Abstract
Many malignancies display heterogeneous features that support cancer progression. Emerging high-resolution methods provide a view of heterogeneity that recognizes the influence of diverse cell types and cell states of the tumor microenvironment. Here we outline a hierarchical organization of tumor heterogeneity from a genomic perspective, summarize the origins of spatially patterned metabolic features, and review recent developments in single-cell and spatially resolved techniques for genome-wide study of multicellular tissues. We also discuss how integrating these approaches can yield new insights into human cancer and emerging immune therapies. Applying these technologies for the analysis of primary tumors, patient-derived xenografts, and in vitro systems holds great promise for understanding the hierarchical structure and environmental influences that underlie tumor ecosystems.
Keywords: in situ, genomics, stroma, hypoxia, metabolism, epigenetics
Tumor heterogeneity and cell plasticity
The cellular mechanisms that underlie malignancy are diverse, plastic, and adaptable. Many tumors display distinct compartments and heterogeneous phenotypes, with tumor progression manifesting aspects of ecosystems that adapt and evolve. This diversity is a key aspect of many cancers that contributes greatly to tumor progression and treatment outcomes [1]. Unfortunately, our current ability to dissect the mechanistic origins of this plasticity remains a persistent challenge. As a result, one major goal for the foreseeable future of cancer biology is to develop new high-resolution technologies capable of dissecting heterogeneous tumors to uncover the unique vulnerabilities operating within each microenvironment.
Genome-wide analysis of bulk tissues has significantly advanced our understanding of carcinogenesis [2], and emerging single-cell variants of these technologies are providing powerful opportunities to reveal new features of complex tissues containing mixed cell types and states. Single-cell and spatially resolved investigations of tumors have the capacity to reveal context-dependent mechanisms and other spatially restricted cues governing tumorigenesis, metastasis, and response to treatment. Hence, techniques capable of characterizing heterogeneous cell states and processes in malignancy have become important tools to identify previously hidden features of cancer.
In this review, we focus on exciting recent genomic and imaging-based technologies that permit high-resolution dissection of cancer processes at the single-cell level. We begin by highlighting the recurrent themes and chief contributors to phenotypic heterogeneity in tumors, and propose a hierarchy of tumor heterogeneity involving cell identities and epigenetic-metabolic states. We then review new high-resolution approaches, addressing both genomic and optical methods to characterize epigenomic and phenotypic states at the single-cell level. Together, these techniques are revealing previously unknown interactions in multicellular tissues that contribute to tumor progression and treatment response.
The hierarchy of tumor heterogeneity
Bulk tumor populations contain several cell types, including malignant cells and non-malignant stromal cells that support or oppose their growth. Although each individual cell within a tumor adopts a gene expression pattern governed by its cell identity, these patterns adapt in response to cell-extrinsic factors and the local microenvironment. The exposure to specific local cues is therefore an important source of heterogeneity for cells sharing the same identity.
These observations led us to propose a general hierarchy describing all sources of heterogeneity operating within a tumor. At the first level, tumor populations are defined in terms of cell type or identity (Figure 1). Cells can be segmented based on characteristic inherited features (e.g. surface markers or cell-specific gene expression patterns), allowing classification of tumors into either malignant cancer cells or non-malignant cells, such as immune cells, fibroblasts, and other stromal cells. These traits can generally be considered irreversible, based on the high epigenetic barrier to altering cell identity [3–5]. At the second tier of the hierarchy, the individual cell types can be further categorized based on cell-type–specific phenotypic states, often influenced by features of the tumor microenvironment. For example, cancer cells may be grouped based on whether they are undergoing oxidative phosphorylation or Warburg metabolism [6]. The variation at this tier reflects cell-type plasticity, and in contrast to the first tier, these features are reversible based on ectopic signals and environmental conditions. Another example is the distinct set of states associated with immune cell activation, as seen in tumor associated macrophages (TAMs), which have different expression patterns and functions based on the pathway of their activation [7]. Yet another example is the transition of cancer cells into more aggressive states that promote invasion and metastasis, often referred to as epithelial-mesenchymal transition (EMT) [8,9]. These and other cell-type–specific state classifications are often associated with important consequences for tumor progression, and play important roles in diagnosis and treatment [9].
Figure 1. The hierarchy of tumor heterogeneity.
(A) Bulk tumor populations can be divided into distinct cell types from different lineages. Cells with similar cell identities are present in distinct microenvironments, which affect their epigenetic-metabolic states. Hence capturing the full functional specialization present in tumors requires finer classification of cells into distinct cell-type–specific states. (B) Gene expression patterns vary spatially within the same cell type based on tumor microenvironment conditions.
The ability of tumor cells to adopt cell-type–specific states has been widely known for decades. A prime example of tumor heterogeneity at the level of cell state can be visualized using fluorodeoxyglucose (FDG), a glucose analog that marks malignant cells by their increased glucose uptake. PET imaging of FDG is widely used in the clinic to map the diverse rates of glucose utilization between and within tumors [10]. The resulting heterogeneity is illustrative of other sources of heterogeneity that also have important functional consequences. For example, in several cancers, differences in tumor progression arise based on varied expression of multi-drug transporters [11], hormone receptors [10,12], cytokines [13–16], and neoantigens [17–19]. In several of these cases, treatments focused on cellular identity alone often result in treatment-resistant phenotypes [12,20]. However, characterizing the entire spectrum of accessible cellular phenotypes provides an avenue to uncover new or shared vulnerabilities [21–23]. Elsewhere in nature, loss of a key player within an ecosystem can lead to destabilization of that ecosystem. Similarly, targeting specific vulnerabilities within portions of heterogeneous tumors may lead to destabilization of the tumor ecosystem, offering new potential therapeutic windows.
Defining the hierarchy of tumor heterogeneity in this way focuses on cell identities and cell states, however many tumors develop distinct genetic changes through clonal or non-clonal evolution [1,24]. For example, a founding cancer cell can give rise to genetically diverse malignant cells through a series of clonal expansions [25–27]. Each of these expansions is linked to the conferral of distinct selective advantages that may vary both in time and space based on the local microenvironment. We posit that selective pressures operating on these cells are relevant to the cancer only insomuch as they alter a phenotypic property of the cells or tumor system. For example, genome-wide profiling of breast cancers [28–31] and glioblastoma [32] reveals far greater diversity at the genetic level than at the transcriptome level. These and many other cancers are routinely separated into a small number of “expression subtypes” despite thousands of diverse genetic changes. Hence, until genetic changes confer an irreversible change in expression patterns [33–35], the genetic diversity of tumor cells can in many ways be considered a variation secondary to cell identity.
