Abstract
Objectives
Older adults show clear deficits in working memory functioning. Here, we investigate the often-reported decline in focus switching, that is, the ability to shift items from the focus of attention into working memory, and back. Specifically, we examined whether equating subjects on early processing (perception and attention) might ameliorate the deficit.
Method
We examined 1-Back and 2-Back performance in younger and older adults, with line segments of different orientation as the stimuli. Stimuli were calibrated depending on each individual’s 75% threshold for 1-Back performance. Subjects made match/mismatch judgments.
Results
After the calibration on 1-Back performance, no age-related differences were found on either accuracy or sensitivity in the 2-Back task. Additionally, when investigating focus-switch trials versus non-focus-switch trials in a random-order 2-Back task, older adults were more efficient at switching the focus of attention than younger adults.
Discussion
These results provide evidence for the view that age-related limitations in focus switching in working memory are caused (at least in part) by changes in early processing (perception and attention), suggesting that (at least some of the) age-related differences in working memory functioning may be due to shifts in trade-off between early processing and memory-related processing.
Keywords: cognition, perception, N-Back, working memory
Introduction
The nature of age-related deficits in working memory has received much attention, and for good reason – there is solid evidence that high-level performance in a host of complex cognitive tasks is associated with measures of working memory capacity (e.g., Conway et al., 2002; Engle, Kane, & Tuholski, 1999; Kemper, Herman, & Lian, 2003; Kyllonen, 1996; Oberauer et al., 2008; Salthouse & Pink, 2008; Unsworth & Spillers, 2010). These correlations are quite respectable in size: In their meta-analysis, Ackerman, Beier and Boyle (2005) concluded that the average correlation between working memory capacity and markers of general fluid ability (g) is .48 (after correcting for unreliability). Working memory functioning declines with advancing age. In a meta-analysis, Bopp and Verhaeghen (2005) found that in complex span tasks that require both passive storage and concurrent active processing older adults’ capacity reached only 74% of younger adults’ capacity. Given the relationship between working memory capacity and fluid cognition, it is not surprising that this age-related decline turns out to be an important contributor to age-related impairments in various aspects of higher-order cognition: The amount of age-related variance in complex cognitive abilities mediated by working memory measures varies between 52% (episodic memory) and 72% (reasoning ability) (Verhaeghen, 2014).
What exactly is the cause of this decline in working memory functioning? In our own work in this area, we honed in on one particular aspect of working memory that appears to be consistently age sensitive, namely the availability of information once it leaves the focus of attention (Vaughan, Basak, Hartman, & Verhaeghen, 2008; Verhaeghen, 2012; Verhaeghen & Basak, 2005; Zhang, Verhaeghen, & Cerella, 2012; see Chen & Li, 2007, and Oberauer, 2002, for similar takes). The focus of attention (Cowan, 1995) is the core buffer of working memory; it holds items available for immediate processing (in common parlance: It holds that what we are currently aware of). Its capacity is typically between one and four items, depending on the task (Verhaeghen, Cerella, & Basak, 2004). If more information is presented than the buffer can handle, the excess is stored away inside an ‘activated’ portion of long-term memory, which we (Verhaeghen et al., 2004) have labeled the outer store (in common parlance often referred to as ‘the back of your mind’). Inside this outer store, items are subject to interference and possibly decay, and can thus get distorted or lost over time.
One task that gives unique insight into this structural bifurcation is the N-Back task. In an N-Back task1, participants are presented with a stream of stimuli (typically letters or digits), one at a time, and are requested to check whether the item currently on the screen matches the item presented N positions in the stream back. In this task, participants pay attention to only a single item at a time, store the intervening item or items into the outer store, and access these items one at a time as needed for comparison with the item on screen. This process of swapping items in and out of the focus of attention has been labeled focus switching (Voigt & Hagendorf, 2002); in the N-Back task, it is engaged as soon as N exceeds 1. Accuracy of retrieval after a focus switch correlates both with tests of working memory capacity and tests of fluid intelligence (Kane, Conway, Miura, & Colflesh, 2007). In our version of the task, we present the stimuli, one at a time, in N columns, left to right. Participants decide whether the current item matches the item previously presented in the same column. This columnization allows for the use of location as an additional retrieval cue, thus lessening the burden on the process of keeping track.
In our laboratory, this paradigm has consistently yielded reliable age-related differences in focus switching: Age-related differences in accuracy are much smaller when N = 1 (i.e., when items do not need to leave the focus of attention) than when N > 1 (i.e., when focus switching is engaged) (Basak & Verhaeghen, 2011; Bopp & Verhaeghen, 2009; Vaughan, Basak, Hartman, & Verhaeghen, 2008; Verhaeghen & Basak, 2005; Verhaeghen & Hoyer, 2007). This result is further confirmed in meta-analysis (Bopp & Verhaeghen, 2018): Across all published studies in the field, the average adult age-related difference for 1-Back accuracy is significant (36 studies; Cohen’s d = 0.50), but about half the size of that for 2-Back accuracy (49 studies; Cohen’s d = 0.97).
These robust findings beg the deeper question about what the underlying cause for this age-related deficit in memory accuracy after a focus switch might be. So far, our lab has investigated three possibilities2. First, it seemed possible that the dual-task nature of focus switching might be to blame. A focus switch is not a single operation – it involves retrieving the old item from the outer store, comparing it with the item currently residing inside the focus of attention, and replacing the old item in the outer store with the current item. Older adults generally have deficits in dual-task processing (for a meta-analysis, see Verhaeghen, Steitz, Sliwinski, & Cerella, 2013); this deficit might drive age-related differences in focus-switching accuracy. When we compared N-Back performance when a full switch was required with N-Back performance when only retrieval, instead of a full switch, was required, we obtained a dual-task cost in both younger and older adults, but this cost did not vary by age (Verhaeghen & Zhang, 2013). This observation is in line with the bulk of the literature on the role of divided attention in working memory aging (for a review, see Kilb & Naveh-Benjamin, 2015).
