Abstract
Although changes in cognitive functions including attention are well documented in aging, the neurobiological basis for such alterations is not fully understood. Increasing evidence points towards the contribution of genetic factors in age-related cognitive decline. However, genetic studies have remained inconsistent in characterizing specific genes that could predict functional decline in aging. Here we utilized next generation RNA sequencing (RNA-seq) to identify patterns of differentially expressed genes in the prefrontal cortex (PFC), a brain region implicated in attention, of young and aged animals that were either cognitively trained or had limited cognitive engagement. Consistent with previous investigations, aging alone was associated with increased expression of genes involved in multiple facets of innate and adaptive immune responses. On the contrary, the expression of immunity-related transcripts was reduced by cognitive engagement. In addition, transcripts across a wide range of cellular processes, including those associated with neuronal remodeling and plasticity, were upregulated by this behavioral manipulation. Surprisingly, aged subjects accounted for higher mean counts of upregulated transcripts and lower mean counts for downregulated transcripts as compared to the young subjects. Because aged rats exhibited lower attentional capacities, it is plausible that transcriptional changes associated with performance in these animals were reflective of compensatory changes that occurred to cope up with the declining integrity of PFC functioning. Interestingly, the effects of both aging and cognitive engagement resulted in an upregulation of transcripts linked to extracellular exosomes, suggesting such extracellular vesicles may moderate a reciprocal gene by environment interaction in order to facilitate the reorganization of PFC circuitry and maintain functionality. Taken together, these findings provide novel insights into the capacities of both cognitive engagement as well as aging to alter gene expression in the PFC, and how the effects of such dynamic factors relate to variation in age-related cognitive abilities.
Keywords: aging, prefrontal cortex, cognition, transcriptomics, rats
1. Introduction
Aging is an inevitable biological process that produces alterations in brain structure and cognitive functions. However, the mechanisms that contribute to age-related cognitive decline are not fully understood. Moreover, it remains unclear why the rate and magnitude of cognitive decline does not occur equally across aged individuals; some display enhanced vulnerability to cognitive decline and associated neurodegenerative disorders, while others exhibit resilience to these conditions. Therefore, it is critical to gain a fundamental understanding of the neurobiological mechanisms responsible for the decline in cognitive abilities that is observed in aging.
Growing evidence suggests that transcriptional regulation may account for such age-related functional deficits. Studies utilizing animal models as well as postmortem human tissues demonstrate altered transcriptional patterns of specific genes across multiple brain regions that are associated with aging and Alzheimer’s disease (AD); in particular, investigations illustrate increased expression of immunity- and inflammation-related genes, as well as reduced expression for genes involved in the maintenance of synapse structure and plasticity, myelin components, mitochondrial function, and neurotrophin signaling (Berchtold et al., 2013; Blalock et al., 2003; Cribbs et al., 2012; Ginsberg, Alldred, & Che, 2012; Ianov et al., 2016; Mangold et al., 2017; Primiani et al., 2014). Moreover, altered expression of these genes is suggested to contribute to decline in cognitive functions observed in aging and age-related neurodegenerative disorders (Blalock et al., 2003; Burger, 2010; Ianov et al., 2016; Saura, Parra-Damas, & Enriquez-Barreto, 2015; Stranahan et al., 2012; Verbitsky et al., 2004). Conversely, gene clusters associated with increased synaptic activity and plasticity are reported to be upregulated in cortical regions during the pre-symptomatic stage of AD, and these changes negatively correlate with performance on the mini-mental state examination (Berchtold et al., 2014; Bossers et al., 2010). In addition, a recent transcriptomic profiling study reported that the increased expression of immediate early genes known to modulate neural activity and synaptic plasticity in the medial prefrontal cortex (mPFC) of age-impaired rats was associated with impaired performance in an operant based, set-shifting task (Ianov et al., 2016). Thus, findings from transcriptomic studies have remained inconsistent in characterizing specific genes that could be linked to age-related alterations in cognitive performance.
Interestingly, evidence from rodent studies indicates that sensory/motor and cognitive stimulation via environmental enrichment alters the expression of genes associated with neuronal structure, synaptic signaling and cognition (Potter, Costa, Cracciola, Hughes, & Arendash, 2005; Rampon et al., 2000). Moreover, activity-dependent regulation of synaptic plasticity is thought to be a critical component in the reorganization of brain networks that is necessary to compensate for age-related changes in cognitive functions (Lalo, Bogdanov, & Pankratov, 2018). Therefore, it is possible that alterations in gene expression in the context of brain aging are reflective of dynamic interactions between the aging process and life-long cognitive experience rather than the effects of aging in isolation. However, any study that examined transcription with aging and cognition or any anti-aging manipulation (exercise, caloric restriction etc.) has not employed this framework.
In the present study, we utilized next generation RNA sequencing (RNA-seq) to identify patterns of differentially expressed genes as a function of age and cognitive experience in the rat brain. Given the importance of the PFC in regulating higher cognitive functions including the executive control of attentional, and the vulnerability of this brain region in aging and AD (Hedden & Gabrieli, 2004; Rossi, Pessoa, Desimone, & Ungerleider, 2009; West, 1996), transcriptomic profiling of the PFC was conducted in young and aged animals that were either cognitively engaged or devoid of any cognitive stimulation.
