Abstract
Purpose
Particulate matter (PM) has been implicated as a risk factor for airway injury. However, the molecular mechanisms remain largely unclear. The goal of this study was to determine whether sirtuin1 (SIRT1), an anti-inflammatory and antiaging protein, protects against PM-induced airway inflammation.
Methods
The effect of SIRT1 on PM-induced airway inflammation was assessed by using in vivo models of airway inflammation induced by PM and in vitro culture of human bronchial epithelial (HBE) cells exposed to PM, resveratrol (SIRT1 activator), or both.
Results
PM-stimulated HBE cells showed a significant decrease in SIRT1 but a notable increase in inflammatory cytokines. SIRT1 gene silencing further enhanced PM-induced expression of inflammatory cytokines. In contrast, resveratrol, a SIRT1 activator, reduced the expression of these cytokines compared with the control cells. In vivo, SIRT1 expression was significantly decreased in lung tissues of PM-exposed mice. Interestingly, resveratrol treatment reversed the enhanced total cells, neutrophils and inflammatory cytokines in PM-induced mice. Moreover, SIRT1 mediated PM-induced inflammatory cytokines expression at least partly through MAPK pathways.
Conclusion
These findings suggest that SIRT1 is involved in the pathogenesis of PM-induced airway inflammation and activation of SIRT1 could prevent airway disorders or disease exacerbations induced by airborne particulate pollution.
Keywords: particulate matter, SIRT1, resveratrol, airway inflammation
Introduction
Ambient air pollution exposure, especially particulate matter (PM), is known to a major risk factor for public health. PM is a widespread air pollutant, which is a mixture of solid particles and liquid droplets in the air.1 After inhalation of particles, the lung is the primary target of airborne pollutants. Epidemiological studies have shown that PM exposure is associated with increased incidence of respiratory diseases such as chronic obstructive pulmonary disease (COPD), asthma and lung cancer.2 However, the detailed mechanisms mediating the adverse effects of PM in these diseases have not yet been fully clarified. Thus, there is an urgent need to find the molecular mechanisms and therapeutic targets in PM-induced airway injury.
Sirtuin 1 (SIRT1), a member of the sirtuin (class III histone deacetylase) family, deacetylates intracellular targets, including transcription factors, signaling molecules, and histones. SIRT1 plays an important role in many pathophysiological processes, including cellular senescence/aging, inflammation, apoptosis/proliferation, and autoimmunity.3 Recent studies have demonstrated that the level and activity of SIRT1 are reduced in cells in vitro and in mouse lungs in vivo exposed to cigarette smoke as well as in lungs of patients with COPD.4 SIRT1 protects against emphysema through forkhead transcription factor (FOXO) 3-mediated reduction of cellular senescence, independently of inflammation.5 SIRT1 suppresses acute lung inflammation during sepsis by controlling inflammasome activation pathway.6 SIRT1 exerted a protective effect against PM2.5-induced oxidative damage by regulating the expression of FOXO3a.7 Moreover, SIRT1 controls coagulation after PM exposure.8 These studies indicated that SIRT1 mediated the pathogenesis of PM-induced diseases. Resveratrol (3,5,4′-trihydroxystilbene), one of dietary polyphenols found in veratrum grandiflorum and richly present in grapes, wine, peanuts, soy, and berries, has been reported to exhibit various bioactivities including antioxidant anti-tumorigenic, and anti-angiogenic effects.9 Recently, SIRT1 has proven to be activated by Resveratrol.9 However, the functions and the detailed mechanisms of how SIRT1 acts in PM-related pulmonary disorders and whether resveratrol protect again PM-induced pulmonary diseases are still unknown.
In the present study, we aimed to investigate the functions of SIRT1 in the regulation of PM-induced inflammation in vitro and in vivo. Our study demonstrated that SIRT1 protects against PM-induced airway inflammation at least partly via MAPK pathways and might suggest that activation of SIRT1 could prevent airway disorders or disease exacerbations induced by airborne particulate pollution.
Materials and methods
Preparation of particle matter samples
Standard reference airborne PM (average diameter: 10.5 μm), which primarily contains polycyclic aromatic hydrocarbons, was purchased from National Institute of Standards and Technology (NIST) Company. PM was suspended and sonicated in phosphate buffered saline (PBS) or saline at a final concentration at 2 μg/μL (mass/volume).
Cell cultures
The human bronchial epithelial (HBE) cells, purchased from American Type Culture Collection (CRL-2741), were used in this study. The cells were grown in RPMI 1640 with 10% fetal bovine serum (FBS), and were routinely maintained at 37°C in a water-saturated atmosphere with 5% CO2. The cells were treated with standard reference airborne PM (standard reference material 1649b, obtained from National Institute of Standards and Technology, Gaithersburg, MD, USA) and resveratrol for 24 h. HBE cells were treated with four concentrations of PM (25, 50, 75, 100 μg/mL) for 24 h or with PM (100 μg/mL) for four times (3, 6, 12, 24 h).
Animal experiments
Male C57BL/6 mice (6–8 week old) were purchased from the Animal Center of Slaccas (Shanghai, China) and were maintained in the animal facility of the laboratory animal center of Guangdong Medical University. PM was suspended and sonicated in saline at 100 μg PM (in 50 μL saline) per day by intratracheal instillation for 3 days. The dose of PM used for the in vitro studies were according the previous studies.10 SIRT1 activator resveratrol (100 mg/kg) was injected intraperitoneally before PM challenge. Control mice received the same volume of saline. Experiments were conducted under protocols using experimental procedures and anesthesia methods approved by the Animal Care and Use Committee at Guangdong Medical University. Our research was also approved by this committee.