A second potential reason for the age-related deficit might be the inability to bind content with context – a source memory deficit where older adults are confused as to exactly what item to retrieve, perhaps as an indicator of a wider age-related binding or associative deficit in working memory (e.g., Chen & Naveh-Benjamin, 2012). We examined this hypothesis by requiring subjects to find a repeating stimulus either within a single series (which requires no source memory) or within each of a set of multiple series (which makes source memory necessary). The added source memory requirement in the second condition led to a cost in both age groups, even when actual memory load was kept constant, but with no age-related differences in this cost (Bopp & Verhaeghen, 2009). Again, this observation is in line with the (scarce) literature on binding deficits in working memory (e.g., Brockmole, Parra, Della Sala, & Logie, 2008).
Third, it seemed possible that in old age, the outer store becomes more susceptible to interference between items. This hypothesis aligns well with the hypothesis that older adults have generalized trouble with resistance to interference (e.g., Hasher & Zacks, 1988; Lustig et al., 2007; West, 1996; Zanto & Gazzaley, 2014; see Verhaeghen, 2011, 2014, for a critical review and a set of meta-analyses on this issue). We examined this proposition by directly examining the build-up of interference over a long run of N-Back items drawn from a small set of possible stimuli, resulting in rapid repeats of specific stimuli. We obtained such a cost, but, again, the cost was identical across age groups (Verhaeghen & Zhang, 2013), in line with our own reviews of the literature (Verhaeghen, 2011, 2014).
In the present paper, we investigate a fourth hypothesis. Perhaps what changes with age is the very nature of the memory representation in the outer store, specifically its resolution, that is, its quality, precision, or fidelity. This hypothesis is, of course, difficult to assess with the usual stimuli used in aging research -- letters, digits, words, or figures, all presented in formats that should allow for perfect read-out – and hence requires stimuli for which actual perceptual/attentional and memory resolution can be assessed at a more detailed level of grain, and at levels of performance clearly below the measurement ceiling. As far as we know, only three studies to date have investigated age-related differences in resolution in (visual) short-term memory (Noack, Lövdén , & Lindenberger, 2012, using locations; Peich, Husain, & Bays, 2013, using slanted lines; Pertzov, Heider, Liang, & Husain, 2015, using memory for locations); none used a paradigm that necessitates focus switching. All three studies found evidence for age-related changes in resolution, and all concluded that there is an age-related deficit in very basic, early processing in visual short-term memory, occurring when all items are processed inside the focus of attention. Because our interest lies with the fate of items stored outside the focus, we will use an N-Back task, which necessitates switching. We elected to use angled lines as stimuli, for three reasons: (a) It is relatively easy to derive individualized thresholds for line orientation discrimination; (b) orientation lends itself easily to presentation in a match-mismatch format; and (c) age differences in line orientation discrimination thresholds have been observed before (Betts, Sekuler, & Bennett, 2006), making this a good task for an age-comparative study.
In the interest of our research question, we made a crucial change to our usual paradigm. Because our focus is on age-related deficits specific to items held inside the outer store, we first equated performance across all subjects for the 1-Back condition, where items remain present inside the focus of attention. We did this by determining individual thresholds for 75% accuracy for line orientation discrimination within a 1-Back task, and applying these individual thresholds to mismatch stimuli. The aim was to equate all participants on the perceptual and attentional requirements for the 1-Back task, so that any differences observed between individuals or groups (including age-related differences) in the 2-Back task would be due to differences in resolution of the representation in the outer store, and not – as would be the case if all participants received the same physical stimuli – a mixture of differences in perceptual noise, attention resolution, and memory fidelity. If the age by N interaction in accuracy survives this manipulation, the conclusion must be that there is a specific age-related deficit in resolution for items held in the outer store; if it does not, then the deficit in memory found in previous studies must be ascribed to differences in perceptual and/or attentional resolution already present in the focus of attention, which magnify once items leave this focus. Note that evidence for a decrease in resolution does not, in the present paradigm, allow us to pinpoint the process or processes associated with this decrease – it could be a matter of defective transfer from the focus into the outer store, maintenance-related degradation, issues during retrieval from the outer store into the focus, or a combination thereof.
One often-overlooked complication is that traditional N-Back paradigms conflate the need for focus switching (our area of interest) with memory load. That is, traditionally, 1-Back tasks are used to measure performance when no focus switching is necessary, and 2-Back tasks are deployed to measure the decrement in performance when the focus-switching requirement is added. Two-Back tasks, however, also by necessity involve a higher working memory load than 1-Back: An extra item needs to be remembered. To disentangle the effects of load and focus switching, we devised an unpredictable 2-Back task (Price, Colflesh, Cerella, & Verhaeghen, 2014). In this task, items are again presented one at a time in 2 virtual columns, but instead of going left-to-right in a predictable sequence, presentation order is randomized; the participant still indicates whether the current item matches the item previously presented in the same column (Fig. 1C presents an illustration). There are two possibilities: Either the location of the current probe matches the location of its predecessor, referencing an item presumably still within the focus (‘non-switch’ trial), or these two locations differ, necessitating a focus-switch process (‘switch’ trial). Non-switch trials are effectively 1-Back trials; switch trials are effectively 2-Back trials. In this unpredictable version, memory load is held constant, because the participant needs to be ready at all times to retrieve the item 2 positions back. This allows us to assess the focus-switch cost more accurately by comparing performance on switch trials with that on non-switch trials.
Figure 1.
