Abstract
Background
Development of an accurate model to predict prognosis for patients with pancreatic neuroendocrine tumors (P-NETs) after surgical resection is urgently needed.
Methods
In the present study, we conducted Cox proportional hazards regression to identify critical prognostic factors for P-NETs by analyzing data from 2174 patients in the Surveillance, Epidemiology, and End Results (SEER) database. Based on the results of multivariate analysis, a novel nomogram was established. Finally, the novel nomogram for P-NETs was validated in a cohort of 81 patients from a Chinese institute.
Results
In the multivariate analysis, age, tumor location, American Joint Committee on Cancer (AJCC) stage, histologic grade, lymph node ratio (LNR) and tumor size were independent risk factors for overall survival (OS) in P-NET patients who underwent radical resection. A nomogram consisting of age, sex, AJCC stage and histologic grade was found to have a concordance index (C-index) of 0.79 for OS in the SEER database, which was significantly higher than the C-index based on the AJCC stage, European Neuroendocrine Tumor Society (ENETS) stage or histologic grade alone. In the validation cohort, the C-index based on the nomogram reached 0.78 for OS. We also defined high-risk (total points >13.5 based on the nomogram) and low-risk populations (total points <13.5 based on the nomogram) in the validation cohort. We found that the actual 5-year recurrence rate in the high-risk group was significantly higher than that in the low-risk group (80.8% vs 23.4%, P<0.001). Kaplan-Meier analysis showed that the 5-year recurrence-free survival (RFS) in the low-risk group was significantly higher than that in the high-risk group (P<0.001).
Conclusion
An AJCC stage- and histologic grade-based model was found to be extremely efficient in predicting survival for patients with P-NETs after surgical resection and deserves further evaluation for future clinical applications.
Keywords: nomogram, P-NET, AJCC stage, grading, overall survival
Introduction
Pancreatic neuroendocrine tumors (P-NETs), arising from cells of the neuroendocrine system, are relatively uncommon.1 Statistical data showed that the incidence of P-NETs has been increasing during the past few decades, which is partly due to improvement in detection.2 Indeed, P-NETs contribute to 1% of pancreatic cancer cases by incidence and 10% of pancreatic cancer cases by prevalence.3 One of the important clinical characteristics of P-NETs is the heterogeneity of the disease:4,5 1) P-NETs can be nonfunctional or functional, based on their origin from different cells; 2) patients with P-NETs may be asymptomatic or have completely different symptoms, as these tumors secrete different biogenic amines or neuropeptides; and 3) P-NETs can be an indolent or aggressive, with distinct biological behavior and prognosis. Thus, it is important to understand the biological and clinical characteristics of P-NET and how they affect the prognosis for specific individuals.
Currently, several staging or grading systems are used to define the extent of disease progression for P-NETs. Two staging systems were developed based on TNM status, namely, the European Neuroendocrine Tumor Society (ENETS) staging classification established by ENETS in 2006 and the American Joint Committee on Cancer (AJCC) staging classification established in 2010.6,7 In comparison with these two staging systems, the 2010 World Health Organization (WHO) classification interprets the grading features for P-NETs.8 However, these staging or grading systems have some limitations in predicting patient prognosis. For example, studies found patients with stage I disease have similar prognosis to patients with stage IIA disease based on the ENETS staging system.
In recent years, few studies have attempted to develop an improved model to predict the prognosis for P-NETs.9,10 However, all of these studies analyzed data from one institute, and none of the models was validated.9,10 In the current study, we identified important prognostic factors and established a novel prognostic nomogram for P-NETs following surgical resection based on the Surveillance, Epidemiology, and End Results (SEER) database, which contains information from a large population. In addition, the novel nomogram was validated using data from a Chinese pancreatic surgery center.
