Abstract
Purpose: Many adult survivors of childhood cancer are at high-risk of developing cardiovascular disease. Cancer therapy may cause damage to the vascular endothelium, thereby initiating atherosclerosis. Atorvastatin has been shown to improve endothelial function independent of reducing cholesterol, as well as reduce/slow arterial stiffness and thickening, yet has never been studied in childhood cancer survivors (CCS).
Methods: Twenty-seven young adult (age 26.8 ± 6.2 years) survivors of childhood acute lymphoblastic leukemia or Non-Hodgkin's lymphoma were randomly assigned (1:1) 40 mg/day of atorvastatin or placebo for 6 months. Brachial artery flow-mediated dilation (FMD), small artery reactive hyperemia index (RHI), arterial stiffness, and carotid artery elasticity/thickness were assessed.
Results: Fifteen participants completed the trial. No significant treatment effect for any vascular outcomes was observed at 6 months; however, a significant decrease in peak FMD (−3.0 [95% confidence interval [CI]: −5.3, −0.7]) and a trending significant decrease in RHI (−0.3 [95% CI: −0.62, 0.01]) was observed in the placebo group, resulting in a trend toward a treatment effects (p < 0.10). No effect on arterial stiffness, carotid arterial elasticity, or thickness was observed.
Conclusion: Six months of atorvastatin treatment did not improve endothelial function or arterial stiffness in young adult CCS. While a trend toward an improvement in endothelial function was present, findings should be interpreted with caution owing to the small number of evaluable participants and subsequent lack of sufficient power. Further research in a larger sample size is needed to fully elucidate the effects of atorvastatin on vascular function. Trial registered at clinicaltrials.gov as NCT01733953.
Keywords: statin, vascular, cardiovascular, trial
Introduction
The prevalence of childhood cancer survivorship has increased over the past three decades.1 More effective surgical interventions, radiotherapy, and risk-stratified chemotherapeutic approaches have led to dramatic improvements in survival rates for many childhood cancers.2–4 These same cancer therapies are also thought to be responsible for many neurocognitive, metabolic, and cardiovascular complications experienced by survivors.5
Although free of cancer, childhood cancer survivors (CCS) are plagued by a higher incidence of abnormal growth and development,6 endocrine disorders,7 premature cardiovascular disease (CVD), and cerebrovascular disease,8–11 as well as declines in neurocognitive function.12–14 Indeed, many adult survivors of childhood cancer are at seven times the risk of dying from CVD compared with the general population.8,15 Most of the increased risk is thought to be the result of the therapies used to treat the cancer, such as chemotherapy and radiation. These therapies likely cause damage to the endothelial cells, which line the arterial wall and, when functioning properly, offer protection from atherosclerosis.16–19 Research has demonstrated that young adult survivors of childhood acute lymphoblastic leukemia (ALL) have endothelial dysfunction compared with healthy controls ∼20 years after receiving cancer treatment.20,21 In addition, adult survivors of childhood ALL have reduced carotid compliance and distensibility (i.e., arterial stiffness) compared with healthy sibling controls.21 Endothelial dysfunction and arterial stiffness are considered early manifestations of atherosclerosis, and therefore may be ideal therapeutic targets to reduce CVD risk.22,23
HMG coenzyme A reductase inhibitors, or statins, are widely used for CVD risk reduction because of their ability to lower circulating low-density lipoprotein (LDL) cholesterol and triglycerides.24 However, there are multiple cholesterol-independent beneficial pleiotropic effects of statins on vascular health,25 including (1) upregulation of endothelial nitric oxide synthase resulting in increased production of nitric oxide26,27; (2) inhibition of arterial smooth muscle cell proliferation28; (3) reduction of arterial stiffness29,30; (4) inflammation31; and (5) oxidative stress.32–34 Therefore, statin treatment, independent of cholesterol lowering, may improve endothelial function and other aspects of vascular health in young adult CCS, which could mitigate the medium- and long-term risk of developing CVD.
Despite the potential for improved vascular health and reduced CVD risk, to our knowledge, statin therapy has never been evaluated in young adult CCS. To improve upon long-term survival outcomes in young adult CCS, trials evaluating the potential benefit of pharmacotherapies are profoundly important to mitigate the therapeutic-induced damage to the vasculature. Detailing the findings of such pharmacologic trials is critical to provide insight into trial conduct, and thereby improve upon future trial design and conduct.
Therefore, the primary objective of this pilot clinical trial was to assess the ability of atorvastatin to improve brachial artery endothelial function (primary endpoint) and other aspects of vascular health, including small artery endothelial function, regional arterial stiffness, and carotid artery elasticity and thickening, in young adult CCS over a 6-month treatment period. The focus of the trial was on survivors of hematologic malignancies, ALL and Non-Hodgkin's lymphoma (NHL). Not only do both ALL and NHL survivors share common treatment exposures (chemotherapy and radiation) but also ALL has been shown to be associated with endothelial impairments. We hypothesized that atorvastatin would significantly increase brachial artery and small artery endothelial function, carotid artery elasticity, as well as reduce arterial thickness in young adult survivors of ALL and NHL compared with placebo.
Study Design and Methods
The study protocol was approved by the University of Minnesota Institutional Review Board (IRB) and the Children's Hospitals and Clinics of Minnesota IRB. Informed consent was obtained from all participants.
