics of the two populations were not provided. The majority of patients did not submit the prescribed two sputum specimens. | ACF: Household contact tracing to identify those with cough ≥ 2 wk increases the number with cough ≥ 2 wk who are screened and the number of smear-positive pulmonary TB cases detected when added to PCF. Contact tracing among neighbors does not. |
| Datiko and Lindtjorn37/2009 | Sidama region Ethiopia, with cough ≥ 2 wk |
Not mentioned | ACF and PCF PCF only |
Number of smear-positive pulmonary TB cases (case detection rate) | Case detection rates: intervention kebeles (ACF and PCF) vs other kebeles (PCF only) 122 of 100,000 vs 69 of 100,000 (P < .001) | The study was cross-sectional, and over a longer period it is likely that PCF would identify a greater proportion of the pulmonary TB cases. Exclusions and criteria for exclusions were not stated. |
ACF: The presence of health extension workers in the community to encourage those with cough ≥ 2 wk to attend the health unit to provide sputum increased the number screened, increased the smear-positive case detection rate when added to PCF, and improved treatment outcomes. |
| Gonzalez-Ochoa et al34/2009 | Household members of home visit patients with cough ≥ 2 wk Las Tunas, Cuba |
Partway through study, limited to individuals at high risk | ACF and PCF PCF only |
Number of smear- and culture-positive pulmonary TB cases |
Case detection rates: 26.2 of 100,000 vs 6.7 of 100,000 |
The ACF and PCF cohort was compared with the previous time period. The ACF protocol was modified partway through the study. Inclusion and exclusion criteria were not stated clearly. Power calculations were not provided. | ACF: Having doctors and nurses check for cough and collecting sputum for smear and culture from other family members with cough ≥ 2 wk during house calls, particularly for those at greater risk (the elderly, heavy alcohol users, ex-inmates, PLWHIV, and the socioeconomically vulnerable), increases the case detection rate compared with results with PCF alone. If screening is limited to those with cough ≥ 3 wk, the prevalence of TB will be greater (P = .0001). |
| Parija et al36/2014 | Individuals with cough ≥ 2 wk Odisha, India |
Not mentioned | Sectors with (ACF and PCF) and without (PCF only) public health campaigns | Number of smear-positive pulmonary TB cases (case detection rate) | In public awareness sectors (ACF and PCF), the number of smear-positive pulmonary TB cases increased 11% over the same time the previous year vs 0.8% increase in PCF-only sectors. The numbers screened increased 87.8% vs 16.6%. | It was unclear whether the study was implemented correctly. The ACF and PCF cohort was compared with the previous time period. Demographic characteristics of the two groups were not provided. Power calculations were not provided. Exclusion criteria were not provided. |
ACF: A public health awareness program encouraging individuals with cough ≥ 2 wk to present for screening (ie, submitting sputum for microscopy), added to PCF, increased the numbers screened and the case detection rate of pulmonary TB. |
| Yassin et al33/2013 | Sidama region, Ethiopia, with health extension workers Hadiya region, Ethiopia, without health extension workers, cough ≥ 2 wk |
Not mentioned | ACF and PCF PCF only |
Number of smear-positive pulmonary TB cases (case detection rate) | The case detection rate increased from 64 to 127 of 100,000 in Sidama, pulmonary TB cases increased 101%, and all TB cases increased 78% vs the previous 15 mo. In Hadiya, the pulmonary TB case detection rate increased 16%. Treatment outcomes also improved in Sidama: 85% were cured and 8% completed therapy vs previous 15 mo when 42% were cured and 35% completed therapy. | Although presented as a comparison study between two regions contemporaneously, most of the comparisons were with the previous time period in the same zone. Demographic characteristics of the two groups were not provided. Exclusion criteria were not provided. | ACF: Health extension workers went house-to-house to collect sputum from those with cough ≥ 2 wk and increased the case detection rate of pulmonary TB and all forms of TB. Adding ACF to PCF increased the case detection rate. Treatment success, a combination of the cure rate and treatment completions, increased with health extension worker involvement. |
| Zachariah et al39/2003 | Household contacts of people with smear-positive pulmonary TB with cough > 3 wk Rural Malawi |
Not mentioned |
ACF and PCF PCF only |
Number of smear-positive pulmonary TB cases |
The prevalence of smear-positive pulmonary TB was 1,735 of 100,000 with ACF vs 191 of 100,000 in the PCF group. | It was unclear whether the study design was implemented correctly. Comparisons were with the previous time period. Unclear whether the subjects were representative of the population. HIV prevalence in Malawi is 9%, and 69% of the index cases were HIV positive. Power calculations were not provided. Exclusion criteria and number excluded were not provided. |
ACF: The households of those with pulmonary TB were visited 1 mo after registration of the index case to identify those with cough > 3 wk. Children ≤ 6 y underwent a chest radiograph and were either treated for active disease or received isoniazid preventive therapy. The addition of ACF to PCF increased the case detection rate. |
ACF = active case finding; PCF =passive case finding.
The seven studies underwent quality assessment (Table 5). Common deficiencies were not defining or correctly implementing the study design, not clearly defining inclusion and exclusion criteria, not calculating or meeting power estimates, not determining whether subjects were representative of the population, and/or not assessing potential treatment confounding variables. One was rated as good,37 five were rated as fair,33, 34, 36, 38, 39 and one was rated as poor35 and will not be discussed. Because of the heterogeneity of the six studies rated as good or fair, and especially the different definitions and implementations of ACF and passive case finding (PCF), the studies could not be synthesized, and an evidence profile could not be developed.