Methods
Patient population and study design
SEER cohort
Data from patients with pathologically confirmed P-NETs were retrieved from the SEER database (from 1988 to 2014) using SEER*Stat version 8.3.4 software. Patient information was uniformly reviewed. Cases were included only if radical resection had been performed. The exclusion criteria were as follows: patients who died within 90 days after operation; patients with unknown TNM stage, unknown tumor size, or unknown tumor grade by pathologic examination; patients with no complete parameters to calculate the metastatic lymph node ratio (LNR), determined using the number of positive regional lymph nodes divided by the number of examined regional lymph nodes. Based on these inclusion and exclusion criteria, 2174 P-NET cases were finally included in the study. Baseline clinicopathologic characteristics including age, sex, TNM stage, tumor location, and histologic grade assessed by tissue differentiation based on hematoxylin and eosin (HE) staining were recorded (Table 1).
Table 1.
Important parameters analyzed in the study
| Parameter | Description |
|---|---|
| AJCC stage | 7th edition, American Joint Committee on Cancer, 2010 |
| ENETS stage | 1st edition, European Neuroendocrine Tumor Society, 2006 |
| WHO grade Histologic grade LNR |
3rd edition, World Health Organization, 2010 Assessed by tissue differentiation based on HE staining Lymph node ratio, calculated using the number of positive regional lymph nodes divided by the number of examined regional lymph nodes |
For detailed searching methods, patient data were collected based on the International Classification of Diseases for Oncology, 2nd and 3rd edition (ICD-O-2/3), for tumors of the pancreas: C25.0–C25.9. The following ICD-O-3 diagnoses were used to identify P-NETs: islet cell adenocarcinoma (8,150), malignant beta-cell tumor (8,151), malignant alpha-cell tumor (8,152), G-cell tumor (8,153), VIPoma (8,155), malignant somatostatinoma (8,156), malignant enteroglucagonoma (8,157), carcinoid tumor (8,240), argentaffin carcinoid tumor (8,241), enterochromaffin cell tumor (8,242), mucocarcinoid tumor (8,243), neuroendocrine carcinoid (8,246), and atypical carcinoid tumor (8,249). TNM information was gathered based on the following codes: 6th edition of the AJCC staging system (2004+), 7th edition if the AJCC staging system (2010+), SS1977 (2004+), collaborative stage (CS) tumor size 2004, CS extension 2004, CS lymph nodes 2004, CS metastases at dx 2004, extent of disease (EOD) 10—extent (1988–2003), EOD 10—nodes (1988–2003), and EOD 10—size (1988–2003).
Validation cohort
A total of 81 P-NET patients from Guangdong Provincial People’s Hospital who met the above inclusion criteria were selected to conduct external validation. Patient clinicopathologic characteristics and clinical prognosis were recorded.
This clinical study was performed strictly according to the declaration of Helsinki and was approved by the ethics committee of Guangdong Provincial People’s Hospital. Written informed consent was obtained from each patient for medical record review and data collection in the medical research.
Statistical analyses
Survival time was calculated from the date of initial diagnosis to the date of death or last follow-up. Recurrence-free survival (RFS) in the validation cohort was verified using Kaplan-Meier curves. Multivariate analysis was performed using Cox proportional hazards regression to evaluate potential risk factors, including age, sex, tumor location, TNM stage, histologic grade, tumor size and LNR. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Cutoff values for tumor size and the LNR were defined according to receiver operating characteristic (ROC) curve analyses. Statistical analysis was accomplished using SPSS 22.0 software (https://www.ibm.com/). For all analyses, P-values <0.05 were defined as statistically significant.
A nomogram based on the results of multivariate analysis was constructed using the rms package in R version 3.4.1 (http://www.r-project.org/). The property of the nomogram was assessed using the concordance index (C-index) and evaluated by comparing nomogram-predicted and observed Kaplan-Meier estimates of survival probability. The bootstraps used for these activities included 1000 resamples. The Rcorrp.cens package in R was used to compare the nomogram and other models. The comparisons were assessed by the C-indexes. A higher C-index indicated greater accuracy for prognostic prediction. To perform external validation of the nomogram, we calculated the total scores for each patient in the validation cohort according to the nomogram. The scores were used as a factor to complete Cox regression analyses for this cohort. Finally, C-indexes and calibration curves were derived based on the Cox regression analysis.