Study population and eligibility criteria
Twenty-seven young adult survivors of childhood ALL and NHL between 18 and 39 years of age, who were treated for cancer before the age of 21 years and who were ≥5 years post-treatment, were eligible to participate. Participants were recruited from the childhood Cancer Survivorship Program at the University of Minnesota and Children's Hospitals and Clinics of Minnesota to participate in the randomized, double-blind, placebo-controlled, pilot clinical trial from 2013 to 2014. Due to historical treatment heterogeneity among Hodgkin's lymphoma and NHL survivors, adult survivors of childhood Hodgkin's lymphoma were not recruited. Cancer diagnosis and treatment details were obtained from medical chart extraction.
Before enrollment, participants underwent detailed screening assessments, including review of family/personal history, current medical status, and medication usage. Initial exclusion criteria included the following: prior bone marrow transplant (BMT); Type 1 or Type 2 diabetes mellitus; LDL ≥130 mg/dL; alanine transaminase (ALT), aspartate transaminase (AST), or creatine kinase (CK) >2 × the upper limit of normal; recent (within 6 months) use of lipid-lowering medication; recent initiation (within 6 months) of antihypertensive medication (stable therapy allowed); recent (within 6 months) use of fibric acid derivatives, lipid-modifying doses of niacin, cyclosporine, or strong CYP3A4 inhibitors; and females who were pregnant or planning to become pregnant. Individuals with elevated LDL-cholesterol were referred for clinical management of dyslipidemia. To combat low enrollment, exclusion criteria were modified midway through enrollment to include participants treated with BMT, as well as participants with baseline LDL ≤160 mg/dL (originally ≤130 mg/dL), to reflect the 2013 American College of Cardiology/American Heart Association Guidelines.24
Experimental design and protocol
The present trial was designed as a randomized, double-blind, placebo-controlled, pilot clinical trial conducted at the University of Minnesota-Twin Cities campus. Participants were recruited, screened, and randomized (1:1) to 40 mg/day of atorvastatin or placebo for 6 months. Randomization was stratified by receipt of BMT. Inpatient testing at baseline and month 6 included assessments of (1) anthropometrics, (2) metabolic panel; (3) vascular function and structure; and (4) safety assessments (blood draw, adverse event review). Participants returned at months 1 and 3 for additional anthropometric assessment, drug compliance (pill count), and review of participant safety. Phone calls were conducted at months 2, 4, and 5 to review interim medical history, changes in medication, assess adverse events, and encourage retention. A data and safety review was conducted after 50% of participants were enrolled, and at the end of the trial. The trial was to be stopped if more than 25% of subjects were withdrawn due to elevated enzyme levels.
Anthropometric and clinical assessments
Weight was measured in the morning following an overnight fast (Model 5002; ST Scale-Tronix, White Plains, NY) in light clothing, without shoes. Height and blood pressure were also measured. Fasting blood samples were obtained for lipids and plasma glucose (analyzed with standard procedures at the Fairview-University Medical Center clinical laboratory).
Vascular assessments
Testing was performed in the Vascular Biology Laboratory in the University of Minnesota Clinical and Translational Science Institute. All the vascular assessments were performed in a quiet, temperature-controlled environment (22°C–23°C) following an overnight fast. Participants abstained from caffeine ingestion, cigarette smoking, and physical activity for 12-hours before vascular assessments. Baseline assessments were performed between 08:00 and 11:00, with 6-month assessments performed within ±2-hours of baseline assessments.
Endothelial function
Following 15 minutes of quiet rest in the supine position, brachial artery flow-mediated dilation (FMD) and reactive hyperemia index (RHI) (EndoPAT 2000; Itamar Medical, Israel) were assessed simultaneously. Vascular images of the left brachial artery were obtained using a conventional ultrasound scanner (Sequoia 512; Siemens, New York, NY) with a 10.0 MHz linear array probe held at a constant distance from the skin and at a fixed point over the brachial artery by a stereotactic arm. In addition, one peripheral arterial tonometry (PAT) finger probe was placed on the index finger of the left hand undergoing hyperemia testing, and a second PAT probe was placed on the contralateral (control), right index finger. Baseline index finger assessments were obtained for 5 minutes before reactive hyperemia. Both probes were inflated to apply a uniform pressure on the fingers (10 mmHg less than diastolic blood pressure) and detect small pulse volume changes throughout the cardiac cycle.
Following baseline measurements of the brachial artery and index fingers of each hand, a blood pressure cuff was placed on the upper forearm of the left arm (immediately distal to the elbow) and inflated to a suprasystolic level for 5 minutes, using techniques previously described.22 The change in brachial artery blood flow and diameter was measured by B-mode ultrasound imaging for 3 minutes after cuff release. The change in pulse amplitude during reactive hyperemia to derive RHI was measured for 5 minutes. Peak FMD was defined as the greatest percent change from resting brachial artery diameter. Intraindividual reproducibility of peak FMD within our laboratory has been demonstrated as a mean difference of 0.53% ± 0.28%. RHI was expressed as the ratio of the hyperemic and the baseline pulse amplitude (corrected for the same ratio on the control finger, thereby controlling for autonomic tone due to vasoconstriction and transient environmental effects). Higher (increased) peak FMD and RHI reflect better (improved) endothelial function.
