Abstract
Purpose:
To evaluate New York State’s mandate that prescribers query the prescription drug monitoring program (PDMP) prior to prescribing Schedule II–IV medications.
Methods:
We conducted an interrupted time series analysis of opioid analgesic prescriptions dispensed to adult New York City (NYC) residents using data from New York State’s PDMP. Our main outcomes were the rate of (a) greater than or equal to five prescriber episodes, (b) greater than or equal to five prescriber and greater than or equal to five pharmacy episodes, and (c) paying for prescriptions with both cash and insurance, per quarter, per 100 000 NYC residents. We defined three periods: (a) the baseline period (January 2011 to July 2012), (b) the anticipatory period (September 2012 to July 2013) after mandate law enactment but before mandate implementation, and (c) the postmandate period (September 2013 to December 2015). For each outcome, we used autoregressive linear regression models to account for correlation in outcomes over time.
Results:
At the end of the postmandate period, the rate of greater than or equal to five prescriber episodes was 58% lower than expected (absolute difference: −17.2 per 100 000 NYC residents; 95% CI, −31.2 to −3.1), the rate of greater than or equal to five prescriber and greater than or equal to five pharmacy episodes was 88% lower than expected (absolute difference: −8.6; 95% CI, −11.0 to −6.3), and the rate of cash and insurance payment episodes was 50% lower than expected (absolute difference: −145.4; 95% CI, −279.4 to −11.6).
Conclusions:
While outcomes were relatively rare, New York State’s PDMP mandate was associated with significant decreases in rates of potentially problematic patterns of opioid analgesic prescriptions.
Keywords: analgesics, health policy, opioid, pharmacoepidemiology, prescription drug diversion, prescription drug misuse, prescription drug monitoring programs
1 |. INTRODUCTION
In the United States in 2016, 42 249 individuals died of a drug overdose involving opioids.1 While heroin and fentanyls are involved in an increasing share of overdose deaths, 40% of overdoses still involve prescription opioid analgesics.1 New York City, a major urban center in the northeast United States, is facing a similar epidemic, and prescription opioid analgesics are still involved in approximately one in five overdose deaths.2
Prescription drug monitoring programs (PDMPs) are state-level databases of dispensed controlled substances that have been authorized in all states. While historically created for public health surveil-lance and law enforcement, a current goal of PDMPs is also to improve prescribing safety and appropriateness by allowing prescribers to see a patient’s complete prescription history.3 While PDMP information alone cannot identify opioid misuse or use disorder, PDMPs can help prescribers identify certain patterns that may suggest risky opioid analgesic use.4–9 In addition, mandates that prescribers query PDMPs may increase prescriber awareness and change prescribing behavior. A recent study found that PDMP mandates were associated with reductions in potentially problematic patterns of opioid analgesic prescriptions filled by Medicare enrollees;10 however, the association among other types of patients has not been described.
To strengthen New York’s PDMP, in August 2012, New York Governor Andrew Cuomo signed the Internet System for Tracking Over-Prescribing Act into law and launched a major statewide outreach campaign. Effective in August 2013, the law mandated prescriber registration and querying of the PDMP system, with limited exceptions, prior to any Schedule II–IV prescription. The new law also rescheduled hydrocodone products to Schedule II in February 2013, required upgrading of the registry’s electronic prescriber access capabilities, and required daily upload of dispensed prescriptions. Previously, the PDMP was rarely used by prescribers, and after the upgrades and mandate, the number of prescriber queries statewide increased from approximately 11 000 per month to over 42 300 per day.11 The goal of the current study was to evaluate the PDMP use mandate in New York City.
2 |. METHODS
We conducted an interrupted time series analysis of prescribing data from an open cohort of approximately 6.6 million adult New York City residents. We obtained data on opioid analgesics (Schedule II–IV) dispensed to adult (age ≥ 18) New York City residents from the New York State Department of Health, Bureau of Narcotic Enforcement, who manage New York’s PDMP. Data on filled prescriptions are uploaded to the PDMP by pharmacies.
From a deidentified surveillance dataset provided by the Bureau of Narcotic Enforcement, we conducted additional data cleaning. This consisted of excluding filled prescriptions with a missing unique prescription identifier, unique patient identifier, or unique pharmacy identifier as well as prescriptions written with a days’ supply of “999.” In addition, we excluded prescriptions written by veterinarians and those written under institutional licenses, which we determined by Drug Enforcement Administration (DEA) professional and license codes.
