We report neurodevelopmental outcomes in 216 infants followed since the time of PCR-confirmed maternal Zika virus (ZIKV) infection in pregnancy during the Rio de Janeiro epidemic of 2015–2016 1,2. Neurodevelopment was assessed by Bayley-III Scales of Infant and Toddler Development (cognitive, language and motor domains) in 146 children and through neurodevelopment questionnaires/neurological examinations in 70 remaining children. Complete eye exams (N=137) and hearing assessments (N=114) were also performed. Below average neurodevelopment and/or abnormal eye or hearing assessments was noted in 31.5% of children between 7 to 32 months of age. Among children assessed by Bayley-III, 12% scored below – 2 SD (score < 70; (a score of 100 ± 2 SD is the range) in at least one domain; 28% scored between −1- –2 SD in any domain (scores < 85–70). Language function was most affected with 35% of 146 children below average. Improved neurodevelopmental outcomes were noted in female children, term babies, children with normal eye exams, and maternal infection later in pregnancy (p=0.01). We noted resolution of microcephaly with normal neurodevelopment in 2 of 8 children; development of secondary microcephaly in 2 other children, and autism spectrum disorder in 3 previously healthy children in the second year of life.
During the 2015–2016 Zika virus (ZIKV) epidemic in Rio de Janeiro, Brazil, we established a prospective cohort of symptomatic pregnant women with RT-PCR confirmed ZIKV-infection. We reported fetal ultrasound findings for the first 88 women enrolled during the Rio de Janeiro epidemic (until 2/2016)1 and subsequently described gestational and infant outcomes for 125 pregnancies until July 20162. All pregnancies came to completion by December 2016. We presently report neurodevelopmental results obtained between 7 to 32 months of age in children from our prospective cohort.
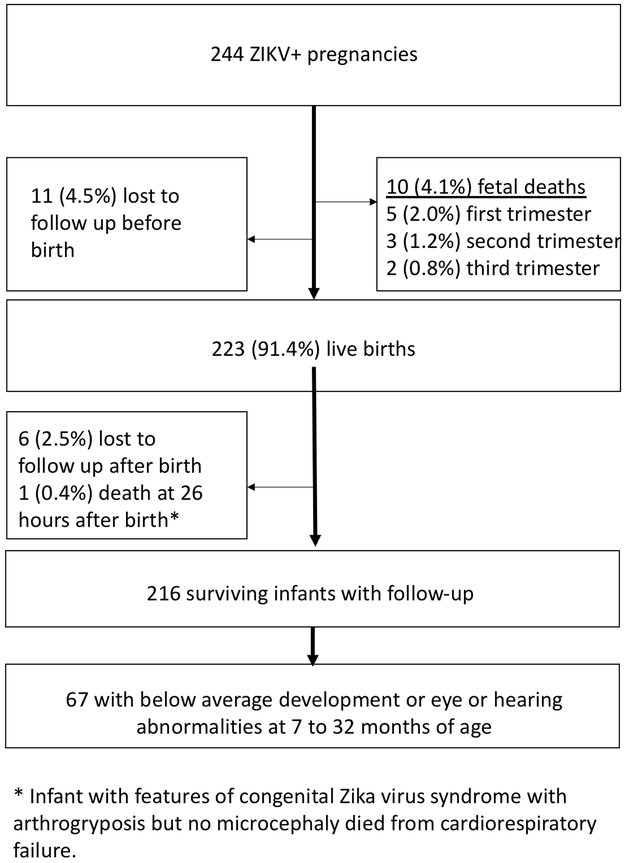
From September 2015 through June 2016, we enrolled 244 pregnant symptomatic women who presented with a rash and tested positive for ZIKV by qualitative RT-PCR of serum or urine. Figure 1 depicts enrollment flow. Among 223 live births, the prematurity rate was 13% and 10 infants (4.6%) were small for gestational age (Table 1). Microcephaly (MC) was identified in 8 of 216 infants with follow-up (3.7%) including 2 infants with secondary MC. Two of the 8MC cases resolved: one infant with proportionate microcephaly developed normal head circumference as he grew; another developed normal head circumference following surgery for cranial synostosis. Both infants had normal neurodevelopmental outcomes, audiometric evaluations and eye exams in the second year of life.