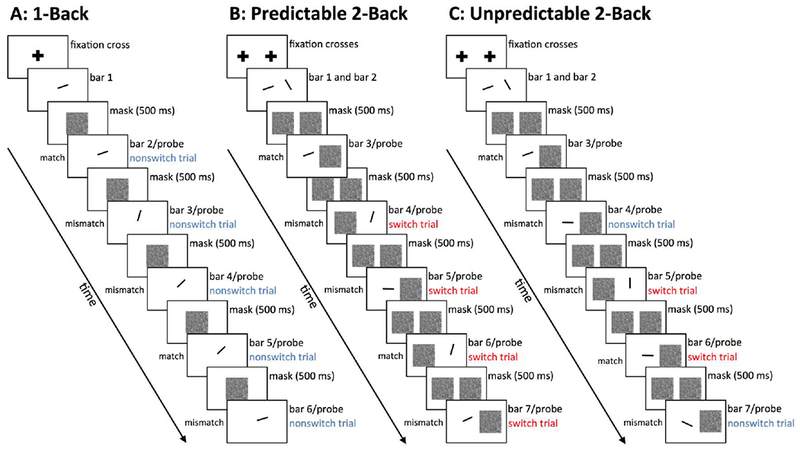
Sequence of events within a block within each condition. Participants were asked to judge whether the probe matches the item previously presented in the same column. Twenty probes were presented per block. Panel A: 1-Back condition; panel B: predictable 2-Back condition; panel C: unpredictable 2-Back condition. In the unpredictable 2-Back condition, trials can be either non-switch trials (probing the column just presented), or switch trials (probing the alternate column). In the predictable 2-Back conditions, all trials are switch trials.
Method
Participants
Participants were 29 younger adults between the ages of 18 and 34 (14 women, Mage = 21.50 years, SD = 4.03), all undergraduate students from the Georgia Institute of Technology, who participated in return for class credit, and 28 older adults between the ages of 64 and 87 (19 women, Mage = 73.90 years, SD = 6.24), recruited from the community, who participated in return for a modest compensation of $10/hour. In our previous research, samples this size have reliably led to the 1-Back versus 2-Back age-related dissociation. Participants were excluded when they self-reported a history of cataracts, glaucoma, macular degeneration, or other vision problems (corrective glasses were not considered an exclusion criterion).
Materials and Procedure
Participants performed 1-Back and 2-Back tasks with slanted bars as the stimuli. Each bar was presented in black inside a virtual circle subtending 4.1° of visual angle; the bar originated from the center of the circle, like a clock hand.
Prior to the main experiment, we determined each participant’s 75% accuracy threshold for line bar orientation in the 1-Back version of the task by using the Bayesian QUEST algorithm (Watson & Pelli, 1983). QUEST is a fast and efficient adaptive psychometric procedure that places each trial at the currently most probable Bayesian estimate of the threshold and yields a final maximum likelihood estimate of the 75% threshold for – in our case – line orientation, expressed in angular degrees, that is, the difference in orientation in two successively presented lines that yields a 75% probability for the participant to correctly identify the mismatch. We used the Quest toolbox as supplied by the Psychtoolbox kit for Matlab (http://docs.psychtoolbox.org/Quest). After initial piloting, the starting value for the threshold estimate was set at 7 angular degrees, and its SD at 4. The algorithm was applied to 24 1-Back trials of 20 stimuli each; the task was run exactly as this task was run in the experiment proper (see next paragraph and Figure 1A), with QUEST making adjustments as needed after each trial. This individually-derived threshold was then used to construct the mismatch stimuli for each participant within the main experiment, as explained in the next paragraph.
For the 1-Back task (Figure 1A), each block started with a 500 ms central fixation cross. The initial stimulus was then presented at fixation until the participant made a key press. Next, the stimulus was replaced by a gray-scale mask (covering the location of the original stimulus) for 500 ms, after which the next stimulus was presented; the task was to determine whether the stimulus was a match or a mismatch. Participants entered their response using the “0” key for match and “1” for mismatch. If a key other than “0” or “1” was pressed, participants received the message “not a valid response” and were returned to the current stimulus. The stimulus remained on screen until the participant made their response, after which the stimulus was replaced by a 500 ms mask, followed by the next test stimulus. Participants were instructed to always compare the current stimulus to the immediately preceding stimulus for a total of 20 to-be-responded-to stimuli per trial. Subjects performed 30 blocks, for a total of 600 responses. Half of those were match, half mismatch; 1/6 of the mismatch trials (50 responses) were rotated either 1, 2, or 3 times the individual’s threshold value, either in a clockwise or counterclockwise direction. We included these different values to potentially uncover interactions between age and difficulty of the perceptual and attentional aspects of the task. We expect the task to be most difficult for 1*threshold and get progressively easier for 2*threshold and 3*threshold; the match stimuli should be particularly easy. If our calibration procedure proved successful, participants’ performance should be matched at 75% for the 1*threshold mismatch stimuli in the 1-Back version of the task.
The 2-Back task was identical to the 1-Back task except for the following changes. Instead of a single, centrally presented stimulus, the initial screen showed two black bars, one at the left of fixation, the other at the right, subtending a total visual angle of 11° (the bars were the same size as those for the 1-Back task). After the mask was presented, the next series of 20 stimuli were presented alternating either predictably or unpredictably on the left or right side of fixation. The mask on the opposite side always remained visible. Once the participant made a key press, both stimuli were covered by masks. The participant was instructed to compare the current test stimulus to the most recent stimulus presented at the same location of the screen.
We implemented two 2-Back conditions, each consisting of 600 responses. One was the standard 2-Back task (Figure 1B), where stimuli alternated between columns in a predictable fashion, left to right. The other was the unpredictable 2-Back task as described in the Introduction (Figure 1C), in which the stimulus would randomly be presented either in the same location as the immediately preceding stimulus (non-switch trial) or in the alternate location (switch trial). 1-Back was always performed before 2-Back, as we have consistently done in our previous work. The experiment was conducted in a single session.