Artery elasticity and thickness
Carotid artery images were obtained by a noninvasive ultrasound scanner (Sequoia 512; Siemens, Mountain View, CA) with a 10.0 MHz linear array probe. Following 15 minutes of quiet rest in the supine position, luminal systolic and diastolic diameters were obtained at a fixed point over the left common carotid artery, ∼1-cm proximal from the carotid bulb. Images were collected at 20 frames/s for 10 seconds to ensure the capture of full arterial diameter change during a cardiac cycle. Systolic and diastolic blood pressures were recorded during the 10-second carotid measurement. Mean diameter through the 10-second cycle was used to calculate carotid compliance and distensibility. Carotid artery intima–media thickness measures were obtained using the same technique. Digital image analysis was performed by the same trained reader, blinded to group assignments. The following formulas for carotid artery compliance and distensibility were used:
Diameter distensibility (DD, %): [ΔD/Dmin] × 100%
Cross-sectional distensibility (CSD, %): [(π × (Dmax/2)2 − π × (Dmin/2)2)/π × (Dmin/2)2] × 100%
Diameter compliance (DC, mm/mmHg): ΔD/ΔP
Cross-sectional compliance 1 (CSC1, mm2/mmHg): [(π × (Dmax/2)2 − π × (Dmin/2)2)/(ΔP)]
Cross-sectional compliance 2 (CSC2, 1/mmHg): [(π × (Dmax/2)2 − π × (Dmin/2)2)/(π × (Dmin/2)2 × ΔP)]
Incremental elastic modulus (IEM, mmHg): 3{1+[π × (maxDiamM/2)2/π × (minDiamM/2)2]}/CSC1
Pulse pressure (ΔP) was calculated as the difference between systolic and diastolic pressures. Diameter change (ΔD) was calculated as the difference in arterial diameter at systolic and diastolic pressures. In addition, Dmax denotes maximum diameter and Dmin denotes minimum diameter. Higher (or increased) DD, CSD, DC, CSC1, and CSC2 reflect better (improved) vascular elasticity, whereas higher (increased) IEM reflects worse (decreased) vascular elasticity.
Regional arterial stiffness was also measured noninvasively by carotid-radial pulse wave velocity (PWV) and aortic augmentation index (AI) (SphygmoCor® System, Sydney, Australia). Carotid-radial PWV and aortic AI were derived by automated algorithms from pressure waveforms obtained by lightly applying a tonometer to the skin. AI is a measure of the relative magnitude of the reflected (or retrograde) pulse wave early in the cardiac cycle. PWV is calculated as distance (meter)/transit time (seconds). The distance was measured between the carotid and radial sites and the sternal notch. The SphygmoCor System measures the time between R-waves of the electrocardiogram and the feet of the pressure and distension wave, respectively, at the measurement site. Higher (increased) AI and PWV reflect higher (increased) arterial stiffening.
Sample size determination
Our goal was to collect preliminary data to determine the treatment effect and variability estimates to inform the design of a larger clinical trial. Our sample size (n = 26) was based upon the number of participants who could be feasibly enrolled in 1-year and the amount of funding available. Based on a previous study,29 atorvastatin improved carotid compliance in overweight/obese adults (0.89–1.15 mm2/mmHg). Using data from statin therapy interventions to improve arterial compliance, a sample size of 13 per group would have 80% power to detect a difference in means of 0.260, assuming that the common standard deviation is 0.230, using a two-group t-test with α = 0.05 one-sided significance level. We anticipated a 20% dropout rate; therefore, a sample size of 26 (13 per group) would yield satisfactory power. Due to higher than expected participant withdrawal, we randomized one additional participant (n = 27).
Statistical analyses
All analyses were performed using SAS v9.2 software (SAS Institute, Inc.). All tests were performed with a significance level of α = 0.05, and p < α signified significance. A generalized linear model was used to assess per-protocol treatment effect on vascular changes from baseline, adjusted for individual baseline values.35,36 Baseline characteristics are expressed as means ± standard deviations, unless otherwise noted. Two-sample t-tests constructed from least-squares means were used to compare overall mean changes from baseline at month 6 (ΔM6) for within-group and between-group treatment effect differences (with 95% confidence intervals [CIs]). The primary analysis was performed on a per-protocol population, which included participants with a treatment compliance level of ≥70% of expected dose compliance. An intent-to-treat analysis was also performed on all randomized participants who completed all 6 months of the study according to treatment assignment.
Results
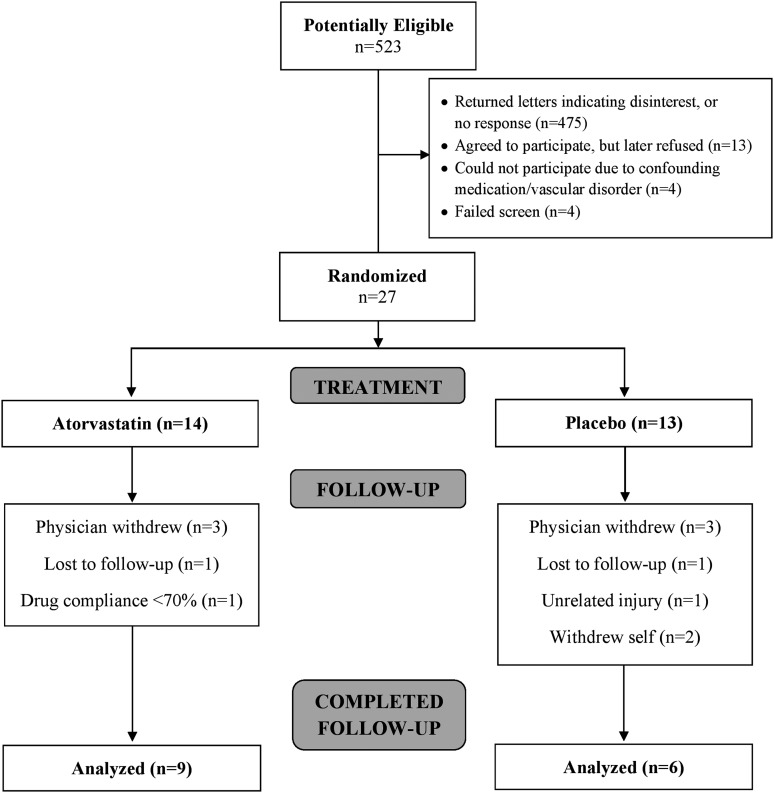
Twenty-seven participants (mean age 26.8 ± 6.2 years; 14 male) with body mass index 31.0 ± 7.5 kg/m2 were randomized. All participants were Caucasian. The Consolidated Standards of Reporting Trials diagram summarizing participant throughput is provided (Fig. 1). Mean baseline demographic and clinical characteristics, as well as baseline vascular characteristics for all randomized participants are presented in Tables 1 and 2, respectively.