Visualizing tumor heterogeneity through single-cell technologies
Recent advances in high-throughput sequencing methods enable measurement of the proportions of mixed cell types and cell-type-specific states that shape the tumor microenvironment. The most popular commercially available single-cell technologies currently rely on microfluidic devices that use either patterned micro-wells for single-cell isolation, or droplet-based barcoding of individual cells, similar to Drop-seq [36], and are extensively reviewed elsewhere [37,38]. Frequently used alternatives to these approaches are based on the clever use of split-pool barcoding schemes [39,40]. Together, these approaches have confirmed that many cancers are characterized by recurrent patterns of cell populations and states [9,19,41–43]. In many cases, the origins of this heterogeneity are thought to be influenced by cytokine production [13–16,44], variable neoantigen presentation [17–19], stromal content [9,42,45], vascularization [46], and other heterogeneous features.
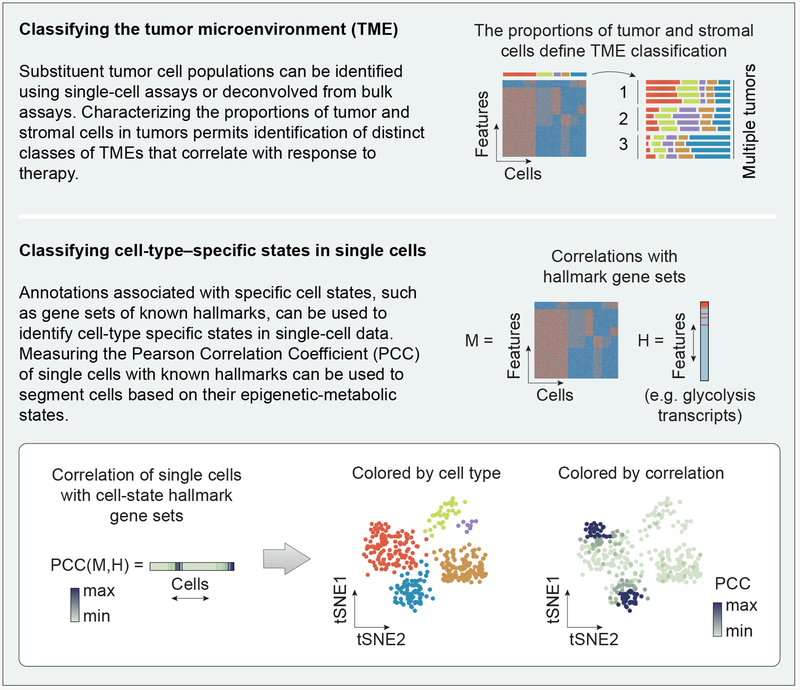
In recent years, a large toolbox of single-cell epigenomic assays has emerged, based on RNA-seq [36], ATAC-seq [47,48] (see Glossary), and more recently, CUT&RUN or ChIC-seq [49,50]. The use of single-cell approaches to characterize cell states within these populations has lagged the measurement of the proportions of distinct cell types, nevertheless, several important examples of cell state changes in cancer are beginning to emerge. One of the strongest signatures is the set of changes referred to as epithelial-mesenchymal transition (EMT), which are associated with a pronounced change of invasiveness and metastatic potential. Using scRNA-seq of colorectal tumors, EMT-like signatures have been found in a portion of cancer-associated fibroblasts (CAFs) [42]. The increased EMT-like signature was observed only in CAFs of the tumor, and not the epithelial cell population. In a separate study relying on ligand-receptor annotations, scRNA-seq data from six syngeneic mouse tumor models was used to deconvolve the complex cell-cell interactions of the tumor microenvironment [51]. This study discovered a correlation between increased tumor growth and tumor cell secretion of key chemokines that may signal other tumor cells or macrophages that express cognate receptors. One recurrent feature of single-cell analytical approaches is the frequent use of inference-based algorithms like SCENICi [52] as well as knowledge-based annotations, for example hallmark gene sets from MSigDBii [53], to segregate cells with similar regulatory activities or phenotypic states into clusters for downstream analysis (Figure 2). Similarly, pathway activities can be derived from transcriptomics data using PROGENyiii, which relies on pathway-responsive gene signatures to define cell states [54].
Figure 2. Visualizing tumor heterogeneity between and within cell types.
Measuring the overall proportion of each cell type in mixed cell populations plays an important role in subtyping tumor microenvironments. Within and between cell types, the epigenetic-metabolic states of cells can be classified by examining correlation to known gene sets associated with specific states.
Conventional single-cell techniques currently rely on dissociated, monodisperse cells. Therefore, these approaches unfortunately require loss of spatial information. Still, such approaches allow for measurement of the cell proportions present in tumor cell populations, which has shown predictive power for classifying tumors based on their distinct microenvironments and their response to therapy (Figure 2) [55,56]. The transcriptomic patterns of cell-type–specific changes are usually less dramatic than those separating cell identities [9], and many cell-type specific changes may be shared between multiple cell types. Therefore, analytical and visualization approaches (e.g. tSNE or variants like UMAPiv [57]) may benefit by being augmented to include hierarchical approaches to map cell-type–specific changes within subpopulations, rather than discriminating cells based on global features. The analysis of global features has the advantage of being mathematically principled, but may fail to classify cell-type–specific phenotypes, because the variations associated with them may be small compared to the higher magnitude differences associated with cell identity, and shared between different cell types. Continued application of these techniques and development of new analytical tools for refinement may yield new avenues for discovery biology and therapeutic intervention.
The spatial hierarchy of tumor heterogeneity
There is a high degree of spatial correlation within tumors because cells with similar phenotypic profiles are often contained within similar microenvironments. The phenotypic diversity of cells is shaped by diverse gradients, including those formed by systemic hormones, local diffusible factors such as TGF-β [58] and Wnt [59], as well as variable nutrient and environmental cues [6], and unknown factors that promote proliferation of cells in close proximity to adipocytes [60]. Furthermore, the heterogeneous tumor immune microenvironment substantially impacts intratumoral diversity and evolution [19] (see Box 1). Altogether, spatial heterogeneity spanning from the cellular level to the tissue level impacts many physiological properties in cancer (Figure 3A). Fortunately, several technologies are ideal for clinical and experimental dissection of these changes.