2. Methods
2.1. Subjects
Male Wistar rats (6 young: 8-10 weeks and 6 retired breeders: 10–12 months; total 12) were procured from Charles River Laboratories (Malvern, PA, USA). Retired breeders were maintained until 22 months of age prior to the initiation of operant behavioral training (see behavioral procedures). Young rats were acquired when the aged rats were ready to be used for experiments and the behavioral procedures for both age groups of animals were started simultaneously. All animals were water regulated by restricting access to a 10-min period in the home cage following each behavioral session. Rats were individually housed and food was available ad-libitum throughout the behavioral training and testing. All experiments were conducted in accordance with the National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee at Temple University.
2.2. Behavioral Procedures
Behavioral training and testing occurred in operant chambers contained within sound-attenuating boxes (Med Associates Inc., St Albans, VT). Young (3 months, N=3) and aged (22 months, N=3) rats were trained in a sustained attention task (SAT) based on procedures described in our previous studies (Parikh et al., 2013; Yegla & Parikh, 2014, 2017). Briefly, the animals were trained to discriminate between randomly and unpredictably occurring signal (illumination of the central panel light that varied at 500ms, 50ms and 25ms) and non-signal (non-illumination) events. Correct response on signal trials (hit, h) and non-signal trials (correct rejection, cr) was rewarded with 0.02 mL water. Incorrect lever press response (signal: miss, m; non-signal: false alarm, fa) was not rewarded (Fig 1A). The houselight remained illuminated throughout the session. Each behavioral session consisted of a pseudo-randomized sequence of signal and non-signal trials with an inter-trial interval of 9±3s and divided into 3 blocks (total trials 162; 54/block). After attaining stable criterion performance (≥70% h500 and ≥70% cr for 3 consecutive sessions), animals were tested for performance in the distractor SAT (dSAT) session that consisted of the presentation of visual distracters (flashing house light; 0.5 Hz) during the second block (Fig 1A). Cognitive performance was analyzed by calculating a) the number of training session required to attain criterion, and b) the proportion of correct responses for signal [(h/h+m)*100] and non-signal trials [(cr/cr+fa)*100].
Figure 1:
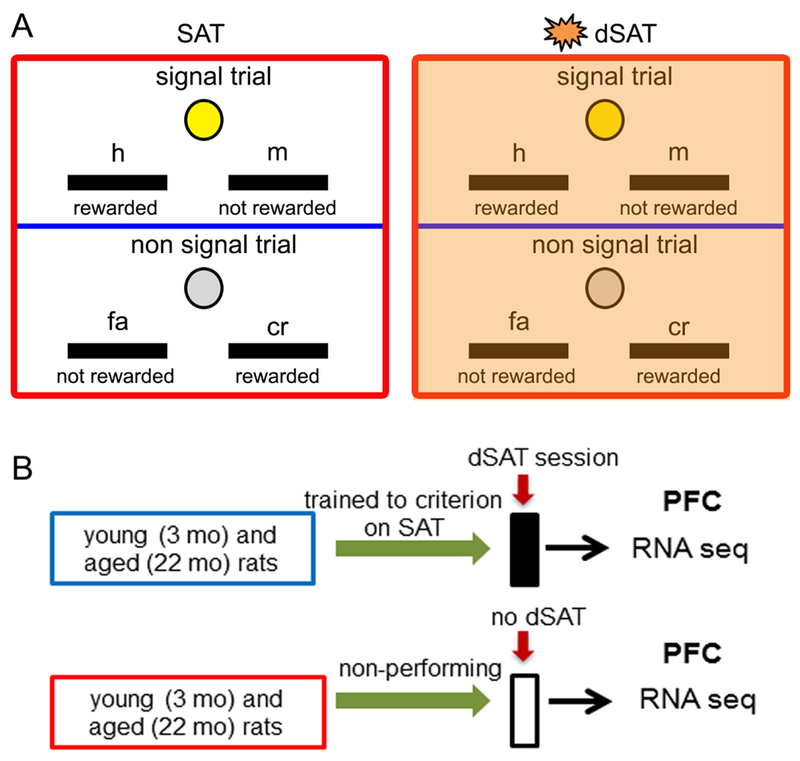
Illustration depicting the rules of the operant sustained attention task (SAT) and experimental design. Rats discriminated between signal (visual cue from the front panel) and non-signal (no cue) trials by exhibiting a correct lever press response that was rewarded. For signal left lever assignment, a left lever press on a signal trial was scored as a “hit” (h) and rewarded; depression of the right lever was scored as a “miss (m)” and was not rewarded. During non-signal trials for the same assignment, a right lever press was scored as a “correct rejection” (cr) and reinforced, while a left lever press was scored as a “false alarm” (fa) and remained unrewarded (A; left). Lever responses for signal and non-signal trials remained counterbalanced across animals. Each behavioral session consisted of pseudo-randomized sequence of signal and nonsignal trials over 3 blocks of 54 trials each. The distractor (dSAT) test session consisted of presentation of visual distractors (flashing house light @ 0.5 Hz) in the 2nd block (A, right). (B) Schematic of experimental design. Young and aged rats trained to criterion on SAT were tested on the distractor (dSAT) behavioral session. At the completion of the test session, brains were immediately removed and PFC tissues were isolated for RNA extraction and RNA-seq analyses. Tissues from another cohort of non-performing animals, housed in standard conditions and exposed to the operant chambers but never trained on the task, were extracted for transcriptomic profiling in a time frame consistent with their performing counterparts.