Figure 1:
Flow diagram of mother-infant pair enrollment and follow-up in the Rio de Janeiro Zika cohort.
Table 1:
Characteristics of ZIKV Exposed Neonates and Neurodevelopmental and Neurosensory Assessments
| Demographics at birth | N=216 | 100% | ||||
|---|---|---|---|---|---|---|
| Mean maternal age at birth and SD | 30.3 years | ± 6.3 years | ||||
| Infant gender | ||||||
| Female | 106 | 49.1% | ||||
| Male | 110 | 50.9% | ||||
| Preterm infants | 28 | 13.0% | ||||
| < 37 to ≥ 35 weeks | 18 | 8.3% | ||||
| < 35 weeks | 10 | 4.6% | ||||
| Small for gestational age | 10 | 4.6% | ||||
| Microcephaly | 8 | 3.7% | ||||
| Primary | 4 | 1.9% | ||||
| Secondary* | 2 | 0.9% | ||||
| Resolved** | 2 | 0.9% | ||||
| Interviews and Bayley III neurodevelopmental assessments at 7 – 32 months of age (n=216) V |
Normal (N) | % | Abnormal (N) | % | % | |
| 154 | 71.3% | 62 | 28.7% | |||
| Bayley-III assessments (N=146) | Between −1 SD and −2 SD (N) |
% | Below −2 SD (N) |
% | ||
| All (N=146) | 87 | 59.6% | 41 | 28.1% | 18 | 12.3% |
| Cognitive (N=146) | 132 | 90.4% | 6 | 4.1% | 8 | 5.5% |
| Language (N=146) | 95 | 65.1% | 34 | 23.3% | 17 | 11.6% |
| Motor (N=146) | 122 | 83.6% | 17 | 11.6% | 7 | 4.8% |
| Other Neurosensory assessments | ||||||
| Hearing (N=114) | 101 | 89% | 13 | 12% | ||
| Funduscopic eye exams (N=137) | 128 | 94% | 9 | 7% | ||
Two infants born with normal head circumference developed microcephaly in the first year of life.
One infant born with proportionate microcephaly developed normal head circumference over time and one infant born with cranial synostosis improved head circumference after surgery.
Neurodevelopmental interviews evaluated time of achievement of developmental stages by an adaptation of the Hammersmith Infant Neurological Examination (HINE) scheme, evaluating neurological exam, motor function and state of behavior in infants from 2 to 36 months. For Bayley-III, The “All” category depicts the lowest score observed in one of the 3 functional domains. One standard deviation (SD) below normal corresponds to a Bayley III composite score below 85; two standard deviations below normal corresponds to a Bayley III composite score below 70.
The present report focuses on 216 infants who had prospective follow-up. All had clinical and neurologic evaluations; 146 infants (67.6%) had Bayley-III assessments. Sixty-seven of 70 remaining 70 children (96%) had normal neurodevelopment questionnaires and neurological examinations (Hammersmith Infant Neurological Examination - HINE). All children were offered Bayley-III assessments. They were not performed if parents declined lengthier neurodevelopmental evaluations (i.e, parents did not wish to stay additional time in clinic or return another day for Bayley-III assessments). In that case neurodevelopment was evaluated through HINE/ neurodevelopmental questionnaires. The age range at the time of the last neurodevelopmental assessment was 7 to 32 months (median age 18 months). Only 5 children had Bayley-III assessments below12 months of age. All HINE assessments were performed in the second year of life.
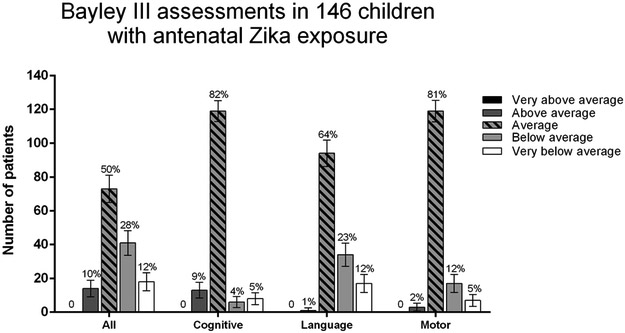
As seen in Table 1 and Figure 2, 71.3% of children (N=154) had normal neurodevelopmental assessments by Bayley-III or HINE evaluations. Fifty-nine of 146 children (40%) had Bayley-III results ≤ −1 SD in at least one of 3 functional domains. When children assessed through HINE and neurodevelopmental questionnaires were included, 62 of 216 antenatally ZIKV-exposed children (28.7%) scored below average in at least one assessment. Among 146 children with Bayley-III results, 28.1% had below average performance (−1SD to - 2SD), and 12.3% performed very below average (<−2SD) in either cognitive, and/or motor and/or language domains. Language development was most affected, with 34.9% of children showing Bayley-III results below −1 SD; 9.6% had delays in cognitive development and 16.4% in motor development. Bayley-III results had a skewed distribution towards lower scores; only 10% of children were above 1 SD in any domain; none had scores above 2 SD. In contrast, 30% of children had below average scores.