Box 1. Tumor immune infiltration.
Tumor infiltration by immune cells is a critically important source of heterogeneity with great significance for emerging immunotherapies. Somatic mutations in tumors generate neoantigens, which influence the tumor microenvironment by recruiting immune cells and altering local cytokine concentrations [17–19, 101–104]. While immune checkpoint blockade has been particularly effective against several malignancies, there remains wide variation in outcomes, with relapse occurring in a third of patients [105]. One potentially confounding issue for immune therapies is that the tumor immune microenvironment contributes both positively and negatively to oncogenic properties [56]. For this reason, identifying patient- and tumor-specific microenvironments represents a promising strategy to reveal the determinants of differential therapeutic response.
Divergent aspects of tumor immune cell heterogeneity are exemplified in breast and lung cancer. In breast cancer, tumor cells secrete cytokines, which recruit inflammatory monocytes that in turn activate gene programs that promote metastasis to the lung [106]. In the breast, positive feedback loops between cancer cells and macrophages stimulate EMT-like patterns to promote metastasis [107]. Interestingly, this effect is context-dependent, as metastasis-associated macrophages can prevent lung metastatic growth by reducing angiogenesis and influencing the extracellular matrix [108]. Altogether, the study of intercellular signaling between tumor and immune cells represents a complex area with profound implications for immune-based therapies.
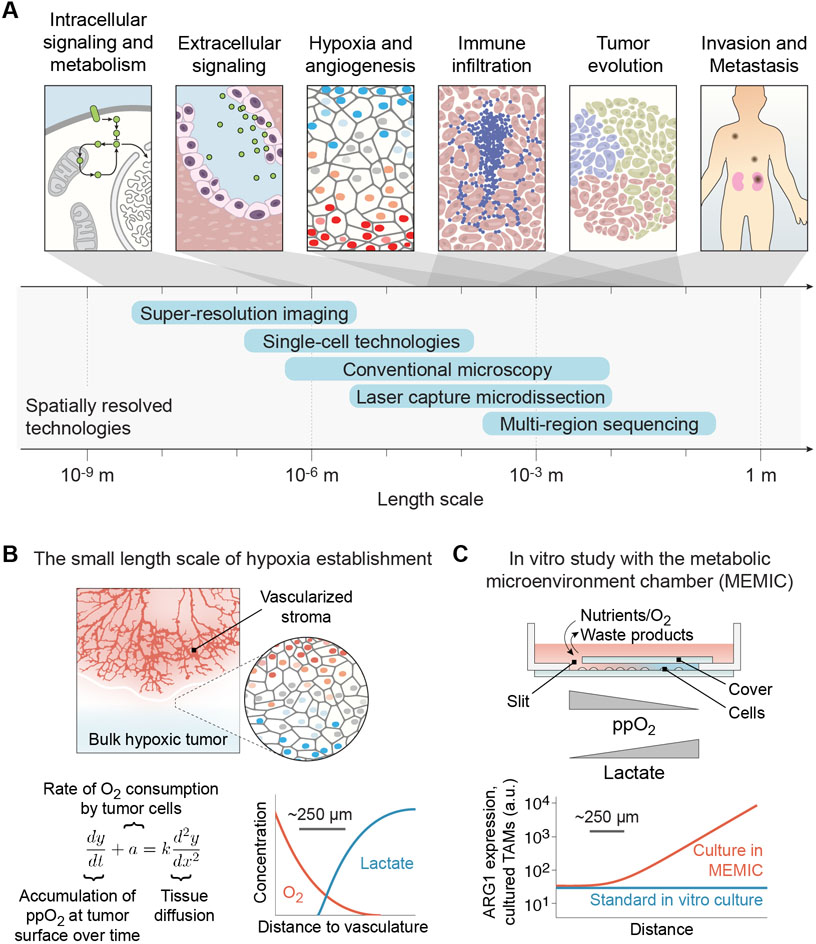
Figure 3. The spatial hierarchy of tumor heterogeneity.
(A) Spatially resolved imaging- and sequence-based technologies provide insight into mechanisms that contribute to cancer biology at multiple levels of scale. (B) The establishment of hypoxia occurs over a short length scale of ~250 μm. Reaction-diffusion equations can model the spatial gradient of diffusible factors near tumor-stromal boundaries. (C) Experimental study of hypoxic gradients in vitro reveals that oxygen tension influences spatial gene expression patterns of tumor associated macrophages (TAMs).
Arguably one of the best models of spatial patterning in cancer is associated with hypoxia. Both mathematical modeling and experimental measures of oxygen perfusion in tumors show that hypoxic or anoxic conditions arise less than 0.5 mm from the vasculature [61]. In cancer, hypoxia arises as tumor cells become further separated from the oxygen-rich blood supply (Figure 3B). Hypoxic metabolic adaptations therefore allow tumor cells to survive low oxygen tension [62]. Inventive engineered systems have allowed researchers to experimentally mimic in vitro the oxygen gradients observed in vivo to study their influence on spatial gene expression. The metabolic microenvironment chamber, or MEMIC, is a chamber for culturing cells with only a small slit through which oxygen, nutrients, and waste products can transit, inducing a spontaneous gradient of these factors (Figure 3C). Culturing TAMs in MEMIC increases arginase 1 expression in response to hypoxia over a 250–500 μm length scale [63]. This hypoxic response of TAMs is required for induction of revascularization, which allows tumors to overcome nutrient poor regions of the tumor. Tumor revascularization in response to hypoxia is a pinnacle of tumor plasticity and survival that results in high spatial heterogeneity over relatively short distances.
At larger length scales, the effects of tumor evolution dominate the spatial hierarchy. As tumors adapt to their developing environments, intratumoral variation of gene expression arises. These mixed cell populations can have different properties, including differential drug response, which can significantly influence therapeutic resistance and clinical management [1,64–67]. In primary clear cell renal cell carcinoma (ccRCC), approximately 75% of driver mutations characterized are subclonal, while VHL mutations are common to all regions of a tumor, illustrating the complex roles played by both driving mutations and tumor evolution [68]. Multiregion sequencing has also demonstrated that spatial limitations and tumor microenvironments cause subclonal mixing of cells, contributing to diversification of the tumor’s mutational landscape. In colorectal tumors, single clonal expansions ultimately result in advanced colorectal tumors with large numbers of mutations due to later selective pressures within the tumor [69]. Altogether, the context-dependent relationships between cell identity and cell states must be investigated at both the local level and also across a tumor.