Immediately after the test session, animals were decapitated for rapid isolation of mPFC that included cingulate, prelimbic and infralimbic regions. Samples were frozen on dry ice for subsequent RNA extraction and high throughput RNA-seq. Another cohort of young (N=3) and aged (A=3) rats served as non-performing control for each age group (Fig 1B). These animals were housed and water regulated in the same conditions. Because performing rats also received water as reward during the operant task, the home cage water access for non-performing rats was kept for a longer duration (i.e. 20-min) to keep the overall water consumption similar. In addition, these animals received the same contextual experience (exposed to the operant chambers) as the performing group, but were never trained on task. Tissues from the non-performing young and aged rats were collected in a time frame consistent with their performing counterparts (Fig 1B).
2.3. RNA extraction, transcriptome sequencing and bioinformatics
Total RNA was extracted from the frozen tissue using RNeasy Kit (Qiagen). Purified RNA (1μg) was used for mRNA isolation and subsequent transcriptome library preparation using the TruSeq Stranded mRNA Library Preparation Kit (Illumina). Libraries were sequenced on the HiSeq 2500 to obtain at least 30 million single (2×50 bp) reads per sample. Sequence data from FASTQ files were aligned to the Ratus Norvegicus (Rnor 6.0) genome for annotation using Bowtie2 and TopHat2 (Kim et al., 2013; Langmead & Salzberg, 2012). Bioconductor package DESeq2 was used for count normalization and analysis (Love, Huber, & Anders, 2014). All raw data was deposited in the NCBI Gene Expression Omnibus database (accession ID: GSE105453).
After implementing the filtering procedure, 15,939 transcripts were compared. A negative binomial regression model with covariates for age, performance (i.e. cognitive engagement), and their interaction, was applied to analyze the data using the following equation: Expression = a + b1*age + b2*performance + g*age*performance; where a is the intercept, b1 is the coefficient of age, b2 is the coefficient of performance, and g is the coefficient of age x performance interaction. The age and performance factors were coded as: young = 0 and aged = 1; non-performing = 0 and performing = 1. Based on these codes, the following 4 expression conditions were obtained: young non-performing expression = a; young performing expression = a + b2; aged non-performing expression = a + b1; aged performing expression = a + b1 + b2 + g. From this model, a contrast representing the main effect of age after adjusting for performance was defined to identify genes most differentially expressed due to age (N = 6/age group; young and aged). The age effect was calculated as: [(aged non-performing expression – young non-performing expression) + (aged performing expression – young performing expression)]/2 = b1+ g/2. The same procedure was adopted for a contrast representing the main effect of performance after adjusting for age to identify differentially expressed transcripts due to cognitive engagement (N = 6/behavioral manipulation group; non-performing and performing). The performance effect was calculated as b2 + g/2. The g interaction coefficient represented the expression difference between the performance effect in aged animals and the performance effect in young animals.
A Wald test was used for differential expression and the nominal p-values were adjusted for multiple comparisons using the false discovery rate (FDR) method (Benjamini & Hochberg, 1995). For significance, a cutoff of FDR<0.1 was imposed. Expression profiles of significant genes were submitted to NIH’s DAVID resources and analyzed according to gene ontology for cellular components and biological processes (Dennis et al., 2003). For interaction effects on differentially expressed genes, a nominal p value with a stringent cutoff (p<0.01) was utilized to select genes for gene enrichment analysis and FDR<0.1 was applied for statistical significance. Behavioral data was analyzed using either one-way or mixed factor ANOVA. Pearson correlation analysis was conducted to examine association between the behavioral measures and gene expression (i.e. normalized mean counts of transcripts). RNA-seq results were validated by performing reverse transcriptase quantitative PCR (qPCR) in a subset of samples (See Supplementary Methods for details on RNA-seq and qPCR).
3. Results
Volcano plots displaying the profiles of 15,939 differentially expressed transcripts in the PFC or rats as a function of age and performance are shown in Fig 2A and 2B. After controlling for cognitive performance (i.e. cognitive engagement), aging altered the expression of 377 genes with a nominal p-value <0.01. Following the application of FDR correction (<0.1), expression changes in 30 genes (24 upregulated, 6 downregulated) reached significance (see Supplementary Tables 1 and 2). A closer examination of data indicated that most of the upregulated transcriptomes were linked to the immune system. These included genes involved in the modulation of the complement pathway, both in the classical pathway (C4b) as well as in the lectin mediated pathway (Clec7a, Clec12a), cytokine signal transduction (Csf2rb, Lrrc66, Irak3) and toll-like receptor signaling (Tlr13). Interestingly, genes indicative of peripheral immune responses were also upregulated, including immunoglobulin signaling (Cd101, Cd22, Cd74) and regulators of adaptive immunity (Pram1, Ncf4). Systematic investigation of functional annotations revealed significant enrichment of the cytokine-mediated signaling pathway (GO:0019221; p=4.21×10−4, FDR=0.06; Supplementary Table 4). A significant age-related downregulation was observed in transcripts associated with membrane proteins (Itm2a, Slc40a1, Tmem14) that facilitated an enrichment of the integral component of membrane (GO:0016021, p=0.03); however, this pathway did not reach significance following FDR correction (Supplementary Table 3).
