Abstract
Background
miR-214-3p has been found to inhibit proliferation and migration in cancer cells. The objective of this study was to determine whether ARHGEF12 is involved in miR-214-3p-mediated suppression of proliferation and migration of pulmonary artery smooth muscle cells (PASMCs).
Material/Methods
PASMCs were cultured under normoxia or hypoxia. miR-214-3p mimics or inhibitors were transiently transfected into PASMCs. Proliferation, apoptosis, and migration of PASMCs were evaluated using MTT assay, flow cytometry, and Boyden chamber apparatus. Western blot analysis was used to examine expression of Rho guanine nucleotide exchange factor 12 (ARHGEF12), c-fos, c-jun, and caspase-3. Luciferase reporter assay was used to test the direct regulation of miR-214-3p on the 3′-untranslated region (UTR) of ARHGEF12.
Results
miR-214-3p was significantly upregulated in hypoxia-treated PASMCs. Knockdown of miR-214-3p significantly attenuated hypoxia-induced proliferation and migration in PASMCs and promoted apoptosis, whereas this effect was aggravated by overexpression of miR-214-3p. In addition, dual-luciferase reporter assay demonstrated that ARHGEF12 is a direct target gene of miR-214-3p. The protein levels of ARHGEF12 were downregulated after knockdown of miR-214-3p in PASMCs. Rescue experiment results indicated that decreased proliferation of PASMCs resulted from knockdown of miR-214-3p were partially reversed by silencing of ARHGEF12 by siRNA. Furthermore, knockdown of miR-214-3p reduced expression of c-jun and c-fos, but increased expression of caspase-3 in PASMCs under hypoxia.
Conclusions
In conclusion, these results indicate that miR-214-3p acts as a novel regulator of hypoxia-induced proliferation and migration by directly targeting ARHGEF12 and dysregulating c-jun and c-fos in PASMCs, and may be a potential therapeutic target for treating pulmonary hypertension.
MeSH Keywords: Hypertension, Pulmonary; MicroRNAs; Rho Guanine Nucleotide Exchange Factors
Background
Pulmonary hypertension (PH) is a progressive cardiovascular disease characterized by abnormal elevation in the pulmonary arterial pressure, and can eventually lead to right ventricular failure and death [1]. A key feature of PH pathology is pulmonary vascular remodeling, involving abnormal proliferation and migration of pulmonary artery smooth muscle cells (PASMCs) [2]. Chronic hypoxia can induce proliferation and migration of PASMCs, and is believed to be an important contributing factor to the development of PH [3]. However, the cellular and molecular mechanisms underlying hypoxia-induced PH are still not well elucidated.
miRNAs are single-stranded non-coding RNAs of approximately 21–23 nucleotides in length. They post-transcriptionally inhibit gene expression by binding to the 3′-untranslated region (UTR) of target mRNAs, and subsequently inducing mRNA degradation or repressing transcription. miRNAs affect a variety of cellular processes such as proliferation, apoptosis, and migration, and play an important role in many diseases such as cancer and cardiovascular diseases [4]. Recently, several miRNAs, including miR-21, miR-204, and miR-130/301, have been reported to regulate proliferation and migration of PASMCs, and contribute to the pathogenesis of PH [5].
miR-214-3p has many important functional roles in tumorigenesis and Alzheimer’s disease [6]. miR-214-3p is upregulated under hypoxia in osteosarcoma cells, and knockdown of miR-214-3p inhibits proliferation, adhesion, and invasiveness in tumor cells [7]. Recent studies showed that miR-214 regulates vascular smooth muscle cell migration, proliferation, and neointima hyperplasia by targeting RNA-binding protein QKI and NCK-associated protein 1 [8,9]. However, the role of hypoxia-related miR-214-3p in PASMCs has not been reported.
Rho GTPase is a master regulator controlling cytoskeletal-dependent functions such as actin cytoskeletal organization, cytokinesis, cell adhesion, and migration [10]. Our previous study reported that the RhoA/Rho-kinase pathway plays a very important role in the development of PH [11,12]. Interestingly, a study showed that Rho guanine nucleotide exchange factor12 (ARHGEF12) can regulate RhoA GTPases [13], but the role of ARHGEF12 is unclear in PASMCs.
Here, we investigated the effect of miR-214-3p on proliferation, apoptosis, and migration of PASMCs under hypoxia and the underlying mechanism. We found that miR-214-3p promoted hypoxia-induced proliferation and migration of PASMCs though targeting ARHGEF12. Conversely, knockdown of miR-214-3p attenuated this effect. Thus, our findings identify a new regulatory pathway for PASMCs involving miR-214-3p and ARHGEF12 in vitro.
