Abstract
A recent clinical trial of a virotherapy approach, consisting of an engineered poliovirus, has provided evidence of apparently durable responses in patients with recurrent glioblastoma. The results of this trial and others indicate that virotherapy might be an effective tool in anticancer immunotherapy. Yet, caution must be exercised until appropriately powered randomized clinical trials truly show efficacy.
Historically, the use of viruses in anticancer therapy has been judged to be a ‘fringe’ and ‘far-fetched’ approach with little credibility outside of a few practitioners of ‘virotherapy’. In the age of precision medicine and targeted therapies, treatments involving relatively untargeted pathogens have not been fashionable in oncology. Over time, however, the tremendous intratumoural genetic and signalling heterogeneity of solid neoplasms, such as glioblastoma, became recognized as an almost insurmountable limitation to therapies targeting one or a few mutations or aberrant pathways1. In this context, the pleiotropic mechanisms of tumour cell-killing engaged by virotherapy are postulated to be advantageous in addressing intratumoural heterogeneity2. In fact, the past 3 years have seen a sudden proliferation of reports of different oncolytic virotherapies being published in very high-impact journals3,4. These studies have revealed that virotherapy can be very effective in turning the tumour microenvironment from an immunosuppressed (cold) to an inflamed (hot) state (FIG. 1). Thus, virotherapy has progressed from being a ‘benchwarming’ player to the new exciting ‘quarterback’ orchestrating the offensive against cancer.
Fig. 1 |. The current hypothesis for anticancer mechanisms of oncolytic virotherapy.
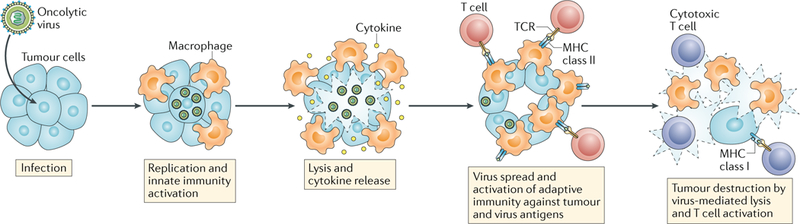
Initial viral infection of tumour cells leads to some degree of direct cytotoxicity, with cell lysis enabling the spread of replicative viruses to surrounding cells. Consequently, the inflammatory response to the infection alters the microenvironment of the tumour from immunologically ‘cold’ to ‘hot’ owing to recruitment and activation of innate and adaptive immune cells capable of mediating anticancer responses. The US National Library of Medicine ClinicalTrials.gov database lists several open clinical trials of different virotherapies for glioblastoma, such as studies using the modified poliovirus PVSRIPO (NCT02986178 and NCT03043391), herpes simplex viruses (NCT02457845, NCT03152318, and NCT02062827), adenoviruses (NCT02798406, NCT03178032, NCT03072134, NCT01811992, NCT02026271, and NCT03576612), retroviruses (NCT02414165), reoviruses (NCT02444546), and vaccinia viruses (NCT03294486). TCR, T cell receptor.
The latest addition to the roster of oncolytic virotherapies is an engineered poliovirus (PVSRIPO) that has recently been tested in a phase I clinical trial involving 61 patients with biopsy-proven recurrent glioblastoma5. The patients did not undergo cytoreductive surgery; instead the tumours were directly infused with PVSRIPO by convection-enhanced delivery. Outcomes were compared with those of a matched historical control group from the same institution. No adverse events directly attributed to neurovirulence were reported; however, 69% of patients had transient neurological complications owing to reactive inflammation and brain oedema following virus administration, although the symptoms were successfully treated with steroids or bevacizumab. The median survival of 12.5 months in PVSRIPO-treated patients was not markedly different from that of the historical control group (11.3 months). Nevertheless, the study was notable for a ‘tail’ of the survival curve plateauing at 21% after 24 months with PVSRIPO, whereas survival in the control group continued to decline from 14% at this time point to <2% beyond 48 months. More significantly, 8 patients in the PVSRIPO group reached the 2-year landmark, 5 of whom also reached the 3-year landmark, and 4 survivors remained alive at 41, 57, 69, and 70 months at the data analysis cut-off date (March 2018)5. These long-term durable responses explain the excitement generated by the trial.