Figure 2:
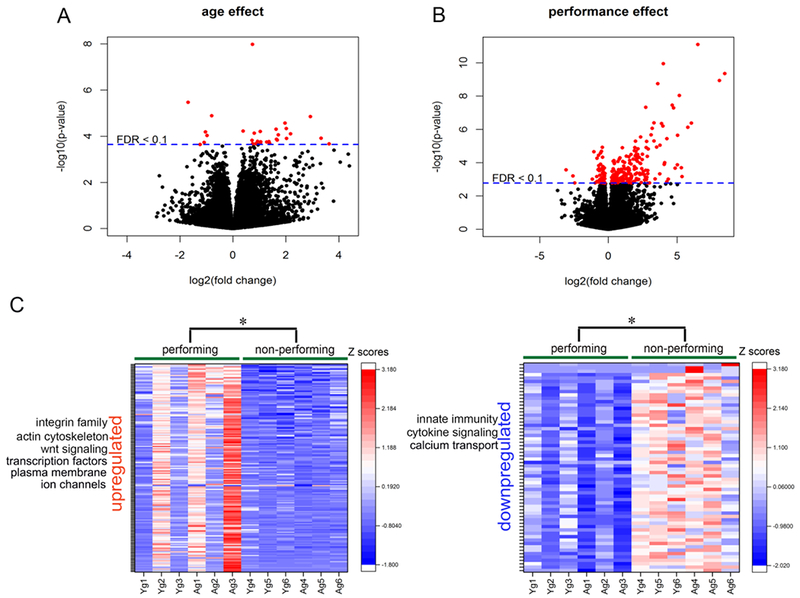
Differential expression of PFC transcriptome as a function of age and cognitive performance (i.e. cognitive engagement). Volcano plots for the differentially expressed genes depicting the effects of age (A) and performance (B), respectively. Genes with FDR-adjusted p-value < 0.1 are depicted as red dots. Positive log2 fold change (right) indicate upregulated transcripts, while negative log2 fold change indicate downregulation. Heat maps of cognitive performance-related changes in gene expression in young and aged rats (left: upregulated; right: downregulated). Each row represents differentially expressed genes. Expression of each gene was converted to standardized Z-scores. Color red represents increasing scores and blue represents decreasing scores.
The effect of performance (i.e. cognitive engagement), after adjusting for the effect of age, was associated with changes in the expression of 623 genes at a nominal p-value<0.01 out of which 247 genes reach significance with FDR<0.1 (185 upregulated, 62 downregulated; see heat maps in Fig 2C and refer Supplementary Tables 5 and 6 for the complete list of transcripts). Cognitive engagement upregulated transcripts implicated in the maintenance of cellular junctions (Itga10, Itga4, Gjb2), activators of Wnt signaling (Wnt5a, Wnt6), as well as components of plasma membrane (Calb2, Cdhr1, Gjb2) and potassium channels (Kcne5, Kcnh6, Kcnh8). Moreover, the expression of genes belonging to the family of integrins (Cdh19, ltgb4, ltga10, Nid1, Spp1), transcription factors (E2f6, Elf2, Foxd1, Foxc2, Nfe2l2, Tfap2b, Twist1) and actin cytoskeleton (Cdk5, Gas213, Lima1) were significantly upregulated. The transcripts associated with immunoglobulin signaling (Emb, Il1rap, Sema4f), innate immunity (S100a9) as well as calcium transport (Cacng5, Calb2, Ccdc109b) were significantly downregulated in performing rats, indicating that cognitive engagement by itself may have the capacity to suppress immunological responses and calcium overload. In addition, the expression of genes associated with synaptic transmission (Dnm1, Nxph3) and ionotropic receptors (Grin2b, Chrna4) were downregulated with cognitive experience. The most significant gene ontology term identified with the upregulated genes using DAVID (see Supplementary Table 8) was integral components of the plasma membrane (GO:0005887, 25 genes, p=6.16×10−6, FDR=0.001) and neuron projection (GO:0043005, 12 genes,p=0.001, FDR=0.09). Downregulated transcripts had enrichment in several GO terms such as extracellular exosomes, synapse, synaptic vesicles and extracellular space (Supplementary Table 7); however, these pathways did not reach significance at FDR cut-off. Interestingly, differences in age appeared to account for the direction of expression associated with performance (i.e. aged animals were significantly more likely to have higher normalized mean counts of transcripts that were significantly upregulated by cognitive engagement (170 out of 185, χ2(1)=129.87, p <0.01) and young animals were significantly more likely to have higher mean counts of downregulated transcripts (58 out of 62), χ2(1)=25.81, p<0.01).