Material and Methods
PASMCs isolation and culture
Primary PASMCs were obtained from rat pulmonary arteries as previous described [14]. Cells were then cultured for 3–5 days in a humidified incubator with 5% CO2 at 37°C. Passage 3–5 cells were used for further experiments. PASMCs were identified by strong immunofluorescent staining of α-actin. Before each experiment, the cells were incubated in serum-free, low-glucose DMEM for 24 h. For normoxia experiments, cells were incubated in a humidified incubator with a constant supply of air containing 21% O2 and 5% CO2 at 37°C. For hypoxia experiments, cells were incubated in a humidified incubator with a supply of a gas mixture containing 3% O2 and 5% CO2.
Transfection
MiR-214-3p mimics, mimic negative controls, inhibitors, inhibitors negative controls, and ARHGEF12 siRNA (si-ARHGEF12) were purchased from Shanghai GenePharma Co., Shanghai, China. Cells were seeded on 6-well plates at a density of 4–5×104 cells/well, and grown to 70% confluence. Cells were transfected with miR-214-3p mimics, inhibitors, or their negative controls using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s instructions. After transfection, cells were cultured in culture medium in normoxia or hypoxia for 48 h. Cells were then harvested and used for the following experiments.
Cell proliferation assay
Proliferation was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. Briefly, cells were plated on 96-well plates at a density of 2×104 cells/well and cultured in normoxia or hypoxia for 24 h. Cells were incubated with MTT dye (20 μl) (MP Biomedicals, Santa Ana, CA) at a final concentration of 5 mg/ml for 4 h. The cells were then dissolved in DMSO, and optical density (OD) was measured at 570 nm in an ELISA plate reader.
Cell apoptosis assay
Apoptosis and cell cycle analysis was performed by flow cytometry as previously reported [15]. Briefly, PASMCs were pretreated with normoxia or hypoxia and were harvested by trypsinization. Cells were suspended at a density of 1×106 cells/ml in 100 μl PBS. Cells were then incubated with fluorescence isothiocyanate (FITC)-conjugated annexin and PI (propidium iodide) for 15 min at 4°C in the dark (Annexin V-FITC & PI Apoptosis Detection Kit, ADL, A0001a). Data were acquired on a BDCalibur device (BD Bioscience, San Jose, CA, USA). The percentage of apoptotic cells was calculated by dividing the total number of cells by the number of apoptotic cells.
Cell migration assay
Cell migration was examined using a Boyden chamber apparatus (Corning USA). Confluent PASMCs were starved in serum-free culture media for 48 h. Cells were incubated for 24 h at 37°C in a 5% CO2 incubator. Cells that did not migrate were removed with a cotton swab. The membranes were then fixed with methanol for 15–20 min and stained with Hemacolor Stain Set for 10 min. The number of cells migrating through the membrane was counted under a light microscope (200×, 5 random fields per well).
Quantitative reverse transcription-PCR
Total RNA was extracted from PASMCs using a miRNeasy Mini Kit (Qiagen, Inc) according to the manufacturer’s instructions. The RNA sample was subsequently treated with RNase-free DNAse-I at room temperature for 20 min and stored at −80°C until use. RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit according to the manufacturer’s instructions. PCR was performed with Fast SYBR® Green Master Mix (Applied Biosystems life technology, NY, USA) on a thermocycler ABI Prism 7300 (Applied Biosystems) in a mixture solution containing 2 μl cDNA, 0.5 μl of each primer, and 10 μl SYBRGreen. The primers used in this study were 5′-TTACAGCAGGCACAGAC-3′ (forward) and 5′-TGGTGTCGTGGAGTCG-3′ (reverse) for miR-214-3p, 5′-GGAGCATCTGGGAATATGGA-3′ (forward) and 5′-TCTTGCAGCTGAGGAATGTG-3′ (reverse) for ARHGEF12, and 5′-AACCTGCCAAGTATGATGAC-3′ (forward) and 5′-TGTTGAAGTCACAGGAGACA -3′ (reverse) for GAPDH as a housekeeping gene. The reaction condition was as follows: 95C for 5 min; 95C for 10 s, 60C for 20 s, and 72°C for 10 s with 40 cycles. The relative expression of miR-214-3p was calculated by normalizing to GAPDH using the 2−ΔΔCt method.
Western blot analysis
PASMCs were lysed in RIPA lysis buffer (Thermo Scientific, USA) supplemented with protease inhibitors (Roche, Switzerland). Cell lysates were centrifuged, and protein concentration was determined using a BCA Protein Assay kit (Thermo Scientific, USA). Proteins (20 μg) were separated by electrophoresis on 4–20% SDS-polyacrylamide gels, and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat dry milk in TBST for 1 h, then incubated with primary antibodies overnight at 4°C. The primary antibodies used in this study targeted c-Jun (Santa Crus, USA), c-fos (Santa Crus, USA), caspase-3 (Bioss, Beijing, China), ARHGEF12 (Santa Cruz, USA), and GAPDH (Santa Crus, USA). The membranes were then incubated with secondary antibodies, goat polyclonal anti-mouse IgG-horseradish peroxidase (HRP), or goat polyclonal anti-rabbit IgG-HRP, at room temperature for 1 h. Bands were visualized using an enhanced chemiluminescence detection kit (Thermo Scientific, USA).