Chemical reagents
Antibodies against SIRT1 (Abcam, MA, USA, Catalog #110304), GAPDH (Sigma-Aldrich, Catalog #SAB2108266), p38 MAPK (CST, MA, USA, Catalog #8690), p-p38 MAPK (CST, MA, USA, Catalog #4511), p-ERK1/2 (CST, MA, USA, Catalog #4370), and ERK (CST, MA, USA, Catalog #4695), Resveratrol (Beyotime Biotechnology, Catalog #SC0276) were used. The specific inhibitor of p38 (SB203580, Selleck.cn, Catalog #S1076) was purchased from Selleck (USA). The specific inhibitor of ERK (U0126,) was purchased from CST(MA, USA, Catalog #9900).
SiRNA/plasmid studies
Two independent siRNAs for knockdown of SIRT1 and a negative control siRNA (scRNA) were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China) and used in this experiment. Nucleotide sequences for siRNAs used were as follows: SIRT1 siRNA-1, 5′-GCGGGAAUCCAAAGGAUAATTUUAUCCUUUGG
AUUCCCGCTT-3′; SIRT1siRNA-2, 5′-GGAGAUGAUAAGAGGCAATT UUGCC
UCUUGAUCAUCUCCTT-3′ and negative control siRNA, sense 5′-UUCUCCGAAC
GUGUCACGUTT-3′, abtisense 5′-ACGUGACACGUUCGGAGAATT-3′. Either SIRT1 siRNA or scRNA was transfected to cells using lipofectamine®3000 (Invitrogen), according to the manufacturer’s protocols with the following minor modifications. Briefly, scRNA or SIRT1siRNA (200 nM) was incubated with 5 μL of lipofectamine®3000 reagent in 250 μL Opti-MEM (GIBCO) for 5 min.
In the plasmid studies, the cells were transfected with SIRT1 plasmid with lipofectamine®3000 reagent when they reached 90% confluence, according to the manufacturer’s protocols. 24 h after plasmid transfection, the transfected cells were exposed to PM.
RNA isolation and quantitative real-time PCR analysis
Total RNA from cells and lung tissues was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The resulting cDNA was used for real time RT-PCR using SYBR Green Master Mix (Takara Biotechnology, Shiga, Japan) on the LightCycler®480II System (Roche Molecular Diagnostics, Pleasanton, CA). The primers are presented in Table 1.
Table 1.
Primer sets for RT-PCR analysis assay
| Genes | Forward | Reverse |
|---|---|---|
| IL-6 (h) | 5ʹ-CCTGAACCTTCCAAAGATGGC-3’ | 5ʹ-TTCACCAGGCAAGTCTCCTCA-3’ |
| IL-8 (h) | 5ʹACTGAGAGTGATTGAGAGTGGAC-3’ | 5ʹAACCCTCTGCACCCAGTTTTC-3’ |
| IL-17A (h) | 5ʹ-TCCCACGAAATCCAGGATGCC-3’ | 5ʹ-CACAGTGGTCCTTCCAGGTT-3’ |
| MUC5AC (h) | 5ʹ-CTTACTCCACCCAAACCTGC-3’ | 5ʹ-CTGTCAACCCCTCTGACCAC-3’ |
| SIRT1 (h) | 5ʹ-GGAGGAGGCATTCGGAAAG-3’ | 5ʹ-TCGTTGGTGTCACACAGAT-3’ |
| GAPDH (h) | 5ʹ-TGTTGCCATCAATGACCCCTT-3’ | 5ʹ-CTCCACGACGTACTCAGCG-3’ |
| IL-6 (m) | 5ʹ-TCTATACCACTTCACAAGTCGGA-3’ | 5ʹ-GAATTGCCATTGCACAACTCTTT-3’ |
| IL-8 (m) | 5ʹ-AAGGCTGGTCCATGCTCT-3’ | 5ʹ-CACAGACATCGTAGCTCTTGAGTG-3’ |
| IL-17A (m) | 5ʹ-TCAGCGTGTCCAAACACTGAG-3’ | 5ʹ-CGCCAAGGGAGTTAAAGACTT-3’ |
| MUC5AC (m) | 5ʹ-CTGTGACATTATCCCATAAGCCC-3’ | 5ʹ-AAGGGGTATAGCTGGCCTGA-3’ |
| SIRT1 (m) | 5ʹ-CACTGTAACTGGGGGCAACT-3’ | 5ʹ-CACTTCTTGTCAGCGTCGAA-3’ |
Abbreviations: h, Human; m, Mouse.
Western blot analysis
Cells lysates and lung tissue homogenates were lysed in RIPA buffer containing protease and phosphatase inhibitors after PM exposed. We prepared total cell lysates by lysing the cells in sodium dodecyl sulfate polyacrylaminde gel electrophoresis (SDS-PAGE) sample loading buffer. Lysates were run on gels and immunoblotted with relevant antibodies using standard methods. GAPDH was used as internal controls for Western blot analysis.
Immunofluorescence
The localization of SIRT1 in the HBE cells was investigated by immunofluorescence. The antibodies that were used in appropriate combinations were rabbit anti-human SIRT1 antibodies (Abcam, MA, USA, Catalog #110304).