In general, aged animals required significantly more SAT training sessions to attain criterion as compared to the young animals (young: 40.33 ± 3.18; aged: 75.66± 11.02; F(1,4) = 3.16, p=0.03; Fig 3A). Analyses of the dSAT testing session across age groups indicated significantly different hit rates as a result of signal duration (F(1, 4)=31.96, p<0.01) as well as block (F(1, 4)= 7.82, p=0.01). Upon further examination, aged animals demonstrated significantly lower hit rates in the post-distracter block during 500ms (ph500) trials (p=0.02; Fig 3B). Together these data indicate that aged rats exhibit learning and attentional impairments which are consistent with our previous work (Parikh, Bernard, Naughton, & Yegla, 2014).
Figure 3.
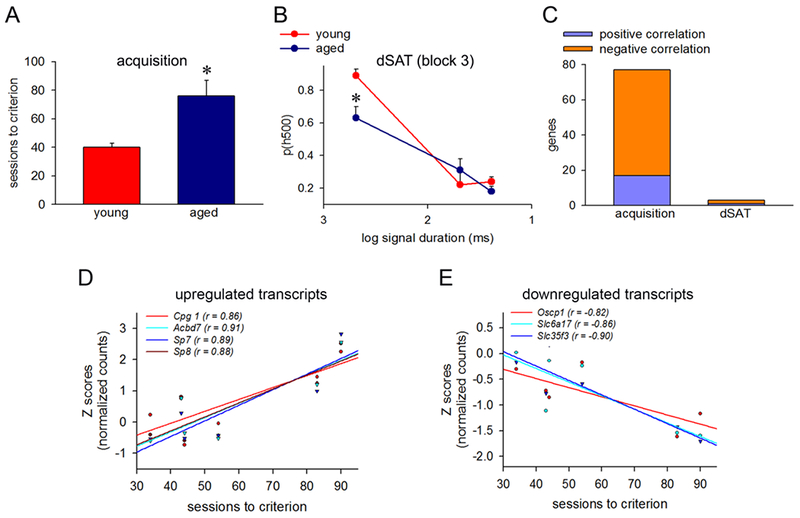
Cognitive performance and its association with differentially expressed transcripts. (A) Aged animals required significantly more SAT training sessions to attain criterion as compared to the young animals. (B) During the dSAT test session, aged animals exhibited impaired ability to detect 500ms signal trials (ph500) in the postdistractor block (block 3) as compared to the young animals. (C) Correlation analyses illustrated that 60 transcripts that were differentially upregulated with cognitive engagement were positively associated with the sessions to criterion while 17 downregulated transcripts exhibited a negative association with this behavioral measure. In addition, 2 upregulated and 1 downregulated transcripts exhibited significant associations with ph500 in block 3 of the dSAT testing session in a similar direction. Scatterplots with trendlines depicting the correlations between normalized counts (Z-scores) and sessions to criterion for representative upregulated (D) and downregulated (E) transcripts. * p<0.05
Next, we asked whether performance-associated changes in gene expression, after adjusting for age, account for differences in cognitive capacities. Correlation analyses illustrated that 60 differentially upregulated genes positively associated with the sessions to criterion while 17 downregulated genes exhibited a negative association with this behavioral measure (Fig 3C–E; Supplementary Table 13). Of note, the upregulated transcripts in this analysis included expression of genes for transcription factors (Sp7, Sp8, Sall1), energy metabolism (Acbd 7, Mcub), oxidative stress (Mgst1) and synaptic plasticity (Cpg1), while downregulated genes included regulators of immune pathways (S100a9) and soluble carrier uptake transporters (Oscp1, Slc6a17, Slc35f3). Surprisingly, three transcripts (2 upregulated: GON7, KEOPS complex subunit homolog and E2F transcription factor 6; 1 downregulated: acyl-CoA thioesterase 4) also exhibited significant association with ph500 in block 3 (post-distractor block) of the dSAT testing session (Supplementary Table 14) illustrating acute performance effects on gene expression.
To further investigate whether differential patterns of prefrontal gene expression between young and aged rats following cognitive engagement might account for differing attentional capacities, we examined interaction effects between age and performance. A total of 44 transcripts (23 upregulated and 21 downregulated; Supplementary Tables 9 and 10) with a stringent nominal p-value cut off (<0.01) were identified for enrichment analysis (see Supplementary Tables 11 and 12). Our data revealed a significant downregulation for astrocyte development (GO:0014002, 3 genes, p=9.2×10−5, FDR=0.02), extracellular matrix (GO:0031012, 4 genes, p=0.001, FDR=0.05) and response to ethanol (GO: 0045471, 4 genes, p=5.4×10−4, FDR =0.07). For upregulated pathways, the most significant enrichment was observed for extracellular exosomes (GO:0070062, 10 genes, p=3.4×10−4, FDR= .01) and extracellular space (GO:0005615, 7 genes, p=0.001, FDR=0.02).