Results
Patient characteristics and survival
In the SEER cohort, a total of 2174 patients were selected according to the inclusion and exclusion criteria. To circumvent the effect of surgical complications on survival, we excluded patients who died within 90 days after surgery. Similarly, 81 patients who underwent radical resection in Guangdong General Hospital were assigned to the validation cohort. Clinicopathologic characteristics in the two cohorts regarding age, sex, tumor size, TNM stage, histologic grade, WHO grade, etc. are listed in Table 2. The WHO grading information for patients was not included in the SEER database. The median and mean follow-up durations were 27 and 39.6 months in the SEER cohort and 60 and 70 months in the validation cohort, respectively. The 3-year and 5-year patient survival rates in the SEER cohort were 85.6% and 72.1%, respectively. For the validation cohort, the 3-year and 5-year patient survival rates were 92.6% and 85.4%, and the 3-year and 5-year RFS rates were 84.2% and 70.3%, respectively.
Table 2.
Clinicopathologic characteristics of P-NET patients in the SEER cohort and validation cohort
| Parameter | SEER cohort (N=2174) | Validation cohort (N=81) |
|---|---|---|
| N (%) | N (%) | |
| Age, years | ||
| ≤60 | 1218 (56) | 63 (78) |
| >60 | 956 (44) | 18 (22) |
| Sex | ||
| Male | 1154 (53) | 49 (60) |
| Female | 1020 (47) | 32 (40) |
| Race | ||
| Caucasian | 1741 (80) | 0 |
| Black | 229 (11) | 0 |
| Other and Unknown | 204 (9.4) | 81 (100) |
| Location | ||
| Head | 692 (32) | 32 (40) |
| Body and Tail | 1103 (51) | 44 (54) |
| Other | 379 (17) | 5 (6) |
| Histologic grade* | ||
| I | 1552 (71) | 37 (46) |
| II | 427 (20) | 31 (38) |
| III or IV | 195 (9.0) | 13 (16) |
| WHO grade | ||
| I | - | 45 (56) |
| II | - | 27 (33) |
| III | - | 9 (11) |
| Stage (AJCC 7th edition) | ||
| I | 976 (45) | 47 (58) |
| II | 810 (37) | 27 (33) |
| III | 45 (2.1) | 1 (1) |
| IV | 343 (16) | 6 (7) |
| Stage (ENETS) | ||
| I | 446 (21) | — |
| II | 569 (26) | — |
| III | 816 (38) | — |
| IV | 343 (16) | — |
| Size (mm) | ||
| <34.5 | 1178 (54) | 49 (60) |
| >34.5 | 996 (46) | 32 (40) |
| LNR | ||
| 0 | 1343 (62) | 73 (90) |
| <0.33 | 475 (22) | 2 (3) |
| >0.33 | 356 (16) | 6 (7) |
| Functional status | ||
| Functional | 278 (13) | 34 (42) |
| Nonfunctional | 1896 (87) | 47 (58) |
Notes: *Histologic grade assessed by tissue differentiation based on HE staining. Abbreviations: AJCC, American Joint Committee on Cancer; ENETS, European Neuroendocrine Tumor Society.
Independent prognostic factors analyzed in the SEER cohort
To identify the critical prognostic factors for P-NETs in a large population, we performed multivariate analysis using the SEER cohort. We used AJCC stages in the regression model instead of ENETS stages because AJCC stages have been shown to have better prognostic value than ENETS stages by the Kaplan-Meier analysis (data not shown). The multivariate analysis results indicated age, tumor location, AJCC stage, histologic grade, LNR and tumor size as independent risk factors for overall survival (OS) in P-NET patients who underwent radical resection (Table 3). In contrast, sex, race and tumor functional status were not statistically significant and were thus not prognostic factors.
Table 3.