Technologies for characterizing multicellular features in malignancy
In addition to emerging single-cell technologies described above, other new technologies are also revealing key insights into malignancy. Below, we review emerging trends for high-resolution methods that have broad application in cancer research.
Advancements in cell sorting and cytometry
Mixed samples can be analyzed at the single cell level in high-throughput fashion using flow cytometry and fluorescence-activated cell sorting (FACS). Modern combinations of cytometry with single-cell genomics and upgraded variations such as CyTOF (Cytometric time-of-flight mass spectrometry) are expected to play an increasingly vital role in defining the heterogeneity of bulk samples.
Though cytometry traditionally defines cell identity through surface markers, it can be augmented with single-cell sequencing approaches to provide additional levels of information. Specifically, using FACS in combination with scATAC-seq links the expression of cell surface markers to changes of the underlying open chromatin landscape [70]. Additionally, CyTOF is a widely used commercially available tool that permits examination of heterogeneous cell states within tumors. CyTOF enables highly multiparametric studies of protein abundance, offering single-cell analysis of up to 50 parameters per cell [71]. One CyTOF study of heterogeneity within high-grade serous ovarian cancer (HGSOC) tumors revealed rare cell populations associated with poorer outcome that were previously missed by bulk sampling. Their identification may lead to new and more specific avenues for therapeutic intervention [72]. By combining CyTOF with proximity ligation assay for RNA (PLAYR), simultaneous high-dimensional single-cell analysis of mRNA and protein can be achieved at a rate of thousands of cells per second, providing insight into the relationship between gene expression and protein abundance [73]. Furthermore, coupling of CyTOF with barcoding of normal lung, tumor tissue, and peripheral blood revealed tumor-specific states of immune cell composition and, importantly, potential immunotherapy strategies for lung adenocarcinoma [74]. Overall, paired analyses using cytometry are advancing the functionality of cell sorting, and yielding a more complete picture of the heterogeneity contained within bulk samples.
Though flow cytometry is invaluable for high-throughput cell typing, its use inherently requires loss of spatial information. However, combining cytometry with other techniques can shed light on spatial heterogeneity as well. NICHE-seq preserves the cell states influenced by surrounding cells or microenvironments, allowing the characterization of these influences on tumor heterogeneity [75]. Here, after in situ labeling of photoactivatable fluorescent markers, FACS is coupled to high-throughput sequencing to study cellular ecosystems in live animal or ex vivo settings. Applying NICHE-seq to melanoma identified niche-specific changes in immune cell localization and expression programs, where different myeloid compositions were associated with different extracellular matrix structures [75]. Spatial cell context can also be preserved when combining CyTOF with immunocytochemistry or immunohistochemistry and high-resolution laser ablation. These combination approaches have achieved spatial resolution of 1 μm in tissue sections, leading to the discovery of new subpopulations within conventional breast cancer subtypes [76]. Clever combinations of existing technologies will continue to advance discovery, providing rich resources with which to test new hypotheses.
Laser-capture microdissection
Laser-capture microdissection (LCM) enables the study of spatial heterogeneity while preserving spatial information. LCM allows for selection and contact-free isolation of cells of interest from distinct tumor regions with high precision [77]. Briefly, cells of interest are microscopically identified on tissue sections prior to laser-assisted isolation. Cells are then isolated using a high-intensity UV laser and collected by laser pressure catapulting. Alternatively, a near-IR laser can be used to melt a thermolabile polymer placed atop the cells of interest, enabling their removal. These regions can be based on morphological features, immunohistochemistry, or expression state. LCM can be paired with genome-wide analyses where spatial information is otherwise lost [77].
Because LCM simply enables dissection of a biological specimen, many downstream applications can be employed to reveal characteristics of cell identity and cell state, including analysis of DNA sequence or copy number, RNA profiling, and mass spectrometry. For example, Topographic Single Cell Sequencing (TSCS) combines LCM, whole-genome amplification, and single-cell DNA sequencing [78]. TSCS revealed that most mutations and copy number variations that contribute to intratumoral heterogeneity of invasive ductal carcinomas (IDC) are a direct result of multiclonal invasion of local ductal carcinomas in situ (DCIS) [78]. Additionally, by pairing LCM with computer-aided microscopic isolation (CAMI), researchers created an automated high-throughput method to analyze cells from tissues or suspensions and automatically guide extraction. CAMI-LCM allows the automatic isolation and subsequent analysis of single cells based on morphology, location, or presence of specific fluorescently-labeled markers [79]. Because LCM preserves cell context, inferences relating cell identity with the surrounding microenvironment can be drawn. Increased integration of LCM with automated high-content systems has great potential to identify new subpopulations that may not be readily detected by routine histopathology.