Immunohistochemistry
The slides were incubated with either a mouse polyclonal against SIRT1 (Proteintech, Catalog #60303–1-Ig). Quantitative measurements of SIRT1 positive cells in the lung tissue were performed according to previously described methods.11 Briefly, SIRT1-positive and -negative cells were counted in each specimen. The cells positive for the SIRT1 antibody staining were expressed as a percentage of total cells. Sections were examined using a light microscope (BX51; Olympus, Japan) and quantified by the Image Pro 6.1 software (Media Cybernetics). At least 10 bronchioles were counted on each slide and the data used for statistical analysis.
Bronchoalveolar lavage fluid (BALF) collection and analysis
Bronchioalveolar lavage was performed as previously described.12 In brief, the lungs were lavaged using a cannula inserted in the trachea and the lungs were instilled with 0.8 mL PBS after exsanguination. Cytospin slides were prepared by Wright-Giemsa staining. Total cell and eosinophil counts were counted under the microscope in a blinded method.
Lung histology
Lung sections were stained with hematoxylin/eosin (HE). Mucus production of goblet cells were assessed by Periodic Acid-Schiff (PAS) staining.12 Sections were examined using a light microscope (BX51; Olympus, Japan) and quantified by the Image Pro 6.1 software (Media Cybernetics). All slides were examined in a random blinded fashion by two independent investigators. At least 10 bronchioles were counted on each slide and the data used for statistical analysis.
Mediator assays
The levels of mouse IL-6 and Keratinocyte-derived Cytokine (KC) in lung homogenate and BALF were measured using ELISA kit (eBioscience, Inc). Minimum detectable dose of mouse IL-6 and KC were determined to be 2 pg/mL and 1 pg/mL, respectively.
Statistical analysis
Normality of distribution was checked with Kolmogorov-Smirnov test. Statistical analyses were performed using the parametric two-tailed Student’s t-test or non-parametric Mann-Whitney U-test for the comparison of two groups, and ANOVA with post-hoc Tukey’s corrections for the comparison of more than two groups. Results are presented as mean ± SD. Data were analyzed with GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). P<0.05 was considered statistically significant.
Results
PM reduces SIRT1 expression in HBE cells
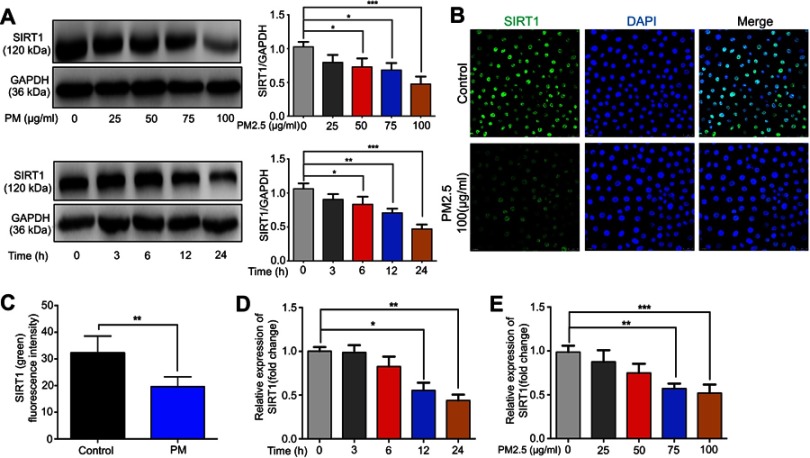
To address the possible role of SIRT1 in PM-induced airway inflammation, we first examined the expression of SIRT1 in HBE cells exposed to PM. We found that treating HBE cells with four concentrations of PM (25, 50, 75 or 100 μg/mL) for 24 h or with four times (3, 6, 12, or 24 h) of PM (100 μg/mL) resulted in a dose- or time-dependent decrease of SIRT1 (Figure 1A–C). As shown in Figure 1D and E, the mRNA expression of SIRT1 was decrease in HBE cells after PM exposed.
Figure 1.
PM exposure induces SIRT1 reduction in HBE cells. (A) Cells were exposed to PM at indicated times or concentrations. Western blot analysis for SIRT1 protein expression in HBE cells treated with PM. (B) Immunofluorescence for SIRT1 protein expression in HBE cells treated with PM (Magnification×400). (C) Semi-quantification results of immunofluorescence. (D and E) RT-PCR analysis for a time course (PM at 100 μg/mL for various times) or dose response (various concentrations of PM for 24 h) of SIRT1 protein expression in HBE cells treated with PM. Data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01, and ***P<0.001.
Abbreviations: PM, Particulate matter; SIRT1, Sirtuin 1; HBE, Human bronchial epithelial.
