Abstract
Background
Hyperammonemia is a common cause of metabolic encephalopathy, mainly related to hepatic cirrhosis. Numerous nonhepatic etiologies exist but they are infrequent and not well known, thus, leading to misdiagnosis and inadequate care. Electroencephalography has a proven diagnostic and prognostic role in comatose patients. Burst suppression is a preterminal pattern found in deep coma states and is rarely associated with metabolic causes.
Case presentation
We report the case of an 81-year-old Caucasian man presenting with rapidly progressive somnolence and mutism. Soon after his arrival in our hospital, he developed profound coma. A comprehensive diagnostic workup was unremarkable except for admission electroencephalography showing diffuse slowing of cerebral activity with an intermittent pattern of burst suppression. He was admitted to our intensive care unit for supportive care where malnutrition-related hyperammonemia was diagnosed. His clinical course was spontaneously favorable and follow-up electroencephalography demonstrated normal cerebral activity.
Conclusions
Nonhepatic hyperammonemia is a rare and potentially reversible cause of encephalopathy. Ammonia level measurement should be part of the diagnostic workup in patients with unexplained coma, particularly in the setting of nutritional deficiencies or nutritional supply. Detection of diffuse and nonspecific mild to moderate slowing of cerebral activity (theta-delta ranges) on electroencephalography is common. In contrast, to the best of our knowledge, burst suppression has never been described in association with hyperammonemia.
Keywords: Hyperammonemia, Coma, Malnutrition, Burst suppression, EEG
Background
Ammonia (NH3+) and its associated acid ammonium (NH4+) result from the catabolism of amino acids (AA). For simplicity, in this case report the term ammonia refers to both forms. In healthy individuals, the main source of ammonia is digestive from intestinal bacterial degradation of proteins and urea. Catabolism of AA continually produces ammonia in human organs. The kidney is another main source, which produces ammonia in the proximal tubular cells in order to buffer H+ in urine. Elimination of ammonia in a healthy individual takes place in the liver, where it is transformed into urea by periportal hepatocytes (the urea cycle). Urea, a nontoxic molecule, is subsequently eliminated in the urine and colon. A second key actor of ammonia metabolism is glutamine synthase (GS), which produces glutamine by incorporating ammonia in glutamate. This enzyme is present in periportal hepatocytes and prevents the release of residual ammonia into the systemic circulation. Produced glutamine is then transported to the small intestine, where ammonia is released, and then carried back to the liver via the portal system. GS is also expressed in muscles, kidneys, and astrocytes acting as an initial adaptive mechanism in case of hyperammonemia. Finally, the kidney can increase ammonia excretion in urine from 30% up to 70% [1–3]. Ammonia gets access to the brain through passive diffusion and mediated transport. It is then combined with glutamate by astrocytes to produce glutamine, which is provided to neurons, reversed back to glutamate and then released as a neurotransmitter. When present in an excessive amount, ammonia has a selective neurotoxicity. Clinical manifestations range from irritability to seizure, coma, cerebral herniation, and death [2]. Hosting the urea cycle, the liver plays an essential role in ammonia detoxification; thus, up to 90% of cases of hyperammonemia are related to hepatic diseases. Apart from hepatic dysfunction, two mechanisms may lead to ammonia excess: increased production and decreased metabolism (Table 1). Ammonia production is raised in cases of exaggerated protein supply (enteral or parenteral), muscle protein catabolism (seizure, trauma, malnutrition), urinary tract infection with urease producing bacteria, and hemato-oncological disorders. Ammonia elimination is altered in cases of inborn errors of metabolism, by medication interfering with urea cycle (for example, valproate), and by portosystemic shunting. Constitutional defects of ammonia metabolism may only be apparent in adulthood, following a precipitating event [3].
Table 1.
Mechanism leading to nonhepatic hyperammonemia: increased production and decreased metabolism
| Overproduction | Undermetabolism |
|---|---|
| Excessive protein supply (enteral or parenteral) | Portosystemic shunting |
| Muscles catabolism: cachexia, starvation, seizure | Medications interfering with urea cycle: valproic acid, carbamazepine, salicylate, glycine, ribavirin |
| Urease producing bacteria urinary tract infections: Proteus mirabilis, Klebsiella species, Escherichia coli, Morganella morganii, Providencia rettgeri, diphtheroids | Inborn error of metabolism: inherited defects of the urea cycle, amino acid transporters, fatty acid oxidation, organic acid disorders |
| Hematological malignancies: multiple myeloma, leukemia |
Electroencephalography (EEG) has a well-recognized role in the evaluation of altered consciousness states. It is a diagnostic and monitoring tool, providing prognostic information. With worsening of metabolic encephalopathies, gradual EEG progression is observed, similar to changes described in drug-induced coma. As the amplitude increases, the dominant EEG frequency declines and the length of flat periods increases toward a completely isoelectric trace [4]. The burst suppression pattern consists of alternative periods of slow waves with high amplitude (the burst phase) and periods of marked activity depression (the suppression phase). It is associated with profound coma of various etiologies (cerebral anoxia, hypothermia, anesthesia, drugs intoxications) [5]. To the best of our knowledge, we report here the first case of an adult with hyperammonemic coma presenting with burst suppression on admission EEG.