Results
Older Adults Have Lower Perceptual and/or Attentional Resolution Than Younger Adults
Thresholds for 75% accuracy as obtained from the QUEST algorithm in the 1-Back task were on average 8.41 angular degrees for younger adults (SD = 4.41), and 13.97 angular degrees for older adults (SD = 5.45). The difference was significant and rather large, t (4.24), p < .001, d = 1.12. Thus, as suspected, older adults have lower resolution for this type of stimulus, even for comparisons taking place within the focus of attention.
2-Back Accuracy Of Older Adults Equals That Of Younger Adults Once 1-Back Performance Is Equated
We performed a 7 (the seven angles of rotation, from zero rotation to 3*threshold rotation in either direction) by 2 (age group) by 2 (1-Back vs. predictable 2-Back) ANOVA. For accuracy, operationalized as proportion correct (Figure 2A), we found no evidence for a main effect of age, F(1, 55) = 0.49, ηp2 = 0.009, p = .49, and none of the interactions involving age were reliable (age by N, F(1, 55) = 0.03, ηp2 = .001, p = .86; age by angle, F (6, 330) = 1.73, ηp2= .030, p = .11; age by N by angle, F (6, 330) = 0.72, ηp2 = .013, p = .64). Thus, when participants are matched on 1-Back performance, age-related working memory differences fail to appear. Note that accuracy for mismatch stimuli presented at ±1*threshold in the 1-Back task was close to 75% for both age groups, showing that our pre-experiment calibration procedure, which aimed to accomplish exactly this, was successful. Clearly, the standard error of the mean is larger than zero, indicating noise in the threshold measurement or drift in the threshold over the course of the experiment, due, perhaps, to a mixture of practice effects and fatigue (if it was one of these two, performance on the 1-Back task would have been higher, respectively lower than what was obtained in the calibration phase); this SEM is not appreciably different between age groups. Additionally, we found main effects of angle, F(6, 330) = 155.62, ηp2 = .739, p < .001 (match trials [i.e., zero deviation] lead to high accuracy, in mismatch trials accuracy monotonically increases as deviation increases), and of N (1-Back yields higher accuracy than 2-Back), F(1, 55) = 133.61, ηp2 = .708. We obtained a significant N by angle interaction, F (6, 330) = 20.52, ηp2 = .272, p < .001 (mainly a dampening of the effects in 1-Back, possibly due to a ceiling effect), but none of the other effects reached significance.
Figure 2.
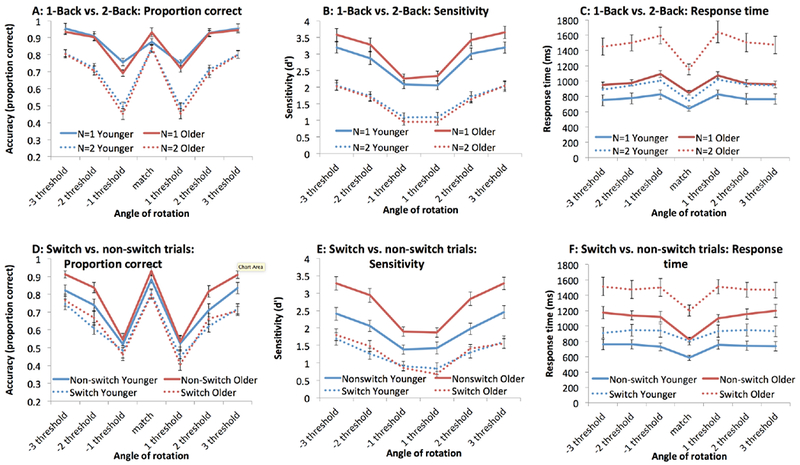
N-back performance after equating subjects on perceptual performance. Panels A-C show 1-back and 2-back performance in terms of proportion correct (panel A), sensitivity (panel B), and response time (panel C). Panels D-F show switch and non-switch trials from the unpredictable 2-back task. This comparison eliminates the memory load confound present in the comparisons in panels A-C. Panels A and B show an absence of age-related effects; panel C shows that older adults have slower access times for 2-back items; panel D and E show an older-adult advantage for non-switch trials; and Panel F shows equivalent age-related differences for both types of trials. Error bars represent standard error of the mean.
These analyses were repeated with sensitivity (d’; calculated as in Macmillan & Kaplan, 1985) as the dependent variable. ANOVA results echo those of the accuracy data: main effects of angle, F(5, 275) = 201.08, ηp2 = .785, p < .001, and of N, F(1, 55) = 104.56, ηp2 = .655, but no main effect of age, F(1, 55) = 0.87, ηp2 = .016, p = .35. There was a significant N by angle interaction, F(5, 275) = 6.91, ηp2 = .112, with a flatter sensitivity by angle profile in 2-Back, but none of the other effects reached significance. Notably, none of the interactions involving age were reliable: age by N, F(1, 55) = 2.73, ηp2 = .047, p = .10; age by angle, (5, 275) = 1.14, ηp2 = .020, p = .34; age by N by angle, F(5, 275) = 0.30, ηp2 = .005, p = .92.
Additionally, response times give an idea of the ease of access to memoranda (Figure 2C). To minimize the effect of outliers in our analyses, we used median RTs within each subject within each condition rather than mean RTs. We found main effects of angle, F(6, 330) = 43.22, ηp2 = .440, p < .001 (fast responses for match trials, and progressively slower RTs as the angle of rotation diminishes), of N (1-Back yields faster RT than 2-Back), F(1, 55) = 50.88, ηp2= 423,355.42, and of age (older adults are slower), F(1, 55) = 15.93, ηp2 = .481, p < .001. There was a significant interaction between N and angle, such that the effects of angle were more outspoken for 2-Back, F(6, 330) = 5.62, ηp2 = .093, p < .001. The interaction between age and N was significant (the age difference was larger for 2-Back than for 1-Back), F (1, 55) = 12.57, ηp2 = .186, p = .001; so was the interaction between age and angle (older adults showed more outspoken effects of angle), F(6, 330) = 2.21, ηp2 = .039, p = .042. The triple interaction was not significant, F(6, 330) = 1.46, ηp2 = .026, p = .193. Only the age by N interaction, but not the effect of angle, survived logarithmic transformation, performed to control for baseline slowing differences between age groups (Faust, Balota, Spieler, & Ferraro, 1999), F(1, 55) = 7.97, ηp2 = .127, p = .007. Thus, older adults are disproportionally slower at accessing items stored in memory once they leave the focus of attention.