FIG. 1.
CONSORT diagram summarizing participant throughput within the trial.
Table 1.
Baseline Demographic and Clinical Characteristics for All Randomized and Study Completing Participantsa
| Randomized | Completed 6-month follow-up | ||||
|---|---|---|---|---|---|
| Overall (N = 27) | Atorvastatin (n = 14) | Placebo (n = 13) | Atorvastatin (n = 9) | Placebo (n = 6) | |
| Age (years) | 26.8 ± 6.2 | 26.6 ± 5.7 | 26.9 ± 6.9 | 28.0 ± 5.7 | 26.4 ± 7.5 |
| Sex (male), n (%) | 14 (51.9%) | 6 (42.9%) | 8 (61.5%) | 3 (33.3%) | 3 (50.0%) |
| Diagnosis age (years) | 7.2 ± 5.2 | 8.0 ± 4.6 | 6.2 ± 5.8 | 7.8 ± 3.9 | 5.0 ± 3.2 |
| Years cancer free (years) | 17.1 ± 7.3 | 16.1 ± 6.6 | 18.2 ± 8.1 | 17.9 ± 7.3 | 18.4 ± 7.6 |
| Diagnosis (n, ALL/NHL) | 19/8 | 10/4 | 9/4 | 6/3 | 5/1 |
| Treatment, n (%) | |||||
| Chemotherapy | 17 (63.0%) | 9 (64.3%) | 8 (61.5%) | 5 (55.6%) | 4 (66.7%) |
| Chemotherapy + radiation | 9 (33.3%) | 4 (28.6%) | 5 (38.5%) | 4 (44.4%) | 2 (33.3%) |
| BMT | 1 (3.7%) | 1 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Weight (kg) | 88.3 ± 22.1 | 89.5 ± 22.8 | 87.0 ± 22.1 | 91.6 ± 21.5 | 72.5 ± 12.3 |
| BMI (kg/m2) | 31.0 ± 7.5 | 31.8 ± 7.9 | 30.1 ± 7.3 | 33.6 ± 7.9 | 25.4 ± 4.8 |
| Systolic BP (mmHg) | 124 ± 15 | 119 ± 13 | 129 ± 14 | 119 ± 15 | 121 ± 14 |
| Diastolic BP (mmHg) | 70 ± 12 | 68 ± 10 | 72 ± 14 | 67 ± 12 | 69 ± 12 |
| Fasting glucose (mg/dL) | 80 ± 17 | 84 ± 11 | 75 ± 21 | 84 ± 11 | 70 ± 31 |
| TC (mg/dL) | 174 ± 35 | 176 ± 41 | 172 ± 29 | 191 ± 33 | 167 ± 27 |
| LDL (mg/dL) | 104 ± 32 | 105 ± 34 | 103 ± 30 | 119 ± 25 | 98 ± 36 |
| VLDL (mg/dL) | 23 ± 9 | 25 ± 10 | 21 ± 8 | 28 ± 9 | 19 ± 7 |
| HDL (mg/dL) | 47 ± 11 | 45 ± 10 | 49 ± 12 | 43 ± 9 | 51 ± 14 |
| Triglycerides (mg/dL) | 116 ± 45 | 12 ± 48.0 | 103 ± 39 | 140 ± 43 | 94 ± 33 |
| TC/HDL ratio | 3.9 ± 1.1 | 4.0 ± 1.2 | 3.7 ± 1.1 | 4.5 ± 1.0 | 3.6 ± 1.3 |
| ALT (U/L) | 39 ± 16 | 37 ± 15 | 41 ± 18 | 35 ± 12 | 28 ± 8 |
| AST (U/L) | 30 ± 10 | 27 ± 12 | 32 ± 9 | 27 ± 9 | 25 ± 2 |
| CK (U/L) | 107 ± 54 | 104 ± 62 | 111 ± 48 | 94 ± 55 | 100 ± 16 |
Values are raw means ± SDs, unless otherwise indicated.
ALL, acute lymphoblastic leukemia; ALT, alanine transaminase; AST, aspartate aminotransferase; CK, creatine kinase; BMI, body mass index; BMT, bone marrow transplant; BP, blood pressure; HDL, high-density lipoproteins; LDL, low-density lipoproteins; NHL, non-Hodgkin's lymphoma; TC, total cholesterol; VLDL, very low-density lipoproteins; SD, standard deviation.
Table 2.