One ACF strategy relied on health extension workers to identify community members with cough ≥ 2 weeks for screening between September 2006 and April 2008 in the Sidama zone in rural Ethiopia.37 In 30 intervention kebeles or neighborhoods, with a population of 178,138, trained health extension workers advised individuals with cough ≥ 2 weeks with or without other symptoms to attend the health units to provide specimens for sputum microscopy.37 Individuals in 21 control kebeles with a population of 118,673 were not provided with this information. The mean case detection rate was 122 of 100,000 in intervention kebeles vs 69 of 100,000 in control kebeles, (P < .001). Moreover, treatment success (cured and completed therapy) rates were better in intervention kebeles (89.3% vs 83.1%; P = .012).37
A subsequent study recruited individuals after the health extension worker program was extended throughout the Sidama zone, and results were compared with the adjacent Hadiya zone that had similar demographic characteristics.33 In Sidama, health education workers went from house-to-house to identify individuals with cough ≥ 2 weeks and collected sputum for microscopy. The program was unavailable in Hadiya, so individuals were obliged to attend health units for assessment and care. Health extension workers identified 49,857 individuals with cough ≥ 2 weeks, and 2,262 (4.5%) were smear positive. Combined with the patients who presented to the health units, 5,090 smear-positive pulmonary TB cases were identified in Sidama. In the previous 15-month period, only 2,534 smear-positive pulmonary TB cases had been identified in Sidama. The health extension worker program resulted in a 101% increase in the case detection rate over the previous 15 months compared with a 16% increase, from 949 to 1,133, in Hadiya during the same period. The pulmonary TB case notification rate was 127 of 100,000 in Sidama vs 69.4 of 100,000 in Hadiya.33
The assumption guiding studies that combine ACF with PCF is that cases identified by means of PCF represent the baseline diagnostic yield and that cases identified by means of ACF account for the additional cases.38 In Lima, Peru, ACF among household contacts of pulmonary TB cases increased the case detection rate from 183 of 100,000 to 914 of 100,000 (P = .02).38 In neighboring households of active cases, ACF did not increase the case detection rate significantly (80 of 100,000 to 221 of 100,000; P = .25).38
A comparative trial of ACF was undertaken in Odisha, India.36 In one-half the districts, village-based health activists underwent a 1-day training program on the signs and symptoms of TB with emphasis on cough ≥ 2 weeks. ACF included a public awareness campaign consisting of a traveling van with announcements by loudspeaker followed by health fairs in targeted sectors encouraging individuals with cough ≥ 2 weeks to provide sputum for microscopy. Compared with the same quarter in the previous year, the number of patients who underwent screening in the intervention sectors increased 87.8% and smear-positive pulmonary TB cases increased by 10.8% compared with 16.6% and 0.8% in the usual practice (ie, PCF, sectors), respectively.
In Las Tunas, Cuba, visiting nurses collected sputum for microscopy and culture from patients and household members with cough ≥ 2 weeks.34 Partway through the study, screening was limited to those with cough and another risk factor for pulmonary TB: the elderly, alcoholics, ex-prisoners, PLWHIV, and the socioeconomically vulnerable. The prevalence of cases was 26.2 of 100,000 in the program vs 6.7 of 100,000 detected by means of PCF at health centers.34
In rural Malawi, patients with pulmonary TB were instructed to tell their household contacts with cough to attend the health unit for screening and served as the PCF cohort.39 In the ACF cohort, health-care workers visited the homes with all active cases to identify any household members with cough for > 3 weeks. Sputum was collected on the spot, and a second specimen was collected for microscopy the following day. Prevalence of pulmonary TB in the PCF cohort was 191 of 100,000 (0.19%) compared with 1,735 of 100,000 (1.74%) in the ACF group.39
Waiting for individuals with symptoms to present for medical care to detect pulmonary TB, also called “PCF,” was the recommended strategy for resource-limited, high-prevalence countries because it is less expensive than actively searching for cases.40, 41, 42 Hard-to-reach undertreated or untreated populations are a reservoir of infection, frustrating efforts at disease eradication.43 However, PCF fails to identify most individuals with pulmonary TB. In 2000, it was estimated that only 27% of new cases with smear-positive sputum were detected, and only 19% were treated successfully.44
ACF programs to identify people suspected of having TB who have not presented for assessment were developed to confirm the diagnosis promptly and initiate therapy.8 Most ACF programs were undertaken in high-prevalence countries and in particularly high-risk groups or among those with poor access to health care such as people living in remote rural areas. These ACF programs actively sought individuals with cough, usually ≥ 2 weeks’ duration, with or without other WHO-endorsed symptoms, and obtained sputum for microscopy, with or without culture or Xpert MTB/RIF testing. Some programs also included a symptom-based questionnaire or chest imaging. Some ACF programs screened contacts of active cases whether or not they were coughing.45
In populations with a high TB prevalence, the addition of ACF to PCF to identify patients with cough ≥ 2 weeks, including household contacts, will increase the number of pulmonary TB cases identified and will identify them earlier.33, 34, 35, 36, 37, 38, 39 Kranzer et al19 published a systematic review that concluded that screening increased the number of pulmonary TB cases diagnosed in the short term, and diagnosed them earlier and with less advanced disease, but it was not clear that treatment outcomes were improved or that transmission rates were reduced. Screening is recommended for PLWHIV and household contacts of active pulmonary TB, but the benefits of wider population-based screening programs remain uncertain.19
If one assumes that individuals identified by means of ACF eventually will present for care, an ACF strategy should identify pulmonary TB cases earlier when disease is less advanced.40 The available data are insufficient to confirm this method. Implementing an ACF strategy to identify individuals with cough ≥ 3 weeks was compared with PCF in South Africa.23 Outcomes were similar, but the earlier initiation of treatment may have resulted in less disease transmission.23 ACF will be more effective only if treatment is started earlier and patients complete therapy.8, 22 As part of a Directly Observed Treatment, Short Course (DOTS) strategy, ACF can increase the number of cases successfully treated.38 This increase may be due to the DOTS component rather than ACF.37, 45 Whether ACF will be effective in environments with a low or middle TB prevalence is unclear.
An important question is whether ACF will affect the pulmonary TB incidence and prevalence. None of the comparative studies demonstrated that ACF provided an added benefit over PCF. However, a Zimbabwean study comparing two ACF strategies reported a subsequent decline in pulmonary TB prevalence after the ACF strategies were implemented.46 No other studies have reported that ACF led to a subsequent reduction in pulmonary TB prevalence.
Suggestions
7. For patients with cough in high TB prevalence populations, we suggest the addition of active case finding (ACF) to passive case finding (PCF) because it may improve outcomes in patients with pulmonary TB (Ungraded Consensus-Based Statement).
8. For patients with cough in high TB prevalence populations, we suggest the addition of ACF to PCF because it may reduce transmission (Ungraded Consensus-Based Statement).
KQ 4: What Is the Most Cost-Effective Strategy to Diagnose TB in Individuals With Cough in High-Prevalence Countries?
A systematic literature review was performed to address the most cost-effective strategy to diagnose a case of TB among individuals with cough ≥ 2 weeks. The PubMed search (Fig 4) identified 111 publications since January 1984, and a search of the SCOPUS database in May 1984 identified a further 28 for a total of 139 publications, of which 19 were selected for full-text review after assessment of the abstracts. Four had information that pertained to this question. Two studies modeled the incremental cost-effectiveness ratio of different screening strategies for TB diagnosis in patients with and those without symptoms.26, 47
The incremental cost-effectiveness ratio of different screening strategies depends on TB prevalence in the population modeled.48 Screening asymptomatic individuals for TB is cost-effective only in very high-prevalence groups.38 ACF with testing of symptomatic individuals seems to be the best approach in most patient populations. In symptomatic individuals, assessment of different diagnostic tests and order of testing were modeled. A modeling study suggested Xpert MTB/RIF testing of individuals with sputum smear-negative results in countries without mycobacterial culture capability would be cost-effective with use of the WHO willingness-to-pay thresholds as opposed to clinical diagnosis.26, 47
Two studies assessed the direct patient costs of investigations into chronic cough, primarily in rural settings in Yemen, Nepal, and China.48, 49 Both articles concluded that direct patient costs were significant, especially when travel to a diagnostic center was required, and suggested that investigations for TB be provided at no or low cost and at the point of care to limit the financial burden of assessment on individuals with chronic cough.48, 49
Suggestions
9. For patients with cough suspected to have pulmonary TB, we suggest that available financial modeling algorithms be used to estimate costs associated with different screening strategies because cost-effectiveness studies have not yet been performed (Ungraded Consensus-Based Statement).