Net Focus-Switching Costs Of Older Adults Equal Those Of Younger Adults Once 1-Back Performance Is Equated
The comparison between 2-Back versus the standard 1-Back task cannot disentangle the influence of focus switching and memory load (see Introduction). In order to isolate the influence of each, participants performed an unpredictable 2-Back task (Figure 1B) in which memory load remained constant but the focus-switch requirement varied. For accuracy (Fig. 2D), we found a significant main effect of angle, F(6, 330) = 102.24, ηp2 = .650, p < .001 (match trials show high accuracy, and accuracy declines with decreasing deviation from a match), and of type of switch (switch trials yield lower accuracy), F (1, 55) = 114.18, ηp2 = .675, p < .001, but not of age, F(1, 55) = 1.38, ηp2 = .024, p = .24. There was a significant age by type of switch interaction, F (1, 55) = 7.43, ηp2 = 0.119, p = .009 – older adults perform, on average, 6 percentage points better than younger adults in non-switch trials, versus 1 percentage point better in switch trials. There was no significant age by angle interaction, F (6,330) = 1.52, ηp2 = 0.025, p = .17, nor was the triple age by angle by type of switch interaction significant, F (6, 330) = 0.16, ηp2 = 0.011, p = .99.
Repeating this analysis with sensitivity (d’) as the dependent measure (Fig. 2E), we obtained a significant main effect of angle, F(5, 275) = 126.85, ηp2 = .698, p < .001, and a main effect of type of switch F(5, 275) = 217.50, ηp2 = .798, p < .001. We now, however, also uncovered a main effect of age, F(1, 55) = 4.54, ηp2 = .076, p = .038, with older adults actually showing higher sensitivity than younger adults (1.99 versus 1.61). There was a significant age by type of switch interaction, F(1, 55) = 24.51, ηp2 = .308, p = < .001: Older adults perform, on average, 0.73 d’ units higher than younger adults in non-switch trials, versus 0.03 in switch trials. There was a significant age by angle interaction, F(5, 275) = 3.80, ηp2 = .065, p = .02, with a monotonic increase in age difference with deviation from match. The triple age by angle by type of switch interaction was not significant, F(5, 275) = 0.76, ηp2 = .014, p = .58.
Switch trials were slower than non-switch trials (Fig. 2F), F(1, 55) = 109.96, ηp2 = 132,159.81, p < .001; there was a main effect of angle, with match trials showing faster RTs, F(6, 330) = 27.93, ηp2 = .337, p < .001; and older adults were slower than younger adults, F (1, 55) = 20.75, ηp2 = .274, p < .001. The only significant interactions were between age and type of trial (older adults were slower still on switch trials compared to non-switch trials), F (1, 55) = 9.43, ηp2 = .146, p = .003; and between age and angle (age differences tended to increase with deviation from match) F(6, 330) = 3.90, ηp2 = .066, p = .001. Neither of these interactions, however, survived logarithmic transformation, F(1, 55) = 1.28, ηp2 = .023, p = .26, for the interaction with type of trial, and F(6, 330) = 2.05, ηp2 = .036, p = .06, for the interaction with angle. Neither the angle by type of trial interaction, F(6, 330) = 1.45, ηp2 = .026, p = .19, nor the triple interaction, F(6, 330) = 1.31, ηp2 = .023, p = .25, reached significance.
Discussion
We examined the reason for the oft-observed age-related deficit in accuracy in a N-Back task when N ≥ 2 compared to the small or nonexistent age-related differences in a 1-Back task. The key manipulation in our experiment was to individually engineer mismatch stimuli to be one, two, or three threshold values away from the original stimulus in the 1-Back version of the task. In doing so, we equated all participants on the perceptual and attention-related aspects of the task, so that any remaining age-related differences in 2-Back would be related to processes associated with the focus-switching requirement. While doing so, we discovered that older adults had a much higher threshold for line orientation than younger adults, with a large effect size (d = 1.12; for similar results, see Betts, Sekuler, & Bennett, 2006).
Our main result is that when participants were equated on 1-Back accuracy, no age differences in the 2–back version of the task appeared. The data points for younger and older adults virtually overlapped; the Bayes factor favoring the null hypothesis was 5.84 for accuracy data, and 4.82 for sensitivity; using the formulas from Table 2 in Jarosz & Wiley, 2014, assuming a unit information prior; Bayes factors for the age by N interaction were 7.39 for accuracy and 1.90 for sensitivity). This result suggests that the age-related differences we (Vaughan et al., 2008; Verhaeghen, 2012; Verhaeghen & Basak, 2005; Zhang et al., 2012) and others (for a meta-analysis, see Bopp & Verhaeghen, 2005) have previously observed in the accuracy of retrieval of items maintained in the outer store of working memory are not, after all, due to processes associated with focus switching (encoding into, maintenance inside or retrieval from working memory), but rather appear to be already present in a version of the task that does not require these working memory processes.