Luciferase reporter assay
To construct a luciferase reporter carrying the 3′ untranslated regions (3′-UTR) of ARHGEF12, the DNA fragments of the 3′-UTRs of ARHGEF12 mRNA containing the putative miR-214-3p binding sequence were amplified by PCR. The fragments were then cloned into the pGL3 luciferase reporter vector. HEK293 cells were transfected with wild-type pGL3-ARHGEF12-3′UTR and mutated pGL3-ARHGEF12-3′UTR with miR-214-3p mimics or miR-negative control, respectively, using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol. At 48 h after transfection, luciferase activity was measured using the dual-luciferase assay system (Promega).
Statistical analysis
Statistical analyses were performed using the SPSS statistics 22.0 software package. Data are presented as mean ± standard deviation (SD). One-way ANOVA was used to compare the differences among groups. P values <0.05 were considered statistically significant.
Results
miR-214-3p expression is upregulated in hypoxia-treated PASMCs
We investigated the effect of hypoxia on the expression of miR-214-3p in rat PASMCs, using QRT-PCR. Hypoxia produced a time-dependent significant upregulation of miR-214-3p expression, and normoxia had no effect on the expression of miR-214-3p (Figure 1A). The expression of miR-214-3p in PASMCs was increased by 15% at 6 h after hypoxia treatment, and increased progressively during hypoxia. The effect of hypoxia on the upregulation of miR-214-3p was most pronounced 48 h after hypoxia treatment.
Figure 1.
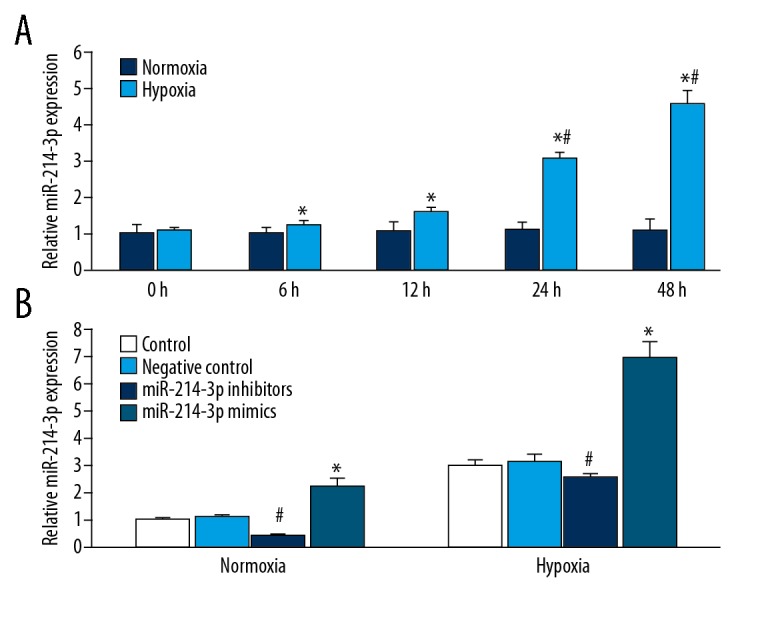
The expression of miR-214-3p in rat PASMCs. QRT-PCR results showing (A) The expression of miR-214-3p was increased in rat PASMCs following hypoxia treatment. n=3; * P<0.05 vs. N 0 h, # P<0.05 vs. H 6 h and H 12 h; (B) QRT-PCR results showing the expression of miR-214-3p in rat PASMCs treated with miR-214-3p mimics or inhibitors following normoxia or hypoxia treatment. n=3; # P<0.05 vs. control, * P<0.05 vs. control.
Knockdown of miR-214-3p inhibits hypoxia-induced proliferation in PASMCs
We examined the effect of miR-214-3p overexpression and knockdown on PASMCs proliferation under normoxia or hypoxia. In PASMCs treated with normoxia or hypoxia, miR-214-3p mimics significantly increased miR-214-3p expression, and miR-214-3p inhibitors significantly reduced miR-214-3p expression (Figure 1B). In addition, under both normoxia and hypoxia, miR-214-3p inhibitors significantly decreased proliferation, and miR-214-3p mimics significantly increased PASMCs proliferation (Figure 2A). Proliferation was significantly greater in PASMCs incubated under hypoxia than those incubated under normoxia. Hypoxia-induced proliferation was inhibited by miR-214-3p inhibitors (Figure 2A).