Baseline Vascular Characteristics for All Randomized Participantsa
| All randomized (n = 27) | Atorvastatin (n = 14) | Placebo (n = 13) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Brachial artery ultrasound | |||||||
| Peak FMD (%) | 22 | 6.7 ± 3.7 | 12 | 6.7 ± 3.4 | 10 | 6.7 ± 4.2 | 0.97 |
| EndoPAT | |||||||
| RHI (%) | 25 | 1.9 ± 0.5 | 12 | 2.1 ± 0.5 | 13 | 1.8 ± 0.5 | 0.13 |
| AI (%) | 25 | −7.6 ± 9.8 | 12 | −6.6 ± 11.7 | 13 | −8.6 ± 7.9 | 0.63 |
| Carotid artery ultrasound | |||||||
| cIMT (mm) | 23 | 0.55 ± 0.05 | 13 | 0.56 ± 0.04 | 10 | 0.55 ± 0.06 | 0.46 |
| cDD (%) | 25 | 9.6 ± 2.3 | 14 | 9.6 ± 2.7 | 11 | 9.6 ± 1.8 | 0.99 |
| cCSD (%) | 25 | 20.1 ± 5.0 | 14 | 20.2 ± 5.8 | 11 | 20.1 ± 4.1 | 0.98 |
| cDC (mm/mmHg × 10−3) | 25 | 11.3 ± 2.5 | 14 | 11.7 ± 3.0 | 11 | 10.8 ± 1.6 | 0.41 |
| cCSC1 (mm2/mmHg × 10−3) | 25 | 121 ± 29 | 14 | 124 ± 35 | 11 | 116 ± 17 | 0.47 |
| cCSC2 (1/mmHg × 10−3) | 25 | 3.7 ± 0.9 | 14 | 3.8 ± 1.1 | 11 | 3.5 ± 0.6 | 0.34 |
| cIEM (mmHg) | 25 | 1527 ± 422 | 14 | 1484 ± 444 | 11 | 1583 ± 405 | 0.57 |
| SphygmoCor | |||||||
| PWV (m/s) | 26 | 7.9 ± 2.0 | 14 | 8.1 ± 2.5 | 12 | 7.6 ± 1.0 | 0.45 |
| Carotid AI (P2/P1) | 18 | 117 ± 19 | 9 | 122 ± 23 | 9 | 112 ± 14 | 0.28 |
| Radial AI (P2/P1) | 25 | 119 ± 25 | 13 | 119 ± 32 | 12 | 119 ± 17 | 0.98 |
Values are listed with sample size (italics) and raw means ± SDs. Data are from all randomized participants (27 total survivors; 14 atorvastatin, 13 placebo) who had interpretable data. All reported brachial and carotid artery measurements performed on the left brachial artery and left common carotid artery.
“c”, carotid artery assessment; AI, augmentation index; CSC1, cross-sectional compliance 1; CSC2, cross-sectional compliance 2; CSD, cross-sectional distensibility; DC, diameter compliance; DD, diameter distensibility; FMD, flow-mediated dilation; IEM, incremental elastic modulus; IMT, intima–media thickness; PWV, pulse wave velocity; RHI, reactive hyperemia index.
Per-protocol analyses of mean change in endothelial function from baseline for all participants who completed the trial are provided (Table 3). The placebo group had a significant within-group decrease in peak FMD, which resulted in a trend toward a significant between-group treatment effect (p = 0.09). The placebo group also had a trend toward a significant within-group decrease in RHI, which similarly resulted in a trend toward a significant between-group treatment effect (p = 0.08). No significant within-group or between-group differences were observed for EndoPAT-derived AI (p = 0.23). Mean change from baseline in artery elasticity and thickness are also provided (Table 3). No within-group differences or between-group treatment effects were observed for any carotid artery assessments of elasticity and thickness, as well as regional arterial stiffness measures as measured by SphygmoCor (all p > 0.05). Similar results were observed in the intent-to-treat analysis.
Table 3.