As noted above, these comparisons between 1-Back and 2-Back results contain a memory load confound (1-Back has a single-item load; 2-Back has a double-item load). We removed this confound by zooming in on the unpredictable 2-Back task, where the memory load is always two items, and comparing non-switch trials (which are effectively 1-Back trials, and do not necessitate a focus switch) with switch trials (which are effectively 2-Back tasks, and do necessitate a focus switch). In this comparison, we did obtain a reliable age by N interaction. Rather than uncovering a deficiency in the switch trials in the older adults, however, this interaction signaled an inverse age effect in the non-switch trials, where older adults in effect outperformed younger adults.
What does this finding signify? There can be at least two possible reasons why an older-age advantage for non-switch trials might be obtained. One possibility – which we will reject – could be a strategic shift. Imagine that younger adults tend to preemptively shift their attention to the item held in the outer store, while the older adults tend to hold on to the last item presented, perhaps as a conservative strategy to keep accuracy high. In that case, older adults would be at an advantage in non-switch trials compared to younger adults, and at a disadvantage in switch trials. We must, however, reject this hypothesis, because it does not fit the response time data. If older adults were not to switch their attention, but younger adults did, access times for older adults should be relatively faster on non-switch trials compared to switch trails; as a consequence, age differences would be smaller for non-switch trials. This turns out not to be the case: There was no age by trial type interaction for response time.
The likelier explanation for the older-age advantage for non-switch trials is an age-related change in the quality of attention itself. That is, the calibration on the perceptual and attentional aspects of stimulus discrimination might free up resources that can now be applied to encoding, maintaining, and/or retrieving a more precise memory representation. Arguably, this effect would be stronger for individuals with larger perceptual difficulties, and hence (on average) affect older adults more than younger adults. Moreover, this sharpening of the representation would not necessarily be reflected in response time.
This interpretation gains weight when considered in the wider context of reports of an increasing integration between perception and cognition with advancing age. That is, while there is no strong evidence suggesting a link between perceptual and cognitive processing in early life or midlife, a number of studies have shown strong interrelationships – a growing dedifferentiation of the two domains -- in old age (e.g., Anstey, Stankov, & Lord, 1993; Lindenberger & Baltes, 1994; Schaie, Baltes, & Strother, 1964). Two reasons for this perception-cognition link have been advanced (Li & Lindenberger, 2002). First, many of the studies on dedifferentiation have also found that sensory functioning and cognitive performance share a large part of the age-related variance, giving rise to the hypothesis that a domain-general mechanism (most often identified as intactness of the neural substrate) might be responsible for the shared effects. This variable is often labeled ‘the common cause’ (e.g., Kiely & Anstey, 2015; Salthouse & Czaja, 2000). Explanations that propose a role for elementary processing speed (e.g., Brown, Brockmole, Gow, & Deary, 2012; Guest, Howard, Brown, & Gleeson, 2015) fall under this category. This interpretation, however, does not fit our data well: Under a common-cause hypothesis, simply eliminating age-related differences in perceptual or attentional processing through a peripheral manipulation, as we did here, should not eliminate differences in cognitive processing, because such manipulations would not have an impact on the purported existing global age-related differences in neural functioning that underly both the perceptual/attentional and cognitive deficits.
A second, causal perspective – the effortfulness hypothesis (Wingfield et al., 2005) – fits our data better. The claim is that deficits in sensory processing might cause age-related differences in cognitive functioning through mechanisms such as resource overlap, resource competition, and/or trade-offs (e.g., Schneider & Pichora-Fuller, 2000). The assumption is that both sensory processing and cognitive processing require the deployment of mental resources. In particular, a higher investment of effort in the initial, perceptual stages of processing may come at the cost of processing resources that would otherwise be available for downstream operations, including effective memory encoding, maintenance, and/or retrieval. Such costs would potentially be exacerbated if the perceptual stage gets successfully resolved, because a lot of the available resources would then be expended at the perceptual stage. The effortfulness hypothesis has so far been tested by adding auditory noise to span tasks (Baldwin & Ash, 2011; Rabbitt, 1968), or by statistically controlling for individual differences in hearing (e.g., Wingfield et al., 2005) or visual acuity (Porto et al., 2016) on measures of span or memory updating. Our experimental elimination of age-related differences in 2-Back performance by equating performance on 1-Back performance – thus equating, we argue, the perceptual and attentional aspects of the task – is compatible with the claims of this effortfulness view. It is, however, not synonymous with it: The stronger test of the hypothesis would be to equate participants on the purely perceptual aspects of the task by using a standard psychophysical procedure such as the two-interval force choice method (e.g., Edden, Muthukumaraswamy, Freeman, & Singh, 2009).
In our previous studies using the N-Back task, we consistently found that age-related differences in the 2-Back task, significant and noticeable, were accompanied by small and often non-significant age-related differences in the 1-Back version. The effortfulness hypothesis makes this understandable: For those with perceptual and/or attention difficulties, high (and hard-fought) levels of perceptual/attentional accuracy would come at a large cost to cognitive processing. In contrast, equating subjects on perceptual and attentional performance, as we did here, should free up additional resources specifically in those who originally performed more poorly on the perceptual/attentional aspects of the task; these resources can now be applied to cognitive processes. Our study, then, adds to the growing literature that shows that changes in more complex aspects of cognition can be ascribed to (or are artifacts of) age-related deficits in less complex/more basic aspects of cognition (e.g., Cerella, Poon & Williams, 1980; Verhaeghen, 2014). What the field has taken for a memory issue, that is, a specific age-related deficit that occurs once items leave attention and need to be retrieved from working memory, might then ultimately just be a consequence of age differences in perceptual and/or attentional resolution.