10. For patients with chronic cough in low income countries, we suggest that strategies for pulmonary TB diagnosis should focus on improved case detection rather than diagnostic testing (Ungraded Consensus-Based Statement).
KQ 5: How Quickly Should Cough Resolve With Effective Antimicrobial Therapy in Patients With Pulmonary TB?
The literature search to determine the expected rate of cough resolution in pulmonary TB identified 322 publications since January 1984 (Fig 5). Among these, 278 had abstracts in English or Spanish and were reviewed. No publication met the criteria to address this KQ. The search did not identify any high-quality studies that evaluated the time required for cough resolution in individuals with TB who were receiving effective anti-TB therapy.
A recently published subanalysis from a small prospective cohort study from Peru quantified cough in 64 patients who were HIV negative and starting treatment for culture-positive pulmonary TB and reported a significant reduction in cough by day 14 of treatment and a further reduction after 2 months of treatment.50 A semiautomated ambulatory cough monitor with a demonstrated sensitivity of 77.5% and specificity of 99.3% identified cough episodes in patients with pulmonary TB. The investigators excluded monitor malfunctions and periods of poor sound quality due to high background noise, and two trained nurses listened to validate the portions of the recording tape with identified cough events. Patients were followed up from just before the treatment start through the first 62 days of therapy. The median number of cough episodes prior to starting treatment was 2.3/h (interquartile range, 1.2-4.1). Cough frequency increased with increasing bacillary load (P < .01). Cough cessation was defined as the time to the first of two consecutive recordings with a cough frequency of ≤ 0.7 cough events per hour during treatment. By day 14, the probability of cough cessation was 42% (95% CI, 25%-64%), and by day 60 the probability was 51% (95% CI, 33%-72%). By day 14, the probability of sputum mycobacterial smear conversion was 26% (95% CI, 17%-39%), and by day 60 the probability was 85% (95% CI, 73%-93%). Sputum culture conversion assessed by means of microscopic observation drug susceptibility was 29% (95% CI, 19%-41%) by day 14 and 94% (95% CI, 85%-98%) by day 60 in this selected cohort of patients with pansensitive pulmonary TB.
Further prospective studies should address this important clinical question. Establishing the appropriate time frame for cough resolution and identifying individuals whose cough persists longer may identify those with persistent infectious state or drug-resistant TB. This method may assist with individualized respiratory isolation management and targeted drug susceptibility testing in resource-limited settings.
KQ 6: Is There a Difference In Cough Duration Between Patients Treated With Drug-Sensitive Cavitary Disease vs Drug-Sensitive Noncavitary Pulmonary TB?
Only 73 citations published since 1984 were identified potentially to answer this question; 51 citations in English and Spanish were reviewed (Fig 6). No publication met the criteria to address this KQ. In conclusion, we found no available prospective, peer-reviewed studies that confidently evaluated differences in cough duration between patients who were treated who had drug-sensitive cavitary pulmonary TB vs those who had drug-sensitive noncavitary pulmonary TB. Future studies should address this clinical question.
Data are insufficient to anticipate the rate of cough resolution in individuals with drug-sensitive pulmonary TB, although one could assume that cough will respond more quickly in the absence of cavitary lung disease. However, one Japanese abstract indirectly addressed this question.51 This prospective study compared cough- and sputum-related quality of life in 64 individuals with pulmonary TB, with both cavitary and noncavitary disease, before and after anti-TB treatment. The median age of the patient cohort evaluated during their hospitalization was 74 years. Their subjects completed the Japanese version of the Leicester Cough Questionnaire and the Cough and Sputum Assessment Questionnaire, designed to evaluate cough and sputum production, at the time of admission and at hospital discharge. Both cough- and sputum-related quality of life in patients with pulmonary TB was impaired but improved after treatment. The Leicester Cough Questionnaire scores correlated positively with the Cough and Sputum Assessment Questionnaire scores (Spearman rank correlation coefficient, 0.430-0.887). Importantly, there was no difference in total Leicester Cough Questionnaire score between patients with cavitary or endobronchial lesions and those without.
KQ 7: In Coughing Patients With Lung Disease, Are There Features That Suggest M avium Complex Lung Disease?
The systematic literature search for individual studies for KQ 7 was conducted using the following databases: MEDLINE via PubMed, Embase, and Scopus with date limitations from January 1, 1984, to April 2014 (Fig 7). The search strategy identified 51 references. Only three articles reported the prevalence of cough in Mycobacterium avium complex (MAC) lung disease, but all were rated as poor quality and were excluded from the evidence review.52, 53, 54
The two controlled trials of MAC lung disease therapy by the British Thoracic Society did not address the prevalence or features of cough.55, 56 Although there is no supporting evidence, one would assume that cough duration, frequency, severity, and likelihood of sputum production increase with more advanced lung disease. Moreover, many with MAC infection have underlying lung disease responsible for, or at least contributing to, their cough.57 In a cohort with mild disease, cough prevalence was 23%.58 The majority of patients with MAC in a French study (71.2%) had underlying lung disease, and 77.6% reported cough.59 There are no cough features predictive of MAC lung disease or predictive of disease due to other nontuberculous mycobacteria. Nodular bronchiectasis, predominantly involving the lingula and/or right middle lobe, is observed primarily in women who are often nonsmokers without a history of antecedent lung disease.52 The majority (86%) with nodular bronchiectasis experienced productive cough and purulent sputum production for an average of 6 months prior to diagnosis and had relatively advanced disease at diagnosis.52 A retrospective review from Seoul, Korea, found that individuals with Mycobacterium intracellulare were more likely to have underlying lung disease and were more likely to experience cough (84% vs 74%; P = .005) than were patients with M avium lung disease.53
In addition to underlying fibrocavitary disease due to previous pulmonary TB or other lung conditions such as sarcoidosis, MAC is frequently cultured from the sputum or bronchoscopy specimens from individuals with a variety of other lung diseases such as COPD, bronchiectasis, and active pulmonary TB.58 It may be challenging to determine whether MAC is contributing to the patient’s symptoms in the presence of underlying lung disease.54 In summary, the reported prevalence of cough varies widely in patients with MAC lung disease. The prevalence will vary with the severity and extent of disease and the presence and severity of comorbid lung disease.