Per-Protocol (≥70% Drug Compliance) Changes in Vascular Health from Baseline in Participants Who Completed the Triala
| Atorvastatin (n = 9) | Placebo (n = 6) | Between-group difference (Ref = placebo) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Baselineb | ΔM6 | n | Baselineb | ΔM6 | n | Treatment effect | ||
| Brachial artery ultrasound | |||||||||
| Peak FMD (%) | 8 | 6.4 ± 4.0 | −0.6 [−2.0, 0.8] | 3 | 7.1 ± 5.2 | −3.0 [−5.3, −0.7]* | 11 | 2.3 [−0.4, 5.0] | 0.09 |
| EndoPAT | |||||||||
| RHI (%) | 9 | 2.17 ± 0.46 | −0.07 [−0.18, 0.32] | 6 | 1.73 ± 0.52 | −0.3 [−0.62, 0.01]† | 15 | 0.37 [−0.05, 0.79] | 0.08 |
| AI (%) | 9 | −5.5 ± 13.3 | −1.6 [−8.7, 5.5] | 6 | −13.1 ± 5.7 | 5.5 [−3.3, 14.3] | 15 | −7.1 [−18.8, 4.5] | 0.23 |
| Carotid artery ultrasound | |||||||||
| cIMT (mm) | 9 | 0.57 ± 0.03 | −0.002 [−0.02, 0.02] | 5 | 0.52 ± 0.07 | −0.004 [−0.03, 0.03] | 14 | 0.002 [−0.04, 0.04] | 0.92 |
| cDD (%) | 9 | 9.0 ± 2.4 | 0.16 [−0.55, 0.86] | 5 | 8.6 ± 1.2 | 0.10 [−0.85, 1.06] | 14 | 0.052 [−1.14, 1.24] | 0.93 |
| cCSD (%) | 9 | 18.8 ± 5.2 | 0.36 [−1.18, 1.90] | 5 | 17.9 ± 2.5 | 0.24 [−1.81, 2.31] | 14 | 0.12 [−2.47, 2.70] | 0.93 |
| cDC (mm/mmHg × 10−3) | 9 | 11.6 ± 3.1 | −0.34 [−1.4, 0.7] | 5 | 11.0 ± 1.4 | 0.56 [−0.8, 2.0] | 14 | −0.90 [−2.66, 0.86] | 0.32 |
| cCSC1 (mm2/mmHg × 10−3) | 9 | 128 ± 35 | −1.3 [−13.8, 11.2] | 5 | 113 ± 18 | 8.4 [−8.5, 25.3] | 14 | −9.7 [−31.1, 11.7] | 0.37 |
| cCSC2 (1/mmHg × 10−3) | 9 | 3.6 ± 1.1 | −0.003 [−0.38, 0.38] | 5 | 3.6 ± 0.4 | 0.18 [−0.33, 0.69] | 14 | −0.19 [−0.82, 0.45] | 0.57 |
| cIEM (mmHg) | 9 | 1554 ± 456 | 146 [−104, 396] | 5 | 1493 ± 163 | 100 [−236, 435] | 14 | 46.6 [−372, 466] | 0.83 |
| SphygmoCor | |||||||||
| PWV (m/s) | 9 | 8.4 ± 2.9 | −0.2 [−1.2, 0.8] | 5 | 7.2 ± 1.3 | −0.9 [−2.2, 0.4] | 14 | 0.8 [−0.9, 2.4] | 0.36 |
| Carotid AI (P2/P1) | 6 | 130 ± 21 | −0.7 [−15.4, 14.1] | 4 | 120 ± 15 | −6.0 [−24.2, 12.2] | 10 | 5.4 [−18.4, 29.1] | 0.66 |
| Radial AI (P2/P1) | 9 | 128 ± 10 | 6.7 [−5.6, 19.1] | 6 | 115 ± 19 | −8.3 [−25.3, 8.6] | 14 | 15.1 [−6.6, 36.7] | 0.17 |
Significant (*p < 0.05) and trending significant (†p < 0.10) within-group changes from baseline are noted. Between-group treatment effect differences are noted with respective p-value. All reported brachial and carotid artery measurements performed on the left brachial artery and left common carotid artery.
Estimates of least-squares means [95% confidence intervals] change from baseline are listed for Month 6 (ΔM6). Data are from participants who completed the 6-month trial and had interpretable vascular measures. Participants in the atorvastatin group were randomly assigned at baseline to receive a 40 mg daily dose of atorvastatin.
Values are raw means ± SDs.
Cumulative adverse events by treatment group are provided (Table 4). Abnormal safety laboratories included all instances where elevated safety laboratories (ALT, AST, or CK) required additional follow-up beyond protocol requirements. Miscellaneous adverse events included reports of headache, insomnia, dizziness, rash, urinary tract infection, and so on. Total drug compliance was not significantly different between atorvastatin and placebo groups (86 ± 11 vs. 83 ± 11%, p = 0.59), respectively.
Table 4.
Intent-to-Treat Summary of Cumulative Adverse Events by Treatment Group for All Randomized Participants
| Atorvastatin (n = 14) | |||
|---|---|---|---|
| AE categories | 1-Month visit | 3-Month visit | 6-Month visit |
| Musculoskeletal | 3 (21%) | 5 (36%) | 6 (43%) |
| Gastrointestinal | 3 (21%) | 3 (21%) | 3 (21%) |
| Fatigue | 0 (0%) | 1 (7%) | 1 (7%) |
| Cognitive | 0 (0%) | 0 (0%) | 0 (0%) |
| Abnormal safety labs | 3 (21%) | 3 (21%) | 3 (21%) |
| Respiratory | 3 (21%) | 7 (50%) | 8 (57%) |
| Miscellaneous | 4 (29%) | 8 (57%) | 11 (79%) |
| Placebo (n = 13) | |||
|---|---|---|---|
| Musculoskeletal | 0 (0%) | 3 (23%) | 4 (31%) |
| Gastrointestinal | 2 (15%) | 2 (15%) | 3 (23%) |
| Fatigue | 1 (8%) | 2 (15%) | 2 (15%) |
| Cognitive | 1 (8%) | 2 (15%) | 2 (15%) |
| Abnormal safety labs | 4 (31%) | 4 (31%) | 4 (31%) |
| Respiratory | 2 (15%) | 5 (38%) | 6 (46%) |
| Miscellaneous | 2 (15%) | 4 (31%) | 6 (46%) |
Discussion
To our knowledge, this is the first trial to evaluate the effect of atorvastatin on arterial health in adult survivors of childhood cancer. While the vascular benefits of statin therapy in different populations are well documented, atorvastatin did not significantly improve endothelial function or arterial stiffness compared with placebo in our pilot trial of young adult survivors of childhood cancer as we had hypothesized. While we observed a significant worsening of endothelial function via peak FMD and a trend toward worsened endothelial function via RHI within the placebo group, the trending significant treatment effect for both peak FMD and RHI should be interpreted with caution due to the notable number of randomized participants that did not finish the trial. Indeed, there is a lack of sufficient power to support the results of our primary and secondary endpoints. Specifically, 5 of the 14 participants randomized to atorvastatin and 7 of the 13 participants randomized to placebo did not complete all 6 months of the intervention. Conservative management of adverse events by the monitoring physicians led to a higher withdrawal rate than originally anticipated, with most of the withdrawals resulting from elevated ALT, AST, or CK levels (greater than 2 × the upper limit of normal). These other factors also contributed to a smaller analyzable sample size: loss to follow-up, self-withdrawal due to time commitment burdens, and poor ultrasound image quality due to participant movement, difficult brachial artery anatomy, or poor circulation.
