Abstract
Organophosphate pesticides are developmental neurotoxicants. We gave diazinon via osmotic minipumps implanted into dams prior to conception, with exposure continued into the second postnatal week, at doses (0.5 or 1 mg/kg/day) that did not produce detectable brain cholinesterase inhibition. We evaluated the impact on acetylcholine (ACh) and serotonin (5-hydroxytryptamine, 5HT) systems in brain regions from adolescence through full adulthood. Diazinon produced deficits in presynaptic ACh activity with regional and sex selectivity: cerebrocortical regions and the hippocampus were affected to a greater extent than were the striatum, midbrain or brainstem, and females were more sensitive than males. Diazinon also reduced nicotinic ACh receptors and 5HT1A receptors, with the same regional and sex preferences. These patterns were similar to those of diazinon given in a much more restricted period (postnatal day 1-4) but were of greater magnitude and consistency; this suggests that the brain is vulnerable to diazinon over a wide developmental window. Diazinon’s effects differed from those of the related organophosphate, chlorpyrifos, with regard to regional and sex selectivity, and more importantly, to the effects on receptors: chlorpyrifos upregulates nicotinic ACh receptors and 5HT receptors, effects that compensate for the presynaptic ACh deficits. Diazinon can thus be expected to have worse neurodevelopmental outcomes than chlorpyrifos. Further, the disparities between diazinon and chlorpyrifos indicate the problems of predicting the developmental neurotoxicity of organophosphates based on a single compound, and emphasize the inadequacy of cholinesterase inhibition as an index of safety.
Keywords: Acetylcholine, Developmental neurotoxicity, Diazinon, Organophosphate pesticides, Serotonin
INTRODUCTION
Organophosphate pesticides impose a worldwide burden of neurodevelopmental disorders nearly equivalent to that of lead and six times that of mercury (Bellinger 2012). Originally, it was thought that all organophosphates acted alike, via their inhibition of cholinesterase (Mileson et al. 1998), the mechanism that produces the overt signs of acute poisoning with these agents. Accordingly, safety thresholds were set based on measurements of cholinesterase activity. Over the last two decades, it has become increasingly obvious that the long-term effects of organophosphate-induced developmental neurotoxicity occurs at exposures below the threshold for effects on this enzyme, involving a family of mechanisms that impair neural cell replication and differentiation, axonogenesis and synaptogenesis, and development of neurotransmitter and neurotrophin systems, ultimately leading to adverse effects on brain structure and behavioral performance (Abreu-Villaça and Levin 2017; Androutsopoulos et al. 2013; Casida and Quistad 2004; Rauh et al. 2012; Slotkin 2005). All organophosphate pesticides share a thiophosphate nucleus that, after metabolic replacement of the sulfur atom with oxygen, binds to the catalytic site of cholinesterase; however, their structures differ substantially in the remaining moieties, raising the likelihood of disparate neurodevelopmental effects at exposures below those necessary for cholinesterase inhibition (Pancetti et al. 2007; Richendorfer and Creton 2015; Slotkin et al. 2006, 2017).
Diazinon was actually the first organophosphate to be identified as a developmental neurotoxicant (Spyker and Avery 1977), although much more attention was paid subsequently to chlorpyrifos (Costa 2006; Mauro and Zhang 2007; Slotkin 2005), in part because of its 40-fold greater overall use (U.S. Geological Survey 2018a, b). We previously characterized the adverse effects of a brief exposure to diazinon in neonatal rats on postnatal days (PN) 1-4, at doses below the threshold for any detectable cholinesterase inhibition (Slotkin et al. 2006), finding neuronal loss and hypergliosis (Slotkin et al. 2008a), long-term impairment of presynaptic activity in acetylcholine (ACh) pathways (Slotkin et al. 2008a), impaired expression of serotonin 5HT1A receptors (Slotkin et al. 2008b), and corresponding deficits in cognitive and emotional behaviors (Roegge et al. 2008; Timofeeva et al. 2008). Importantly, we found a number of distinct differences between diazinon and comparable exposures to chlorpyrifos, including opposite effects on neural cell packing density (Garcia et al. 2001; Slotkin et al. 2008a), and differing impacts on ACh and 5HT pathways (Aldridge et al. 2004; Slotkin et al. 2001, 2008a, b). For the neurotransmitter effects, the chief differences fell into two broad classes. First, although both chlorpyrifos and diazinon produced long-term deficiencies in ACh synaptic activity, chlorpyrifos showed compensatory upregulation of α4β2 nicotinic ACh receptors (nAChRs), an effect that would serve to offset the impairment of presynaptic ACh function (Slotkin et al. 2013). In contrast, diazinon exposure in the same developmental period evoked decreases in α4β2 nAChRs (Slotkin et al. 2008a). Second, with chlorpyrifos, we found long-term upregulation of 5HT1A and 5HT2 receptors, and showed that the cognitive deficiencies resulting from the ACh defect was offset by 5HT replacement of what would ordinarily be ACh-dependent functions (Aldridge et al. 2005a, b). Diazinon reduced 5HT1A receptor expression (Slotkin et al. 2008b), which again, would contribute to a worsened functional outcome compared to chlorpyrifos, conclusions that were confirmed in comparative studies of cognitive and emotional behaviors (Aldridge et al. 2005a; Levin et al. 2001; Roegge et al. 2008; Timofeeva et al. 2008). Third, the regional and sex selectivities of chlorpyrifos and diazinon differed (Aldridge et al. 2004; Dam et al. 1999; Slotkin et al. 2008a, b; Slotkin and Seidler 2005); surprisingly, for some indices, females showed greater adverse effects with diazinon as compared to males, a result that is at odds with typical neurotoxicant effects (Kern et al. 2017).
In the current study, we extended the developmental period of diazinon exposure to encompass all of gestation and into the second postnatal week, instead of just a brief, four-day neonatal window. This was done to address the key questions of whether a more prolonged exposure period produces the same or different patterns of effects, and if a similar effect, whether there is a greater magnitude with longer exposures. We already know that the adverse effects of chlorpyrifos on ACh and 5HT systems differ substantially when exposures occur prenatally vs. postnatally (Aldridge et al. 2004; Dam et al. 1999; Qiao et al. 2003, 2004). If diazinon likewise shows temporal specificity, we might expect to see a different pattern of effects with continuous perinatal exposure as compared to short-term neonatal treatment. Furthermore, if the disparities in the effects of diazinon and chlorpyrifos reflect distinctive critical periods, then a more prolonged period of diazinon exposure might lessen their differences. Finally, this extended exposure thus encompasses stages of rat brain development that correspond to the entire gestational period in humans (Rodier 1988).