KQ 8: In Coughing Patients With Lung Disease, Are There Features to Suggest Fungal Infection or Paragonimiasis?
The 2014 PubMed database search identified 283 citations, and a further 19 were identified in the Scopus database, for a total of 302 citations (Fig 8). The 42 Pneumocystis jirovecii citations are not addressed in this section.
Among the remaining articles, others were excluded for a variety of reasons: review article, single case report, or small case series of < 10 subjects. Some addressed other conditions (ie, not fungal lung diseases, not about lung disease, fungal disease in other species) or did not address cough, leaving 54 citations for further assessment. Most of the remainder were case series of patients with aspergillosis, blastomycosis, coccidioidomycosis, cryptococcal disease, histoplasmosis, mucormycosis, paracoccidioidomycosis, or penicilliosis infections. Only three were comparative studies, albeit of poor quality, and were excluded from the evidence review.60, 61, 62
The prevalence of cough has been reported in patients, with and without an immunocompromising condition, in a variety of fungal diseases of varying severities: Aspergillus, blastomycosis, coccidioidomycosis, Cryptococcus, histoplasmosis, Mucor, paracoccidioidomycosis, and penicilliosis.63, 64, 65, 66, 67, 68, 69, 70 The reported cough prevalence has varied from approximately 25% to 100%, but the reported prevalence was approximately 50% in the three comparative trials.60, 61, 62 The prevalence of hemoptysis also varied widely. None of the studies identified any features that are characteristic of cough due to any of the fungal diseases.
Paragonimiasis is contracted by consuming raw or undercooked crabs or crayfish infected with a Paragonimus species. Initial symptoms are usually gastrointestinal: diarrhea, abdominal pain, fever, eosinophilia, and hepatosplenomegaly. Pleuropulmonary involvement usually occurs in the chronic phase, manifesting as cough, sputum production, and hemoptysis.71 No published prospective studies or comparative trials of cough due to paragonimiasis were identified. In some tropical regions, between 10% and 15% of those with chronic cough have paragonimiasis.72, 73, 74, 75, 76 Cases occasionally are reported from the Mississippi River Basin in individuals who consume raw crayfish.77
Limitations
We did not plan to limit our review to high-prevalence populations, but most of the reports and virtually all of the comparative reports were from high-risk populations and from low-income countries. Because the comparative reports described data from individuals at high risk in low-income countries, the recommendations were developed for those populations. The lack of inclusion of health-care practitioners from high-prevalence, low- and low-middle- income countries and the lack of input from patients at high risk in the development of these recommendations are obvious deficiencies. The relative dearth of data to answer the KQs was also an important limitation of this report.
Conclusions
The objective of this report was to update the 2006 ACCP clinical practice guideline for cough due to TB and other chronic infections.11 An international panel of experts was assembled to develop management guidelines from a systematic review of the literature. A rigorous evidence-based process, under the direction of the CHEST methodologists, was used to review the relevant literature. Eight appropriate and topical PICO questions were created to guide the literature review. The 2006 guidelines were based on the available literature to 2003, whereas the current guideline updated the literature to 2014 and in the case of KQs 1 and 3, to February 2016, and in the case of KQ 2, to March 2017. The literature addressing these PICO questions was largely of low quality. However, the panel agreed that in countries with a high TB prevalence, most individuals at high risk and household contacts with cough ≥ 2 weeks do not have pulmonary TB. The literature did not provide strong evidence that there is an optimal cough duration for when to screen for TB. We found no evidence that the evidence that the WHO- or Practical Approach to Lung Health in South Africa-endorsed cough ≥ 2 weeks is superior to ≥ 1, 3, or 4 weeks. Screening of patients with cough for < 2 weeks will increase the number of pulmonary TB cases identified earlier but also will increase the cost of investigations.
In PLWHIV, it is essential to exclude active TB prior to initiating ART or isoniazid preventive therapy. Cough has a fair negative predictive value in PLWHIV but not in all subpopulations (eg, pregnant PLWHIV and PLWHIV already receiving ART) and lacks the sensitivity and specificity for a confident diagnosis or to exclude pulmonary TB. Screening for any WHO-endorsed symptom rather than only cough increases the diagnostic sensitivity for TB.10, 27, 28, 29, 30, 31, 32 The presence of other WHO-endorsed symptoms in addition to cough increases the likelihood that a patient has pulmonary TB, but it is still too low to confirm a diagnosis of pulmonary TB in resource-limited settings. However, the negative predictive value (ie, the absence of any WHO-endorsed symptom in PLWHIV in high-prevalence countries who are not receiving ART, not close contacts of active cases, and are not pregnant) can help to exclude those who do not require additional testing for pulmonary TB. The addition of chest radiography to WHO-endorsed symptom-based screening considerably increases the diagnostic reliability.
The addition of ACF to PCF can increase the number of cases identified but should be combined with prompt treatment in a DOTS program to improve outcomes and hopefully reduce disease transmission. Screening strategies involving asymptomatic individuals are only cost-effective in high-prevalence countries. Financial support should be provided to encourage patients with cough to present for screening, and those with pulmonary TB should have treatment provided free of charge.
One report addressing the effect of pulmonary TB treatment on cough was identified.50 There was a statistically significant decline in cough frequency after patients had been receiving treatment for 14 days, and there was a further reduction in cough frequency after patients had been receiving treatment for 2 months.50 Despite expectations to the contrary, we did not find studies that directly addressed whether cough resolves more slowly with effective antimicrobial therapy in patients with pulmonary TB with cavitary lung involvement than in patients with pulmonary TB with noncavitary pulmonary disease.
Cough is common in patients with lung disease due to MAC, other nontuberculous mycobacteria, fungal diseases, and paragonimiasis, but we did not find evidence that addresses whether there are cough features that will distinguish these conditions from each other, pulmonary TB, or other lung diseases. The reported prevalence of cough in these conditions varied widely likely because of differences in disease severity and the presence of underlying lung disease.
Areas for Future Research
To fill the gaps in knowledge that were identified, the following studies should be performed in the future:
-
•
In resource-limited settings with a high TB prevalence, prospective longitudinal studies with new point-of-care diagnostics tools to determine the optimal and most cost-effective timing to screen patients with cough for pulmonary TB will help determine their role and the optimal time for testing in patients with cough and other TB-related symptoms.