Another possible factor that may have influenced our findings was the inconsistent time of day that vascular testing was performed. Peak FMD is lower in the morning,37–39 which may be the result of acute increases in augmented sympathetic activation,40,41 hemodynamic changes,42 neurohormonal factors, and increases in coagulation43 shortly after waking. Similar findings of increased arterial stiffness via pulse wave analysis have been reported in the morning hours.44,45 While early morning testing was not always a viable option for participants due to conflicting work schedules or longer commutes, we aimed to decrease both inter- and intraindividual variability in testing times as much as possible.
A meta-analysis of 46 randomized controlled trials recently demonstrated that statin therapy improves endothelial function, as assessed by FMD (standardized mean difference 0.68 [95% CI: 0.46, 0.90; p < 0.001]), as well as venous occlusion plethysmography and coronary infusion of acetylcholine, in a wide array of populations.25 Additional studies in overweight and obese adults have also reported arterial destiffening with 80 mg/day of atorvastatin following 30 days30 and 12-weeks29 of treatment. While we did not observe such an effect in our small pilot trial, our small sample size limits our ability to draw confident conclusions from these trial results.
Nonetheless, this is the first trial to ever test the effect of statin therapy in adult survivors of childhood cancer. While further research in a larger sample size is needed to fully elucidate the effects of atorvastatin on vascular function, the findings of this study are important for the design of future drug trials in adult survivors of childhood cancer. In addition, given that CVD outcomes among adult survivors of childhood cancer vary by cancer severity, treatment modalities, sex, age at treatment, and number of years since treatment,46–48 future trials should strive to achieve a sufficient sample size to allow for stratification by these factors.
Successful recruitment, enrollment, and retention of adolescent and young adult survivors of childhood cancer for randomized clinical drug trials are extremely difficult. Subsequently, the clinical and research infrastructure for these particular cancer survivors would benefit greatly from more effective systems to promote life-long relationships with clinicians and investigators. Improvements in care-delivery models across the lifespan would help ensure reliable access to survivors of childhood cancer allowing for more efficient research recruitment and enrollment. The most impactful clinical research related to pharmacologic interventions for adolescent and young adult survivors of childhood cancer will hinge on investigators' ability to design drug trials that are sufficiently powered with larger sample sizes. This will be a great challenge but efforts to continually develop and successfully execute randomized clinical trials for adolescent and young adult survivors of childhood cancer must continue and build upon the lessons learned from studies such as this.
Acknowledgments
This study was supported by funding from the Children's Cancer Research Fund Hodder Chair (to J.A.R.); the National Center for Advancing Translational Sciences of the National Institutes of Health Award (UL1TR000114); as well as the American Heart Association Pre-Doctoral Fellowship Award: 0410034Z (to K.L.M.).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki 1964, and its later amendments or comparable ethical standards.
Clinical Trial Registration
This trial is registered on www.clinicaltrials.gov (NCT01733953).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. SEER Pediatric Monograph. Bethesda, MD: Nattional Cancer Institute; 1999 [Google Scholar]
- 2. Smith MA, Ries LAG. Childhood cancer: incidence, survival, and mortality. In: Pizzo PAI, Poplack DG. (Eds). Principles and practice of pediatric oncology (fourth edition). Philadelphia, PA: Lippincott Williams and Wilkins; 2002; pp. 1–12 [Google Scholar]
- 3. Ries LAG, Eisner MP, Kosary CL. SEER cancer statistics review 1975–2000. Bethesda, MD: National Cancer Institute; 2003 [Google Scholar]
- 4. Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113(9):2575–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oeffinger KC, Mertens AC, Skylar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82 [DOI] [PubMed] [Google Scholar]
- 6. Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88(10):4731–9 [DOI] [PubMed] [Google Scholar]
- 7. Nandagopal R, Laverdiere C, Mulrooney D, et al. Endocrine late effects of childhood cancer therapy: a report from the Children's Oncology Group. Horm Res. 2008;69:65–74 [DOI] [PubMed] [Google Scholar]
- 8. Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–72 [DOI] [PubMed] [Google Scholar]
- 9. Oeffinger KC, Buchanan GR, Eshelman DA, et al. Cardiovascular risk factors in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2001;23(7):424–30 [DOI] [PubMed] [Google Scholar]
- 10. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–82 [DOI] [PubMed] [Google Scholar]
- 11. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. Br Med J. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moleski M. Neuropsychological, neuroanatomatical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch Clin Neuropsychol. 2000;350:1535–48 [PubMed] [Google Scholar]