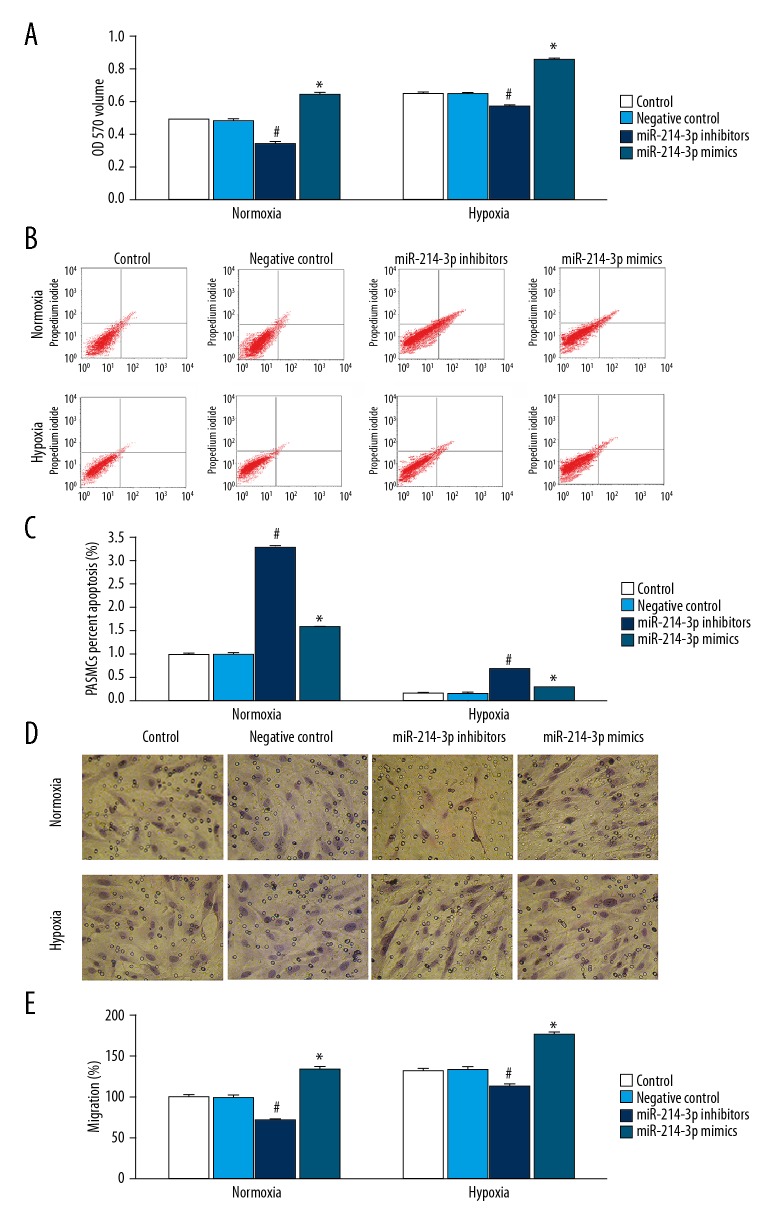
Figure 2.
Knockdown of miR-214-3p inhibited proliferation and migration and promoted apoptosis of PASMCs. PASMCs treated with miR-214-3p control, negative control, mimics, or inhibitors were cultured in normoxia or hypoxia for 24 h. (A) The proliferation was assessed using MTT assay; (B, C) The apoptosis of PASMCs assessed by flow cytometry; (D, E) Quantification of the migration of PASMCs by Transwell. n=3; # P<0.05 vs. control, * P<0.05 vs. control.
Knockdown of miR-214-3p promotes PASMCs apoptosis
We then investigated the effect of miR-214-3p overexpression and knockdown on PASMCs apoptosis under normoxia or hypoxia. Under both normoxia and hypoxia, miR-214-3p inhibitors significantly increased apoptosis, and miR-214-3p mimics produced the same effect (Figure 2B, 2C). The rate of apoptosis was significantly lower in PASMCs treated with hypoxia than in those treated with normoxia. Hypoxia-induced apoptosis was increased by miR-214-3p mimics and miR-214-3p inhibitors (Figure 2B, 2C).
Knockdown of miR-214-3p inhibits hypoxia-induced PASMCs migration
We then investigated the effect of miR-214-3p overexpression and knockdown on PASMCs migration under normoxia and hypoxia. Under both normoxia and hypoxia, miR-214-3p inhibitors significantly decreased cell migration, and miR-214-3p mimics significantly increased cell migration (Figure 2D, 2E). In comparison to normoxia, hypoxia increased cell migration in PASMCs. Hypoxia-induced cell migration was inhibited by miR-214-3p inhibitors (Figure 2D, 2E).