-
•
Trials that couple ACF with prompt treatment through DOTS programs should be undertaken to evaluate whether they improve outcomes and reduce TB prevalence in high-prevalence populations.
-
•
Cost-effectiveness studies are essential to optimize cost-effectiveness in low- and middle-income jurisdictions to maximize the impact of diagnostics and treatment in high-prevalence settings.
-
•
In consideration of the increasing proportion of pulmonary TB cases with drug-resistant TB, prospective trials to define the expected time for resolution of cough with effective therapy, in patients with prior TB treatments and/or areas of high MDR-TB prevalence, would be helpful to optimize patient care, especially in resource-limited jurisdictions where routine drug susceptibility testing is not readily available.
-
•
Prospective studies in patients with cough with novel point-of-care diagnostics would be helpful for early detection and characterization of pulmonary infections due to MAC, other nontuberculous mycobacteria, fungal diseases, and paragonimiasis for prompt optimization of treatment selection and management.
Acknowledgments
Author contributions: S. K. F. wrote the initial draft of the manuscript. All authors had full access to the data and contributed to the key question development. P. E., D. A. F. and S. K. F. independently reviewed all of the manuscripts and articles. B. I. drafted the methods section and performed the grading of the evidence. All authors critically reviewed the manuscript for important intellectual content, participated in writing the final manuscript through suggested revisions, agreed with all suggestions and recommendations, and approved the final draft.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. K. F. has no financial conflicts of interest regarding the contents of this article. However, he has received clinical trial research funding from Boehringer-Ingelheim, GlaxoSmithKline, Insmed-Synertec, and Novartis and advisory board/speaker bureau fees from Boehringer-Ingelheim, GlaxoSmithKline, and Novartis. R. S. I. has no financial or intellectual conflicts of interest regarding the content of this article. Moreover, although R. S. I. is the Editor in Chief of CHEST, the review and all editorial decisions regarding this manuscript were made independently by others. None declared (P. E., D. A. F., B. I.).
Collaborators: Todd M. Adams, MD (Webhannet Internal Medicine Associates of York Hospital, Moody, ME), Kenneth W. Altman, MD, PhD (Baylor College of Medicine, Houston, TX), Elie Azoulay, MD, PhD (University of Paris, Paris, France), Alan F. Barker, MD (Oregon Health & Science University, Portland, OR), Surinder S. Birring, MBChB, MD (Division of Asthma, Allergy and Lung Biology, King’s College London, Denmark Hill, London, England), Fiona Blackhall, MD, PhD (University of Manchester, Department of Medical Oncology, Manchester, England), Donald C. Bolser, PhD (College of Veterinary Medicine, University of Florida, Gainesville, FL), Louis-Philippe Boulet, MD, FCCP (Institut universitaire de cardiologie et de pneumologie de Québec, Quebec [IUCPQ], QC, Canada), Sidney S. Braman, MD, FCCP (Mount Sinai Hospital, New York, NY), Christopher Brightling, MBBS, PhD, FCCP (University of Leicester, Glenfield Hospital, Leicester, England), Priscilla Callahan-Lyon, MD (Adamstown, MD), Anne B. Chang, MBBS, PhD, MPH (Royal Children’s Hospital, QLD, Australia), Andréanne Coté, MD (Institut universitaire de cardiologie et de pneumologie de Québec [IUCPQ], Quebec, QC, Canada), Terrie Cowley (The TMJ Association, Milwaukee, WI), Paul Davenport, PhD (Department of Physiological Sciences, University of Florida, Gainesville, FL), Satoru Ebihara, MD, PhD (Department of Rehabilitation Medicine, Toho University School of Medicine, Tokyo, Japan), Ali A. El Solh, MD, MPH (University at Buffalo, State University of New York, Buffalo, NY), Patricio Escalante, MD, MSc, FCCP (Mayo Clinic, Rochester, MN), Stephen K. Field, MD (University of Calgary, Calgary, AB, Canada), Dina Fisher, MD, MSc (University of Calgary, Respiratory Medicine, Calgary, AB, Canada), Cynthia T. French, PhD, FCCP (UMass Memorial Medical Center, Worcester, MA), Peter Gibson, MBBS (Hunter Medical Research Institute, NSW, Australia), Philip Gold, MD, MACP, FCCP (Loma Linda University, Loma Linda, CA), Cameron Grant, MB ChB, PhD (University of Auckland School of Medicine, Auckland, New Zealand), Susan M. Harding, MD, FCCP (Division of Pulmonary, Allergy and Critical Care Medicine Department of Medicine, University of Alabama at Birmingham, Birmingham, AL), Anthony Harnden, MB ChB, MSc (University of Oxford, Oxford, England), Adam T. Hill, MB ChB, MD (Royal Infirmary and University of Edinburgh, Edinburgh, Scotland), Richard S. Irwin, MD, Master FCCP (UMass Memorial Medical Center, Worcester, MA), Peter J. Kahrilas, MD (Feinberg School of Medicine, Northwestern University, Chicago, IL), Joanne Kavanagh, MBChB, (Division of Asthma, Allergy and Lung Biology, King’s College London, Denmark Hill, London, England), Karina A. Keogh, MD (Mayo Clinic, Rochester, MN), Kefang Lai, MD, PhD (First Affiliated Hospital of Guangzhou Medical College, Guangzhou, China), Andrew P. Lane, MD (Johns Hopkins University School of Medicine, Baltimore, MD), Kaiser Lim, MD (Mayo Clinic, Rochester, MN), J. Mark Madison, MD, FCCP, (UMass Memorial Medical Center, Worcester, MA), Mark A. Malesker, PharmD, FCCP (Creighton University School of Pharmacy and Health Professions, Omaha, NE), Stuart Mazzone, PhD, FCCP (University of Melbourne, VIC, Australia), Lorcan McGarvey, MD (The Queen’s University Belfast, Belfast, Northern Ireland), Alex Molasoitis, PhD, MSc, RN (Hong Kong Polytechnic University, Hong Kong, China), Abigail Moore, BM BCh, (University of Oxford, Oxford, England), M. Hassan Murad, MD, MPH (Mayo Clinic, Rochester, MN), Mangala Narasimhan, DO, FCCP (Hofstra-Northwell Health, Manhasset, NY), Huong Q. Nguyen, PhD, RN (Kaiser Permanente, Pasadena, CA), Peter Newcombe, PhD (School of Psychology University of Queensland, QLD, Australia), John Oppenheimer, MD (University of Medicine and Dentistry of New Jersey-Rutgers University), Marcos I. Restrepo, MD, MSc, FCCP (South Texas Veterans Health Care System, San Antonio, TX), Mark Rosen, MD, Master FCCP (Icahn School of Medicine at Mount Sinai, New York, NY), Bruce Rubin, MEngr, MD, MBA (Virginia Commonwealth University, Richmond, VA), Jay H. Ryu, MD, FCCP (Mayo Clinic, Rochester, MN), Sonal Singh, MD, MPH (UMass Memorial Medical Center, Worcester, MA), Jaclyn Smith, MB ChB, PhD (University of Manchester, Manchester, England), Susan M. Tarlo, MBBS, FCCP (Toronto Western Hospital, Toronto, ON, Canada), Julie Turmel, PhD (Institut universitaire de cardiologie et de pneumologie de Québec, Quebec [IUCPQ], QC, Canada), Anne E. Vertigan, PhD, MBA, BAppSc (SpPath) (John Hunter Hospital, NSW, Australia), Gang Wang, MD, PhD (Sichuan University, West China Hospital, Chengdu, China), Miles Weinberger, MD, FCCP (University of Iowa Hospitals and Clinics, Iowa City, IA), Kelly Weir, MS Path, PhD (Menzies Health Institute Queensland/Griffith and Gold Coast, Australia)
Other contributions: We thank Nancy Harger, MLS, and Judy Nordberg, MLS, education and clinical services librarians working in the University of Massachusetts Medical School Library in Worcester, MA, who undertook all the searches for these systematic reviews. We also thank Sheena Patel, MPH, for her helpful critical review of our manuscript.