SIRT1 inhibits pm-induced inflammatory cytokines in HBE cells
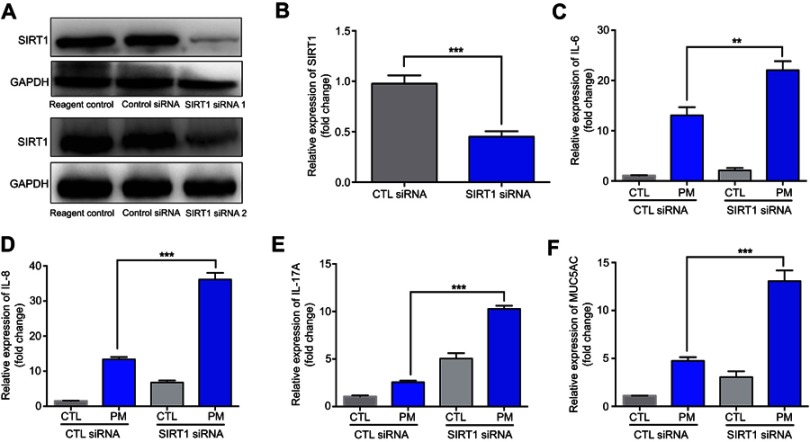
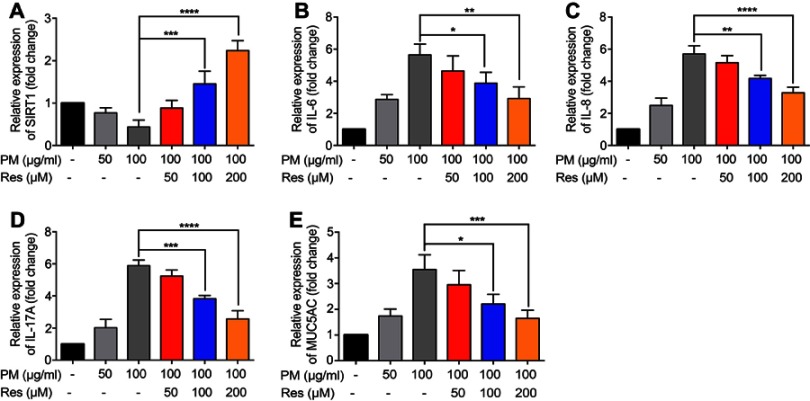
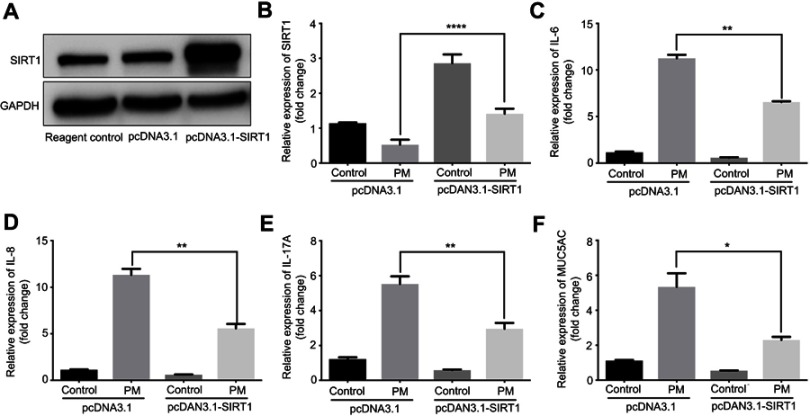
Previous studies have found that PM induces inflammatory cytokine production.1,2 Therefore, we next investigated the role of SIRT1 gene silencing on PM-induced inflammatory cytokines in HBE cells. The mRNA levels of IL-6, IL-8, and IL-17A were significantly increased in PM-induced HBE cells. SIRT1 gene silencing further increased PM-induced inflammatory cytokines compared with the control cells that received control siRNA (Figure 2A–E). SIRT1 gene silencing also significantly increased the expression of Mucin 5AC (MUC5AC) (Figure 2F). To confirm these findings, overexpression of SIRT1 experiment was done using SIRT1 plasmid. We found that SIRT1 overexpression significantly reduced PM-induced inflammatory cytokines compared with the control cells (Figure S1). We next sought to determine whether SIRT1 activator resveratrol attenuated inflammatory cytokines caused by PM. To address this, HBE cells were treated with PM and/or Resveratrol. We found that PM-induced mRNA transcripts of IL-6, IL-8, IL-17A, and mucin production were significantly attenuated by resveratrol treatment (Figure 3). Collectively, these results suggested that SIRT1 suppressed PM-induced inflammatory response and mucin production.
Figure 2.
SIRT1 gene silence increases PM-induced inflammatory cytokines in HBE cells. Cells were transfected with control (CTL) siRNA or SIRT1 siRNA for 24 h, and then were treated with PM (100 μg/mL) for 24 h to measure the levels of IL-6, IL-8, IL-17A, and MUC5AC.(A) SIRT1 expression was measured by Western blot. (B-F) The mRNA levels of IL-6, IL-8, IL-17A, and MUC5AC were measured by RT-PCR. Data are presented as mean ± SD of three independent experiments. **P<0.01 and ***P<0.001.
Abbreviations: SIRT1, Sirtuin 1; PM, Particulate matter; HBE, Human bronchial epithelial; MUC5AC, Mucin 5AC.
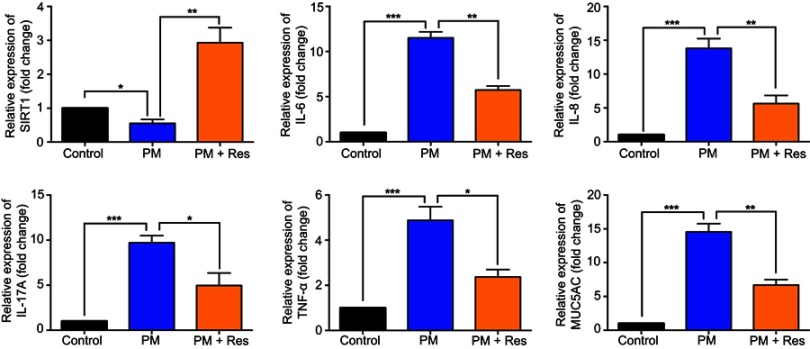
Figure 3.
Resveratrol (Res) inhibits PM-induced inflammatory cytokines in HBE cells. HBE cells were treated with SIRT1 activator resveratrol (Res) together with PM for 24 h. (A-E) The mRNA levels of IL-6, IL-8, IL-17A, and MUC5AC were measured by RT-PCR. Data are presented as Mean ± SD of three independent experiments. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Abbreviations: PM, Particulate matter; HBE, Human bronchial epithelial; SIRT1, Sirtuin 1; MUC5A, Mucin 5AC.