To study the impact of the PDMP use mandate, we selected a study period of 5 years, from January 2011 to December 2015. We chose this period to provide enough data for analysis and to avoid some contemporaneous interventions that could impact opioid analgesic prescribing, including the reformulation of extended-release oxycodone (2010) and a law limiting prescription duration for acute pain (2016). We defined three periods for this study: (a) the baseline period (Q1 2011 to Q2 2012, six quarters), (b) the anticipatory period (Q4 2012 to Q2 2013, three quarters), after the law was enacted but before the mandate became effective, and (c) the postmandate period (Q4 2013 to Q4 2015, nine quarters). We excluded the quarter in which the law was enacted (Q3 2012) and the quarter in which the mandate became effective (Q3 2013).
We used three outcome measures of potentially problematic prescriptions at the New York City resident level: (a) greater than or equal to five prescribers in the same quarter, (b) greater than or equal to five prescribers and greater than or equal to five pharmacies in the same quarter, and (c) paying for prescriptions with both cash and insurance in the same quarter.9,12 We calculated the rate of New York City residents with each measure in each quarter, per 100 000 New York City residents.
To account for population changes over time, we directly standardized all rates by 5-year age group (18–24, 25–29, 30–34, etc), sex, and borough of residence (Bronx, Brooklyn, Manhattan, Queens, Staten Island) to the 2010 New York City population. In each calendar year, we classified patients into age groups based on their mean age across all filled prescriptions. We also determined patients’ borough of residence by identifying the most common borough of residence across all filled prescriptions in the calendar year. For those with an equal number of filled prescriptions in more than one borough, we used the last borough in which a prescription was filled.
3 |. STATISTICAL ANALYSIS
First, we estimated the prevalence of each outcome at the start of the study period. Next, we analyzed outcomes using linear autoregressive models (SAS PROC AUTOREG) to account for correlation in outcomes over time. We created a separate model for each outcome measure. Each model had five independent variables: (a) quarter, a continuous variable representing the study quarter; (b) anticipatory period, a dichotomous variable that equals 0 prior to the anticipatory period and 1 after the start of the period; (c) anticipatory period quarter, a continuous variable representing the number of quarters after the start of the anticipatory period, equal to 0 prior to the period and after the end of the period; (d) postmandate, a dichotomous variable that equals 0 prior to the mandate and 1 after the start of the mandate; and (e) postmandate quarter, a continuous variable indicating the number of quarters after the start of the mandate, equal to 0 prior to the mandate. For each outcome, we tested for autocorrelation using the generalized Durbin-Watson test. If detected, we used the autoregressive error model with the appropriate order. We accounted for seasonality by adding quarter indicator variables to all models.
For each outcome measure, we calculated the relative and absolute difference between the observed and expected values at the end of the anticipatory period and at the end of the postmandate period. We estimated the expected values of the outcome measures, if the mandate had not occurred, by projecting the baseline trend forward to the end of the anticipatory period and to the end of the postmandate period. We calculated 95% confidence intervals using the bootstrap method.13 All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
4 |. RESULTS
Of all adult New York City residents filling an opioid analgesic at the start of the study period (Q1 2011), 0.4% represented greater than or equal to five prescriber episodes, 0.1% were greater than or equal to five prescriber and greater than or equal to five pharmacy episodes, and 3.6% were cash and insurance episodes. During the baseline period, there was no significant trend in the rate of greater than or equal to five prescriber episodes, a significant upward trend in the rate of greater than or equal to five prescriber and greater than or equal to five pharmacy episodes (0.13 per 100 000 per quarter; 95% CI, 0.06 to 0.2), and no significant trend in cash and insurance episodes (Table 1).
TABLE 1.