Multivariate analysis of prognostic factors in the SEER cohort
| Parameter | HR (95% CI) | P-value |
|---|---|---|
| Age, years | ||
| ≤60 | 1 | |
| >60 | 1.41 (1.09–1.83) | 0.009 |
| Sex | ||
| Male | 1 | |
| Female | 0.80 (0.62–1.04) | 0.090 |
| Location | ||
| Head | 1 | |
| Body and Tail | 0.57 (0.42–0.77) | <0.001 |
| Other | 0.83 (0.58–0.33) | 0.328 |
| Histologic grade* | ||
| I | 1 | |
| II | 1.50 (1.08–2.08) | 0.017 |
| III or IV | 4.47 (3.30–6.50) | <0.001 |
| Stage (AJCC 7th edition) | ||
| I | 1 | |
| II | 1.58 (0.97–2.59) | 0.068 |
| III | 2.52 (1.18–5.40) | 0.017 |
| IV | 4.57 (2.79–7.53) | <0.001 |
| Size (mm) | ||
| <34.5 | 1 | |
| >34.5 | 1.72 (1.26–2.35) | 0.001 |
| LNR | ||
| 0 | 1 | |
| <0.33 | 1.14 (0.79–1.65) | 0.448 |
| >0.33 | 1.45 (1.02–2.06) | 0.037 |
Notes: *Histologic grade assessed by tissue differentiation based on HE staining. Abbreviations: CI, confidence interval; HR, hazard ratio; AJCC, American Joint Committee on Cancer; ENETS, European Neuroendocrine Tumor Society.
A novel prognostic nomogram for OS
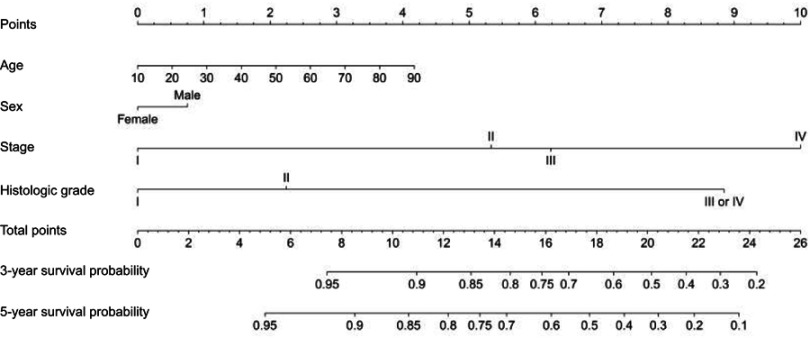
Based on the results of multivariate analysis, we integrated the significant prognostic factors in different combinations to establish a satisfactory nomogram for P-NETs (Table 4). Sex was also included as a baseline parameter. We found that a nomogram consisting of age, sex, AJCC stage and histologic grade was appropriate (Figure 1). The C-index of the nomogram for OS prediction was 0.79 (95% CI 0.74–0.83). Addition of other risk factors, including tumor location, LNR and tumor size, did not significantly improve the C-index (Table 4). For calibration, the 3-year and 5-year survival probabilities showed an optimal agreement between nomogram prediction and actual observation (Figure 2).
Table 4.
Comparison of different models for OS prediction in the SEER cohort
| Model | C-index | 95% CI |
|---|---|---|
| Age + Sex + AJCC stage + Histologic grade | 0.79 | 0.74–0.83 |
| Age + Sex + AJCC stage + Histologic grade + LNR | 0.79 | 0.74–0.83 |
| Age + Sex + Histologic grade + LNR + Size | 0.77 | 0.73–0.82 |
| AJCC stage only | 0.71 | 0.67–0.75 |
| ENETS stage only | 0.70 | 0.65–0.74 |
| Histologic grade only | 0.72 | 0.67–0.74 |
Abbreviations: C-index, concordance index; CI, confidence interval; OS, overall survival.
Figure 1.