Endorsements: This guideline has been endorsed by the American Association of Respiratory Care (AARC).
Role of sponsors: CHEST was the sole supporter of these guidelines, this article, and the innovations addressed within.
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://www.chestnet.org/Guidelines-and-Resources/Guidelines-and-Consensus-Statements/CHEST-Guidelines.
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Contributor Information
Stephen K. Field, Email: sfield@ucalgary.ca.
CHEST Expert Cough Panel:
Todd M. Adams, Kenneth W. Altman, Elie Azoulay, Alan F. Barker, Surinder S. Birring, Fiona Blackhall, Donald C. Bolser, Louis-Philippe Boulet, Sidney S. Braman, Christopher Brightling, Priscilla Callahan-Lyon, Anne B. Chang, Andréanne Coté, Terrie Cowley, Paul Davenport, Satoru Ebihara, Ali A. El Solh, Patricio Escalante, Stephen K. Field, Dina Fisher, Cynthia T. French, Peter Gibson, Philip Gold, Cameron Grant, Susan M. Harding, Anthony Harnden, Adam T. Hill, Richard S. Irwin, Peter J. Kahrilas, Joanne Kavanagh, Karina A. Keogh, Kefang Lai, Andrew P. Lane, Kaiser Lim, J. Mark Madison, Mark A. Malesker, Stuart Mazzone, Lorcan McGarvey, Alex Molasoitis, Abigail Moore, M. Hassan Murad, Mangala Narasimhan, Huong Q. Nguyen, Peter Newcombe, John Oppenheimer, Marcos I. Restrepo, Mark Rosen, Bruce Rubin, Jay H. Ryu, Sonal Singh, Jaclyn Smith, Susan M. Tarlo, Julie Turmel, Anne E. Vertigan, Gang Wang, Miles Weinberger, and Kelly Weir
References
- 1.World Health Organization. Global Tuberculosis Report: 2015. 20th ed. Geneva, Switzerland: WHO Press.http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1. Accessed August 18, 2016.
- 2.World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. http://www.who.int/tb/tbscreening/en/. Accessed August 18, 2016. [PubMed]
- 3.English R.G., Bachmann M.O., Bateman E.D. Diagnostic accuracy of an integrated respiratory guideline in identifying patients with respiratory symptoms requiring screening for pulmonary tuberculosis: a cross-sectional study. BMC Pulm Med. 2006;6:22. doi: 10.1186/1471-2466-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Bank. World Bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed December 10, 2017.
- 5.Sanchez A., Gerhardt G., Natal S. Prevalence of pulmonary tuberculosis and comparative evaluation of screening strategies in a Brazilian prison. Int J Tuberc Lung Dis. 2005;9(6):633–639. [PubMed] [Google Scholar]
- 6.Boehme C.C., Nicol M.P., Nabeta P. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steingart K.R., Schiller I., Horne D.J., Pai M., Boehme C.C., Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;(1) doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu E., Cheng S., Wang X., Hu D., Zhang T., Chu C. A systematic review of the investigation and management of close contacts of tuberculosis in China. J Public Health (Oxf) 2010;32(4):461–466. doi: 10.1093/pubmed/fdq032. [DOI] [PubMed] [Google Scholar]
- 9.Abebe D.S., Bjune G., Ameni G., Biffa D., Abebe F. Prevalence of pulmonary tuberculosis and associated risk factors in Eastern Ethiopian prisons. Int J Tuberc Lung Dis. 2011;15(5):668–673. doi: 10.5588/ijtld.10.0363. [DOI] [PubMed] [Google Scholar]
- 10.Kim L., Heilig C.M., McCarthy K.D. Symptom screen for identification of highly infectious tuberculosis in people living with HIV in Southeast Asia. J Acquir Immune Defic Syndr. 2012;60(5):519–524. doi: 10.1097/QAI.0b013e318256b3db. [DOI] [PubMed] [Google Scholar]
- 11.Rosen M.J. Chronic cough due to tuberculosis and other infections: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):197S–201S. doi: 10.1378/chest.129.1_suppl.197S. [DOI] [PubMed] [Google Scholar]
- 12.Lewis S.Z., Diekemper R.L., French C.T., Gold P.M., Irwin R.S., for the CHEST Expert Panel Methodologies for the development of the management of cough: CHEST guideline and expert panel report. Chest. 2014;146(5):1395–1402. doi: 10.1378/chest.14-1484. [DOI] [PubMed] [Google Scholar]
- 13.Balshem H., Helfand M., Schünemann H.J. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Andrews J.C., Schunemann H.J., Oxman A.D. GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Bastos L.G.V., Fonseca L.S., Mello F.C.Q., Ruffino-Netto A., Golub J.L., Conde M.B. Prevalence of pulmonary tuberculosis among respiratory symptomatic subjects in an out-patient primary health unit. Int J Tuberc Lung Dis. 2007;11(2):156–160. [PubMed] [Google Scholar]
- 16.Ngadaya E.S., Mfinanga G.S., Wandwalo E.R., Morkve O. Detection of pulmonary tuberculosis among patients with cough attending outpatient departments in Dar es Salaam, Tanzania: does duration of cough matter? BMC Health Serv Res. 2009;9:112. doi: 10.1186/1472-6963-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santha T., Garg G., Subramani R. Comparison of cough of 2 and 3 weeks to improve detection of smear-positive tuberculosis cases among out-patients in India. Int J Tuberc Lung Dis. 2005;9(1):61–68. [PubMed] [Google Scholar]
- 18.Menzies D., Alvarez G.G., Khan K. Treatment of latent tuberculosis infection. In: Menzies D., editor. Canadian Tuberculosis Standards. 7th ed. Public Health Agency of Canada; Ottawa, ON: 2014. pp. 125–152. [Google Scholar]
- 19.Kranzer K., Afnan-Holmes H., Tomlin K. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17(4):432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 20.Lawn S.D., Kerkhoff A.D., Vogt M., Ghebrekristos Y., Whitelaw A., Wood R. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54(8):1071–1079. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A.C., Golb J.E., Cavalcante S.C. Controlled trial of active tuberculosis case finding in a Brazilian favela. Int J Tuberc Lung Dis. 2010;14(6):720–726. [PMC free article] [PubMed] [Google Scholar]
- 22.Golub J.E., Mohan C.I., Comstock G.W., Chaisson R.E. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9(11):1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 23.Den Boon S., Verver S., Lombard C.J. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: the additional value of active case finding. Epidemiol Infect. 2008;136(10):1342–1349. doi: 10.1017/S0950268807000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luetkemeyer A.F., Firnhaber C., Kendall M.A., for the AIDS Clinical Trials Group A5295 and Tuberculosis Trials Consortium Study 34 Team Evaluation of Xpert MTB/RIF versus AFB smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis. 2016;62(9):1081–1088. doi: 10.1093/cid/ciw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Global Tuberculosis Report: 2013. Geneva, Switzerland: WHO Press. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed August 18, 2018.