Figure 2: Bayley-III assessments in 146 children between the ages of 7 to 32 months.
Very above average: > 2 SD, score > 131; Above average: 1 to 2 SD, score 116 to 130; Average: −1 to 1 SD, score 85 to 115; Below average: −1 to – 2 SD, score 84 to 70; Very below average: < - 2 SD, score < 70.
Populational percentages are shown at the top of each bar.
Although 4.6% of infants were small for gestational age at birth, only 2 of 216 infants (1%) had failure to thrive on follow-up (Z-scores < −2). Eye exams were abnormal in 9 of 137 infants (7%) and included chorioretinal atrophy, macular hypoplasia, optic nerve hypoplasia, optic nerve pallor, pigment mottling and increased optic cup. Hearing deficits were noted in 13 of 114 infants (12%). Sixty-eight of 216 children (31.5%) had neurodevelopment below average in at least one domain, and/or eye and/or hearing abnormalities. (Figures 1, 2 and Table 1). Among 18 children with very below average performance (12%), 6 were microcephalic and 3 (2.1%) developed autism spectrum disorder (ASD). The 2 children with resolving microcephaly had average scores on Bayley-III assessments in the second year. Three children with ASD were normal initially and developed ASD in year 2.
Parameters associated with a Bayley-III score below −1 SD in 146 children included prematurity (OR: 5.41; 95%CI 1.66–20.56, p<0.01), and an abnormal funduscopic exam (OR: 20.35; 95%CI 3.02, 412.72). Female gender was a protective risk factor (OR: 0.36; 95%CI 0.16–0.78) against a below average Bayley-III score. (Supplemental Tables 1-2An abnormal funduscopic exam was associated with a Bayley-III score below −2 SD (OR: 7.92; 95%CI 1.24–48.89). Statistically significant associations with maternal age, education, mode of delivery, infant birth weight, and hearing function were not seen.
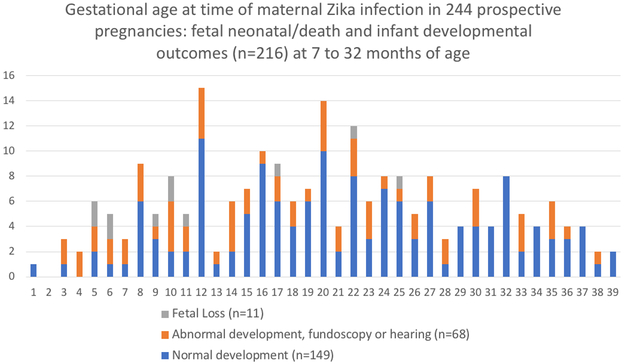
An earlier gestational age at the time of ZIKV infection was a significant predictor of below average neurodevelopment (Supplemental Table 2/Figure 3). Later gestational age at the time of ZIKV infection was less likely associated with below average neurodevelopment, fetal loss, and microcephaly (Supplemental Table 2). For each trimester ZIKV infection was delayed, risk for below average development decreased by 46% (OR=0.54, adjusted; 95%CI 0.34, 0.86). Eye and hearing abnormalities were not associated with gestational age of ZIKV infection. Fetal loss was noted in women infected as late as 25 weeks (Figure 3).
Figure 3: Gestational age at time of maternal Zika virus infection in 244 pregnancies.
Adverse pregnancy and infant outcomes by week of maternal ZIKV infection during gestation. Adverse pregnancy outcomes are fetal loss. Infant outcomes include below average/ abnormal neurodevelopment and/or abnormal eye and/or abnormal hearing assessments between 7 to 32 months of age.