PVSRIPO possesses several biological features that might explain its anticancer properties6. First, the virus rapidly hijacks the cell’s synthetic machinery upon infection and is, therefore, very efficient at killing tumour cells. Second, a transgenic molecular switch ensures selective replication in cancer cells and not nonmalignant cells of the central nervous system. Third, entry of the virus into cells is dependent on CD155, a membrane protein overexpressed by tumour cells. Fourth, upon infection, the virus triggers a potent innate immune response mediated by type 1 interferons (IFNs). This response is innocuous to the PVSRIPO virus itself, which produces a protease that disrupts host cell protein synthesis while selective viral translation is maintained, thereby enabling continued viral replication and spread; however, IFN response does result in a localized inflammatory reaction and the recruitment of activated neutrophils and macrophages to the tumour micro-environment. Finally, PVSRIPO infects antigen-presenting cells but does not lyse them, inducing a protracted inflammatory milieu and activation of adaptive immunity, including cytotoxic T lymphocyte responses against tumour antigens.
At first glance, the potential survival benefit associated with PVSRIPO seems surprising and very exciting, perhaps exceeding the results obtained with other experimental agents in similar clinical settings. However, published results from trials of other viro-therapies also demonstrate that a small subset of patients have durable clinical responses. For instance, the investigators of a very similar trial3, in which patients with recurrent glioblastoma underwent biopsy sampling and then intratumoural injection of a tumour cell-selective adenovirus (DNX-2401), reported that 5 of 25 patients (20%) were alive at the 3-year landmark. In 2004, we published a phase I multi-institutional clinical trial in patients with recurrent malignant glioma using a tumour cell-selective adenovirus (ONYX-015)7. Remarkably, 2 of 24 patients (8%) included in the trial are alive and in remission >16 years after treatment (S. B. Tatter, personal communication), although both had a histological diagnosis of recurrent anaplastic astrocytoma. Other trials investigating alternative virotherapy strategies have recently shown an encouraging number of patients with prolonged survival. For example, the non-lytic, replicating, cytosine deaminase-expressing retrovirus vocimagene amiret-rorepvec (Toca 511), combined with Toca FC (an extended release formulation of the 5-fluorouracil prodrug 5-fluorocytosine), was superior to chemotherapy with lomus-tine alone in the treatment of recurrent glioblastoma, with 12 of 43 patients (28%) alive at the 2-year landmark; however, the virotherapy was combined with tumour reresection, which is known to confer an additional survival advantage, and, in this case, the virus acted as a facilitator of local delivery of chemotherapy, rather than by inducing direct tumour lysis8. Other responders have been reported after viral-based gene therapy administered as an adjuvant in the standard-of-care treatment of patients with newly diagnosed glioblastoma9 or combined with radiation in those with recurrent glioblas-toma10. Thus, a key question is whether the existence of populations of long-term survivors is typical of PVSRIPO or is independent of the virotherapy of choice, possibly owing to the general immunostimulatory effects of infection by the viruses, or alternatively is reflective of a small subset of patients who might have a durable response to any particular treatment. In fact, a second question relates to the characteristics that distinguish responders from nonresponders. In the trial of PVSRIPO5, signals hinted that methylation of the MGMT promoter and a tumour cross-sectional area <873 mm2 characterized responders. In the Toca 511 trial8, differences between the tumour tran-scriptome of responders and nonresponders were reported. Clearly more research on predictive biomarkers of responsiveness to virotherapies is warranted.
As with the findings of any phase I trial, cautious optimism is needed regarding the potential clinical utility of PVSRIPO or other virotherapies. Larger, randomized trials (for example, NCT02986178) will establish whether PVSRIPO is a ‘franchise player’ in anticancer therapy — or just another ‘journeyman’. Yet, a single-shot therapy that can potentially actuate multiple anticancer mechanisms (direct cytotoxicity, induction of cytokines and danger signals, activation of innate immunity, and activation of effector T cell immunity; FIG. 1) does provide a possible solution to the problem of intratumoural heterogeneity; therein lies the most exciting justification for virotherapy against cancer.
Acknowledgements
E.A.C. acknowledges research support by the US NIH National Cancer Institute under awards 2P01CA163205, CA069246-20, and P50CA165962. P.P. acknowledges research support by the US National Institute of Neurological Disorders and Stroke under award 5K08NS101091. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing interests
E.A.C. is a consultant for Advantagene and DNAtrix. He has also previously been a consultant for Tocagen and Ziopharm Oncology. P.P. declares no competing interests.
The ClinicalTrials.gov database: www.clinicaltrials.gov
References
- 1.McGranahan N & Swanton C Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27, 15–26 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Lawler SE et al. Oncolytic viruses in cancer treatment: a review. JaMa Oncol. 3, 841–849 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Lang FF et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 36, 1419–1427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjardins A et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MC et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. TranslMed. 9, eaan4220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiocca EA et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 10, 958–966 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Cloughesy TF et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl Med. 8, 341ra375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheeler LA et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 18, 1137–1145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markert JM et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 22, 1048–1055 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