- 26.Vassall A., van Kampen S., Sohn H. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS One. 2011;8(11):e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanifa Y., Fielding K.L., Charalambous S. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16(9):1252–1259. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann C.J., Variava E., Rakgokong M. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One. 2013;8(4):e62211. doi: 10.1371/journal.pone.0062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaCourse S.M., Cranmer L.M., Matemo D. Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J Acquir Immune Defic Syndr. 2016;71(2):219–227. doi: 10.1097/QAI.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi S., Cavanaugh J.S., Shiraishi R.W. Performance of clinical screening algorithms for tuberculosis intensified case finding among people living with HIV in western Kenya. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad Khan F., Verkuijl S., Parrish A. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28(10):1463–1472. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangaka M.X., Wilkinson R.J., Glynn J.R. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis. 2012;55(12):1698–1706. doi: 10.1093/cid/cis775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassin M.A., Datiko D.G., Tulloch O. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcomes in southern Ethiopia. PLoS One. 2013;8(5):e63174. doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Ochoa E., Brooks J.L., Matthys F., Caliste P., Armas L., van der Stuyft P. Pulmonary tuberculosis case detection through fortuitous cough screening during home visits. Trop Med Int Health. 2009;14(2):131–135. doi: 10.1111/j.1365-3156.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- 35.Valenca M.S., Scaini J.L.R., Abileira F.S., Goncalves C.V., von Groll A., Silva P.E.A. Prevalence of tuberculosis in prisons: risk factors and molecular epidemiology. Int J Tuberc Lung Dis. 2015;19(10):1182–1187. doi: 10.5588/ijtld.15.0126. [DOI] [PubMed] [Google Scholar]
- 36.Parija D., Patra T.K., Kumar A.M.V. Impact of awareness drives and community-based active tuberculosis case finding in Odisha, India. Int J Tuberc Lung Dis. 2014;18(9):1105–1107. doi: 10.5588/ijtld.13.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datiko D.G., Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS One. 2009;4(5):e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becerra M.C., Pachao-Torreblanca I.F., Bayona J. Expanding tuberculosis case detection by screening household contacts. Public Health Rep. 2005;120(3):271–277. doi: 10.1177/003335490512000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zachariah R., Spielmann M.P., Harries A.D. Passive versus active case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7(11):1033–1039. [PubMed] [Google Scholar]
- 40.Murray C.J.L., Styblo K., Rouillon A. Tuberculosis. In: Jamiston D.T., Mosley W.H., Measham A.R., Bobadilla J.L., editors. Disease Control Priorities in Developing Countries. Oxford University Press; New York, NY: 1993. pp. 233–259. [Google Scholar]
- 41.World Health Organization. Global Tuberculosis Control: Surveillance, Planning, Financing: WHO Report 2004. Geneva, Switzerland: World Health Organization. http://apps.who.int/iris/bitstream/10665/42889/2/9241562641.pdf. Accessed January 2, 2018.
- 42.Obermeyer Z., Abbott-Klafter J., Murray C.J.L. Has the DOTS strategy improved case finding or treatment success? PLoS One. 2008;3(3):e1721. doi: 10.1371/journal.pone.0001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav R.P., Nishikiori N., Satha P., Eang M.T., Lubell Y. Cost-effectiveness of a tuberculosis active case finding program targeting household and neighbourhood contacts in Cambodia. Am J Trop Med Hyg. 2014;90(5):866–872. doi: 10.4269/ajtmh.13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dye C., Watt C.J., Bleed D. Low access to a highly effective therapy: a challenge for international tuberculosis control. Bull World Health Organ. 2002;80(6):437–444. [PMC free article] [PubMed] [Google Scholar]
- 45.Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS One. 2009;4(5):e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbett E.L., Bandason T., Duong T. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376(9748):1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishikiori N., van Weezenbeek C. Target prioritization and strategy selection for active case-finding of pulmonary tuberculosis: a tool to support country-level project planning. BMC Public Health. 2013;13:97. doi: 10.1186/1471-2458-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramsay A., Al-Agbhari N., Scherchand J. Direct patient costs associated with tuberculosis diagnosis in Yemen and Nepal. Int J Tuberc Lung Dis. 2010;14(2):165–170. [PubMed] [Google Scholar]
- 49.Xu B., Diwan V.K., Bogg L. Access to tuberculosis care: what did chronic cough patients experience in the way of healthcare-seeking? Scand J Public Health. 2007;35(4):396–402. doi: 10.1080/14034940601160664. [DOI] [PubMed] [Google Scholar]
- 50.Proaño A., Bravard M.A., López J.W. Tuberculosis Working Group in Peru. Dynamics of cough frequency in adults undergoing treatment for pulmonary tuberculosis. Clin Infect Dis. 2017;64(9):1174–1181. doi: 10.1093/cid/cix039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki T, Tanaka Y, Watanabe H, et al. Cough- and sputum-related quality of life in pulmonary tuberculosis [abstract A5530]. Paper presented at: American Thoracic Society Conference; May 17, 2016; San Francisco, CA.