Advanced methods that combine genomics with high spatial resolution
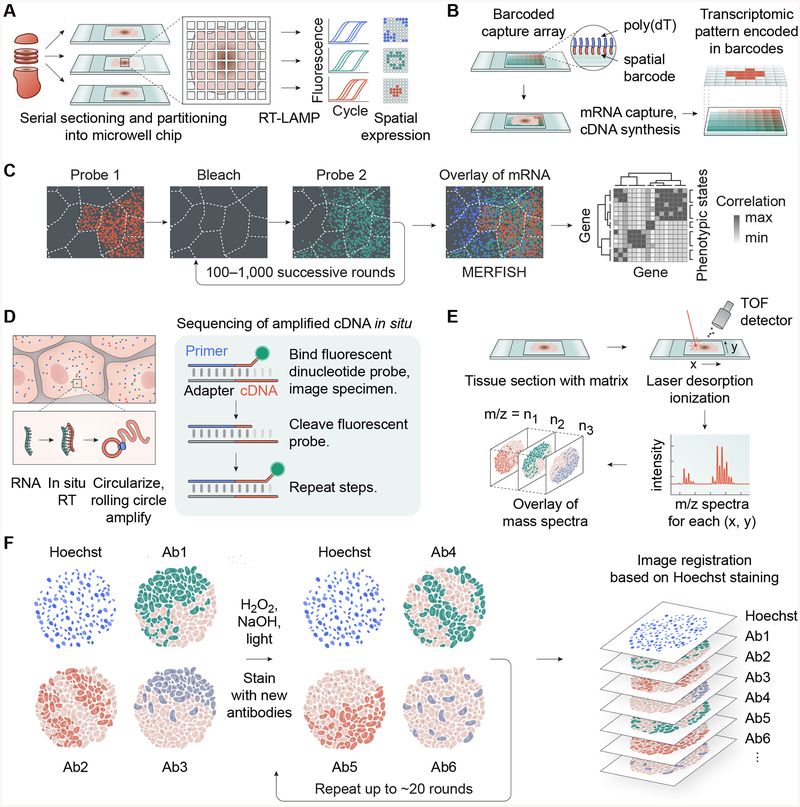
Several advanced technologies have been developed to perform genome-wide expression studies in situ, permitting genomic assessment and preservation of local tumor microenvironment landmarks. In one such plate-based assay, tissue cryosections are partitioned into a microwell chip where picoliter-scale reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) reactions take place (Figure 4A). These reactions are subsequently measured using a fluorescence plate reader to reveal spatially variable cell states based on expression patterns of key genes [80]. In another method, referred to as “spatial transcriptomics” by the authors, tissue sections are positioned on glass slides affixed with oligo(dT) primers containing unique spatial barcodes for transcript mapping (Figure 4B). Subsequent fluorescent visualization or RNA-seq analysis demonstrated spatial transcriptomic heterogeneity while preserving histological context in breast cancer tissue [81,82]. Interestingly, this approach highlighted that only specific regions of the tumor had engaged an EMT-like program. A higher-resolution variation of this approach called Slide-seq substitutes barcoded oligo(dT) primers with barcoded 10-μm beads. Slide-seq affixes these beads to slides to provide a spatial index onto which tissue cryosections are placed [83]. Following reverse transcription, tissue digestion, and library amplification, the spatial expression profiles are computationally reconstructed. Slide-seq has been employed to spatially map individual cell types in brain cryosections, as well as the different cell states induced in response to injury [83].
Figure 4. Advanced technologies enable genomic characterization of malignant processes in situ.
(A) Highly resolved RT-LAMP assay using microwells enables quantitative assessment of RNA expression changes while preserving spatial information. (B) Spatial transcriptomics using a spatially barcoded poly(dT) capture probes. (C) MERFISH enables high-dimensional investigation of transcription states using error-robust single-molecule FISH counting of transcripts. (D) FISSEQ enables in-situ Sanger-like sequencing while preserving tissue structure. (E) MALDI-IMS enables in situ mass spectrometric analysis of proteins and metabolites with better than single-cell resolution. (F) Principle of multi-round, multiplexed tissue immunofluorescence (MxIF and CycIF).
Highly multiplexed single-molecule visualization of the number and distribution of transcripts has also been achieved in cells and tissue sections using successive rounds of FISH (fluorescent in situ hybridization) labeling. Up to 1001 unique mRNAs can be imaged and quantified via MERFISH (multiplexed error-robust single-molecule fluorescent in situ hybridization). Here, cryosections or cells fixed on coverslips undergo repeated rounds of FISH labeling and imaging with a set of RNA probes, allowing for the construction of high-dimensional gene regulatory networks with intercellular and intracellular spatial resolution of individual molecules in tissues (Figure 4C) [84]. The spatial resolution of MERFISH can be increased by combining with expansion microscopy [85], a method of physical sample expansion that results in increased distances between single molecules. Furthermore, MERFISH is also compatible with immunofluorescence staining of subcellular structures, allowing mRNAs to be correlated to specific cell compartments [85]. Another sequential FISH technique, seqFISH, uses a standard confocal microscope with a fluorescent barcoding approach, enabling the detection and subcellular localization of tens of thousands of genes within single cells. Application of seqFISH to tissue sections allows the identification cell-cell interactions such as localized expression of ligand-receptor pairs within neighboring cells [86].
Another technique, FISSEQ (fluorescent in situ RNA sequencing) permits RNA localization of over 8,000 genes in cells and tissues through in situ amplification and sequencing of cDNA (Figure 4D). This method permits 3D fluorescent visualization and identification of mRNA transcripts in cells, while preserving tissue architecture [87]. By preserving tissue context, in situ approaches allow high-resolution characterization of cell states relative to molecular and phenotypic spatial landmarks.
Single-cell resolution of protein/metabolite abundance
Spatially resolved visualization of proteins, lipids, metabolites and posttranslational modifications associated with tumorigenic properties can be achieved in tissue sections through matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS, Figure 4E). For example, MALDI-IMS has been used to characterize diverse metabolic states of malignancy with high spatial resolution, including components of glycolysis and the TCA cycle [88,89]. MALDI-IMS has also revealed highly vascularized regions of ccRCC tumors through detection of proteins representative of increased tumor vascularization [90]. Additionally, MALDI-IMS enabled identification of key histone modifications enriched in surrounding normal tissue relative to the tumor, thereby visualizing the underlying tumor-specific epigenetic states.
New enzymatic histochemistry approaches allow spatial characterization of metabolic signatures in complex tissue samples with single-cell resolution [91]. Using consecutive tissue slides, multiple enzymatic activities can be visualized and quantified by enzyme histochemistry and automated whole-tissue histocytometry. This approach allowed comparison of single-cell metabolic states of distinct immune cell populations in healthy versus tumor colon tissue [91]. Interestingly, the microenvironment was found to significantly influence cells of similar identity: Glycolytic activity of tumor-associated macrophages is significantly decreased compared to their counterparts in normal tissue. Together, in situ protein and metabolic assays permits spatial characterization of heterogeneous activities otherwise inaccessible through conventional IHC.
Conventional immunofluorescent detection of proteins in tissues has been limited by the number of fluorescent channels on a microscope. However, multiplexed fluorescence microscopy (MxIF) [92] and a related form, cyclic immunofluorescence (CycIF) [93] are imaging approaches that use multi-round staining with standard reagents and equipment to achieve high-dimensional immunofluorescence images. MxIF and CycIF can detect upwards of 60 proteins through sequential antibody probing, imaging, and fluorophore bleaching to create highly multiplexed images of tumor tissue at the single-cell level (Figure 4F). In tissues, tissue-based CycIF (t-CycIF) has been used to compare protein expression across a pancreatic tumor section, where CD45+ immune cells were found to be highly infiltrated within tumor tissue compared to adjacent normal pancreas [94].