Knockdown of miR-214-3p inhibited ARHGEF12 expression by targeting the 3′-UTRs
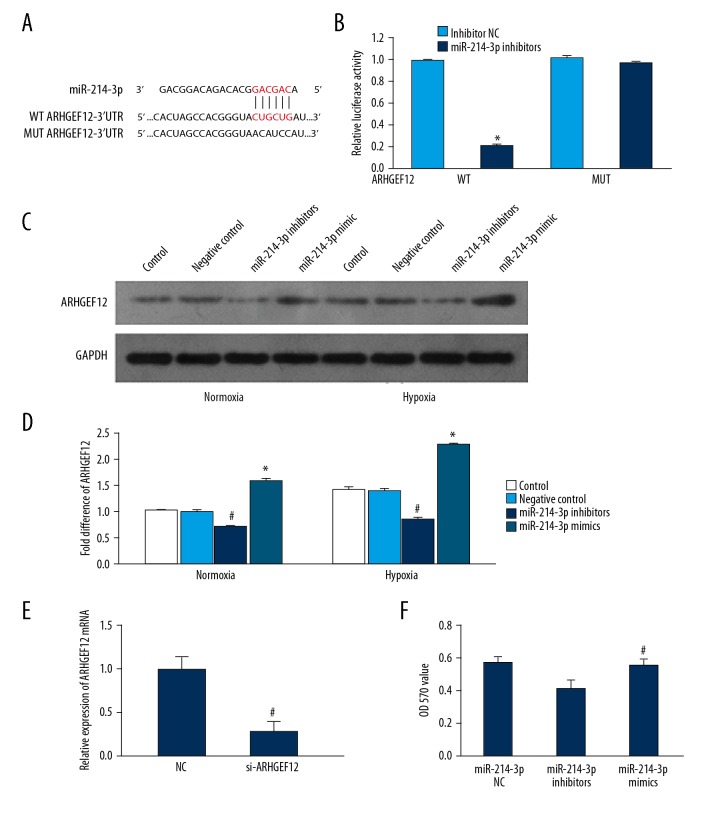
To identify the putative targets of miR-214-3p, we used 3 established miRNA target prediction algorithms: TargetScan5.1, miRbase, and miRGene. All these programs indicated that the 3′-UTRs of ARHGEF12 mRNA contained a putative miR-214-3p binding site. We cloned a luciferase reporter plasmid containing the 3′UTRs of the ARHGEF12 mRNA, and measured luciferase activity in HEK293 cells transfected with the reporter plasmid and miR-214-3p inhibitors. Luciferase activity was significantly lower in cells transfected with miR-214-3p inhibitors and ARHGEF12-3′UTR plasmids than in those transfected with ARHGEF12-3′UTR plasmids (Figure 3A, 3B), suggesting that the 3′UTR of ARHGEF12 is the target of miR-214-3p.
Figure 3.
miR-214-3p targeted the 3′-UTR of ARHGEF12. (A, B) Luciferase reporter assay was performed in HEK293 cells transfected with wild-type ARHGEF12-3′UTR or mutant ARHGEF12-3′UTR and miR-214-3p mimics or negative control. * P<0.05 vs. NC. NC – negative control; WT – wild-type; MUT – mutated; (C, D) The effect of miR-214-3p on the expression of ARHGEF12 in PASMCs; # P<0.05 vs. control, * P<0.05 vs. control. (E) The expression of ARHGEF12 mRNA was specifically decreased by si-ARHGEF12; # P<0.05 vs. control; (F) Specific silencing of ARHGEF12 promoted proliferation of PASMCs. # P<0.05 vs. miR-214-3p inhibitors group.
Western blot analysis showed that hypoxia treatment significantly increased ARHGEF12 expression in PASMCs when compared to normoxia treatment. In addition, miR-214-3p inhibitors significantly decreased ARHGEF12 expression in PASMCs following normoxia or hypoxia, while miR-214-3p mimics produced the opposite effect (Figure 3C, 3D).
Silencing of ARHGEF12 by siRNA promotes proliferation in PASMCs
As a proof of principle, siRNA targeting the mRNA of ARHGEF12 (si- ARHGEF12) was performed. As shown in Figure 3E, expression of ARHGEF12 was significantly reduced on mRNA when PASMCs were treated with si-ARHGEF12. Proliferation of PASMCs was found to be significantly increased when assessed by the MTT treated with miR-214-3p inhibitors+si-ARHGEF12 compared to miR-214-3p inhibitors (Figure 3F).