Our study design was modeled on our previous work with chlorpyrifos and diazinon. Because administration involved an extended period, we delivered diazinon using osmotic minipumps, implanted prior to mating, so as to avoid the stress of repeated handling. We then evaluated the impact on ACh and 5HT systems from adolescence through adulthood, assessed in brain regions comprising all the major ACh and 5HT projections and their corresponding cell bodies. For ACh, we evaluated the concentration of presynaptic high-affinity choline transporters (hemicholinium-3 [HC3] binding), the activity of choline acetyltransferase (ChAT), and the concentration of α4β2 nAChRs. High-affinity choline transporters and ChAT are both constitutive components of ACh nerve terminals but they differ in their regulatory mechanisms and hence in their functional significance. ChAT is the enzyme that synthesizes ACh, but is not regulated by nerve impulse activity, so that its presence provides an index of the development of ACh projections (Slotkin 2008). In contrast, HC3 binding to the choline transporter is directly responsive to neuronal activity (Klemm and Kuhar 1979), so that comparative effects on HC3 binding and ChAT enable the characterization of both the development of cholinergic innervation and presynaptic impulse activity. For that determination, we calculated the HC3/ChAT ratio as an index of presynaptic activity relative to the number of cholinergic nerve terminals (Slotkin 2008). The α4β2 nAChR is the most abundant subtype in the mammalian brain and regulates the ability of ACh systems to release other neurotransmitters involved in reward, cognition and mood (Dani and De Biasi 2001). For 5HT systems, we focused on 5HT1A and 5HT2 receptors, subtypes that play major roles in 5HT-related mental disorders, including depression (Maes and Meltzer 1995) and that are known targets for organophosphate-induced developmental neurotoxicity (Aldridge et al. 2004; Slotkin et al. 2006, 2008b; Slotkin and Seidler 2005).
MATERIALS AND METHODS
Animal treatments.
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Animal Care and Use Committee and in accordance with all federal and state guidelines. Sprague-Dawley rats were shipped by climate-controlled truck (transportation time < 1 hr) and were allowed to acclimate to the housing facility for two weeks prior to treatment. At 9 weeks of age, each female was implanted with a Type 2ML4 Alzet® osmotic minipump, inserted subcutaneously on the back. The pumps were implanted under anesthesia (60 mg/kg ketamine + 0.15-0.50 mg/kg dexmedetomidine given i.p.; followed post-implant by 0.15 mg/kg atipamezole + 5 mg/kg ketoprofen given s.c. and topical bupivacaine) and the animals were allowed to recover for three days. Mating was then initiated by including a male rat in the cage for a period of 5 days. Although the pumps are marketed as a four-week delivery device, it actually takes approximately 35 days for the reservoir to be exhausted completely (information supplied by the manufacturer). Because the insemination date varied among different mating pairs, exposure thus began 7-3 days prior to fertilization and terminated between PN8-11.
There were three treatment groups, each comprising 9-14 dams: control (dimethylsulfoxide vehicle); and two concentrations of diazinon dissolved in dimethylsulfoxide, calibrated to deliver 0.5 or 1 mg/kg/day at the start of the infusion period. Because body weights increased with gestation, the dose rate fell by approximately one-third by the end of gestation and then rose back toward the original values with the postpartum weight loss. In studies of short-term exposure, these diazinon doses have been previously shown to elicit developmental neurotoxicity directed toward ACh and 5HT systems, without overt signs of toxicity, and to be below the threshold for any detectable cholinesterase inhibition (Slotkin et al. 2006, 2008a, b). Further, we performed preliminary studies to ensure that prolonged treatment with the chosen dose rates did not have significant effects on fertility, maternal weight gain, litter size or postnatal growth.
Parturition occurred during gestational day 22, which was also taken as PN0, and litters were culled on PN1 to 8-10 pups to ensure standard nutrition. Whole brains from the culled animals were analyzed for cholinesterase activity. For the remaining pups, weaning occurred on PN21. On PN30, 60, 100 and 150, animals were decapitated and brain regions were dissected for determination of ACh and 5HT synaptic markers: frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum, midbrain and brainstem. The two cortical regions were sectioned at the midline and the right half used for the current determinations. The left halves of the cortical regions were reserved for future studies, along with the cerebellum, which is sparse in ACh and 5HT projections. Tissues were frozen in liquid nitrogen and stored at −80°C until assayed. Each treatment group comprised 5-9 animals of each sex at each age point, with each litter contributing no more than one male and one female to any of the determinations at a given age.
Assays.
Assays were conducted on each individual tissue, so that each determination represented a value from the corresponding brain region of one animal. The techniques were identical to those used in our previous studies (Slotkin et al. 2006, 2008a, b), and accordingly, will be described only in brief. For the cholinesterase assays, tissues were thawed and homogenized (Polytron, Brinkmann Instruments, Westbury, NY, USA) in ice-cold 50 mM Tris (pH 7.4), and aliquots of the homogenate were withdrawn for measurement of total protein (Smith et al. 1985) and cholinesterase activity (Ellman et al. 1961) using acetylthiocholine iodide as a substrate. Activity was calculated relative to total protein. Assays were conducted on six animals of each sex for each treatment group.
For the other assays, tissues were thawed and homogenized (Polytron) in 79 volumes of ice-cold 10 mM sodium-potassium phosphate buffer (pH 7.4). Aliquots of the homogenate were assayed for ChAT using 50 μM [14C]acetyl-coenzyme A as a substrate and activity was determined as the amount of labeled ACh produced relative to tissue protein. For binding measurements, the cell membrane fraction was prepared from the same tissue homogenate and aliquots were assayed for: (1) HC3 binding, using a ligand concentration of 2 nM [3H]HC3 with or without 10 μM unlabeled HC3 to displace specific binding; (2) nAChR binding, using 1 nM [3H]cytisine with or without 10 μM nicotine as a displacer; (3) 5HT1A receptor binding with 1 nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin, displaced with 100 μM 5HT; and (4) 5HT2 receptor binding with 0.4 nM [3H]ketanserin, displaced with 10 μM methylsergide. Ligand binding was calculated relative to the membrane protein concentration.
Some of the regions had insufficient amounts of tissue to permit all assays to be performed. Accordingly, we did not obtain values for nAChRs in the striatum, nor for the 5HT receptors in either the striatum or hippocampus.
Data analysis.
The initial statistical comparisons were conducted by a global ANOVA (data log-transformed because of heterogeneous variance among regions, measures and ages) incorporating all the variables and measurements in a single test so as to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. The variables in the global test were treatment (vehicle, diazinon 0.5 mg/kg/day, diazinon 1 mg/kg/day), brain region, age and sex, with multiple dependent measures (hereafter, designated simply as “measures”): HC3 binding, ChAT activity and nAChR binding for the ACh synaptic makers; 5HT1A and 5HT2 receptor binding for the 5HT synaptic markers. For both transmitter systems, the dependent measures were treated as repeated measures, since all the determinations were derived from the same sample. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control or from each other. Significance was assumed at the level of p < 0.05, two-tailed.