Prognostic nomogram for P-NET. This nomogram consists of eight lines. The first line is the point line for each variable axis. Then the next four lines are variable axes, including “Age”, “Sex”, “Histologic Grade” and “Stage”. For an individual patient, a vertical line is made between each variable axe and the point line, which determines the number of point for each variable. After summing these points to get the total points, a vertical line can be drawn between the “Total Points” line and “3-year survival probability” or “5-year survival probability” line to determine the probability of 3 or 5-year survival, respectively. Stage, American Joint Committee on Cancer (AJCC) staging classification established by AJCC in 2010; Histologic Grade is assessed by tissue differentiation based on HE staining.
Figure 2.

The calibration curve for predicting patient survival at 3 years (A) and 5 years (B) in the SEER cohort. Nomogram-predicted probability of overall survival is labeled on the x-axis; actual overall survival is labeled on the y-axis.
To further evaluate the benefit of the novel model, we compared the predictive power for patient prognosis between the nomogram and other single prognostic factors using the SEER cohort. The C-index for OS prediction was 0.72 based on histologic grade, which was significantly lower than the C-index based on the nomogram (0.79, P<0.01). Moreover, the C-index using the nomogram (0.79) was significantly higher than the C-index using staging systems, including the AJCC staging system (C-index=0.71, P<0.001) and the ENETS staging system (C-index=0.70, P<0.001). Therefore, the novel nomogram established in the study showed higher predictive power for OS than any other prognostic factors or widely used staging system.
Validation of the novel nomogram for OS and RFS
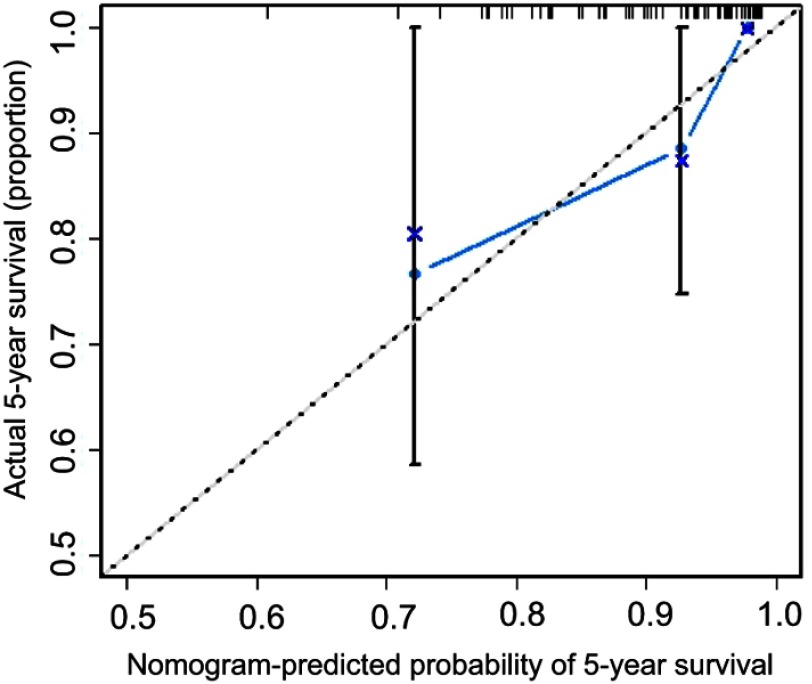
We conducted a validation study using patient data from Guangdong General Hospital. The C-index for the nomogram in the validation cohort was 0.78 (95% CI, 0.70 to 0.95). The calibration curve showed good agreement between nomogram prediction and actual observation for the 5-year survival probability (Figure 3). In addition, we calculated the C-index for AJCC stage, histologic grade and WHO grade using the validation cohort, which were 0.60, 0.68 and 0.69, respectively. These values were significantly lower than the C-index for the nomogram (P<0.001 vs AJCC stage; P<0.001 vs histologic grade; and P<0.001 vs WHO grade). These findings indicate that our novel nomogram had a strong predictive power for OS in the validation cohort.
Figure 3.