- 52.Prince D.S., Peterson D.D., Steiner R.M. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 53.Koh W.J., Jeong B.H., Jeon K. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012;142(6):1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 54.Raju B., Schluger N.W. Significance of respiratory isolates of Mycobacterium avium complex in HIV-positive and HIV-negative patients. Int J Infect Dis. 2000;4(3):134–139. doi: 10.1016/s1201-9712(00)90074-2. [DOI] [PubMed] [Google Scholar]
- 55.Research Committee of the British Thoracic Society First randomized trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax. 2001;56(3):167–172. doi: 10.1136/thorax.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins P.A., Campbell I.A., Banks J., Gelder C.M., Prescott R.J., Smith A.P. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunistic mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax. 2008;63(7):627–634. doi: 10.1136/thx.2007.087999. [DOI] [PubMed] [Google Scholar]
- 57.Clague H.W., El-Ansary E.H., Hopkins C.A., Roberts C. Pulmonary infection with opportunistic mycobacteria on Merseyside 1974-1983. Postgrad Med J. 1986;62(727):363–368. doi: 10.1136/pgmj.62.727.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubo K., Yamazaki Y., Hachiya T. Mycobacterium avium-intracellulare pulmonary infection in patients without known predisposing lung disease. Lung. 1998;176(8):381–391. doi: 10.1007/pl00007620. [DOI] [PubMed] [Google Scholar]
- 59.Dailloux M., Abalain M.L., Laurain C., for the French Mycobacteria Study Group Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. Eur Respir J. 2006;28(6):1211–1215. doi: 10.1183/09031936.00063806. [DOI] [PubMed] [Google Scholar]
- 60.Freitas R.M., Prado R., Prado F.L. Pulmonary paracoccidioidomycosis: radiology and clinical-epidemiological evaluation. Rev Soc Bras Med Trop. 2010;43(6):651–656. doi: 10.1590/s0037-86822010000600010. [DOI] [PubMed] [Google Scholar]
- 61.Gerson S.L., Talbot G.H., Lusk E., Hurwitz S., Strom B.L., Cassileth P.A. Invasive pulmonary aspergillosis in adult acute leukemia: clinical clues to its diagnosis. J Clin Oncol. 1985;3(8):1109–1116. doi: 10.1200/JCO.1985.3.8.1109. [DOI] [PubMed] [Google Scholar]
- 62.Mootsikapun P., Srikulbutr S. Histoplasmosis and penicilliosis: comparison of clinical features, laboratory findings and outcome. Int J Infect Dis. 2006;10(1):66–71. doi: 10.1016/j.ijid.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad T., Ahmed S.W., Hussain N., Rais K. Clinical profile and postoperative outcome in patients with simple and complex aspergilloma of lung. J Coll Physicians Surg Pak. 2010;20(3):190–193. [PubMed] [Google Scholar]
- 64.Baumgardner D.J., Halsmer S.E., Egan G. Symptoms of pulmonary blastomycosis: Northern Wisconsin, United States. Wilderness Environ Med. 2004;15(4):250–256. doi: 10.1580/1080-6032(2004)015[0250:sopbnw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Petersen L.R., Marshall S.L., Barton-Dickson C. Coccidioidomycosis among workers at an archeological site, Northeastern Utah. Emerg Infect Dis. 2004;10(4):637–642. doi: 10.3201/eid1004.030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andama A.O., den Boon S., Meya D., for the International HIV-associated Opportunistic Pneumonias (IHOP) Study Group Prevalence and outcomes of cryptococcal antigenemia in HIV-seropositive patients hospitalized for suspected tuberculosis in Uganda. J Acquir Immune Defic Syndr. 2013;63(2):189–194. doi: 10.1097/QAI.0b013e3182926f95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arango M., Castaneda E., Agudelo C.I., for the Colombian Histoplasmosis Study Group Histoplasmosis: results of the Colombian national survey, 1992-2008. Biomedica. 2011;31(3):344–356. doi: 10.1590/S0120-41572011000300007. [DOI] [PubMed] [Google Scholar]
- 68.Casotti J.A.S., Motta T.Q.R., Ferreira Junior C.U.G., Cerutti Jr C. Disseminated histoplasmosis in HIV positive patients in Espírito Santo state, Brazil: a clinical-laboratory study of 12 cases (1999-2001) Braz J Infect Dis. 2006;10(5):327–330. doi: 10.1590/s1413-86702006000500005. [DOI] [PubMed] [Google Scholar]
- 69.Deesomchok A., Tanprawate S. A 12-case series of Penicillium marneffei pneumonia. J Med Assoc Thai. 2006;89(4):441–447. [PubMed] [Google Scholar]
- 70.McAdams H.P., de Christenson M.R., Strollo D.C., Patz E.F., Jr. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol. 1997;168(6):1541–1548. doi: 10.2214/ajr.168.6.9168721. [DOI] [PubMed] [Google Scholar]
- 71.Mukae H., Taniguchi H., Matsumoto N. Clinicoradiologic features of pleuropulmonary Paragonimus westermani on Kyusyu Island, Japan. Chest. 2001;120(2):514–520. doi: 10.1378/chest.120.2.514. [DOI] [PubMed] [Google Scholar]
- 72.Moyou-Somo R., Kefie-Arrey C., Dreyfuss G., Dumas M. An epidemiologic study of pleuropulmonary paragonimiasis among pupils in the peri-urban zone of Kumba town, Meme Division, Cameroon. BMC Public Health. 2003;3:40. doi: 10.1186/1471-2458-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belizario V., Jr., Totanes F.I., Asuncion C.A. Integrated surveillance of pulmonary tuberculosis and paragonimiasis in Zamboanga del Norte, the Philippines. Pathog Glob Health. 2014;108(2) doi: 10.1179/2047773214Y.0000000129. 950-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rekka Devi K., Narain K., Mahanta J. Active detection of tuberculosis and paragonimiasis in the remote areas in North-Eastern India using cough as a simple indicator. Pathog Glob Health. 2013;107(3):153–156. doi: 10.1179/2047773213Y.0000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Odermatt P., Habe S., Manichanh S. Paragonimiasis and its intermediate hosts in a transmission focus in Lao People’s Democratic Republic. Acta Trop. 2007;103(2):108–115. doi: 10.1016/j.actatropica.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 76.Jeon K., Koh W.J., Kim H. Clinical features of recently diagnosed pulmonary paragonimiasis in Korea. Chest. 2005;128(3):1423–1430. doi: 10.1378/chest.128.3.1423. [DOI] [PubMed] [Google Scholar]
- 77.Diaz J.H. Boil before eating: paragonimiasis after eating raw crayfish in the Mississippi River Basin. J La State Med Soc. 2011;163(5):261–266. [PubMed] [Google Scholar]