Data were compiled as means and standard errors. To enable ready visualization of treatment effects across different regions, ages and measures, the results are given as the percent change from control values but statistical procedures were always conducted on the original data, with log transforms because of heterogeneous variance as noted above. In addition, the log-transform evaluates the treatment differences as a proportion to control values, rather than as an arithmetic difference. This was important because of technical limitations: on any single day, we could conduct assays for all treatment groups and both sexes, but for only one region at one age point. Accordingly, representing the data as proportional differences (percent control) enables a full comparison of treatment effects and treatment interactions with all the other variables, even though absolute values for the controls cannot be compared across regions and ages (since assays for each region and age point were run on separate days). Graphs were scaled to encompass the different dynamic ranges of the changes in the various parameters. The original values for each set of determinations appear in the Supplemental Tables.
Materials.
Animals were purchased from Charles River Laboratories (Raleigh, NC, USA) and osmotic minipumps (model 2ML4) were from Durect Corp. (Cupertino, CA, USA). Diazinon was obtained from Chem Service (West Chester, PA, USA). PerkinElmer Life Sciences (Boston, MA, USA) was the source for [3H]HC3 (specific activity, 125 Ci/mmol), [3H]cytisine (specific activity 35 Ci/mmol), [3H]8-hydroxy-2-(di-n-propylamino)tetralin (specific activity, 135 Ci/mmol), [3H]ketanserin (63 Ci/mmol) and [14C]acetyl-coenzyme A (specific activity 6.7 mCi/mmol). Methylsergide was obtained from Sandoz Pharmaceuticals (E. Hanover, NJ, USA) and all other chemicals came from Sigma-Aldrich (St. Louis, MO, USA).
RESULTS
Maternal, litter and growth effects.
None of the treatments had any significant effect on maternal weight gain during or after pregnancy or on the proportion of dams giving birth (data not shown). Likewise, litter size and sex ratio were unaffected: control (n=14), 11.7 ± 0.6 pups per litter, 53 ± 3% male; diazinon 0.5 mg/kg/day (n=9), 11.7 ± 0.6 pups per litter, 46 ± 8% male, diazinon 1 mg/kg/day (n=11), 11.4 ± 0.5 pups per litter, 52 ± 5% male. The offspring displayed no significant treatment-related changes in body weight throughout adolescence and adulthood (Table S1). For brain region weights, there was a slight (2%) overall increase in the group receiving the higher dose of diazinon that was statistically significant (p < 0.01) compared either to the control or low dose diazinon groups (Table S1).
Cholinesterase inhibition.
There were no significant changes in brain cholinesterase activity, measured on the day after birth (i.e. while diazinon was still being administered): control 47 ± 1 nmol/mg protein/min, diazinon 0.5 mg/kg/day 46 ± 1, diazinon 1 mg/kg/day 48 ± 2. Values are reported for males and females combined (n=12 per treatment group) because of the absence of a significant main effect of sex or an interaction of treatment × sex. Power testing indicated we would have been able to detect as little as a 6% difference as statistically significant.
Global statistical analyses of ACh synaptic markers.
Because nAChRs were not determined in one of the brain regions (striatum), there were two different ways of performing global statistical analyses for ACh synaptic markers. First, we examined all three markers (HC3 binding, ChAT activity, nAChR binding) across five of the regions, excluding the striatum. We identified a significant main effect of treatment (p < 0.0001) as well as interactions of treatment × measure (p < 0.0001), treatment × sex (p < 0.0003), treatment × measure × sex (p < 0.05), and treatment × measure × sex × age (p < 0.05). Second, we excluded the nAChR measurements and evaluated HC3 binding and ChAT activity across all six regions, and likewise found a significant treatment main effect (p < 0.0001) and interactions of treatment × measure (p < 0.0001), treatment × sex (p < 0.005), and treatment × region (p < 0.02). Accordingly, we evaluated each of the measures separately for main treatment effects and interactions of treatment with other variables.
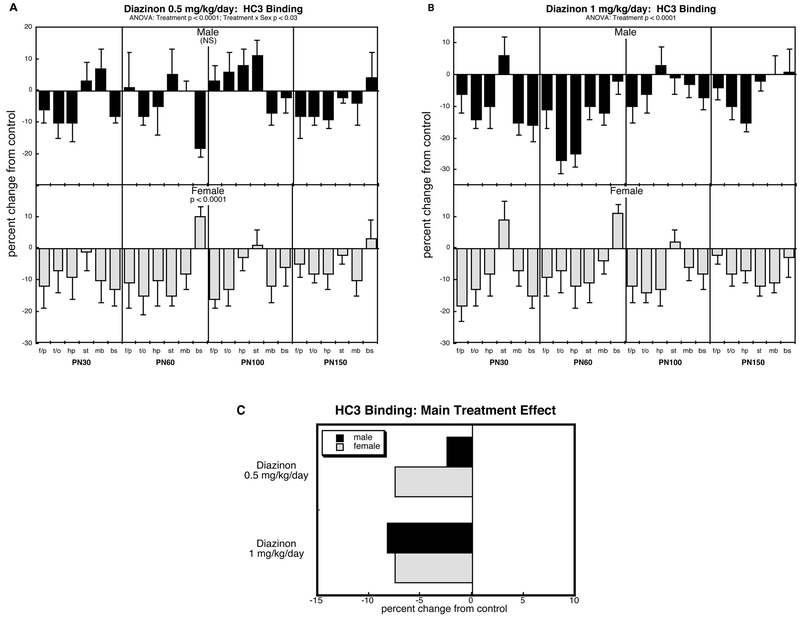
HC3 binding (Figure 1).
Figure 1.
Effects of perinatal diazinon exposure on HC3 binding: (A) 0.5 mg/kg/day, (B) 1 mg/kg/day. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table S2. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests were carried out only where justified by interactions of treatment with other variables. Panel (C) shows the simple main treatment effects, collapsed across age and region. Abbreviations: f/p, frontal/parietal cortex; t/o, temporal/occipital cortex; hp, hippocampus; st, striatum; mb, midbrain; bs, brainstem; NS, not significant.
Across all groups, ANOVA showed a significant main treatment effect (p < 0.0001) that depended on sex (treatment × sex, p < 0.02). Consequently, we examined each diazinon treatment group for differences from control and interaction of treatment × sex; although age and region were still retained as a factor in the statistical analysis, any age- or region-related interactions were ignored because of the absence of a significant interaction with treatment in the higher-order test.
The lower dose of diazinon evoked a sex-selective overall reduction in HC3 binding, with significant deficits in females but not males (Figure 1A). At the higher dose, the sex selectivity was no longer evident, with decreases appearing in both males and females (Figure 1B). Because of the complexity of the results, we developed a simplified graphical representation of the data, calculating the mean values for main treatment effects, collapsed across region and age (Figure 1C). This streamlined picture dilutes the effects seen for specific regions or ages by averaging them with data points for which there was no effect or an opposite effect, so that the absolute magnitude becomes smaller; in addition, the variability term is no longer meaningful, since values are collapsed across factors that interact with treatment and that contribute to the overall variance. Despite these limitations, there were obvious overall patterns that correspond to the net outcomes presented in Figures 1A and 1B. Both treatments produced reductions in FIC3 binding, but at the lower dose, the effect was bigger in females than in males.