4. Discussion
Our RNA-seq dataset identified age-related upregulated expression of several distinct genes associated with multiple facets of CNS and peripheral immune responsiveness in the PFC, including transcripts associated with the classical and lectin-mediated complement pathways, pro-inflammatory cytokines, toll-like receptor and immunoglobulin signaling. These data are consistent with previous studies demonstrating increases in the innate and adaptive immune responsiveness in brain with aging and AD (Berchtold et al., 2013; Cribbs et al., 2012; Ianov et al., 2016; Ianov, Riva, Kumar, & Foster, 2017; Nikas, 2013; Van Eldik et al., 2016).
Assessment of transcriptomic variation as a direct consequence of cognitive engagement (i.e. after adjusting for the effect of age) revealed upregulated expression of a host of genes that are involved in the activation of Wnt signaling, modulation of extracelluar matrix (such as integrins, cyclin-dependent kinase 5) and regulators of transcription (e.g. eukaryotic initiation factor 2α and forkhead transcription factors). These cellular processes are linked to the maintenance of neural connectivity and synapse formation, cellular remodeling and structural plasticity (Dowling, Yu, & Fuchs, 1996; Klann, Anti on, Banko, & Hou, 2004; Kume, 2008; Park & Shen, 2012; Schuman & Murase, 2003). Conversely, downregulated transcripts as a result of cognitive engagement included genes related to peripheral immune response, (i.e. a pattern of expression opposite to the observed effect of age), those genes associated with synaptic transmission as well as calcium transport, including Dynamin1, NMDA receptor subunit 2B and Calbindin 2. Given the evidence that enriched environmental training alters the expression of genes associated with neuronal growth, synapse morphology, and structural plasticity (Potter et al., 2005; Rampon et al., 2000; Thiriet et al., 2008), it is possible that cognitive engagement associated with distinct stages of the attentional task training in our study might have exerted beneficial effects on PFC structure and function. It should be noted that although both performing and non-performing rats were water regulated and consumed similar amount of water overall during the duration of experiments, it is only the performing rats who received water in the operant boxes. Thus, we cannot rule out if any gene expression changes observed in the cognitively engaged group might have occurred due to the availability of reward in the training environment.
The most striking observation of our investigation was that the effects of cognitive engagement on alterations in gene expression were age-dependent. Notably, aged-performing subjects exhibited higher read counts of ~92% of upregulated transcripts and lower read counts of ~94% of the downregulated transcripts as compared to the young rats. Interestingly, the expression of upregulated transcripts associated with synaptic plasticity, oxidative stress and bioenergetics predicted poor attentional performance, while downregulated transcripts associated with the immune system were associated with better performance. In addition, aged rats in general exhibited slower learning and reduced attentional capacities as compared to their young counterparts. Therefore, it is tempting to speculate that transcriptional changes associated with cognitive performance in aged rats were more reflective of compensatory changes that might have occurred to cope up with the decline in the integrity of PFC structure in aging. This interpretation is consistent with the resource modulation hypothesis, which posits that genetic effects on brain structure and function are magnified in aging, such that performance variability in aging might ultimately be a result of dynamic, gene-environment interactions (Lindenberger et al., 2008; Papenberg, Lindenberger, & Backman, 2015).
While it is not possible to parse out precisely when during the course of cognitive training/testing the transcriptional changes might have occurred in the PFC of performing rats, our correlation analyses provided some insights into the temporal precedence of these changes. Specifically, we noted that 77 differentially expressed transcripts (60 upregulated and 17 downregulated) were associated with SAT acquisition/learning performance (i.e. sessions to criterion), while only 3 transcripts were associated with the ability to detect signals in the post-distractor block in the dSAT given on the last day of testing. Based on these data, we can infer that stable gene expression changes were predominantly associated with cognitive engagement, and that only a small portion of differentially-expressed transcripts reflected acute changes in performance on the testing day.
Notably, examination of age by performance interaction effects on transcriptomic profiling revealed an upregulation of gene clusters associated with extracellular exosome and extracellular space. It is noteworthy that enrichment of these clusters was downregulated with cognitive engagement (see Results), supporting our interpretations that transcriptomic changes associated with cognitive performance were potentially influenced by age in an inverse manner. Extracellular exosomes are known to transport molecules and genetic material, including miRNAs, directly between cell bodies; in turn, they are capable of influencing gene expression in recipient cells as well as regulating intercellular communication between neurons and glia, mediating microglial immune response, and modulating synaptic function (Budnik, Ruiz-Canada, & Wendler, 2016; Fruhbeis, Frohlich, & Kramer-Albers, 2012). Interestingly, recent evidence suggests that aged senescent cells increase the secretion of extracellular exosomes that release miRNAs that regulate pro-inflammatory response in aging (Robbins, 2017). Moreover, these vesicles are known to transport various cytotoxic proteins, including amyloid-β, hyperphosphorylated tau, α-synuclein and prion-like proteins that are known to be associated with age-related neuropathological conditions (Iraci, Leonardi, Gessler, Vega, & Pluchino, 2016). Therefore, it is plausible that extracellular exosome signaling may moderate the dynamic interactions between aging and cognitive performance by altering specific genetic and cellular processes that facilitate the reorganization of PFC networks; consequently, higher expression of genes associated with this pathway may predict age-related differences in attentional capacities. Further studies that examine cell-specific profiling are warranted to determine the precise contribution of neurons and glia to the observed transcriptomic changes.