Concluding Remarks
The rapid pace of technological advances in genomics and microscopy has resulted in an explosion of new tools for cancer research. These tools are providing powerful opportunities to improve diagnoses, develop more effective therapies, and understand the complexity of cancer in new detail. In particular, the intersection of sequencing and optical methods is providing multi-level information required to investigate altered function in both space and time. Coupling these approaches with complementary tools, such as small-molecule probes [95] or environmentally selective optical reporters [96], may help to identify how conditions of the tumor microenvironment impact tumor progression and metastasis with high spatiotemporal resolution.
An important challenge is to apply these tools to uncover the recurrent patterns of cell types and states that contribute to disease progression and therapeutic response (see Outstanding Questions). Classification and systematization of tumor microenvironments is becoming increasingly valuable as a clinical diagnostic [97]. As a result, high-resolution tumor mapping may increasingly serve an important role in clinical diagnosis and treatment. Given their robustness, high scalability, and familiarity to pathologists, cyclic immunostaining approaches such as MxIF and CycIF may provide the fastest plausible path to using single-cell data in clinical trials. However, plummeting sequencing costs in the last decade have supported an avalanche of data-rich assays whose output is encoded by DNA sequence. In either case, spatially encoded and other highly multiplexed single-cell technologies are poised to reveal many new insights, as precision oncology increasingly focuses on interactions between specific cell types and states in situ. We therefore envision a future in which tumor biopsies are routinely examined for cell-type-specific states using high-resolution techniques, in addition to screening for mutations affecting tumor suppressors and oncogenes.
Outstanding Questions Box.
What is the best way to categorize the diverse and heterogeneous phenotypes in a population of malignant and non-malignant cells?
Can single-cell visualization tools be improved by focusing on hierarchical cell-type–specific classifications rather than global genome-wide changes?
How can single-cell assays be combined to relate single-cell phenotypes to their genetic and epigenetic-metabolic states?
What are the most important and clinically actionable features of single-cell data?
What specimen size and resolution are needed for high-resolution techniques to reveal therapeutic vulnerabilities?
Are there patterns or characteristic responses of the tumor ecosystem to perturbation?
What are the interactions between the tumor microenvironment and common genetic changes to tumor suppressors and oncogenes?
As genomic methods are augmented to provide single-cell resolution, the preservation of spatial information remains a major challenge. The trend towards increased integration of genomics and microscopy suggests that their joint application will play an essential role in the study of malignancy. Several emerging super-resolution imaging techniques (e.g., single-plane illumination microscopy [98] and lattice light-sheet microscopy [99]) permit fast high-resolution 3D imaging of live samples, including immune and circulating tumor cells. These optical techniques and many variants [100] can also provide important insights into dynamic, spatially regulated processes that contribute to malignancy and tumor biology.
Our understanding of the tumor microenvironment, immune infiltration, and stemness, is becoming increasingly comprehensive thanks to many innovative quantitative techniques that can capture the heterogeneity of cell states in tumors. These technologies are expected to complement emerging immune-based and related therapies [56] by revealing currently unknown multicellular interactions that promote cancer.
Table 1.
High-resolution technologies for the study of tumor ecosystems.
| Technology | Applications to cancer | Strengths | Limitations / constraints | References | |
|---|---|---|---|---|---|
| Genomic and unbiased approaches | Nanowell microfluidic devices | High-throughput single-cell analysis of subpopulations | Ease of use /
established technology |
Relatively expensive, loss of spatial resolution | [47] |
| Droplet-based single-cell sequencing | [36,51] | ||||
| Split-pool based approaches | No complex equipment required | Protocols still in development, loss of spatial resolution | [39,40,48] | ||
| MERFISH | Spatially resolved RNA expression in tissues and cells | Applicable to cells and tissue sections; high-resolution | Limited to 1001 unique mRNAs | [84,85] | |
| Slide-seq and “spatial transcriptomics” | Applicable to tissues | Specialized oligomer arrays needed, limited
resolution of “spatial transcriptomics” is overcome in Slide- seq |
[81–83] | ||
| seqFISH | Tens of thousands of
distinct transcripts detected |
Specialized primary and readout probes needed | [86] | ||
| FISSEQ | High-resolution and preserves spatial information | Expensive equipment; not ideal for low-abundance transcripts | [87] | ||
| Multi-region sequencing | Addresses large-
scale heterogeneity |
Spatial information retained | Low spatial resolution | [17,19,24–27,64–69] | |
| Laser-Capture Microdissection (LCM) | Isolation of cell(s) based on microscopic visualization | Native spatial information and cell state retained | Automation required for high-throughput analyses; | [77–79] | |
| MALDI-IMS | Spatial proteomics, metabolomics | Label-free, preserves spatial information | Semi-quantitative | [88–90] | |
| Candidate-based approaches | Mass cytometry - Flow cytometry coupled with time-of-flight (CyTOF) | Single-cell proteomics |
Quantitative singlecell analysis of proteins; | Currently limited to measurement of ~50 parameters per cell | [71–74,76,106] |
| Immunohistochemistry (IHC) |
Visualization of heterogeneity of specific cancer markers | Retains tumor niche information | Limited by compatible antibodies and FISH probes | [76] | |
| Cyclic Immunofluorescence (CycIF) |
Visualization of ~60 proteins in tissue samples | Uses standard equipment and reagents | Number of cycles limited by sample integrity, limited to compatible antibodies | [92–94] | |
| Histocytometry | Combines microscopy and multiplexed antibody staining | Provides spatial and contextual information | Antibody availability and compatibility | [91] | |
| Fluorescence-activated cell sorting (FACS) | High-throughput characterization of bulk samples | Widely available commercially | Limited by antibodies available; limited to a few markers | [70,75] | |
| Light-sheet microscopy | Non-invasive imaging | Low phototoxicity | Expensive equipment and training required | [99,100] |
Highlights.
Recent developments in single-cell approaches have provided an avalanche of data regarding tumor heterogeneity in many tumor settings. There remains a great need to systematize and categorize these data to yield biological insights about tumor function.