We would like to stress that we are not implying that all age-related deficits in cognition, or even just in working memory, are ultimately attributable to changes in perception and/or attention. The mechanism we propose here is not a direct causal cascade, but an indirect effect of potential resource sharing between the perceptual, attention, and cognitive stages of working memory processing. We do maintain that our work suggests that age-related deficits in cognitive processes should not be assumed a priori, but that it makes eminent sense to explore lower-level explanations for what manifests as a cognitive issue whenever possible. Therefore, extension of this work to other tasks and more direct indices of neural processing would be desirable. Specifically, we assume this work would apply to other working memory tasks that require shifting from processing to maintaining memoranda, such as operation span or reading span, but likely also to simpler tasks where trade-offs between stimulus encoding and maintenance seem likely, such as delayed match-to-sample tasks.
Acknowledgments
This work was supported by the National Institutes of Health under grant AG-16201.
Footnotes
This task is often considered an updating task (e.g., Schmiedek, Hildebrandt, Lövdén, Wilhelm, & Lindenberger, 2009). We make a distinction between the processes of focus switching and of updating per se -- the former concerns access, the latter requires replacing the content of the outer store with new content. These two processes are dissociable, as shown in training studies (Jain, 2018; Price, Colflesh, Cerella, & Verhaeghen, 2014).
The cited studies all explicitly examine age-related differences in focus switching using the N-Back paradigm, or closely related paradigms. Other mechanisms proposed for age-related changes in more global aspects of working memory include associative deficits (e.g., Chen & Naveh-Benjamin, 2012) and potential deficits in memory refreshing (e.g., Loaiza, Rhodes, & Anglin, 2015). At present, it remains an open question whether the age-related issues with memory refreshing found in some studies (e.g., Fanuel, Plancher, Monsaingeon, Tillmann, & Portrat, 2017; Loaiza, et al., 2015) are related to, or even reducible to, age-related differences in focus switching.
Contributor Information
Paul Verhaeghen, School of Psychology, Georgia Institute of Technology.
Shriradha Geigerman, School of Psychology, Georgia Institute of Technology.
Haoxiang Yang, Industrial Engineering and Management Sciences, Northwestern University.
Alejandra C. Montoya, School of Psychology, Georgia Institute of Technology
Dobromir Rahnev, School of Psychology, Georgia Institute of Technology.
References
- Anstey K, Stankov L, & Lord S (1993). Primary aging, secondary aging, and intelligence. Psychology and Aging, 8, 562–570. [DOI] [PubMed] [Google Scholar]
- Baldwin CL, & Ash IK (2011). Impact of sensory acuity on auditory working memory span in young and older adults. Psychology and Aging, 26(1), 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak C, & Verhaeghen P (2011). Aging and switching the focus of attention in working memory: Age differences in item availability, but not item accessibility. Journals of Gerontology: Psychological Sciences, 66, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, & Bennett PJ (2007). The effects of aging on orientation discrimination. Vision Research, 47, 1769–1780. [DOI] [PubMed] [Google Scholar]
- Bopp KL, & Verhaeghen P (2005). Aging and verbal memory span: A meta-analysis. The Journals of Gerontology: Psychological Sciences, 60, 223–233. [DOI] [PubMed] [Google Scholar]
- Bopp KL, & Verhaeghen P (2009). Working memory and aging: Separating the effects of content and context. Psychology and Aging, 24, 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp KL, & Verhaeghen P (2018). Aging and N-Back performance: A meta-analysis. Journals of Gerontology: Psychological Sciences. [DOI] [PubMed] [Google Scholar]
- Brockmole JR, Parra MA, Della Sala S, & Logie RH (2008). Do binding deficits account for age-related decline in visual working memory?. Psychonomic Bulletin & Review, 15, 543–547. [DOI] [PubMed] [Google Scholar]
- Brown LA, Brockmole JR, Gow AJ, & Deary IJ (2012). Processing speed and visuospatial executive function predict visual working memory ability in older adults. Experimental Aging Research, 38, 1–19. [DOI] [PubMed] [Google Scholar]
- Cerella J, Poon LW, & Williams DH (1980). Age and the complexity hypothesis In Poon LW (Ed.), Aging in the 1980s (pp. 332–340). Washington, DC: American Psychological Association. [Google Scholar]
- Chen T, & Li D (2007). The roles of working memory updating and processing speed in mediating age-related differences in fluid intelligence. Aging, Neuropsychology and Cognition, 14, 631–646. [DOI] [PubMed] [Google Scholar]
- Chen T, & Naveh-Benjamin M (2012). Assessing the associative deficit of older adults in long-term and short-term/working memory. Psychology and Aging, 27, 666–682. [DOI] [PubMed] [Google Scholar]
- Cowan N (1995). Attention and memory: An integrated framework. New York: Oxford University Press. [Google Scholar]
- Edden RA, Muthukumaraswamy SD, Freeman TC, & Singh KD (2009). Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. Journal of Neuroscience, 29, 15721–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanuel L, Plancher G, Monsaingeon N, Tillmann B, & Portrat S (2018). Temporal dynamics of maintenance in young and old adults. Annals of the New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, & Ferraro FR (1999). Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological Bulletin, 125, 777–799. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, & D’Esposito M (2007). Top-down modulation and normal aging. Annals of the New York Academy of Sciences, 1097, 67–83. [DOI] [PubMed] [Google Scholar]
- Guest D, Howard C, Brown LA, & Gleeson H (2015). Aging and the rate of visual information processing. Journal of Vision, 15, 1–25. [DOI] [PubMed] [Google Scholar]
- Hasher L, & Zacks RT (1988). Working memory, comprehension, and aging: a review and a new view In Bower GH (Ed.), The psychology of learning and motivation (Vol. 22, pp. 193–225). San Diego, CA: Academic Press. [Google Scholar]
- Jain S (2018). Understanding the training and transfer effects in N-Back training (unpublished doctoral dissertation). Georgia Institute of Technology, Atlanta. [Google Scholar]