Knockdown of miR-214-3p reduced c-jun and c-fos expression, but increased caspase-3 expression in PASMCs
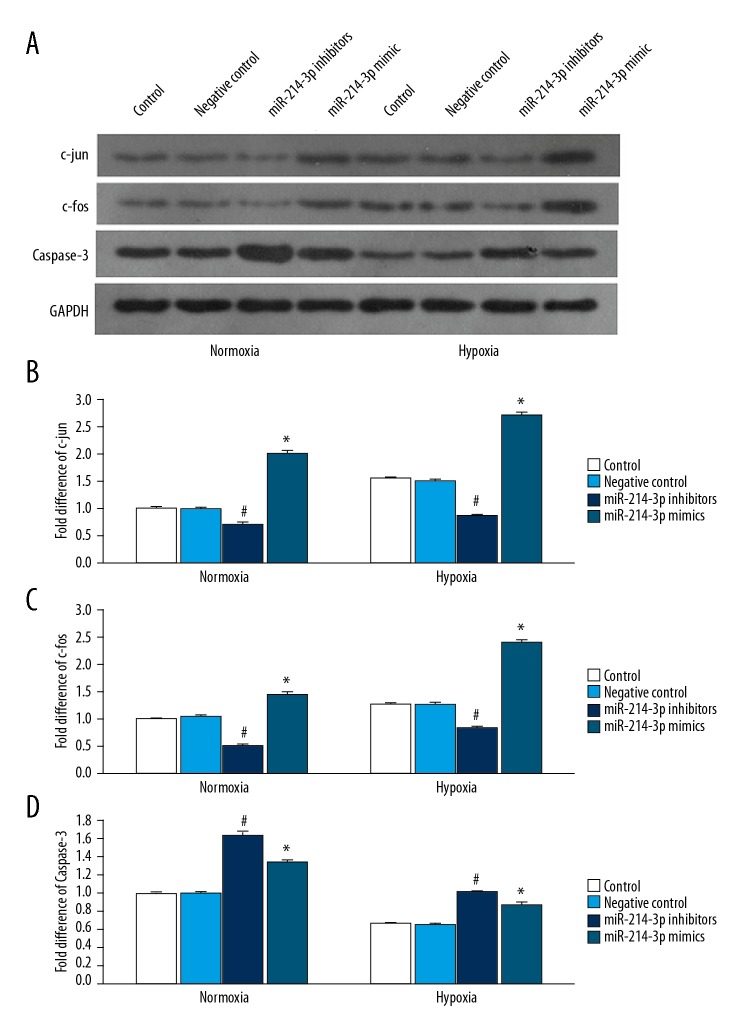
We also examined the effect of miR-214-3p on c-jun, c-fos, and caspase-3 expression in PASMCs. Western blot analysis showed that miR-214-3p inhibitors significantly decreased c-jun and c-fos expression, but increased caspase-3 expression in PASMCs following normoxia or hypoxia (Figure 4A–4D). miR-214-3p mimics produced the opposite effect on c-jun and c-fos, and the same effect on caspase-3. In addition, hypoxia treatment significantly increased c-jun and c-fos expression and significantly decreased caspase-3 expression in PASMCs when compared to normoxia treatment. miR-214-3p inhibitors abolished hypoxia-induced changes in expression of these proteins.
Figure 4.
The effect of miR-214-3p on the expression of c-jun, c-fos, and caspase-3 in PASMCs. (A) Representative Western blot showing the expression of c-jun, c-fos, and caspase-3 in PASMCs treated with miR-214-3p control, negative control, mimics, or inhibitors following normoxia or hypoxia exposure for 24 h. GAPDH was used as a loading control; Quantification of the expression of c-jun (B), c-fos (C) and caspase-3 (D). n=3; # P<0.05 vs. control, * P<0.05 vs. control.
Discussion
In the present study, we investigated the effect of miR-214-3p on proliferation, apoptosis, and migration of rat PASMCs under hypoxic conditions. We found that miR-214-3p was time-dependently upregulated in hypoxia-treated PASMCs. Hypoxia induced proliferation, inhibited apoptosis, and promoted cell migration in PASMCs. Knockdown of miR-214-3p inhibited the effect of hypoxia on PASMCs, suggesting that hypoxia may induce proliferation, apoptosis inhibition, and migration of PASMCs by upregulating miR-214-3p. Furthermore, we found that the 3′-UTRs of ARHGEF12 mRNA contained a putative miR-214-3p binding site. Luciferase reporter assays confirmed that miR-214-3p inhibited ARHGEF12 expression by targeting the 3′-UTRs. Taken together, our findings suggest that miR-214-3p regulates hypoxia-induced proliferation, apoptosis, and migration of PASMCs by directly targeting ARHGEF12.
Several studies have demonstrated that hypoxia induces upregulation of miR-214-3p expression [16,17]. Similarly, in the present study, we found that hypoxia upregulated miR-214-3p in rat PASMCs, but the mechanisms underlying hypoxia-induced upregulation of miR-214-3p remain unclear. Further experiments are required to elucidate the mechanisms underlying hypoxia-induced upregulation of miR-214-3p in PASMCs.