Infants were followed since the time of maternal antenatal infection until the second and third years of life, allowing investigation of longer term neurodevelopmental outcomes than previously recorded. We noted 8 cases of MC (3.7%): 2 developed postnatally and 2 resolved over time, one spontaneously and the other following corrective surgery for craniosynostosis. Congenital ZIKV has been associated with early fusion of sutures3.
A concern following in utero ZIKV exposure was whether infants born without obvious structural brain abnormalities would have normal development2, 5-15. It appears that this is not a safe assumption. Nearly 1/3 of infants had findings impacting future development regardless of head size. A Bayley-III score below −2 SD (less than 70) in any functional domain is the accepted cut-off value for severe developmental delay16. A Bayley-III score between −1 to – 2 SD (70–84) is the cut-off value associated with risk of developmental delay17. In the general population, a normal distribution of neurodevelopmental scores would be expected, when using a standardized tool such as Bayley-III. We did not see a Bell shaped curve when we plotted results for ZIKV-in utero exposed children. Instead, there was a skewed distribution to the right; no children had scores above 2 SD from the norm, whereas 12% of children had scores below – 2 SD. A higher rate of below average performance on the Bayley-III language scale as compared to the cognitive scale was noted; this is consistent with studies performed in preterm and very low birth weight infants where the Bayley-III scale seriously underestimated developmental delay by age 2 years in one study18 and underdiagnosed developmental delay when compared to Bayley-II.19 If this is the case in our population, neurodevelopmental results could actually be worse than reported.
In a previous report comparing neuroimaging findings with neurodevelopmental performance in 94 children, we noted a significant association between normal results on brain imaging and higher Bayley-III scores4. Nevertheless, neuroimaging failed to predict severe developmental delay in 2% of children and normal development in 16%4. We surmise that non-structural or non-specific findings identified in brain imaging in the first month of life following in utero ZIKV exposure are not necessarily predictive of subsequent suboptimal development, nor are poor growth measures or non-specific early clinical findings. In our original report of 125 pregnancies, 42% of children had abnormal clinical and or imaging findings around the time of birth2. In the present analysis of 216 children evaluated as late as 32 months of age, 31.5% had below average performance in developmental assessments and/or abnormal neurosensorial function.
Interestingly, 24 of 49 children (49%) with abnormal findings in early infancy in our previous report 2 had normal assessments in their second or third years of life. This includes children with early functional abnormalities such as seizures and hypotonia, as well as non-specific neuroimaging findings in the neonatal period. Conversely, a normal exam at birth did not guarantee future normal development; 17 of 68 children (25%) with normal assessments in infancy in our earlier report 2 had below average neurodevelopmental assessments and/or abnormal hearing or ophthalmologic assessments on follow-up. This includes 3 children who developed ASD in the second year of life. Autism has been reported to have higher prevalence in congenital infections; ASD was as high as 5% in children with congenital rubella syndrome20 and ismore prevalent in children with congenital CMV. It has been postulated that neuroimmune modulation may play a role in the genesis of autism in infants exposed to congenital ZIKV infection21.
In our analyses attempting to identify potential predictors of abnormal development, we found significant associations with abnormal eye exams, prematurity, male gender and gestational age at infection. Female infants are known to have improved outcomes following serious perinatal infections as compared to males22. Women infected in later periods of pregnancy were less likely to have fetal loss, or have infants with structural brain defects, or below normal development. In a prior study we did not find associations between abnormal infant findings following in utero ZIKV exposure and maternal disease severity during pregnancy, but it is noteworthy to mention that all infants in our cohort were born to women with symptomatic ZIKV infection23. Since there is no evidence to suggest that children of women with asymptomatic ZIKV infection have better outcomes than those of symptomatic women, close follow-up of all antenatally ZIKV-exposed children is warranted.
The longitudinal nature of our unique infant cohort with RT-PCR-confirmed maternal ZIKV infection during pregnancy allows us to estimate the frequency of specific clinical features in ZIKV-exposed infants. The frequency of eye manifestations was 7% and hearing deficits 12%. Prior studies including our own 24-29 reported higher rates of eye findings in infants with congenital ZIKV infection. It is important to remember that some studies were not prospective in design and were subject to referral bias.