Changes in quarterly rates of potentially problematic patterns of opioid analgesic prescriptions per 100 000 adult New York City residents, 2011–2015
| Outcome | Baseline Period | Anticipatory Period | Postmandate Period | |||
|---|---|---|---|---|---|---|
| Intercept (95% CI) |
Trend (95% CI) |
Immediate Difference (95% CI) |
Difference in Trenda (95% CI) |
Immediate Difference (95% CI) |
Difference in Trenda (95% CI) |
|
| ≥5 prescribers | 26.7 (25.1 to 28.3) | 0.1 (−0.3 to 0.6) | −2.6 (−6.2 to 1.0) | −0.04 (−1.6 to 1.5) | −10.6 (−14.4 to −6.9)*** | −0.4 (−0.9 to 0.07) |
| ≥5 prescribers and ≥5 pharmacies | 7.3 (7.0 to 7.6) | 0.13 (0.06 to 0.2)** | −1.3 (−1.9 to −0.6)** | −0.2 (−0.5 to 0.05) | −5.0 (−5.6 to −4.4)*** | −0.3 (−0.3 to −0.2)*** |
| Cash and insurance payment | 221.9 (206.2 to 237.5) | 3.3 (−1.0 to 7.5) | −3.5 (−38.0 to 31.0) | −1.3 (−15.8 to 13.2) | −46.3 (−82.4 to −10.2)* | −10.6 (−15.4 to −5.8)** |
Refers to the absolute difference in trend, relative to the baseline period.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
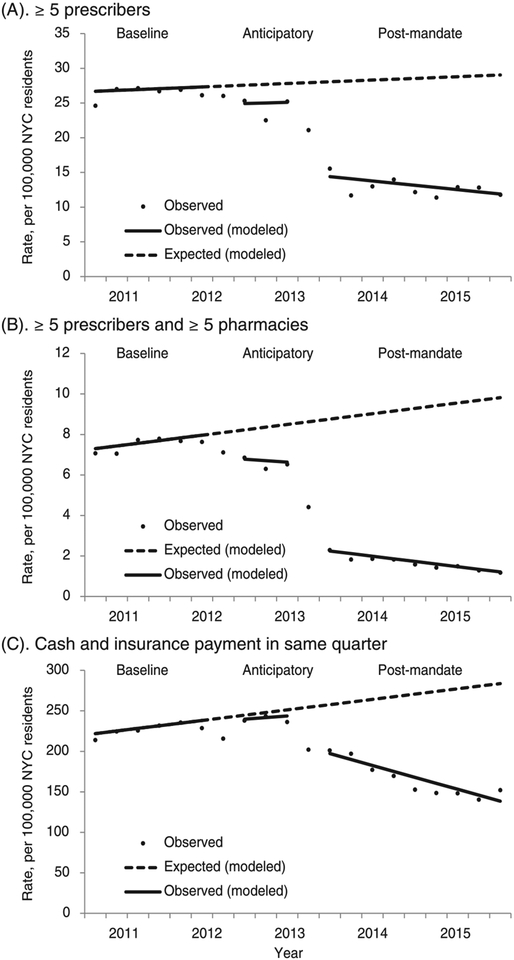
Comparing the anticipatory period with the baseline period, we did not find significant differences in trends for any outcome. At the end of the anticipatory period, the rate of greater than or equal to five prescriber and greater than or equal to five pharmacy episodes was 22% lower than expected (absolute difference: −1.9 per 100 000; 95% CI, −3.3 to −0.5; Table 2 and Figure 1). We did not find a significant difference between observed and expected rates of other outcomes at the end of the anticipatory period.
TABLE 2.
Difference in quarterly rates of potentially problematic patterns of opioid analgesic prescriptions, per 100 000 adult New York City residents, 2011–2015
| Outcome | Starting Value (95% CI) |
End of Anticipatory Period (Observed Versus Expected) | End of Postmandate Period (Observed Versus Expected) | ||
|---|---|---|---|---|---|
| Relative Difference (95% CI) |
Absolute Difference (95% CI) |
Relative Difference (95% CI) |
Absolute Difference (95% CI) |
||
| ≥5 prescribers | 26.7 (25.1 to 28.3) | −9 (−37 to 23) | −2.7 (−11.1 to 5.7) | −58 (−97 to −13) | −17.2 (−31.2 to −3.1) |
| ≥5 prescribers and ≥5 pharmacies | 7.3 (7.0 to 7.6) | −22 (−37 to −6) | −1.9 (−3.3 to −0.5) | −88 (−100 to −68) | −8.6 (−11.0 to −6.3) |
| Cash and insurance payment | 221.9 (206.2 to 237.5) | −2 (−33 to 32) | −7.5 (−88.4 to 75.5) | −50 (−89 to −5) | −145.4 (−279.4 to −11.6) |
Bold denotes P ≤ 0.05.