Case presentation
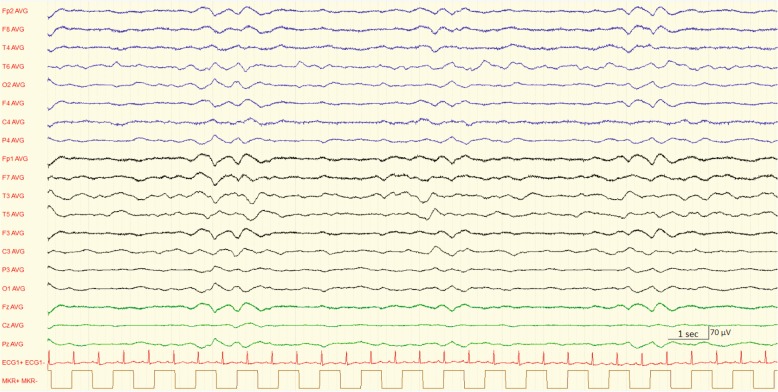
An 81-year-old Caucasian man known only for benign prostatic hyperplasia was referred for loss of appetite and poor food intake, with rapidly progressing somnolence and mutism. On admission at our emergency department, he developed a deep coma state. A physical examination revealed an alteration of consciousness (Glasgow Coma Scale 4/15) without any other relevant signs. Routine laboratory values were in the normal range. A urine culture showed no bacterial growth and toxicology screening was negative. A head computed tomography (CT) scan was normal and cerebrospinal fluid analysis showed mildly raised proteins (664 mg/l), with no white cells. Non-convulsive status epilepticus was suspected, and he was treated with a loading dose of levetiracetam (1600 mg intravenously administered) before being admitted to our intensive care unit (ICU). The EEG performed on admission (Fig. 1) showed a diffuse slow activity with an intermittent burst suppression pattern. In the absence of any other evident cause for coma, his ammonium level was measured and proved to be high (259 μmol/l, normal value < 60), with subsequent confirmation. Supportive care was initiated, and evolution at 48 hours showed complete clinical recovery and normalization of blood ammonia level. A follow-up EEG demonstrated normal cerebral activity. The etiological assessment following clinical recovery revealed low blood levels of several essentials AA pointing to malnutrition-related hyperammonemia. He was satisfied with the care he received.
Fig. 1.
Electroencephalography at admission with burst suppression pattern: periods of slow waves (the burst) alternate with period of electroencephalography activity suppression (LoFil 0.53 Hz, Hifil 70 Hz)
Discussion and conclusions
We describe the case of a patient presenting with a rapidly progressive coma state and an unusual burst suppression EEG pattern. An initial diagnostic workup failed to reveal any evident etiology, including hepatic dysfunction. Dosing of ammonia level allowed us to diagnose nonhepatic hyperammonemic encephalopathy (NHHE). Although this rare condition is usually associated with a poor prognosis [6], our patient’s clinical course with supportive care was marked by complete clinical recovery. This case highlights the necessity of including ammonia level measurement in the diagnostic workup of unexplained coma, even in the absence of hepatic dysfunction. Early diagnosis is important as it may be lethal if unrecognized and untreated. Severe hepatic hyperammonemic encephalopathy and NHHE usually induce a nonspecific diffuse symmetric slowing of electrographic cerebral activity ranging from theta to delta waves. The only report we found of burst suppression was in a 13-year-old girl with high-dose (> 50 mg/kg) valproic acid-induced hyperammonemic encephalopathy. However, causal association was not assessable due to many confounding factors, particularly the administration of high-dose and combined anti-epileptic drugs (midazolam, lorazepam, fosphenytoin, and phenobarbital) before EEG was performed [7]. We therefore believe that our case describes the first association between such an EEG pattern and hyperammonemic encephalopathy, moreover, with spontaneous complete recovery. Although probably rare, this combination is certainly underreported, either because measurement of ammonia levels is not routinely performed, or because EEG and ammonia measurement are usually not done simultaneously.
Acknowledgements
The authors are grateful to Dr Guillermo Toledo, neurologist, for EEG images and interpretation.
Abbreviations
- AA
Amino acids
- EEG
Electroencephalography
- GS
Glutamine synthase
- NHHE
Nonhepatic hyperammonemic encephalopathy
Authors’ contributions
AL conceived and drafted the first version of the paper. MP contributed in drafting the paper. TF revised the manuscript. AL, MP, and TF were in charge of the patient care. All authors read and approved the final manuscript.
Funding
No funding sources.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antonio Leidi, Email: antoleidi@hotmail.com.
Marisa Pisaturo, Email: Marisa.Pisaturo@ghol.ch.
Thierry Fumeaux, Email: Thierry.Fumeaux@ghol.ch.
References
- 1.Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24:169–181. doi: 10.1007/s11011-008-9122-5. [DOI] [PubMed] [Google Scholar]
- 2.Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012;49:214–228. doi: 10.1258/acb.2011.011206. [DOI] [PubMed] [Google Scholar]
- 3.Cichoż-Lach H, Michalak A. Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J Gastroenterol. 2013;19:26–34. doi: 10.3748/wjg.v19.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009;50:38–39. doi: 10.1111/j.1528-1167.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakusic A, Sabov M, McCambridge AJ, Rabinstein AA, Singh TD, Mukesh K, et al. Features of Adult Hyperammonemia Not Due to Liver Failure in the ICU. Crit Care Med. 2018;46:e897–e903. doi: 10.1097/CCM.0000000000003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherian KA, Legatt AD. Burst Suppression Pattern on Electroencephalogram Secondary to Valproic Acid-Induced Hyperammonemic Encephalopathy. Pediatr Neurol. 2017;73:88–91. doi: 10.1016/j.pediatrneurol.2016.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.