One of our study limitations is that parents of children who are healthy-appearing are often reluctant to consent to further evaluations. Zika virus can be a highly stigmatizing diagnosis. Thirty-two percent of the children in this study had neurologic/ developmental functions assessed through an adapted version of the Hammersmith Infant Neurological Examination scheme which included a detailed neurodevelopmental questionnaire in lieu of Bayley-III. Another study limitation is that we did not have a control group of children who underwent neurodevelopmental testing in parallel. Because ZIKV was highly epidemic in Rio de Janeiro, and because of the diagnostic challenges for detection of antenatal ZIKV exposure (asymptomatic/ unidentified maternal infection), we could not establish a control group in whom we could confidently demonstrate absence of antenatal ZIKV exposure. Nevertheless, since the ZIKV epidemic has subsided, there are not many cohort studies of children with PCR confirmed antenatal ZIKV exposure with extended longer term follow-up. Our data demonstrates there are subtle but nevertheless important neuro-repercussions of antenatal ZIKV exposure arising over time beyond severe structural brain defects and microcephaly noted at birth. These children need to be monitored far beyond early infancy. In our opinion, all children born to mothers with ZIKV infection during pregnancy should be followed longitudinally in the first 3 years of life by a multidisciplinary team with bi-annual neurodevelopmental, ophthalmologic and auditory evaluations even if early evaluations were normal. As Zika is a recently recognized congenital infection, it remains to be seen if future repercussions such as learning disabilities, further hearing loss or other problems can affect exposed children during school age years, so yearly assessments until age 7 years should be performed.
Our neurodevelopmental findings in children have been corroborated by animal studies, including a recent study by Zhao et al (personal communication) demonstrating abnormal neurologic functions in mice following ZIKV infection, including features of ASD. These mice had perturbations in social interaction and showed signs of depression, impaired learning and memory, in addition to severe motor defects. Similar to the children in our study, following infection with an Asian strain of ZIKV, mice demonstrated significant impairment of visual cortical function, circuit organization and experience-dependent plasticity. Long term outcomes may be influenced by more subtle inflammation-immune responses mediated by ZIKV, raising the possibility that early interventions could improve the neurodevelopmental trajectory of children exposed to ZIKV in utero, a possibility that requires further investigation.
Methods (Online)
Study Population
In this cohort study, pregnant women at any week of gestation who presented to the acute febrile illness clinic at the Fundação Oswaldo Cruz with a rash as previously described were enrolled 2. Infants from PCR confirmed30 ZIKV-positive mothers were followed prospectively.
Study Oversight
The study protocol was approved by the institutional review boards at Fundação Oswaldo Cruz (Fiocruz) and the University of California, Los Angeles. Participants provided written informed consent. The authors vouch for the accuracy and completeness of the data and the analyses and for the fidelity of the study to the protocol.
Infant Clinical Assessments
Anthropometric measures at birth were obtained for all live births and subsequent visits as previously described 2. Microcephaly (MC) at birth (primary) was defined as head circumference Z-score < −2 (moderate) and < −3 (severe). Secondary MC developed postnatally31. Detailed eye exams were performed by pediatric ophthalmologists as previously described24-26. Hearing assessments were performed through brainstem evoked response audiometry (BERA)2, 4.
Neurodevelopmental Assessments
All infants had detailed neurologic assessments with accompanying neurodevelopmental interviews performed concurrently. Bayley Scales of Infant and Toddler Development, third edition, Bayley-III32 were performed by trained personnel for 3 domains: cognitive, language (receptive and expressive) and motor (fine and gross). Bayley-III was chosen as the developmental tool validated cross-culturally in Brazil33. Reported results are for the latest Bayley-III assessment performed for each child, ranging from 7 to 32 months of age. A Bayley-III score within 1 standard deviation (SD) above or below the norm of 100 was considered normal for that specific domain. A score between 1 to 2 SD above the norm (116 to 130) was considered above average and 1 to 2 SD below the norm (< 85 to 70) moderately below average. A score above 2 SD from the norm (> 130) was considered very above average and 2SD below the norm very below average (< 70). The scoring system is consistent with previously published developmental studies16, 34- 37. In the absence of Bayley-III, time of achievement of developmental stages was investigated by an adaptation of the Hammersmith Infant Neurological Examination (HINE) scheme, to evaluate neurological exam, motor function and state of behavior in infants from 2 to 36 months38-40. Main psychomotor milestones were included in our interview. Gross motor skills included emergence of head control, onset of sitting free, standing and walking freely. Time of appearance of babbling, single words (for single words at least 5–10 words with a semantic role), responsive smiling and index finger pointing towards an object were obtained from parental interview and patient observation.