FIGURE 1.
Changes in potentially problematic patterns of opioid analgesic prescriptions, New York City, 2011–2015. A, ≥5 prescribers. B, ≥5 prescribers and ≥5 pharmacies. C, Cash and insurance payment in same quarter
Comparing the postmandate period with the baseline period, we did not find a significant difference in trend for greater than or equal to five prescriber episodes, but there was a significantly lower trend for greater than or equal to five prescriber and greater than or equal to five pharmacy episodes (−0.3 per 100 000 per quarter; 95% CI, −0.3 to −0.2) and cash and insurance episodes (−10.6 per 100 000 per quarter; 95% CI, −15.4 to −5.8). At the end of the postmandate period, the rate of greater than or equal to five prescriber episodes was 58% lower than expected (absolute difference: −17.2 per 100 000; 95% CI, −31.2 to −3.1), and the rate of greater than or equal to five prescriber and greater than or equal to five pharmacy episodes was 88% lower than expected (absolute difference: −8.6; 95% CI, −11.0 to −6.3). The rate of cash and insurance episodes was 50% lower than expected (absolute difference: −145.4 per 100 000; 95% CI, −279.4 to −11.6).
5 |. DISCUSSION
While outcomes were relatively rare, we found that implementation of a strict PDMP mandate was associated with a significantly lower rate of three potentially problematic prescribing patterns. In addition, we found evidence of a change in outcomes prior to when the mandate became effective. Our findings suggest that PDMP mandates, and the educational and outreach campaigns that precede them, can change prescriber behavior.
Through reductions in overall prescribing and potentially problematic prescribing,14,15 PDMPs may improve patient outcomes such as opioid misuse, use disorder, and overdose; however, this has not been established directly.16 Reductions in prescribing after PDMP implementation may not necessarily indicate more appropriate or judicious prescribing. Whether PDMPs have a positive or negative effect on patient outcomes depends on what specifically happens when prescribers change their treatment plan based on PDMP information. If PDMP information leads prescribers to diagnose opioid use disorder and link patients with treatment, then outcomes may improve. In contrast, if PDMP information leads prescribers to abruptly discontinue opioid prescribing without tapering, or diagnosing and treating any underlying opioid use disorder, then PDMPs could potentially push patients to use illicit opioids and outcomes may worsen. Further research should determine how prescribers respond to information from PDMPs and the effects of these decisions on patient outcomes.
This study has several limitations. First, we used observational data without a comparison group, and therefore, causation cannot be determined. Acceleration in preexisting national trends toward lower opioid analgesic prescribing and lower rates of multiple prescriber episodes that coincided with the PDMP mandate may explain, at least in part, our findings.17,18 However, we did find significant changes in outcomes that were temporally linked with the PDMP mandate. Second, this study only included data from New York City and therefore may not be generalizable to other areas including less urban areas that may have lower rates of multiple prescriber and pharmacy episodes.19 However, with a total population of approximately 8.5 million, New York City is an important population center and is larger than all but 11 states. Third, the Internet System for Tracking Over-Prescribing Act also led to rescheduling of hydrocodone products to Schedule II during the anticipatory period, and due to the contemporaneous nature of this policy with the PDMP mandate, we could not separate any contribution that rescheduling may have had on outcomes. However, our analysis included all Schedule II–IV opioid analgesic prescriptions, and therefore, shifts in prescribing from hydrocodone to other opioid analgesics would not be expected to bias our results. Fourth, we had a limited number of quarters in the baseline (six) and anticipatory period (three), which may have increased the uncertainty around our estimates. Fifth, while PDMP data represent a census of prescriptions filled in New York State by New York City residents, the completeness of prescription reporting from other states is not known. Finally, there may be misclassification of payment method when pharmacists upload this information at the time of dispensing.
In conclusion, we found that New York’s PDMP mandate was associated with reductions in potentially problematic patterns of opioid analgesic prescriptions among New York City residents. However, the impact of the mandate on patient outcomes, other than patterns of filled prescriptions, is not known. States implementing PDMP mandates to reduce harms from opioid analgesics should track both prescribing outcomes and patient outcomes such as opioid misuse, use disorder, and overdose.