Downregulation of miR-214 inhibits proliferation and glycolysis in non-small-cell lung cancer cells via downregulating the expression of hexokinase 2 and pyruvate kinase isozyme M2 [18]. In this study, we found that knockdown of miR-214-3p inhibited hypoxia-induced proliferation in PASMCs. Since expression of transcription factors c-fos and c-jun is increased under hypoxia and promotes proliferation, we further examined the effect of miR-214-3p on c-fos and c-jun expression in PASMCs under hypoxia. We found that hypoxia significantly increased expression of these transcription factors in PASMCs. Consistent with our findings, several studies reported that hypoxia increases expression of c-fos and c-jun in PASMCs [19]. Furthermore, we found that hypoxia-induced upregulation of c-fos and c-jun was significantly inhibited by knockdown of miR-214-3p, suggesting that miR-214-3p inhibits hypoxia-induced proliferation by suppressing c-fos and c-jun expression in PASMCs.
Hypoxia is reported to inhibit apoptosis in PASMCs [20,21]. Consistent with these studies, we found that hypoxia reduced the percentage of apoptotic PASMCs. Knockdown of miR-214-3p significantly inhibited hypoxia-induced suppression of apoptosis in PASMCs, significantly increasing the percentage of apoptotic cells. miR-214-3p-induced apoptosis is reported to be caspase-dependent in neural apoptosis in injured spinal cords [22]. In this study, we found that hypoxia inhibited caspase-3 expression, and knockdown of miR-214-3p promoted hypoxia-induced expression of caspase-3 in PASMCs. Our findings suggest that knockdown of miR-214-3p increases apoptosis of PASMCs under hypoxia via a caspase-3-dependent mechanism.
Increased migration of PASMCs and endothelial cells contributes to pulmonary vascular remodeling. Several studies have shown that hypoxia induces migration of PASMCs [23]. Consistent with these studies, we also found that hypoxia increased PASMCs migration. miR-214-3p is reported to promote the migration and invasion of osteosarcoma cells [7]. Furthermore, we found that knockdown of miR-214-3p inhibited hypoxia-induced PASMCs migration. Since miR-214-3p expression was upregulated in hypoxia-treated PASMCs, miR-214-3p downregulation may mediate hypoxia-induced migration in PASMCs, thus contributing to pulmonary vascular remodeling in PH.
The RhoA/RhoA kinase signaling pathway plays an important role the pathogenesis of PH, and inhibiting the RhoA/RhoA kinase pathway is a promising therapy for the treatment of PH [11,24]. Guanine exchange factors (GEF) are known to couple Gα subunits and Rho signaling, and there are multiple GEFs that can potentially function in platelet signal transduction from Gα subunits to RhoA [25]. Leukemia-associated RhoGEF (ARHGEF12) plays a critical role in the RhoA/RhoA kinase pathway [25]. However, the role of ARHGEF12 in PH is not yet clear. In this study, we found that hypoxia increased ARHGEF12 expression. In addition, we demonstrated that miR-214-3p inhibited hypoxia-induced PASMCs proliferation and migration, and reduced ARHGEF12 expression by targeting the 3′-UTRs, suggesting that miR-214-3p plays an important role in pulmonary vascular remodeling by directly regulating ARHGEF12 expression.
Some limitations of the present study should be taken into consideration. First, we only performed in vitro experiments. If a hypoxia PH animal model experiment had been used, the conclusion of this article would be more convincing to reveal the mechanism in PH. Second, obtaining data on PH patients could be helpful in gaining a comprehensive understanding of the role of microRNA-214-3p, but we could not collect the data on PH patients under current conditions. Third, although GAPDH was stably expressed as an internal reference in our study, some studies used the normalization of microRNA expression Rnu6 (small nuclear RNA). In our future experiments, we plan to assess whether GAPDH or small-nucleolar RNAs is more suitable. Finally, we did not use microarray analysis to examine the global expression of microRNA in this study. We plan to perform research on the role of microRNA-214-3p in PH to overcome these limitations in the future.
Conclusions
To the best of our knowledge, this study demonstrates for the first time that hypoxia upregulates miR-214-3p expression, which in turn increases ARHGEF12 level, thereby promoting PASMCs proliferation and migration. Targeting miR-214-3p may be a promising therapy for PH. Further studies are needed to confirm the role of miR-214-3p in animals, and to test whether this miRNA may have other potential targets.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81560694, 81760015, and 81100037), the “Special and Joint Program” of Yunnan Provincial Science and Technology Department and Kunming Medical University (No. 2018FE001(-2-6) and 2017FE467(-094)), Yunnan health training project of high level talents (No. D-201627), and Young Academic and Technical Leaders of Yunnan Province (No. 2017HB053)
Conflict of interests
None.