Resveratrol attenuates pm-induced airway inflammation in vivo
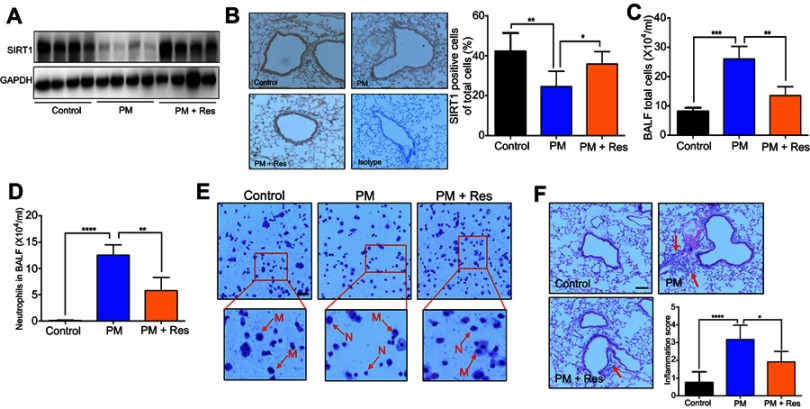
We next investigated the effect of resveratrol in regulation of airway inflammation in vivo. Mice were treated with PM and/or resveratrol, as described in the methods. We observed that although there was a significant decrease in SIRT1 expression in the lungs of PM-exposed WT mice, SIRT1 reduction was not observed in resveratrol-treated mice (Figure 4A and B). Total inflammatory cells and neutrophils in the BALF were significantly increased in PM-exposed mice. In contrast, mice treated with resveratrol before instillation of PM showed a significant decrease in lung inflammation versus mice only exposed to PM (Figure 4C–E). Histological analysis further confirmed that PM-induced airway inflammation was significantly ameliorated by resveratrol treatment (Figure 4F). PM-induced inflammatory cytokines such as IL-6, IL-8 and mucin production were also significantly reduced in mice treated with resveratrol (Figure 5A–F). Changes in protein expression in BALF and lung tissues were consistent with that of the mRNA levels (Figure S2).
Figure 4.
Resveratrol (Res) inhibits PM-induced airway inflammation in mice. Mice were instilled intratracheally with normal saline (NS), PM or PM plus resveratrol (Res) for 3 d, and the mice were sacrificed after 24 h. (A) Western blot analysis for SIRT1 protein expression in lung tissues of mice. (B) IHC analysis for representative images of lung tissues of mice stained with SIRT1 protein (red arrows; Magnification×400) and semi-quantified SIRT1 expression of the IHC staining. (C) The total inflammatory cells and the number of neutrophils (D) in the BALF were measured. (E) Representative images of macrophages and neutrophils in the BALF were analyzed (red arrows; Magnification×400) using Giemsa staining. (F) Representative images of lung tissue stained with H&E (Magnification×400) and Semi-quantified inflammation score of the H&E staining (red arrows). Data are representative of three independent studies (n=5–6 mice per group per study). Results are expressed as mean ± SD. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Abbreviations: M, macrophage; N: neutrophils; PM, Particulate matter; SIRT1, Sirtuin 1; IHC, Immunohistochemistry; BALF, Bronchoalveolar lavage fluid; H&E, Hematoxylin-eosin.
Figure 5.
Resveratrol (Res) reduces airway inflammation in response to PM exposure. The levels of IL-6, IL-8, IL-17A, TNF-α, and MUC5AC in lung tissue were analyzed by RT-PCR. Data are representative of three independent studies (n=5–6 mice per group per study). Results are expressed as mean ± SD. *p<0.05, **p<0.01, and ***p<0.001. Abbreviations: PM, Particulate matter; MUC5AC, Mucin 5AC; RT-PCR, Reverse Transcription-Polymerase Chain Reaction.
SIRT1 suppress pm-induced inflammation response partly via the activation of MAPK pathways
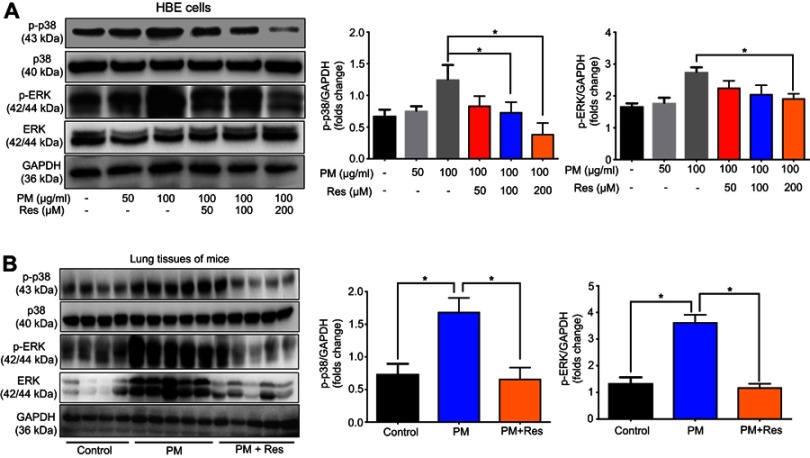
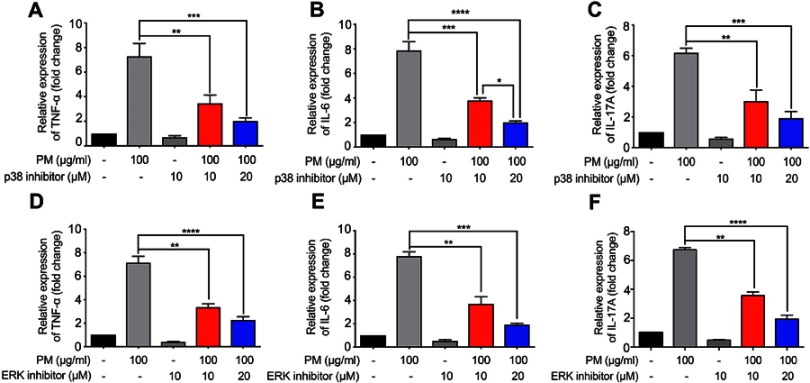
The mitogen-activated protein kinase (MAPK) family is well known to play a key role in mediating inflammatory responses.11 To assess whether MAPK pathways are involved in SIRT1 suppression of PM-induced inflammatory responses, the effect of SIRT1 on activation of p38-MAPK and ERK1/2 was evaluated by Western blotting. Stimulation of HBE cells with PM resulted in a transient phosphorylation of p38-MAPK and ERK1/2 (Figure 6A). In addition, activation of SIRT1 by resveratrol significantly decreased the PM-induced activation of MAPK pathways in HBE cells (Figure 6A). In vivo, we observed that p38-MAPK and ERK were activated in the lung tissues of PM-induced mice. Moreover, resveratrol-treated mice showed a significant decrease the activation of MAPK pathways versus mice only exposed to PM (Figure 6B). To further confirm PM-induced activation of MAPK pathways mediate PM-induced inflammation, p38-MAPK inhibitor SB203580 and ERK inhibitor U0126 were used in our study. We found that inflammatory cytokines were significantly increase in PM-stimulated HBE cells. In contrast, p38-MAPK inhibitor and ERK inhibitor significantly attenuated the PM-induced cytokine production in HBE cells (Figure 7). Collectively, these data suggested that the functions of SIRT1 in regulation of PM-induced inflammation and mucus production are mediated, at least in part, by the MAPK pathways.