The calibration curve for predicting patient survival at 5 years in the validation cohort. Nomogram-predicted probability of overall survival is labeled on the x-axis; actual overall survival is labeled on the y-axis.
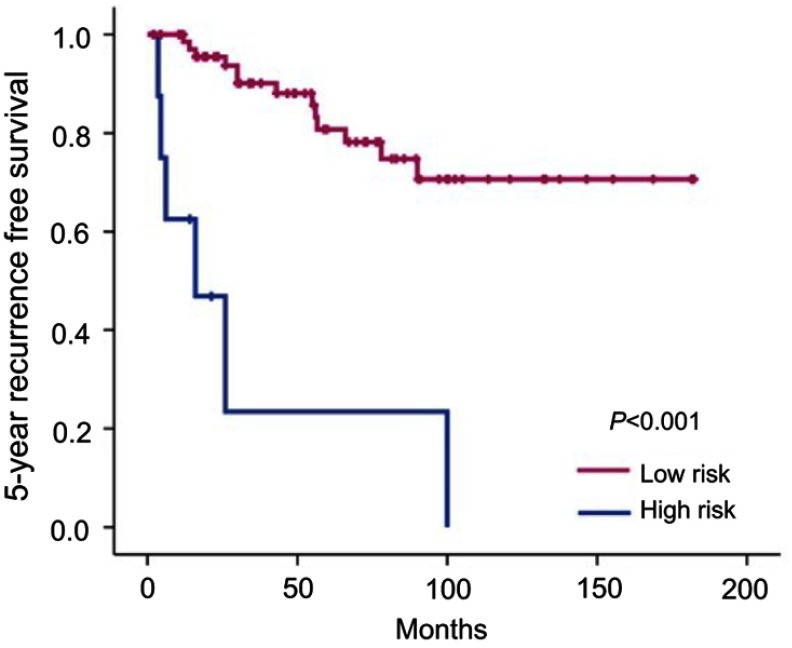
To investigate whether the calculated score based on the nomogram is associated with RFS, we assessed the total points for each patient in the validation cohort based on the nomogram. Patients in the validation cohort were divided into a high- and low-risk group based on the predicted 5-year survival probability. Since the 5-year survival rate of P-NET patients following surgery is reported to be approximately 75%,6 we defined low risk as a 5-year survival probability of more than 75% (total points <13.5 based on the nomogram) and high risk as a 5-year survival probability of less than 75% (total points >13.5 based on the nomogram). We found that the actual 5-year recurrence rate in the high-risk group was significantly higher than that in the low-risk group (80.8% vs 23.4%, P<0.001). Finally, we performed Kaplan-Meier analysis to evaluate RFS in the high- and low-risk groups. Indeed, the 5-year RFS in the low-risk group was significantly higher than that in the high-risk group (P<0.001, Figure 4). Therefore, the current nomogram is valuable in predicting both OS and RFS in the validation cohort.
Figure 4.
Kaplan-Meier curves showing the 5 year RFS in high risk and low risk group in the validation cohort. High risk was defined as total points >13.5 by the nomogram and low risk as total points <13.5 by the nomogram.
Discussion
P-NET is a heterogeneous disease that may result in different prognoses in different patients even after radical resection. Many studies have attempted to identify factors to accurately predict patient prognosis.11 However, most studies lack representativeness because they are based on small samples from single institutes. In the current study, by analyzing a large population in the SEER database, we identified critical prognostic factors for P-NETs. Importantly, we established a novel nomogram to help predict patient survival following surgical resection.