Many key features of malignancy are mediated through interactions between different cell types and the influence of the local tumor microenvironment.
We describe a conceptual framework for analyzing the hierarchy of tumor heterogeneity involving cell types and cell-type–specific states.
The interactions of tumor development, progression, and varying microenvironments give rise to a spatial hierarchy of tumor heterogeneity.
New technologies for in situ genomics enable genome-wide study of tumor features while preserving spatial information of microenvironment features.
Acknowledgments
We apologize to the many authors whose relevant work we could not cite due to space constraints. We thank J. Rosen, X. Chen, and K. Cermakova (Baylor College of Medicine), and W. Wang (M.D. Anderson Cancer Center) for helpful feedback. This work was supported by NIH grant R00CA187565 (H.C.H.), CPRIT grant RR170036 (H.C.H.), the V Foundation grant V2018–003 (H.C.H.), and Gabrielle’s Angel Foundation for Cancer Research (H.C.H.).
Glossary
- ATAC:
A high-throughput genome-wide assay for chromatin accessibility
- CUT&RUN:
A sensitive alternative to ChIP-seq that uses micrococcal nuclease (MNase) fused to protein A
- CyTOF:
A mass spectrometry analog to flow cytometry that measures the single-cell abundance of many protein targets
- Multiregion sequencing:
Dissection of a tumor into separate discrete regions to map the variation of its features over space
- tSNE:
An approach to plot high-dimensional genome-wide data that places similar cells close together on a 2D graph
- UMAP:
A fast plotting procedure similar to tSNE that better preserves global structure, and whose output can be used for further downstream analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no competing financial interests.
Resources
References
- 1.Turajlic S et al. (2019). Resolving genetic heterogeneity in cancer. Nat. Rev. Genet doi: 10.1038/s41576-019-0114-6 [DOI] [PubMed] [Google Scholar]
- 2.Corces MR et al. (2018). The chromatin accessibility landscape of primary human cancers. Science 362, eaav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flavahan WA et al. (2017). Epigenetic plasticity and the hallmarks of cancer. Science 357, eaal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messerschmidt DM et al. (2014). DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 28, 812–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang Y-S et al. (2011). Stem cells and reprogramming: breaking the epigenetic barrier? Trends Pharmacol. Sci 32, 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hensley CT et al. (2016). Metabolic Heterogeneity in Human Lung Tumors. Cell 164, 681–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez FO and Gordon S (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson DA et al. (2015). Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puram SV et al. (2017). Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 171, 1611–1624.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurland BF et al. (2017). Estrogen Receptor Binding (18 F-FES PET) and Glycolytic Activity (18 F-FDG PET) Predict Progression-Free Survival on Endocrine Therapy in Patients with ER + Breast Cancer. Clin. Cancer Res. 23, 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patch A-M et al. (2015). Whole-genome characterization of chemoresistant ovarian cancer. Nature 521, 489–94 [DOI] [PubMed] [Google Scholar]
- 12.Li Q et al. (2018). Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses. Nat. Commun 9, 3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niyongere S et al. (2019). Heterogeneous expression of cytokines accounts for clinical diversity and refines prognostication in CMML. Leukemia 33, 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somasundaram R et al. (2017). Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat. Commun 8, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaayvaz M et al. (2018). Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun 9, 3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bocci F et al. (2019). Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc. Natl. Acad. Sci. U. S. A 116, 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGranahan N et al. (2016). Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevanović S et al. (2017). Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 356, 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal R et al. (2019). Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S et al. (2013). Endocrine-Therapy-Resistant ESR1 Variants Revealed by Genomic Characterization of Breast-Cancer-Derived Xenografts. Cell Rep. 4, 1116–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birsoy K et al. (2013). MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet 45, 104–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camarda R et al. (2016). Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med 22, 427–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bader DA et al. (2018). Mitochondrial pyruvate import is a metabolic vulnerability in androgen receptor-driven prostate cancer. Nat. Metab 1, 70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlinger M et al. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med 366, 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamal-Hanjani M et al. (2017). Tracking the Evolution of Non-Small-Cell Lung Cancer. N. Engl. J. Med 376, 2109–2121 [DOI] [PubMed] [Google Scholar]
- 26.Williams MJ et al. (2018). Quantification of subclonal selection in cancer from bulk sequencing data. Nat. Genet 50, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caswell-Jin JL et al. (2019). Clonal replacement and heterogeneity in breast tumors treated with neoadjuvant HER2-targeted therapy. Nat. Commun 10, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russnes HG et al. (2017). Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters. Am. J. Pathol 187, 2152–2162 [DOI] [PubMed] [Google Scholar]
- 29.Nik-Zainal S et al. (2016). Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis C et al. (2012). The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koboldt DC et al. (2012). Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan CW et al. (2013). The somatic genomic landscape of glioblastoma. Cell 155, 462–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodges C et al. (2016). The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med 6, a026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodges HC et al. (2018). Dominant-negative SMARCA4 mutants alter the accessibility landscape of tissue-unrestricted enhancers. Nat. Struct. Mol. Biol 25, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanton BZ et al. (2017). Smarca4 ATPase mutations disrupt direct eviction of PRC1 from chromatin. Nat. Genet 49, 282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macosko EZ et al. (2015). Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levitin HM et al. (2018). Single-Cell Transcriptomic Analysis of Tumor Heterogeneity. Trends in cancer 4, 264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navin NE (2015). The first five years of single-cell cancer genomics and beyond. Genome Res. 25, 1499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg AB et al. (2018). Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusanovich DA et al. (2018). The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature 555, 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng C et al. (2017). Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 169, 1342–1356.e16 [DOI] [PubMed] [Google Scholar]
- 42.Li H et al. (2017). Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet 49, 708–718 [DOI] [PubMed] [Google Scholar]
- 43.Bartoschek M et al. (2018). Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun 9, 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jahani-Asl A et al. (2016). Control of glioblastoma tumorigenesis by feed-forward cytokine signaling. Nat. Neurosci 19, 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa A et al. (2018). Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 33, 463–479.e10 [DOI] [PubMed] [Google Scholar]