Figure 6.
Resveratrol (Res) suppresses PM-induced inflammation response in HBE cells and mice via MAPK pathways. (A) HBE cells were treated with PM or resveratrol (Res), and the protein levels of MAPK pathways were measured by Western blot. (B) Mice were instilled intratracheally with normal saline (NS), PM or PM plus resveratrol respectively for 3 days, and the mice were sacrificed after 24 h. The protein levels of MAPK pathways were measured by Western blot. Data are representative of three independent studies. Data are representative of three independent studies. Results are expressed as mean ± SD. *p<0.05.
Abbreviations: PM, Particulate matter; HBE, Human bronchial epithelial; MAPK, Mitogen-activated protein kinase.
Figure 7.
MAPK inhibitors suppress PM-induced inflammation response in HBE cells. (A–I) p38 inhibitor SB203580 and ERK inhibitor U0126 were added 2 h prior PM exposed and cells were collected after 24 h. The levels of TNF-α, IL-6 and, IL-17A were analyzed by RT-PCR. Data are representative of three independent studies. Results are expressed as mean ± SD. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Abbreviations: MAPK, Mitogen-activated protein kinase; PM, Particulate matter; HBE, Human bronchial epithelial; RT-PCR, Reverse Transcription-Polymerase Chain Reaction.
Discussion
In the present study, we demonstrated that SIRT1 expression was decreased in HBE cells and lung tissues after PM exposure, suggesting that SIRT1 is involved in the pathogenesis of PM-induced airway inflammation. We further clarified that SIRT1 activators such as resveratrol inhibited PM-induced inflammatory responses in HBE cells and in mouse airways, partly via inhibition of the MAPK pathways. Collectively, our results suggest that activation of SIRT1 (eg, resveratrol) may represent a therapeutic strategy for airway disorders or disease exacerbations induced by airborne particulate pollution.
PM is a key indicator of air pollution brought into the air by a variety of natural and human activities. PM consists of a heterogeneous mixture of solid and liquid particles suspended in air that varies continuously in size and chemical composition in space and time.13 The sources of PM are complex and include combustion, transportation, factory emissions, agriculture, and natural sources. Exposure to PM has been identified as an increased incidence of respiratory diseases, which are associated with increased hospital admissions, emergency room visits, morbidity and mortality. PM exposure induced necroptosis in HBE cells and in mouse lungs and specific molecule inhibitors of necroptosis, markedly reduced PM-induced inflammatory cytokines in HBE cells.14 However, the cause of this condition is complex and the detailed mechanisms mediating the adverse effects of PM in respiratory diseases remain largely poor unclear. SIRT1 is the most extensively studied sirtuin and is involved in silencing the chromatin of the mating locus.15,16 SIRT1 negatively regulates transcription factors in the nucleus by the deacetylation of modified lysine residues on histones, transcription factors, and other non-histone proteins.4 SIRT1 can suppress inflammation, apoptosis and inhibit senescence, which are hallmarks of several chronic inflammatory diseases.15 Yao et al showed that SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Caito et al demonstrated that oxidants/aldehydes covalently modify SIRT1, decreasing enzymatic activity and marking the protein for proteasomal degradation, which has implications in inflammatory conditions.5 SIRT1 levels were reduced in macrophages and lungs of smokers and patients with COPD.4 Although SIRT1 has been shown to a metabolic NAD1-dependent protein/histone deacetylase that regulates proinflammatory mediators, it is not known whether SIRT1 regulates airway inflammation and mucin production in response to PM. In this study, we found that SIRT1 expression was decreased and resulted in inflammatory cytokines and mucus production in PM-induced HBE cells and lung tissues. Moreover, the functions of SIRT1 in regulation of PM-induced inflammation and mucus production are mediated, at least in part, by the MAPK pathways. It might suggest that activation of SIRT1 could prevent the development of PM induced-airway inflammation.