- 13. Kaemingk KI, Carey ME, Moore IMK, et al. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychol. 2004;10:14–23 [DOI] [PubMed] [Google Scholar]
- 14. Anderson F, Kunin-Batson A. Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr Blood Cancer. 2009;52:159–64 [DOI] [PubMed] [Google Scholar]
- 15. Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer. 2007;48(7):723–6 [DOI] [PubMed] [Google Scholar]
- 16. Lüscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20(Suppl II):II3–10 [PubMed] [Google Scholar]
- 17. Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol. 2001;12:383–9 [DOI] [PubMed] [Google Scholar]
- 18. Drexler H. Factors involved in the maintenance of endothelial function. Am J Cardiol. 1998;82:3S–4S [DOI] [PubMed] [Google Scholar]
- 19. Halcox JPJ, Deanfield JE. Endothelial cell function testing: how does the method help us in evaluating vascular status? Acta Paediatr Suppl. 2004;446:48–54 [DOI] [PubMed] [Google Scholar]
- 20. Dengel DR, Ness KK, Glasser SP, et al. Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30(1):20–5 [DOI] [PubMed] [Google Scholar]
- 21. Dengel DR, Kelly AS, Zhang L, et al. Signs of early sub-clinical atherosclerosis in childhood cancer survivors. Pediatr Blood Cancer. 2014;61:532–7 [DOI] [PubMed] [Google Scholar]
- 22. Celermajer D, Sorensen K, Gooch V, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5 [DOI] [PubMed] [Google Scholar]
- 23. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95 [DOI] [PubMed] [Google Scholar]
- 24. Stone N, Robinson J, Lichtenstein A, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;1–84 [DOI] [PubMed] [Google Scholar]
- 25. Reriani MK, Dunlay SM, Gupta B, et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2011;18(5):704–16 [DOI] [PubMed] [Google Scholar]
- 26. Matsuno H, Takei M, Hayashi H, et al. Simvastatin enhances the regeneration of endothelial cells via VEGF secretion in injured arteries. J Cardiovasc Pharmacol. 2004;43:333–40 [DOI] [PubMed] [Google Scholar]
- 27. Ota H, Eto M, Kano M, et al. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30:2205–11 [DOI] [PubMed] [Google Scholar]
- 28. Axel DI, Riessen R, Runge H, et al. Effects of cerivastatin on human arterial smooth muscle cell proliferation and migration in transfilter cocultures. J Cardiovasc Pharmacol. 2000;35(4):619–29 [DOI] [PubMed] [Google Scholar]
- 29. Orr JS, Dengo AL, Rivero JM, et al. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension. 2009;54(4):763–8 [DOI] [PubMed] [Google Scholar]
- 30. Ratchford EV, Gutierrez J, Lorenzo D, et al. Short-term effect of atorvastatin on carotid artery elasticity: a pilot study. Stroke. 2011;42(12):3460–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207 [DOI] [PubMed] [Google Scholar]
- 32. Suzumura K, Yasuhara M, Tanaka K, et al. An in vitro study of the hydroxyl radical scavenging property of fluvastatin, an HMG-CoA reductase inhibitor. Chem Pharm Bull. 1999;47:1010–2 [DOI] [PubMed] [Google Scholar]
- 33. Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96 [DOI] [PubMed] [Google Scholar]
- 34. Singh U, Devaraj S, Jialal I, et al. Comparison effect of atorvastatin (10 versus 80 mg) on biomarkers of inflammation and oxidative stress in subjects with metabolic syndrome. Am J Cardiol. 2008;102(3):321–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med. 1992;11:1685–704 [DOI] [PubMed] [Google Scholar]
- 36. Senn S. Change from baseline and analysis of covariance revisited. Stat Med. 2006;25:4334–44 [DOI] [PubMed] [Google Scholar]
- 37. Bau PFD, Bau CHD, Naujorks AA, et al. Diurnal variation of vascular diameter and reactivity in healthy young men. Braz J Med Biol Res. 2008;41:500–3 [DOI] [PubMed] [Google Scholar]
- 38. Kollias GE, Stamatelopoulos KS, Papaioannou TG, et al. Diurnal variation of endothelial function and arterial stiffness in hypertension. J Hum Hypertens. 2009;23:597–604 [DOI] [PubMed] [Google Scholar]
- 39. Otto ME, Svatikova A, Barretto RB, et al. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109:2507–10 [DOI] [PubMed] [Google Scholar]
- 40. Muller JE, Tofler GH, Verrier RL. Sympathetic activity as the cause of the morning increase in cardiac events: a likely culprit, but the evidence remains circumstantial. Circulation. 1995;91:2508–9 [DOI] [PubMed] [Google Scholar]
- 41. Hijmering ML, Stroes ES, Olijhoek J, et al. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–8 [DOI] [PubMed] [Google Scholar]
- 42. Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–90 [DOI] [PubMed] [Google Scholar]
- 43. Tofler GH, Brezinski D, Schafer AI, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–8 [DOI] [PubMed] [Google Scholar]
- 44. Papaioannou TG, Karatzis EN, Papamichael CM, et al. Circadian variation of arterial pressure wave reflections. Am J Hypertens. 2006;19:259–63 [DOI] [PubMed] [Google Scholar]
- 45. Bodlaj G, Berg J, Biesenbach G. Diurnal variation of pulse wave velocity assessed non-invasively by applanation tonometry in young healthy men. Yonsei Med J. 2007;48:665–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shankar S, Marina N, Hudson M, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396 [DOI] [PubMed] [Google Scholar]
- 47. Buizer A, de Sonneville L, van den Heuvel-Eibrink M, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr Blood Cancer. 2005;45:281–90 [DOI] [PubMed] [Google Scholar]
- 48. van der Pal H, van Dalen E, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–55 [DOI] [PubMed] [Google Scholar]