- 46.Stamatelos SK et al. (2019). Tumor Ensemble-Based Modeling and Visualization of Emergent Angiogenic Heterogeneity in Breast Cancer. Sci. Rep 9, 5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buenrostro JD et al. (2015). Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cusanovich DA et al. (2015). Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348, 910–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hainer SJ et al. (2019). Profiling of Pluripotency Factors in Single Cells and Early Embryos. Cell doi: 10.1016/j.cell.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ku WL et al. (2019). Single-cell chromatin immunocleavage sequencing (scChIC-seq) to profile histone modification. Nat. Methods 16, 323–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar MP et al. (2018). Analysis of Single-Cell RNA-Seq Identifies Cell-Cell Communication Associated with Tumor Characteristics. Cell Rep. 25, 1458–1468.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aibar S et al. (2017). SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liberzon A et al. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubert M et al. (2018). Perturbation-response genes reveal signaling footprints in cancer gene expression. Nat. Commun 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venteicher AS et al. (2017). Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, eaai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binnewies M et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med 24, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becht E et al. (2018). Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol 37, 38–47 [DOI] [PubMed] [Google Scholar]
- 58.Oshimori N et al. (2015). TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 160, 963–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tammela T et al. (2017). A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 545, 355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nickel A et al. (2018). Adipocytes induce distinct gene expression profiles in mammary tumor cells and enhance inflammatory signaling in invasive breast cancer cells. Sci. Rep 8, 9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milotti E et al. (2017). Pulsation-limited oxygen diffusion in the tumour microenvironment. Sci. Rep 7, 39762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakazawa MS et al. (2016). Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmona-Fontaine C et al. (2017). Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl. Acad. Sci. U. S. A 114, 2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boutros PC et al. (2015). Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet 47, 736–45 [DOI] [PubMed] [Google Scholar]
- 65.Miller CA et al. (2016). Aromatase inhibition remodels the clonal architecture of estrogen-receptor-positive breast cancers. Nat. Commun 7, 12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrissy AS et al. (2017). Spatial heterogeneity in medulloblastoma. Nat. Genet 49, 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turajlic S et al. (2018). Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell 173, 581–594.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerlinger M et al. (2014). Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat. Genet 46, 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sottoriva A et al. (2015). A Big Bang model of human colorectal tumor growth. Nat. Genet 47, 209–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Litzenburger UM et al. (2017). Single-cell epigenomic variability reveals functional cancer heterogeneity. Genome Biol. 18, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spitzer MH and Nolan GP (2016). Mass Cytometry: Single Cells, Many Features. Cell 165, 780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez VD et al. (2018). Commonly Occurring Cell Subsets in High-Grade Serous Ovarian Tumors Identified by Single-Cell Mass Cytometry. Cell Rep. 22, 1875–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frei AP et al. (2016). Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat. Methods 13, 269–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lavin Y et al. (2017). Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 169, 750–765.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medaglia C et al. (2017). Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science 358, 1622–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giesen C et al. (2014). Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–22 [DOI] [PubMed] [Google Scholar]
- 77.Espina V et al. (2006). Laser-capture microdissection. Nat. Protoc 1, 586–603 [DOI] [PubMed] [Google Scholar]
- 78.Casasent AK et al. (2018). Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing. Cell 172, 205–217.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brasko C et al. (2018). Intelligent image-based in situ single-cell isolation. Nat. Commun 9, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ganguli A et al. (2018). Pixelated spatial gene expression analysis from tissue. Nat. Commun 9, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ståhl PL et al. (2016). Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 [DOI] [PubMed] [Google Scholar]
- 82.Jemt A et al. (2016). An automated approach to prepare tissue-derived spatially barcoded RNA-sequencing libraries. Sci. Rep 6, 37137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriques SG et al. (2019). Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moffitt JR et al. (2018). Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang G et al. (2018). Multiplexed imaging of high-density libraries of RNAs with MERFISH and expansion microscopy. Sci. Rep 8, 4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eng C-HL et al. (2019). Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature 568, 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JH et al. (2014). Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ly A et al. (2016). High-mass-resolution MALDI mass spectrometry imaging of metabolites from formalin-fixed paraffin-embedded tissue. Nat. Protoc 11, 1428–43 [DOI] [PubMed] [Google Scholar]
- 89.Dilillo M et al. (2017). Ultra-High Mass Resolution MALDI Imaging Mass Spectrometry of Proteins and Metabolites in a Mouse Model of Glioblastoma. Sci. Rep 7, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spraggins JM et al. (2016). Next-generation technologies for spatial proteomics: Integrating ultra-high speed MALDI-TOF and high mass resolution MALDI FTICR imaging mass spectrometry for protein analysis. Proteomics 16, 1678–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller A et al. (2017). Exploring Metabolic Configurations of Single Cells within Complex Tissue Microenvironments. Cell Metab. 26, 788–800.e6 [DOI] [PubMed] [Google Scholar]
- 92.Gerdes MJ et al. (2013). Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc. Natl. Acad. Sci. U. S. A 110, 11982–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin J-R et al. (2015). Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat. Commun 6, 8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin J-R et al. (2018). Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. Elife 7, 8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cermakova K and Hodges HC (2018). Next-Generation Drugs and Probes for Chromatin Biology: From Targeted Protein Degradation to Phase Separation. Molecules 23, 1958, doi: 10.3390/molecules23081958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Danhier P et al. (2015). Combining Optical Reporter Proteins with Different Half-lives to Detect Temporal Evolution of Hypoxia and Reoxygenation in Tumors. Neoplasia 17, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y and Chen L (2016). Classification of Advanced Human Cancers Based on Tumor Immunity in the MicroEnvironment (TIME) for Cancer Immunotherapy. JAMA Oncol. 2, 1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Genovese G et al. (2017). Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature 542, 362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu T et al. (2018). Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kubota SI et al. (2017). Whole-Body Profiling of Cancer Metastasis with Single-Cell Resolution. Cell Rep. 20, 236–250 [DOI] [PubMed] [Google Scholar]
- 101.Tran E et al. (2014). Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Allen EM et al. (2015). Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tran E et al. (2015). Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rizvi NA et al. (2015). Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ribas A and Wolchok JD (2018). Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Linde N et al. (2018). Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su S et al. (2014). A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 25, 605–20 [DOI] [PubMed] [Google Scholar]
- 108.Celus W et al. (2017). Loss of Caveolin-1 in Metastasis-Associated Macrophages Drives Lung Metastatic Growth through Increased Angiogenesis. Cell Rep. 21, 2842–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]