- Jarosz AF, & Wiley J (2014). What are the odds? A practical guide to computing and reporting Bayes factors. The Journal of Problem Solving, 7, 2–9. [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, & Colflesh GJH (2007). Working memory, attention control, and the N-back task: A cautionary tale of construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33, 615–622. [DOI] [PubMed] [Google Scholar]
- Kiely K & Anstey K 2015, Common cause theory in aging, in Pachana NA (ed.), Encyclopedia of Geropsychology, Springer Science + Business Media, Singapore. [Google Scholar]
- Kilb A, & Naveh-Benjamin M (2015). The effects of divided attention on long-term memory and working memory in younger and older adults: Assessment of the reduced attentional resources hypothesis In Logie RH & Morris RG (Eds.), Current issues in memory. Working memory and ageing (pp. 48–78). New York, NY, US: Psychology Press. [Google Scholar]
- Li KZ, & Lindenberger U (2002). Relations between aging sensory/sensorimotor and cognitive functions. Neuroscience & Biobehavioral Reviews, 26, 777–783. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, & Baltes PB (1994). Sensory functioning and intelligence in old age: a strong connection. Psychology and Aging, 9, 339–355. [DOI] [PubMed] [Google Scholar]
- Loaiza VM, Rhodes MG, & Anglin J (2013). The influence of age-related differences in prior knowledge and attentional refreshing opportunities on episodic memory. Journals of Gerontology: Psychological Sciences, 70, 729–736. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, & Zacks RT (2007). Inhibitory deficit theory: Recent developments in a “new view” In Gorfein DS & MacLeod CM (Eds.), The place of inhibition in cognition (pp. 145–162). Washington, DC: American Psychological Association. [Google Scholar]
- Macmillan NA, & Kaplan HL (1985). Detection theory analysis of group data: Estimating sensitivity from average hit and false-alarm rates. Psychological Bulletin, 98, 185–199. [PubMed] [Google Scholar]
- Noack Hannes, Martin Lövdén Florian Schmiedek, and Lindenberger Ulman. “Cognitive plasticity in adulthood and old age: gauging the generality of cognitive intervention effects.” Restorative Neurology and Neuroscience, 27: 435–453. [DOI] [PubMed] [Google Scholar]
- Oberauer K (2002). Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition, 28, 411–421. [PubMed] [Google Scholar]
- Peich M-C, Husain M, & Bays PM (2013). Age-related decline of precision and binding in visual working memory. Psychology and Aging, 28, 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertzov Y, Heider M, Liang Y, & Husain M (2015). Effects of healthy ageing on precision and binding of object location in visual short term memory. Psychology and Aging, 30, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto FH, Tusch ES, Fox AM, Alperin BR, Holcomb PJ, & Daffner KR (2016). One of the most well-established age-related changes in neural activity disappears after controlling for visual acuity. Neuroimage, 130, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Colflesh GJH, Cerella J, & Verhaeghen P (2014). Making working memory work: The effects of extended practice on focus capacity and the processes of updating, forward access, and random access. Acta Psychologica, 148, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PMA (1968). Channel capacity, intelligibility and immediate memory. Quarterly Journal of Experimental Psychology, 20, 241–248. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, & Czaja S (2000). Structural constraints on process explanations in cognitive aging. Psychology and Aging, 15, 44–55. [DOI] [PubMed] [Google Scholar]
- Schaie KW, Baltes P, & Strother CR (1964). A study of auditory sensitivity in advanced age. Journal of Gerontology, 19, 453–457. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Hildebrandt A, Lövdén M, Wilhelm O, & Lindenberger U (2009). Complex span versus updating tasks of working memory: The gap is not that deep. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Schneider BA, & Pichora-Fuller MK (2000). Implications of perceptual deterioration for cognitive aging research In Craik FIM and Salthouse TA (eds). The handbook of aging and cognition, 2nd ed., (pp. 155–219). Mahwah, NJ: Erlbaum. [Google Scholar]
- Vaughan L, Basak C, Hartman M & Verhaeghen P (2008). Aging and working memory inside and outside the focus of attention: Dissociations of availability and accessibility. Aging, Neuropsychology, and Cognition, 15, 703–724. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P (2012). Working memory still working: Age-related differences in working memory and executive control In: Ohta N and Naveh-Benjamin M (Eds.), Memory and Aging, Psychology Press. [Google Scholar]
- Verhaeghen P (2014). The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences. New York: Oxford University Press. [Google Scholar]
- Verhaeghen P, & Basak C (2005). Aging and switching of the focus of attention in working memory: Results from a modified N-Back task. Quarterly Journal of Experimental Psychology (A), 58, 134–154. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J, & Basak C (2004). A working memory workout: How to change to size of the focus of attention from one to four in ten hours or less. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30, 1322–1337. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, & Hoyer WJ (2007). Aging, focus switching and task switching in a continuous calculation task: Evidence toward a new working memory control process. Aging, Neuropsychology, and Cognition, 14, 22–39. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P & Zhang Y (2013). What is still working in working memory in old age: Dual tasking and resistance to interference do not explain age-related item loss after a focus switch. Journals of Gerontology, 68, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt S, & Hagendorf H (2002). The role of task context for component processes in focus switching. Psychologische Beiträge, 44, 248–274. [Google Scholar]
- Watson AB, & Pelli DG (1983). QUEST: A Bayesian adaptive psychometric method. Attention, Perception, & Psychophysics, 33(2), 113–120. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Tun PA, & McCoy SL (2005). Hearing loss in older adulthood what it is and how it interacts with cognitive performance. Current Directions in Psychological Science, 14, 144–148. [Google Scholar]
- Zanto TP,& Gazzaley A (2014). Attention and aging In: Nobre AC & Kastner S (Eds.), Handbook of Attention (pp. 927–971). Oxford, UK: Oxford University Press. [Google Scholar]
- Zhang Y, Verhaeghen P, Cerella J (2012). Working memory at work: How the updating process alters the nature of memory encoding. Acta Psychologia, 139, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