Resveratrol exhibits a wide range of beneficial characteristics including anti-inflammatory, anti-aging, and anti-oxidative properties. Recent studies indicate that resveratrol may protect against age-associated neurodegeneration and improve cognitive impairments in mouse models of Alzheimer’s disease (AD) and in human patients.3,4,17 Resveratrol may protect bronchial epithelial cells from cigarette smoke-induced apoptosis in vitro by preventing mitochondrial dysfunction, and mitofusin 2 may be associated with the anti-apoptotic functions of resveratrol in HBE cells.18 Resveratrol can increase the activity of human SIRT1 by as much as eight-fold through reducing of the Km value for acetylated substrate.19 The process by which resveratrol activated SIRT1 was found to be fluorophore-dependent, and resveratrol was further identified as a direct activator of SIRT1.19 Resveratrol increased hippocampal SIRT1 expression in an animal model of isoflurane-induced cognitive impairment.20 Zhao et al reported that intracerebroventricular injection of resveratrol in old mice for one week increased SIRT1 activation and markedly improved the long-term memory and long-term potentiation (LTP) formation on hippocampal slices.21 Resveratrol has anti-oxidative, anti-inflammatory, anti-apoptotic and autophagy regulating effects by increasing SIRT1 level.17 In our study, we found that resveratrol can inhibited inflammatory cytokines and mucus production by upregulating SIRT1 expression. Accumulating studies have demonstrated that there were other mechanisms underlying PM-induced airway inflammation, such as DNA damage, cell death, reactive oxygen species (ROS), and MAPK pathways.11 In our current study, we found that SIRT1 suppression of PM-induced airway inflammatory is related to MAPK pathways in vitro and in vivo. Collectively, these results indicated that SIRT1 activators such as resveratrol inhibit PM-induced inflammatory responses, likely via inhibition of MAPK pathways.
Limitations
The present study does have some limitations. First, we performed in vitro experiments with HBE cells in our study. However, the mechanisms of PM-induced inflammatory infiltrates are complicated, other cells such as alveolar epithelium cells, macrophages, may be also involved in PM-induced airway inflammation. We cannot sure that resveratrol has the same effects on inflammatory cells as it did with HBE cells; Second, because resveratrol is not a specific activator of SIRT1, SIRT1 transgenic mice will be needed to further study the role of SIRT1 in the pathogenesis of PM-induced airway inflammation. Third, The dose of PM used for the in vitro studies were according the previous studies.10 In these studies, HBE cells were treated with PM at 100 μg/mL. However, PM has been shown to cause cell death at doses above 50 μg/mL. To assessed whether the dose of PM2.5 in our study cause cell death, cell activity was analyzed using CCK8. We found that PM did not cause HBE cell death at dose of 100 μg/mL and at 24 h. Our results were consistent with previous studies. The reason for the discrepancies of the results, the PM has been shown to cause cell death at doses above 50 μg/mL, is unclear. However, it may be due to differences in cell types, difference source of PM2.5, in vitro and in vivo experiments.
Conclusion
In summary, the present study demonstrated that SIRT1 plays an important role in the regulation of PM-induced airway inflammation and might suggest that activation of SIRT1 could airway disorders or disease exacerbations induced by airborne particulate pollution.
Supplementary materials
SIRT1 overexpression suppresses PM-induced inflammation response in HBE cells. Cells were transfected with Control or SIRT1 plasmid and were stimulated with PM. (A) SIRT1 expression was measured by Western blot. (B-F) The mRNA levels of SIRT1, IL-6, IL-8, IL-17A, and MUC5AC were assessed by RT-PCR.Data are representative of three independent experiments. Results are expressed as mean ± SD. *p<0.05, **p<0.01, and ****p<0.0001.
Abbreviations: SIRT1, Sirtuin 1; PM, Particulate matter; HBE, Human bronchial epithelial.
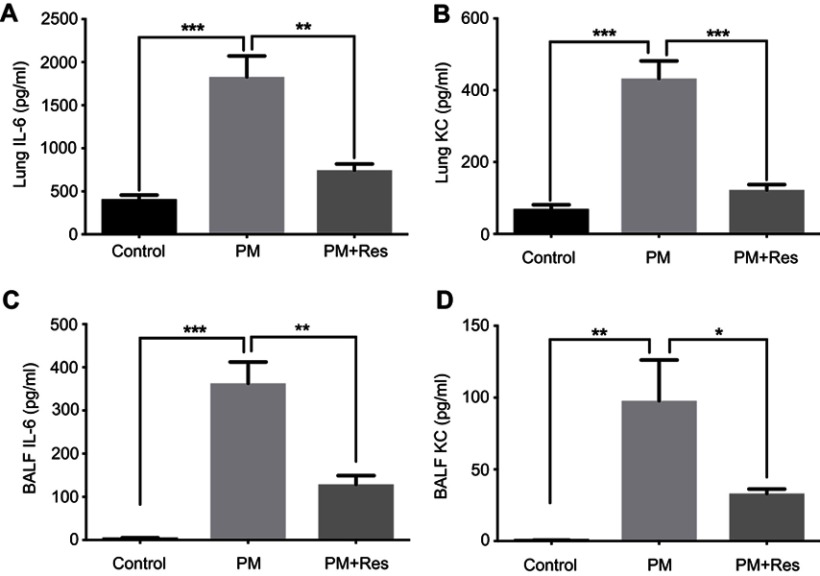
Resveratrol (Res) suppresses PM-induced airway inflammation. WT mice were exposed to PM for 3 days. Lungs and BALF were isolated 1 day after the last PM challenge. The inflammatory cytokines in the lung homogenate (A and B) and BALF (C and D) were assessed by using an enzyme-linked immunosorbent assay. Data are representative of three independent studies (n=5–6 mice per group per study). Results are expressed as mean ± SD. *p<0.05, **p<0.01, and ***p<0.001.