References
- 1.Marra AM, Bossone E, Salzano A, et al. Biomarkers in pulmonary hypertension. Heart Fail Clin. 2018;14:393–402. doi: 10.1016/j.hfc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 2.de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev. 2016;21:239–57. doi: 10.1007/s10741-015-9519-2. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ Res. 2006;99:675–91. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 4.Dlouha D, Hubacek JA. Regulatory RNAs and cardiovascular disease – with a special focus on circulating microRNAs. Physiol Res. 2017;66:S21–38. doi: 10.33549/physiolres.933588. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Li L. Modulation of miRNAs in pulmonary hypertension. Int J Hypertens. 2015;2015 doi: 10.1155/2015/169069. 169069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Li Q, Liu C, et al. MiR-214-3p attenuates cognition defects via the inhibition of autophagy in SAMP8 mouse model of sporadic Alzheimer’s disease. Neurotoxicology. 2016;56:139–49. doi: 10.1016/j.neuro.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Miao M, Wang Z. miR-214-3p promotes the proliferation, migration and invasion of osteosarcoma cells by targeting CADM1. Oncol Lett. 2018;16:2620–28. doi: 10.3892/ol.2018.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afzal TA, Luong LA, Chen D, et al. NCK associated protein 1 modulated by miRNA-214 determines vascular smooth muscle cell migration, proliferation, and neointima hyperplasia. J Am Heart Assoc. 2016;5:e004629. doi: 10.1161/JAHA.116.004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Li Z, Yang M, et al. MicroRNA-214 regulates smooth muscle cell differentiation from stem cells by targeting RNA-binding protein QKI. Oncotarget. 2017;8:19866–78. doi: 10.18632/oncotarget.15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu A, Zuo C, He Y, et al. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-beta1 signaling. J Clin Invest. 2015;125:1228–42. doi: 10.1172/JCI77656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing XQ, Gan Y, Wu SJ, et al. Rho-kinase as a potential therapeutic target for the treatment of pulmonary hypertension. Drug News Perspect. 2006;19:517–22. doi: 10.1358/dnp.2006.19.9.1050426. [DOI] [PubMed] [Google Scholar]
- 12.Xing XQ, Gan Y, Wu SJ, et al. Statins may ameliorate pulmonary hypertension via RhoA/Rho-kinase signaling pathway. Med Hypotheses. 2007;68:1108–13. doi: 10.1016/j.mehy.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Springelkamp H, Iglesias AI, Cuellar-Partida G, et al. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum Mol Genet. 2015;24:2689–99. doi: 10.1093/hmg/ddv027. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WF, Xiong YW, Zhu TT, et al. MicroRNA let-7g inhibited hypoxia-induced proliferation of PASMCs via G0/G1 cell cycle arrest by targeting c-myc. Life Sci. 2017;170:9–15. doi: 10.1016/j.lfs.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Liu L, Bai M, et al. Hypoxia-induced activation of Twist/miR-214/E-cadherin axis promotes renal tubular epithelial cell mesenchymal transition and renal fibrosis. Biochem Biophys Res Commun. 2018;495:2324–30. doi: 10.1016/j.bbrc.2017.12.130. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Zhang W, Wang Y, et al. Hypoxia-induced miR-214 expression promotes tumour cell proliferation and migration by enhancing the Warburg effect in gastric carcinoma cells. Cancer Lett. 2018;414:44–56. doi: 10.1016/j.canlet.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Zhang M, Jiang H, et al. Down-regulation of miR-214 inhibits proliferation and glycolysis in non-small-cell lung cancer cells via down-regulating the expression of hexokinase 2 and pyruvate kinase isozyme M2. Biomed Pharmacother. 2018;105:545–52. doi: 10.1016/j.biopha.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Biasin V, Chwalek K, Wilhelm J, et al. Endothelin-1 driven proliferation of pulmonary arterial smooth muscle cells is c-fos dependent. Int J Biochem Cell Biol. 2014;54:137–48. doi: 10.1016/j.biocel.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Xia S, Tai X, Wang Y, et al. Involvement of Gax gene in hypoxia-induced pulmonary hypertension, proliferation, and apoptosis of arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2011;44:66–73. doi: 10.1165/rcmb.2008-0442OC. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Fan K, Wang P, et al. Carvacrol induces the apoptosis of pulmonary artery smooth muscle cells under hypoxia. Eur J Pharmacol. 2016;770:134–46. doi: 10.1016/j.ejphar.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Fan Y, Wu Y. Tetramethylpyrazine alleviates neural apoptosis in injured spinal cord via the downregulation of miR-214-3p. Biomed Pharmacother. 2017;94:827–33. doi: 10.1016/j.biopha.2017.07.162. [DOI] [PubMed] [Google Scholar]
- 23.Yi B, Cui J, Ning J, et al. cGMP-dependent protein kinase Ialpha transfection inhibits hypoxia-induced migration, phenotype modulation and annexins A1 expression in human pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2012;418:598–602. doi: 10.1016/j.bbrc.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 24.Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355–63. doi: 10.1517/14728222.2012.671811. [DOI] [PubMed] [Google Scholar]
- 25.Zou S, Teixeira AM, Yin M, et al. Leukaemia-associated Rho guanine nucleotide exchange factor (LARG) plays an agonist specific role in platelet function through RhoA activation. Thromb Haemost. 2016;116:506–16. doi: 10.1160/TH15-11-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]