There is no doubt that higher age at diagnosis, more advanced stage and higher histologic grade are strongly associated with worse survival, as shown in our study and others.12,13 Nevertheless, there is controversy regarding whether functional and nonfunctional tumors have different impacts on prognosis.14 Some investigators argue that functional tumors have more favorable prognosis than nonfunctional tumors, based on observed differences in their studies.15 However, unlike functional tumors, nonfunctional tumors tend to be diagnosed during later life because no symptoms are observed in the initial stages of disease.14 Thus, nonfunctional tumors may be larger or have more advanced stage or grade than functional tumors in some studies, which can lead to some bias.16 In our study, no significant difference was observed regarding tumor size, stage or grade between functional and nonfunctional P-NETs (data not shown). Multivariate analysis showed that the functional status was not an independent prognostic factor for P-NETs. Thus, we concluded that the functional status had no effect on P-NET patient survival, which was consistent with other studies.17,18
At present, two staging systems and the WHO grading system are widely used in the clinic to help evaluate disease progression for P-NETs. For the first time, we combined the AJCC staging and histologic grading parameters to develop a novel nomogram for OS in P-NET patients. We established the novel model by integrating age, sex, AJCC stage and tumor histologic grade. The nomogram was proven to have the following advantages: 1) it has a higher predictive power than AJCC or ENETS stage alone, which is shown in both the SEER cohort and the validation cohort; 2) it is a simple model to calculate and to be used in the clinic, as it only contains four parameters all of which are easy to obtain; 3) it can be used to define a high-risk population, with total points >13.5; and 4) it was developed using a western database and validated using an eastern database, which indicates that it is applicable across races. Therefore, the novel nomogram established in our study has very good predictive power. We recommend that patients with higher total points (>13.5 based on the nomogram) should be closely followed up due to a high risk of recurrence. Further, it will be interesting to investigate whether adjuvant therapy following surgical resection is beneficial for these high-risk patients in future studies.
Lymph node metastasis is an important prognostic factor associated with disease progression.19 Conventionally, the number of positive lymph nodes is used to define the status of lymph node metastasis, as in the AJCC and ENETS staging systems. However, in recent years, the LNR has been shown to be a more powerful prognostic factor than the number of positive lymph nodes in P-NETs.20 Our study also proved that the LNR was an independent prognostic factor for P-NETs based on multivariate analysis. Unfortunately, addition of the LNR to our model (C-index=0.79) did not significantly improve the predictive power, suggesting that although the number of positive lymph nodes included in the AJCC stage has value for the nomogram, the LNR provides no additional advantage.
In recent years, two studies developed predictive nomograms for P-NETs based on data from single institutes. Ellison et al demonstrated that a nomogram consisting of age, sex, and Ki-67 expression has a C-index of 0.74.9 In another study, Ye et al described a nomogram based on two factors, mitotic rate and functional status.10 Nonetheless, these studies had small sample sizes, and the models they developed were not validated in other populations. Despite their limitations, both studies suggested that grading information is important for predicting prognosis in P-NET patients, consistent with our results. The difference is that the other studies used Ki-67 expression or mitotic rate to represent grading information, whereas we used histologic grade assessed by tissue differentiation based on HE staining. Although we could not analyze the significance of Ki-67 expression or mitotic rate in our study due to limited information in the SEER database, it will be interesting in the future to see which of these factors alone or in combination has the greatest power for predicting survival in P-NET patients.
There are some limitations in our study, which is retrospective in nature based on the SEER database. One of the limitations is that information on tumor recurrence after surgical resection is not provided in the SEER database. This may have led to some limitations in evaluating outcomes for P-NET. Second, we did not evaluate the validity of the WHO grading system, also due to the lack of data in the SEER database. Third, the surgical information provided by the SEER database is rough, especially for patients with distant metastasis (AJCC stage IV). Although a fraction of patients were reported to undergo surgery for metastatic disease, the details regarding radical or palliative procedures are not provided, which might lead to bias. In addition, as a missing piece of data, the effects of genetic heterogeneity or syndrome on the prognosis of P-NETs have not been well evaluated. A good predictive model should consider multidimensional information regarding the tumor, microenvironment and host. Future studies are needed to address these issues.