Abbreviations: PM, Particulate matter; WT, Wild type; BALF, Brochoalveolar lavage fluid; KC, Keratinocyte-derived Cytokine.
Acknowledgments
Financial support was provided by the National Natural Science Foundation of China (81670025), the Natural Science Foundation of Guangdong Province China (2018A0303130269), and the Doctoral Startup Found-ation of the Affiliated Hospital of Guangdong Medical University (Tianwen Lai).
Disclosure
The authors declare that they have no conflicts of interest regarding this manuscript.
References
- 1.Sacks JD, Stanek LW, Luben TJ, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119(7):446–454. doi: 10.1289/ehp.1002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. doi: 10.1016/S0140-6736(16)31597-5 [DOI] [PubMed] [Google Scholar]
- 3.Rahman I, Kinnula VL, Gorbunova V, Yao H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med. 2012;54(Suppl):S20–S28. doi: 10.1016/j.ypmed.2011.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):861–870. doi: 10.1164/rccm.200708-1269OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao H, Sundar IK, Ahmad T, et al. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2014;306(9):L816–L828. doi: 10.1152/ajplung.00323.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J. Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L847–L853. doi: 10.1152/ajplung.00274.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Duan Z, Liu X, Yuan Y. N-acetyl-l-cysteine ameliorates the PM2.5-induced oxidative stress by regulating SIRT-1 in rats. Environ Toxicol Pharmacol. 2018;57:70–75. doi: 10.1016/j.etap.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, Liu MC, Liang M, Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood. 2012;119(10):2422–2429. doi: 10.1182/blood-2011-04-350413 [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Feng J, Zhang R, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017;2017:4602715. doi: 10.1155/2017/4602715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZH, Wu YF, Wang PL, et al. Autophagy is essential for ultrafine particle-induced inflammation and mucus hyperproduction in airway epithelium. Autophagy. 2016;12(2):297–311. doi: 10.1080/15548627.2015.1124224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai T, Wu D, Chen M, et al. YKL-40 expression in chronic obstructive pulmonary disease: relation to acute exacerbations and airway remodeling. Respir Res. 2016;17:31. doi: 10.1186/s12931-016-0338-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai T, Tian B, Cao C, et al. HDAC2 suppresses IL17A-mediated airway remodeling in human and experimental modeling of COPD. Chest. 2018;153(4):863–875. doi: 10.1016/j.chest.2017.10.031 [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 14.Xu F, Luo M, He L, et al. Necroptosis contributes to urban particulate matter-induced airway epithelial injury. Cell Physiol Biochem. 2018;46(2):699–712. doi: 10.1159/000488726 [DOI] [PubMed] [Google Scholar]
- 15.Caito S, Rajendrasozhan S, Cook S, et al. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. Faseb J. 2010;24(9):3145–3159. doi: 10.1096/fj.09-151308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:113. doi: 10.1042/BJ20070140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao W, Dou Y, Li A. Resveratrol boosts cognitive function by targeting SIRT1. Neurochem Res. 2018;43(9):1705–1713. doi: 10.1007/s11064-018-2586-8 [DOI] [PubMed] [Google Scholar]
- 18.Song C, Luo B, Gong L. Resveratrol reduces the apoptosis induced by cigarette smoke extract by upregulating MFN2. PLoS One. 2017;12(4):e0175009. doi: 10.1371/journal.pone.0175009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200 [DOI] [PubMed] [Google Scholar]
- 20.Li XM, Zhou MT, Wang XM, et al. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. J Mol Neurosci. 2014;52(2):286–293. doi: 10.1007/s12031-013-0141-2 [DOI] [PubMed] [Google Scholar]
- 21.Zhao YN, Li WF, Li F, et al. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435(4):597–602. doi: 10.1016/j.bbrc.2013.05.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SIRT1 overexpression suppresses PM-induced inflammation response in HBE cells. Cells were transfected with Control or SIRT1 plasmid and were stimulated with PM. (A) SIRT1 expression was measured by Western blot. (B-F) The mRNA levels of SIRT1, IL-6, IL-8, IL-17A, and MUC5AC were assessed by RT-PCR.Data are representative of three independent experiments. Results are expressed as mean ± SD. *p<0.05, **p<0.01, and ****p<0.0001.
Abbreviations: SIRT1, Sirtuin 1; PM, Particulate matter; HBE, Human bronchial epithelial.
Resveratrol (Res) suppresses PM-induced airway inflammation. WT mice were exposed to PM for 3 days. Lungs and BALF were isolated 1 day after the last PM challenge. The inflammatory cytokines in the lung homogenate (A and B) and BALF (C and D) were assessed by using an enzyme-linked immunosorbent assay. Data are representative of three independent studies (n=5–6 mice per group per study). Results are expressed as mean ± SD. *p<0.05, **p<0.01, and ***p<0.001.
Abbreviations: PM, Particulate matter; WT, Wild type; BALF, Brochoalveolar lavage fluid; KC, Keratinocyte-derived Cytokine.