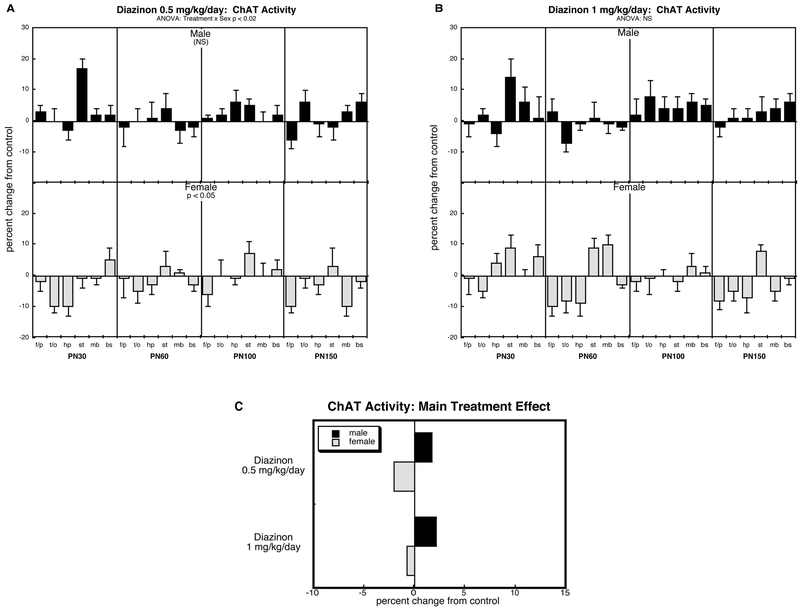
ChAT activity (Figure 2).
Figure 2.
Effects of perinatal diazinon exposure on ChAT activity: (A) 0.5 mg/kg/day, (B) 1 mg/kg/day. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table S3. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests were carried out only where justified by interactions of treatment with other variables. Panel (C) shows the simple main treatment effects, collapsed across age and region. Abbreviations: f/p, frontal/parietal cortex; t/o, temporal/occipital cortex; hp, hippocampus; st, striatum; mb, midbrain; bs, brainstem; NS, not significant.
Comparing all treatment groups, ANOVA identified a treatment × sex interaction (p < 0.03). Accordingly, we examined each treatment for sex-selective treatment effects, but not for treatment interactions with age or region. As before, we retained age and region in the statistical analysis but ignored any resultant interactions of treatment with these factors because the interactions were absent in the higher order test.
In contrast to the robust effects on HC3 binding, diazinon elicited only minor changes in ChAT activity. At 0.5 mg/kg/day, females showed small but statistically significant overall reductions, whereas males were spared and even showed slight (nonsignificant) increases (Figure 2A). The same patterns were present at the 1 mg/kg/day dose but were not statistically different from controls (Figure 2B). Flowever, the effects of the two diazinon dose groups were also not statistically distinguishable from each other, so that it is not appropriate to conclude that there was a nonmonotonic dose-response curve; further, when the two diazinon doses were compared collectively against controls, the increase in males also became statistically significant (p < 0.04), distinguishing it from the decrease seen in females. Again, these patterns are most readily seen by examining the main treatment effects collapsed across region and age (Figure 2C): slight reductions in females and slight increases in males.
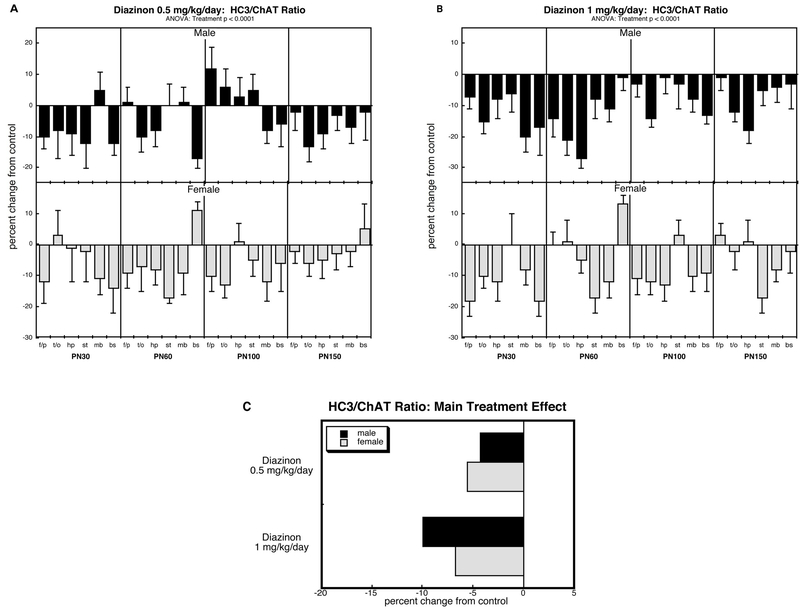
HC3/ChAT ratio (Figure 3).
Figure 3.
Effects of perinatal diazinon exposure on the HC3/ChAT ratio: (A) 0.5 mg/kg/day, (B) 1 mg/kg/day. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table S4. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests were not carried because of the absence of interactions of treatment with other variables. Panel (C) shows the simple main treatment effects, collapsed across age and region. Abbreviations: f/p, frontal/parietal cortex; t/o, temporal/occipital cortex; hp, hippocampus; st, striatum; mb, midbrain; bs, brainstem.
ANOVA for the HC3/ChAT ratio identified a main treatment effect (p < 0.0001) but no significant interactions of treatment with the other factors. Accordingly, we examined the diazinon groups only for their overall treatment differences from controls, including the other factors in the lower-order statistical tests but ignoring any resultant interactions of treatment with the other factors.
The low dose of diazinon elicited an overall decrease in the HC3/ChAT ratio that was equally evident in males and females (Figure 3A). The higher dose intensified this effect (p < 0.003 comparing the two diazinon doses to each other; Figure 3B). The collapsed view of the main treatment effects readily displayed the deficits present in both sexes and the larger net decrease seen at the higher dose (Figure 3C).
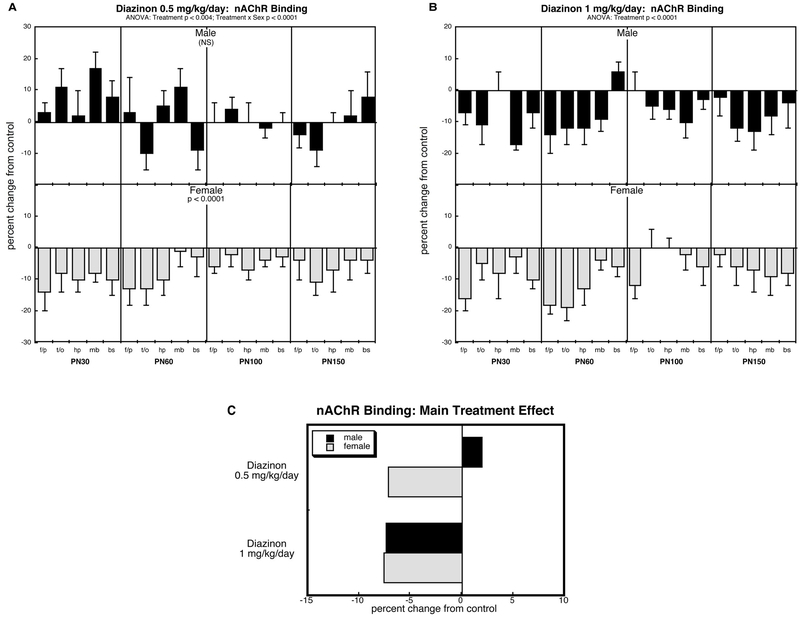
nAChR binding (Figure 4).