KEY POINTS.
At baseline, New York City residents filling potentially problematic prescriptions were relatively rare (0.1%–3.6% of those filling any opioid analgesic prescription).
New York State’s mandate that prescribers query the prescription drug monitoring program (PDMP) prior to prescribing any Schedule II–IV medication was associated with reductions in potentially problematic opioid analgesic prescriptions filled by New York City residents.
Reductions in potentially problematic prescriptions may not necessarily lead to improved patient outcomes (eg, opioid use disorder and overdose); states implementing PDMP mandates should track patient outcomes.
ACKNOWLEDGEMENTS
This research was supported by funding from the National Institute on Drug Abuse (K08DA043050). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in design or conduct of the study or the decision to publish study results.
Funding information
National Institute on Drug Abuse, Grant/Award Number: K08DA043050
Footnotes
ETHICS STATEMENT
This study was considered exempt by the New York City Department of Health and Mental Hygiene Institutional Review Board.
Prior presentations: This work was presented in part at the 2017 Addiction Health Services Research Meeting (Madison, WI) and at the 2018 AcademyHealth Annual Research Meeting (Seattle, Washington).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants–United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paone D, Nolan ML, Tuazon E, Blachman-Forshay J. Unintentional drug poisoning (overdose) deaths in New York City, 2000–2016. New York City Department of Health and Mental Hygiene: Epi Data Brief (89); June 2017. [Google Scholar]
- 3.Perrone J, Nelson LS. Medication reconciliation for controlled substances–an “ideal” prescription-drug monitoring program. N Engl J Med. 2012;366(25):2341–2343. [DOI] [PubMed] [Google Scholar]
- 4.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22): 2613–2620. [DOI] [PubMed] [Google Scholar]
- 5.Peirce GL, Smith MJ, Abate MA, Halverson J. Doctor and pharmacy shopping for controlled substances. Med Care. 2012;50(6):494–500. [DOI] [PubMed] [Google Scholar]
- 6.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796–801. [DOI] [PubMed] [Google Scholar]
- 7.Dilokthornsakul P, Moore G, Campbell JD, et al. Risk factors of prescription opioid overdose among colorado medicaid beneficiaries. J Pain. 2016;17(4):436–443. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Wilsey B, Bohm M, et al. Defining risk of prescription opioid overdose: pharmacy shopping and overlapping prescriptions among long-term opioid users in medicaid. J Pain. 2015;16(5): 445–453. [DOI] [PubMed] [Google Scholar]
- 9.Becker WC, Fenton BT, Brandt CA, et al. Multiple sources of prescription payment and risky opioid therapy among veterans. Med Care. 2017;55(Suppl 7 Suppl 1):S33–S36. [DOI] [PubMed] [Google Scholar]
- 10.Buchmueller TC, Carey C. The effect of prescription drug monitoring programs on opioid utilization in medicare. Am Econ J Econ Pol. 2018;10(1):77–112. [Google Scholar]
- 11.Prescription Drug Monitoring Program Training and Technical Assistance Center. PDMP prescriber use mandates: characteristics, current status, and outcomes in selected states. Available at: http://www.pdmpassist.org/pdf/Resources/Briefing_on_mandates_3rd_revision_A.pdf Accessed July 3, 2018. (Archived by WebCite® at http://www.webcitation.org/70e2VxYLJ).
- 12.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled substance prescribing patterns–Prescription Behavior Surveillance System, Eight States, 2013. MMWR Surveill Summ. 2015;64(9):1–14. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen H, Schackman BR, Aden B, Bao Y. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to medicaid enrollees. Health Aff (Millwood). 2017;36(4):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao Y, Pan Y, Taylor A, et al. Prescription Drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff (Millwood). 2016;35(6):1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink DS, Schleimer JP, Sarvet A, et al. Association Between prescription drug monitoring programs and nonfatal and fatal drug overdoses: a systematic review. Ann Intern Med. 2018;168(11): 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simeone R Doctor shopping behavior and the diversion of prescription opioids. Subst Abus. 2017;11:1178221817696077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald DC, Carlson KE. The ecology of prescription opioid abuse in the USA: geographic variation in patients’ use of multiple prescribers (“doctor shopping”). Pharmacoepidemiol Drug Saf. 2014;23(12): 1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]