In conclusion, radical resection is the only curative treatment for P-NET patients. However, distinct clinical and molecular characteristics in different individuals lead to different outcomes after curative resection. We described a novel nomogram combining the AJCC stage and histologic grade, which has a satisfactory predictive power for P-NETs following surgical resection and might be a good model for application in the clinic. Based on these findings, more molecular and genetic information concerning the heterogeneity of P-NETs should be explored to facilitate individualized treatment for P-NETs in the future.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (project No. 81702783 and 81672475) and the Natural Science Foundation of Guangdong Province (project No. 2017A030310574)
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, Yao J. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol. 2011;4:29. doi: 10.1186/1756-8722-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han X, Zhang C, Tang M, et al. The value of serum chromogranin A as a predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. Eur J Gastroenterol Hepatol. 2015;27:527–535. doi: 10.1097/MEG.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 6.Luo G, Javed A, Strosberg JR, et al. Modified staging classification for pancreatic neuroendocrine tumors on the basis of the American Joint Committee on Cancer and European Neuroendocrine tumor society systems. J Clin Oncol. 2017;35:274–280. doi: 10.1200/JCO.2016.67.8193 [DOI] [PubMed] [Google Scholar]
- 7.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–833. doi: 10.1038/modpathol.2010.58 [DOI] [PubMed] [Google Scholar]
- 8.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e [DOI] [PubMed] [Google Scholar]
- 9.Ellison TA, Wolfgang CL, Shi C, et al. A single institution’s 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg. 2014;259:204–212. doi: 10.1097/SLA.0b013e31828f3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye L, Ye H, Zhou Q, et al. A retrospective cohort study of pancreatic neuroendocrine tumors at single institution over 15 years: new proposal for low- and high-grade groups, validation of a nomogram for prognosis, and novel follow-up strategy for liver metastases. Int J Surg. 2016;29:108–117. doi: 10.1016/j.ijsu.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 11.Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–7803. doi: 10.1158/1078-0432.CCR-08-0734 [DOI] [PubMed] [Google Scholar]
- 12.Wei IH, Harmon CM, Arcerito M, Cheng DF, Minter RM, Simeone DM. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann Surg. 2014;260:1088–1094. doi: 10.1097/SLA.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29:3044–3049. doi: 10.1200/JCO.2011.35.1817 [DOI] [PubMed] [Google Scholar]
- 14.Wang SE, Su C-H, Kuo Y-J, et al. Comparison of functional and nonfunctional neuroendocrine tumors in the pancreas and peripancreatic region. Pancreas. 2011;40:253–259. doi: 10.1097/MPA.0b013e3181f94cc4 [DOI] [PubMed] [Google Scholar]
- 15.Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: review of 125 patients. J Gastrointest Surg. 1998;2:472–482. [PubMed] [Google Scholar]
- 16.Sallinen V, Haglund C, Seppanen H. Outcomes of resected nonfunctional pancreatic neuroendocrine tumors: do size and symptoms matter? Surgery. 2015;158:1556–1563. doi: 10.1016/j.surg.2015.04.035 [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum DJ, Turrini O, Ewald J, et al. Pancreatic neuroendocrine tumor: a multivariate analysis of factors influencing survival. Eur J Surg Oncol. 2014;40:1564–1571. doi: 10.1016/j.ejso.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 18.Fischer L, Kleeff J, Esposito I, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Brit J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051 [DOI] [PubMed] [Google Scholar]
- 19.Conrad C, Kutlu OC, Dasari A, et al. Prognostic value of lymph node status and extent of lymphadenectomy in pancreatic neuroendocrine tumors confined to and extending beyond the pancreas. J Gastrointest Surg. 2016;20:1966–1974. doi: 10.1007/s11605-016-3243-7 [DOI] [PubMed] [Google Scholar]
- 20.Liu P, Zhang X, Shang Y, et al. Lymph node ratio, but not the total number of examined lymph nodes or lymph node metastasis, is a predictor of overall survival for pancreatic neuroendocrine neoplasms after surgical resection. Oncotarget. 2017;8:89245–89255. doi: 10.18632/oncotarget.19184 [DOI] [PMC free article] [PubMed] [Google Scholar]