Figure 4.
Effects of perinatal diazinon exposure on nAChR binding: (A) 0.5 mg/kg/day, (B) 1 mg/kg/day. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table S5. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests were carried only where justified by interactions of treatment with other variables. Panel (C) shows the simple main treatment effects, collapsed across age and region. Abbreviations: f/p, frontal/parietal cortex; t/o, temporal/occipital cortex; hp, hippocampus; mb, midbrain; bs, brainstem; NS, not significant.
Across all groups, ANOVA identified a main effect of treatment on nAChR binding (p < 0.0001), as well as a treatment × sex interaction (p < 0.0001). Accordingly, we examined each treatment for differences from control and for the interaction of treatment with sex, but not for interactions with age or region; again, age and region were still retained as a factor in the statistical analysis, but any resultant age- or region-related interactions were ignored because of the absence of a significant interaction in the higher-order test.
The lower dose of diazinon elicited a sex-selective decrease in nAChR binding: males were spared whereas females showed a significant overall decrease (Figure 4A). Raising the dose to 1 mg/kg/day evoked deficits in both males and females (Figure 4B). Again, these were readily seen by graphing the main treatment effects collapsed across region and age (Figure 4C).
Global statistical analyses of 5HT receptor binding.
Across both 5FIT receptor subtypes and all treatment groups, global ANOVA identified a main effect of treatment (p < 0.0001) as well as interactions of treatment × subtype (p < 0.0001), treatment × sex (p < 0.05), treatment × region (p < 0.01), treatment × subtype × sex (p < 0.01), treatment × subtype × age (p < 0.0001) and treatment × subtype × sex × region × age (p < 0.002). Since treatment interacted with subtype, and given that the other factors interacted with those two elements, we separated the analysis by receptor subtype to look for treatment effects and interactions of treatment with the other factors.
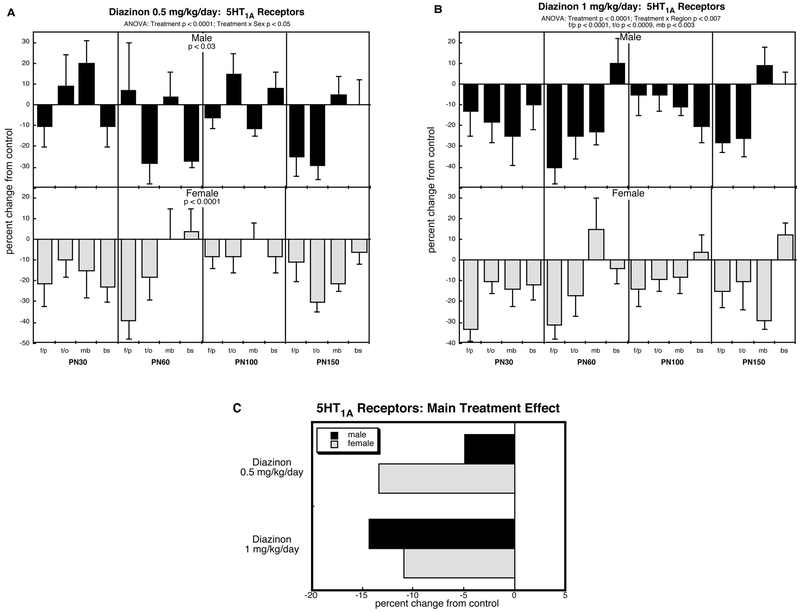
5HT1A receptors (Figure 5).
Figure 5.
Effects of perinatal diazinon exposure on 5HT1A receptor binding: (A) 0.5 mg/kg/day, (B) 1 mg/kg/day. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table S6. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests were carried out only where justified by interactions of treatment with other variables. Panel (C) shows the simple main treatment effects, collapsed across age and region. Abbreviations: f/p, frontal/parietal cortex; t/o, temporal/occipital cortex; mb, midbrain; bs, brainstem.
Across all groups, ANOVA identified a main effect of treatment on 5HT1A receptors (p < 0.0001) as well as interactions of treatment × sex (p < 0.02), treatment × region (p < 0.03) and treatment × sex × region × age (p < 0.007). Accordingly, we examined each treatment for differences from control and for interactions of treatment with the other factors.
At the lower dose of diazinon, both sexes showed significant deficits in 5HT1A receptors but with a greater effect in females compared to males (Figure 5A). At the higher dose, the deficits became equivalent in males and females (no treatment × sex interaction) but there were regional selectivities superimposed on the overall decreases; (Figure 5B); significant deficits were greatest in the cerebrocortical regions, lesser in the midbrain, and not significant in the brainstem.
The main treatment effects collapsed across region and age readily show the major deficits in 5HT1A receptors, with sex-selectivity at the low dose, but not the high dose of diazinon (Figure 5C).
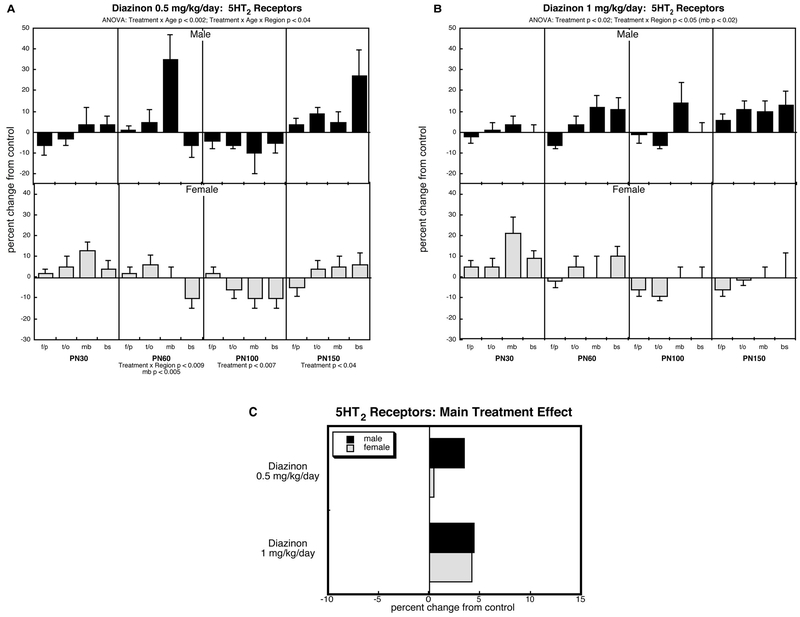
5HT2 receptors (Figure 6).