Although our study revealed insights into the genomic basis of age-related cognitive decline, one of the limitations of our investigation was the low sample size that restrained statistical inference concerning the age x performance interaction effects on gene enrichment analyses. Another limitation of the present study was that expression profiling was conducted only in male subjects which make it difficult to generalize the effects of age and cognitive engagement on prefrontal transcriptome to both sexes.
Supplementary Material
Highlights:
Aging increased the expression of innate and adaptive immunity genes.
Expression of immunity-related transcripts was reduced with cognitive engagement.
Cognitive experience upregulated transcripts associated with remodeling/plasticity
Effects of cognitive engagement on prefrontal transcriptomics were age-dependent.
Aging and performance interacted to upregulate exosome-associated transcripts.
Acknowledgements
This work was supported by a grant from the National Institute on Aging (AG046580) to VP. We thank Dr. Thomas C. Foster (University of Florida, Gainesville) for helpful comments and suggestions on the final draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None.
References
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. doi: 10.2307/2346101 [DOI] [Google Scholar]
- Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, & Cotman CW (2013). Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and alzheimer’s disease. Neurobiol Aging, 34(6), 1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold NC, Sabbagh MN, Beach TG, Kim RC, Cribbs DH, & Cotman CW (2014). Brain gene expression patterns differentiate mild cognitive impairment from normal aged and alzheimer’s disease. Neurobiol Aging, 35(9), 1961–1972. doi : 10.1016/j.neurobiolaging.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, & Landfield PW (2003). Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci, 23, 3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossers K, Wirz KT, Meerhoff GF, Essing AH, van Dongen JW, Houba P, et al. (2010). Concerted changes in transcripts in the prefrontal cortex precede neuropathology in alzheimer’s disease. Brain, 133(Pt 12), 3699–3723. doi: 10.1093/brain/awq258 [DOI] [PubMed] [Google Scholar]
- Budnik, V., Ruiz-Canada, C., & Wendler, F. (2016). Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci, 17(3), 160–172. doi: 10.1038/nrn.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C (2010). Region-specific genetic alterations in the aging hippocampus: Implications for cognitive aging. Front Aging Neurosci, 2, 140. doi: 10.3389/fnagi.2010.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, et al. (2012). Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. Journal of Neuroinflammation, 9(1), 179. doi: 10.1186/1742-2094-9-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. (2003). David: Database for annotation, visualization, and integrated discovery. Genome Biol, 4(5), P3. doi: 10.1186/gb-2003-4-5-p3 [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, & Fuchs E (1996). Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol, 134(2), 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, & Kramer-Albers EM (2012). Emerging roles of exosomes in neuron-glia communication. Front Physiol, 3, 119. doi: 10.3389/fphys.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, & Che S (2012). Gene expression levels assessed by ca1 pyramidal neuron and regional hippocampal dissections in alzheimer’s disease. Neurobiol Dis, 45(1), 99–107. doi: 10.1016/j.nbd.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JD (2004). Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci, 5(2), 87–96. doi: 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Ianov L, Rani A, Beas BS, Kumar A, & Foster TC (2016). Transcription profile of aging and cognition-related genes in the medial prefrontal cortex. Frontiers in Aging Neuroscience, 5(113). doi: 10.3389/fnagi.2016.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianov L, Riva A, Kumar A, & Foster TC (2017). Dna methylation of synaptic genes in the prefrontal cortex is associated with aging and age-related cognitive impairment. Frontiers in Aging Neuroscience, 9(249). doi: 10.3389/fnagi.2017.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraci N, Leonardi T, Gessler F, Vega B, & Pluchino S (2016). Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci, 17(2), 171. doi: 10.3390/ijms17020171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, & Salzberg SL (2013). Tophat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology, 14(4), R36. doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Antion MD, Banko JL, & Hou L (2004). Synaptic plasticity and translation initiation. Learn Mem, 11(4), 365–372. doi: 10.1101/lm.79004 [DOI] [PubMed] [Google Scholar]