Figure 6.
Effects of perinatal diazinon exposure on 5HT2 receptor binding: (A) 0.5 mg/kg/day, (B) 1 mg/kg/day. Data represent means and standard errors, presented as the percent change from control values; complete original data are shown in Supplement Table S6. Multivariate ANOVA for each treatment appears at the top of the panels. Lower-order tests were carried only where justified by interactions of treatment with other variables. Panel (C) shows the simple main treatment effects, collapsed across age and region. Abbreviations: f/p, frontal/parietal cortex; t/o, temporal/occipital cortex; mb, midbrain; bs, brainstem.
Across all groups, ANOVA identified effects on 5HT2 receptors, characterized by interactions of treatment × age (p < 0.02) and treatment × region × age (p < 0.05). Accordingly, we examined each treatment for differences from control, focusing on interactions with region and age, but not with sex; although sex was still retained as a factor in the statistical analysis, any sex-related interactions were ignored because of the absence of a significant interaction in the higher-order test.
In contrast to the robust deficits seen for diazinon’s effects on 5HT1A receptors, the effects on 5HT2 receptors were in the opposite direction and were more modest in magnitude. At an exposure of 0.5 mg/kg/day, there were age- and region-specific increases (Figure 6A) which became more consistent (significant main treatment effect) at the higher dose (Figure 6B). The collapsed view of the main treatment effects displays the overall net increases in 5HT2 binding evoked by diazinon exposure (Figure 6C).
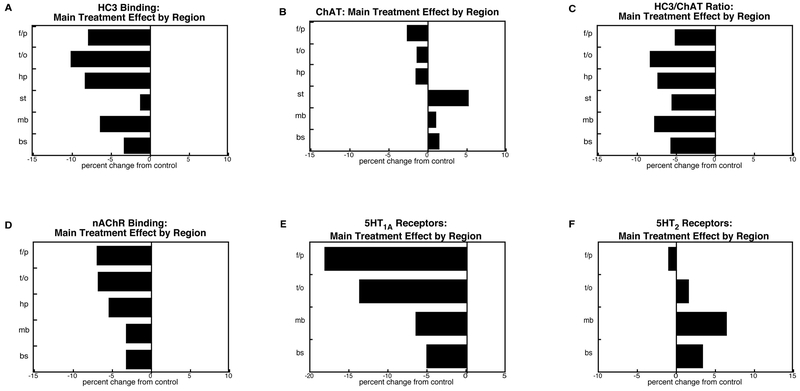
Regional selectivity (Figure 7).
Figure 7.
Regional selectivity of diazinon, displayed as the main treatment effect collapsed across dose, sex and age: (A) HC3 binding, (B) ChAT activity, (C) HC3/ChAT ratio, (D) nAChR binding, (E) 5HT1A receptors, (F) 5HT2 receptors. Regional selectivity was statistically significant across the presynaptic ACh markers (HC3, ChAT), as evidenced by a treatment × region interaction (p < 0.02); values were significantly different from control for frontal/parietal cortex (f/p, p < 0.03), temporal/occipital cortex (t/o, p < 0.0004) and hippocampus (hp, p < 0.003) but not for striatum (st), midbrain (mb) and brainstem (bs). Likewise, for the adaptive receptor markers (nAChR binding, 5HT1A receptors, 5HT2 receptors), regional selectivity was apparent (treatment × region interaction, p < 0.02), with significant differences for frontal/parietal cortex (p < 0.0001), temporal/occipital cortex (p < 0.004) and midbrain (p < 0.05), but not brainstem.
In the analyses above, we separated the data into males and females, and subdivided into individual biomarkers. Whereas this made the sex selectivity of many of the effects readily discernible, it obscured the regional selectivity (treatment × region interaction) that had been apparent in the higher-order tests, that had combined values across sexes and the different measures: ACh biomarkers, p < 0.02 for the interaction; 5HT receptor biomarkers, p < 0.01. To visualize the regional differences, we collapsed the diazinon treatment effects across dose, age and sex and then reevaluated the statistical outcomes grouped by target mechanisms that shared the same regional determinations: presynaptic ACh markers (HC3, ChAT; all six regions), and the “adaptive” receptor responses (nAChR binding, 5HT receptor subtypes; statistical analysis across the four regions for which all three were determined). For the presynaptic ACh markers (HC3, Figure 7A; ChAT, Figure 7B), there was a significant treatment × region interaction (p < 0.02), with a clear pattern of deficits predominating in the cerebrocortical regions and hippocampus, lesser effects in the midbrain and brainstem, and least in the striatum. Thus, although the deficit in the HC3/ChAT ratio was fairly uniform across all the regions (Figure 7C), there were regional differences in the mechanism underlying the subnormal values. For frontal/parietal cortex, temporal/occipital cortex and hippocampus, both HC3 binding and ChAT were reduced, but the effect on HC3 binding was much larger. For the midbrain and brainstem, there were smaller effects on HC3 binding superimposed on a slight increase in ChAT, leading to a similar drop in the HC3/ChAT ratio. For the striatum, an increase in ChAT was the predominant contributor to the reduced ratio.
For the adaptive receptor biomarkers, there was also a significant treatment × region interaction (p < 0.02), connoting regional selectivity of diazinon’s effects. As with the ACh presynaptic markers, the reductions in nAChRs (Figure 7D) and 5HT1A receptors (Figure 7E) were most prominent in the cerebrocortical regions as opposed to the midbrain and brainstem. For the 5HT2 subtype, the regional disparities reflected elevations in the latter two regions, but not in the cerebrocortical areas (Figure 7F).
DISCUSSION
Our results point to four main conclusions. First, continuous perinatal diazinon exposure produced ACh and 5HT deficits that were more consistent and intense than those seen when exposure was restricted to a four-day window in the postnatal period (Slotkin et al. 2008a, b), indicating that vulnerability extends over a wider developmental span. Second, despite the greater effects of continuous perinatal diazinon exposure, the basic pattern of effects resembled that of the shorter, postnatal treatment paradigm. On a regional basis, this was characterized by targeting of cerebrocortical regions and the hippocampus, but sparing of the striatum and of earlier-developing regions, the midbrain and brainstem. With brief postnatal treatment, it could be argued that the exposure occurred after the spike of neurogenesis and neurodifferentiation for the spared regions (Rodier 1988). However, in the current study, the exposure continued throughout all phases of prenatal and early postnatal neurodevelopment, thus bracketing neurogenesis and neurodifferentiation for all the brain areas, and yet we still saw the same regional sparing. This indicates that diazinon specifically targets the regulation of ACh and 5HT synaptic activity in neural pathways that project to cerebrocortical regions and the hippocampus, again pointing to underlying defects in the regulation of synaptic activity; future studies will need to address the underlying mechanisms for regional selectivity.