- Kume T (2008). Foxc2 transcription factor: A newly described regulator of angiogenesis. Trends Cardiovasc Med, 18(6), 224–228. doi: 10.1016/j.tcm.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Bogdanov A, & Pankratov Y (2018). Diversity of astroglial effects on aging- and experience-related cortical metaplasticity. Frontiers in Molecular Neuroscience, 11(239). doi: 10.3389/fnmol.2018.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, & Salzberg SL (2012). Fast gapped-read alignment with bowtie 2. Nat Methods, 9(4), 357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, & Backman L (2008). Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci, 2(2), 234–244. doi: 10.3389/neuro.01.039.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, & Anders S (2014). Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol, 15(12), 550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold CA, Wronowski B, Du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, & Freeman WM (2017). Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. Journal of Neuroinflammation, 14, 141. doi: 10.1186/s12974-017-0920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikas JB (2013). Inflammation and immune system activation in aging: A mathematical approach. Scientific reports, 3, 3254. doi: 10.1038/srep03254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenberg G, Lindenberger U, & Backman L (2015). Aging-related magnification of genetic effects on cognitive and brain integrity. Trends Cogn Sci, 19(9), 506–514. doi: 10.1016/j.tics.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Parikh V, Bernard CS, Naughton SX, & Yegla B (2014). Interactions between abeta oligomers and presynaptic cholinergic signaling: Age-dependent effects on attentional capacities. Behav Brain Res, 274, 30–42. doi: 10.1016/j.bbr.2014.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Howe WM, Welchko RM, Naughton SX, D’Amore DE, Han DH, et al. (2013). Diminished trka receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur J Neurosci, 37(2), 278–293. doi: 10.1111/ejn.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, & Shen K (2012). Wnts in synapse formation and neuronal circuitry. Embo j, 31(12), 2697–2704. doi: 10.1038/emboj.2012.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, & Hodges JR (1999). Attention and executive deficits in alzheimer’s disease. A critical review. Brain, 122 (Pt 3), 383–404. doi: 10.1093/brain/122.3.383 [DOI] [PubMed] [Google Scholar]
- Potter H, Costa DA, Cracciola JR, Hughes T, & Arendash GW (2005). Environmental enrichment prevents cognitive decline and induces memory-related gene expression in a mouse model of alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 1(1), S99. doi: 10.1016/j.jalz.2005.06.343 [DOI] [Google Scholar]
- Primiani CT, Ryan VH, Rao JS, Cam MC, Ahn K, Modi HR, et al. (2014). Coordinated gene expression of neuroinflammatory and cell signaling markers in dorsolateral prefrontal cortex during human brain development and aging. PLOS ONE, 9(10), e110972. doi: 10.1371/journal.pone.0110972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, et al. (2000). Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA, 97(23), 12880–12884. doi: 10.1073/pnas.97.23.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, & Davatzikos C (2003). Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci, 23(8), 3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD (2017). Extracellular vesicles and aging. Stem CellInvestig, 4, 98. doi: 10.21037/sci.2017.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, & Ungerleider LG (2009). The prefrontal cortex and the executive control of attention. Exp Brain Res, 192(3), 489–497. doi: 10.1007/s00221-008-1642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. (2004). Thinning of the cerebral cortex in aging. Cereb Cortex, 14(7), 721–730. doi: 10.1093/cercor/bhh032 [DOI] [PubMed] [Google Scholar]
- Saura CA, Parra-Damas A, & Enriquez-Barreto L (2015). Gene expression parallels synaptic excitability and plasticity changes in alzheimer’s disease. Frontiers in Cellular Neuroscience, 9(318). doi: 10.3389/fncel.2015.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, & Murase S (2003). Cadherins and synaptic plasticity: Activity-dependent cyclin-dependent kinase 5 regulation of synaptic beta-catenin-cadherin interactions. Philos Trans R Soc Lond B Biol Sci, 358(1432), 749–756. doi: 10.1098/rstb.2002.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Martin B, Chadwick W, Park S-S, Wang L, Becker KG, et al. (2012). Metabolic context regulates distinct hypothalamic transcriptional responses to antiaging interventions. International journal of endocrinology, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Rojas C, & Inestrosa NC (2018). Loss of canonical wnt signaling is involved in the pathogenesis of alzheimer’s disease. Neural Regen Res, 13(10), 1705–1710. doi: 10.4103/1673-5374.238606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriet N, Amar L, Toussay X, Lardeux V, Ladenheim B, Becker KG, et al. (2008).Environmental enrichment during adolescence regulates gene expression in the striatum of mice. Brain Res, 1222, 31–41. doi: 10.1016/j.brainres.2008.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2012). Aging and functional brain networks. Mol Psychiatry, 17(5), 471, 549–458. doi: 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik LJ, Carrillo MC, Cole PE, Feuerbach D, Greenberg BD, Hendrix JA, et al. (2016). The roles of inflammation and immune mechanisms in alzheimer’s disease. Alzheimers Dement (N Y), 2(2), 99–109. doi: 10.1016/j.trci.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitsky M, Yonan AL, Malleret G, Kandel ER, Gilliam TC, & Pavlidis P (2004). Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn Mem, 11(3), 253–260. doi: 10.1101/lm.68204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL (1996). An application of prefrontal cortex function theory to cognitive aging. Psychol Bull, 120(2), 272–292. [DOI] [PubMed] [Google Scholar]
- Yegla B, & Parikh V (2014). Effects of sustained prongf blockade on attentional capacities in aged rats with compromised cholinergic system. Neuroscience, 261, 118–132. doi: 10.1016/j.neuroscience.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegla B, & Parikh V (2017). Developmental suppression of forebrain trka receptors and attentional capacities in aging rats: A longitudinal study. Behav Brain Res, 335, 111–121. doi: 10.1016/j.bbr.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