The third main conclusion is that females were more sensitive than males, a distinction that runs opposite to that of most other neurotoxicants, for which males are typically more vulnerable (Kern et al. 2017); again, this mirrors the pattern seen with short-term postnatal exposure (Slotkin et al. 2008a, b), and the reasons for this specificity need to be explored. Fourth, the regional and sex selectivities seen for diazinon did not match those obtained with chlorpyrifos exposure, whether given in prenatal or postnatal periods (Aldridge et al. 2004; Dam et al. 1999; Qiao et al. 2003, 2004; Slotkin and Seidler 2005, 2007). Even more critically, our earlier work with chlorpyrifos showed compensation for the presynaptic deficits in ACh function, in the form of upregulation of nAChRs and 5HT receptors (Aldridge et al. 2004, 2005a; Slotkin et al. 2013; Slotkin and Seidler 2005). With diazinon exposure, not only did these compensatory effects not occur, but the receptors were actually downregulated, effects that would serve to augment the adverse consequences of presynaptic ACh deficits. The differences between diazinon and chlorpyrifos further point to a lack of dependence of their neurodevelopmental effects on their common attribute as cholinesterase inhibitors. Indeed, the organophosphate doses used in both sets of studies were below the threshold for any detectable cholinesterase inhibition.
Globally, perinatal diazinon exposure impaired the development of ACh presynaptic activity, evidenced by reductions in the HC3/ChAT ratio in every brain region (Figure 7C). The deficits reflected a much greater, negative impact on HC3 binding compared to ChAT. We can thus rule out the possibility that diazinon simply leads to loss of ACh neurons, which would have produced a parallel loss in both markers. Instead, there appears to be a functional defect in the regulation of ACh presynaptic activity, since HC3 binding is responsive to neural activity but ChAT is not (Slotkin 2008). Indeed, superimposed on this general pattern, there were specific regional differences in the contribution of HC3 and ChAT components (Figure 7A, 7B). The cerebrocortical regions and hippocampus displayed the largest reductions in HC3 binding, whereas slight increases in ChAT contributed to the lowered ratio in the other regions, especially the striatum. The same regional disparities carried over into the expression of nAChRs and 5HT receptors: greater deficits for nAChRs and 5HT1A receptors in cerebrocortical regions compared to midbrain and brainstem (Figure 7D, 7E), and compensatory upregulation of 5HT2 receptors only in the latter two regions (Figure 7F). Taken together, this demonstrates a clear regional pattern of vulnerability: greatest deficits in regions with high ACh and 5HT synaptic terminal density (cerebrocortical regions, hippocampus) but lesser effects in earlier-developing regions enriched in neuronal cell bodies for these transmitter systems (midbrain, brainstem). This is exactly the same pattern seen when diazinon administration was restricted to a brief postnatal window (Slotkin et al. 2008a, b), indicating that the regional selectivity is a characteristic of this particular organophosphate, and not reflective of regional differences in a critical window of vulnerability. Further, the fact that there is regional selectivity specific to each marker indicates that diazinon does not simply repress expression of the ACh- and 5HT-related proteins, as then, the regions would all show the same defects.
Perhaps the most important part of our findings is the distinction between the effects of diazinon as compared to our earlier results for the effects of chlorpyrifos on the same biomarkers (Aldridge et al. 2004, 2005a; Slotkin et al. 2013). First, both sets of studies utilized exposures that had little or no impact on cholinesterase activity; in the present study, we found no reduction in cholinesterase, and could have detected an effect as small as a 6% reduction, well below the 70% inhibition threshold required to elicit functional cholinergic hyperstimulation (Clegg and van Gemert 1999). Our studies with chlorpyrifos were likewise conducted with exposures at or below the threshold for barely-detectable cholinesterase inhibition (Qiao et al. 2002; Song et al. 1997). With the matching of exposures on the basis of the cholinesterase biomarker, both organophosphates had deleterious effects on the development of ACh presynaptic activity but they differed substantially in regional- and sex-selectivity, and most critically, in their abilities to elicit compensatory changes in nAChRs and 5HT receptors. Chlorpyrifos produced much greater loss of ACh presynaptic activity in males, without discernible regional selectivity (Slotkin et al. 2013); as seen here, the lower dose of diazinon affected females to a greater extent than males for both HC3 binding (Figure 1A) and ChAT (Figure 2A), superimposed on a distinct regional hierarchy (Figures 7A, 7B). But the biggest differences between the two organophosphates were seen for the compensatory receptor responses. Whereas chlorpyrifos produces upregulation of nAChRs and 5HT receptors (Aldridge et al. 2004, 2005a; Slotkin et al. 2013), diazinon elicited significant overall decreases in nAChRs and the 5HT1A receptor subtype, effects that would serve to exacerbate the presynaptic functional deficits. Unlike the effects of chlorpyrifos, these two receptor changes again showed preference for females (Figures 4A, 5A). Slight upregulation was detected for the 5HT2 subtype but the magnitude of the effect was modest compared to the loss of the other two receptors. The critical differences between the main treatment effects of chlorpyrifos and diazinon are summarized in Table 1, along with a comparison of short-term (PN1-4) diazinon to continuous perinatal exposure as studied in the present paper. The latter indicates an intensification of diazinon’s effects with the more prolonged exposure, but the overall same pattern with one exception (sex selectivity for 5HT1A receptors).
TABLE 1.
Comparative Main Treatment Effects of Chlorpyrifos and Diazinon
| Chlorpyrifos PN1-4 (Aldridge et al. 2004; Slotkin et al. 2013) | Diazinon PN1-4 (Slotkin et al. 2008a,b) | Perinatal Diazinon (this study) | |
|---|---|---|---|
| HC3 binding | ↓↓ male effect > female |
↓↓ female effect > male |
↓↓ female effect > male |
| ChAT activity | ↓ female effect > male |
↓ female effect > male |
↓ female effect > male |
| HC3/ChAT ratio | ↓↓ male effect > female |
↓ | ↓↓ |
| nAChR binding | ↑↑ male effect > female |
↓ female effect > male |
↓↓ female effect > male |
| 5HT1A receptors | ↑↑ male effect > female |
↓ male effect > female |
↓↓ female effect > male |
Interestingly, the distinctions between chlorpyrifos and diazinon are equally demonstrable with in vitro models of neurodifferentiation (Slotkin and Seidler 2008, 2009): whereas both organophosphates impair emergence of the ACh phenotype, chlorpyrifos promotes overall expression of nAChR subunits and 5HT receptors, whereas diazinon largely suppresses them. This points to direct mechanistic differences between the effects of the two organophosphates on neural development. In any case, our results lead to a prediction of more deleterious neurobehavioral deficits after diazinon exposure as compared to chlorpyrifos; behavioral studies to confirm this prediction are nearing completion.
Finally, the disparities between the effects of diazinon and chlorpyrifos emphasize the fact that the developmental neurotoxicity of organophosphates is not “one-size-fits-all.” More specifically, at exposures below the threshold for cholinesterase inhibition, there are distinct dissimilarities in regional- and sex-selectivities, and in the adaptive changes that serve to offset presynaptic ACh deficits. Consequently, it is problematic to set safety thresholds for one organophosphate on the basis of another, especially if such comparisons are based on an inadequate biomarker such as cholinesterase inhibition.
Supplementary Material
Highlights.
Diazinon was given from gestation through postnatal week 2
Exposures were below the threshold for cholinesterase inhibition
Acetylcholine (ACh) and serotonin (5HT) systems were impaired into adulthood
ACh presynaptic neuronal activity, ACh and 5HT receptors were all reduced
Diazinon worse than chlorpyrifos, which shows compensatory receptor upregulation
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [grant number ES010356.
Abbreviations:
- 5HT
serotonin (5-hydroxytryptamine)
- Ach
acetylcholine
- ANOVA
analysis of variance
- ChAT
choline acetyltransferase
- HC3
hemicholinium-3
- nAChR
nicotinic acetylcholine receptor
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: TAS has received consultant income in the past three years from Pardieck Law (Seymour, IN), Gjording Fouser (Boise, ID), Thorsnes Bartolotta McGuire (San Diego, CA), Walgreen Co. (Deerfield, IL) and Cracken Law (Dallas, TX).
REFERENCES
- Abreu-Villaça Y and Levin ED (2017) Developmental neurotoxicity of succeeding generations of insecticides. Environ. Intl 99, 55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ and Slotkin TA (2005a) Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 113, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ and Slotkin TA (2005b) Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health Perspect. 113, 1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ and Slotkin TA (2004) Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 112, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsopoulos VP, Hernandez AF, Liesivuori J and Tsatsakis AM (2013) A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology 307, 89–94. [DOI] [PubMed] [Google Scholar]
- Bellinger DC (2012) Comparing the population neurodevelopmental burdens associated with children’s exposures to environmental chemicals and other risk factors. Neurotoxicology 33, 641–643. [DOI] [PubMed] [Google Scholar]
- Casida JE and Quistad GB (2004) Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol 17, 983–998. [DOI] [PubMed] [Google Scholar]
- Clegg DJ and van Gemert M (1999) Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J. Toxicol. Environ. Health 2, 211–255. [DOI] [PubMed] [Google Scholar]
- Costa LG (2006) Current issues in organophosphate toxicology. Clin. Chim. Acta 366, 1–13. [DOI] [PubMed] [Google Scholar]
- Dam K, Garcia SJ, Seidler FJ and Slotkin TA (1999) Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev. Brain Res. 116, 9–20. [DOI] [PubMed] [Google Scholar]
- Dani JA and De Biasi M (2001) Cellular mechanisms of nicotine addiction. Pharmacol. Biochem. Behav 70, 439–446. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Anders V and Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol 7, 88–95. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Crumpton TL and Slotkin TA (2001) Does the developmental neurotoxicity of chlorpyrifos involve glial targets? Macromolecule synthesis, adenylyl cyclase signaling, nuclear transcription factors, and formation of reactive oxygen in C6 glioma cells. Brain Res. 891, 54–68. [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Homme KG, King PG, Bjorklund G, Chirumbolo S and Geier MR (2017) Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol. Exp. 77, 269–296. [PubMed] [Google Scholar]
- Klemm N and Kuhar MJ (1979) Post-mortem changes in high affinity choline uptake. J. Neurochem 32, 1487–1494. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ and Slotkin TA (2001) Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev. Brain Res. 130, 83–89. [DOI] [PubMed] [Google Scholar]
- Maes M and Meltzer H (1995) The serotonin hypothesis of major depression In: Bloom FE, Kupfer DJ, Bunney BS, Ciaranello RD, Davis KL, Koob GF, Meltzer HY, Schuster CR, Shader RI and Watson SJ (Eds), Psychopharmacology: The Fourth Generation of Progress, Raven Press, New York, pp. 933–944. [Google Scholar]
- Mauro RE and Zhang L (2007) Unique insights into the actions of CNS agents: lessons from studies of chlorpyrifos and other common pesticides. CNS Agents Med. Chem. 7, 183–199. [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG and Wallace KB (1998) Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol. Sci 41, 8–20. [DOI] [PubMed] [Google Scholar]
- Pancetti F, Olmos C, Dagnino-Subiabre A, Rozas C and Morales B (2007) Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase. J. Toxicol. Env. Health B 10, 623–630. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Abreu-Villaça Y, Tate CA, Cousins MM and Slotkin TA (2004) Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Dev. Brain Res. 148, 43–52. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S and Slotkin TA (2002) Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ. Health Perspect. 110, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM and Slotkin TA (2003) Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ. Health Perspect. 111, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA and Peterson BS (2012) Brain anomalies in children exposed to a common organophosphate pesticide. Proc. Natl. Acad. Sci 109, 7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendorfer H and Creton R (2015) Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology 49, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM (1988) Structural-functional relationships in experimentally induced brain damage. Prog. Brain Res. 73, 335–348. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA and Levin ED (2008) Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res. Bull. 75, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S and Seidler FJ (2017) Diazinon and parathion diverge in their effects on development of noradrenergic systems. Brain Res. Bull. 130, 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA (2005) Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos In: Gupta RC (Ed), Toxicity of Organophosphate and Carbamate Pesticides, Elsevier Academic Press, San Diego, pp. 293–314. [Google Scholar]
- Slotkin TA (2008) If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol. Teratol 30, 1–19. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED and Seidler FJ (2008a) Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ. Health Perspect. 116, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Infante A and Seidler FJ (2013) Prenatal dexamethasone augments the sex-selective developmental neurotoxicity of chlorpyrifos: implications for vulnerability after pharmacotherapy for preterm labor. Neurotoxicol. Teratol 37, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA and Seidler FJ (2001) Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 902, 229–243 [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED and Seidler FJ (2008b) Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res. Bull. 75, 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2005) The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev. Brain Res. 158, 115–119. [DOI] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2007) Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod. Toxicol 23, 421–427. [DOI] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2008) Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol 233, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA and Seidler FJ (2009) Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res. Bull. 78, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED and Seidler FJ (2006) Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 114, 1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ and Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal. Biochem 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S and Slotkin TA (1997) Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol 145, 158–174. [DOI] [PubMed] [Google Scholar]
- Spyker JM and Avery DL (1977) Neurobehavioral effects of prenatal exposure to the organophosphate diazinon in mice. J. Toxicol. Environ. Health 3, 989–1002. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA and Levin ED (2008) Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Teratol 30, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Geological Survey. (2018a) Estimated Annual Agricultural Pesticide Use: Pesticide Use Maps - Chlorpyrifos. http://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2015&map=CHLORPYRIFOS&hilo=L&disp=Chlorpyrifos [accessed 16 Jan 2019].
- U.S. Geological Survey. (2018b) Estimated Annual Agricultural Pesticide Use: Pesticide Use Maps - Diazinon. http://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2012&map=DIAZINON&hilo=L&disp=Diazinon [accessed 16 Jan 2019]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.