Statistical Analysis
A multivariate logistic regression was used to examine the associations between development (N=146) and medical predictors (prematurity, sex, size for gestional age, and cesarean delivery), clinical outcomes (ZIKV hearing and eye abnormalities), and demographic predictors (mothers’ age at delivery and education level). Associations between gestational age at infection and clinical outcomes, including hearing (N=114), neurodevelopment (N=216), fetal loss (N=233), mirocephaly (N=216), and fundoscopic evaluation (n=137), were explored by multivariate logistic regression. Covariates (including maternal age at delivery, prematurity, birth weight, cesarean delivery, mother’s education level, child’s gender, hearing abnormality, eye abnormality) were examined for colinearity, using chi-square for nominal covariates, and correlation analysis for two continuous variables. Potential confounding variables were individually examined for the association of both the outcome and the predictive factor then evaluated by stepwise regression analysis. Covariates that demonstrated an association with both the outcome and predictive variable, and changed the crude beta regression by 10% from the unadjusted analysis in at least one analysis were included in all models. These included maternal age at birth, child’s gender, prematurity, size for gestional age, C-section, mother’s educational level. When examining developmental delay as an outcome, analyses were further adjusted for hearing and eye abnormalities. Odds ratios, 95% confidence intervals, and 2-sided p-values were reported. Analyses were conducted using the statistical package R (R version 3.0.1, The R Foundation for Statistical Computing, www.r-project.org).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Acknowledgments:
This study was supported by the Departamento de Ciência e Tecnologia (DECIT/ 25000.072811/2016-17) do Ministério da Saúde do Brasil (P.B., M.E.M.); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/ 88887.116627/2016–01; Brazilian National Council for Scientific and Technological Development CNPq/441098/2016–9; CNPq307282/2017) (P.B., M.E.M.) ; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/ E_18/2015TXB (M.E.M.); FAPERJ/239224/E032018 CNE (M.E.M.); FAPERJ/ E_26/202.862/2018 CNE) (P.B.); Fondation Mérieux; ZikAlliance 734548 (P.B.); the Thrasher Research Fund (20164370) (K.N.S., K.A.); the Bill and Melinda gates Foundation Grand Challenges Explorations (OPP112887) (P.B.M.); the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health AI28697 (K.N.S.), AI1259534–01 (K.N.S., P.B., G.C.), AI140718–01 (K.N.S., P,B.), the National Eye Institute (NEI) of the National Institutes of Health AI129847–01 (K.N.S., I. T, P.B.) and the United Kingdom’s Department for International Development (M.E.M.) and the ZikaPlan (Preparedness Latin American Network) (M.E.M.).
We thank the women who enrolled in this study and the Fiocruz Zika Field Team who rendered our work possible.
Footnotes
Competing Interest Statement
The authors declare no competing interests.
Contributor Information
Karin Nielsen-Saines, David Geffen UCLA School of Medicine, Los Angeles
Patrícia Brasil, Fundação Oswaldo Cruz, Rio de Janeiro
Tara Kerin, David Geffen UCLA School of Medicine, Los Angeles
Zilton Vasconcelos, Fundação Oswaldo Cruz, Rio de Janeiro
Claudia Raja Gabaglia, Biomedical Research Institute of Southern California, Oceanside
Luana Damasceno, Fundação Oswaldo Cruz, Rio de Janeiro
Marcos Pone, Fundação Oswaldo Cruz, Rio de Janeiro
Liege M. Abreu de Carvalho, Fundação Oswaldo Cruz, Rio de Janeiro
Sheila M. Pone, Fundação Oswaldo Cruz, Rio de Janeiro
Andrea A. Zin, Fundação Oswaldo Cruz, Rio de Janeiro
Irena Tsui, David Geffen UCLA School of Medicine, Los Angeles
Tania Regina S. Salles, Fundação Oswaldo Cruz, Rio de Janeiro
Denise Cotrim da Cunha, Fundação Oswaldo Cruz, Rio de Janeiro
Roozemerie Pereira Costa, Fundação Oswaldo Cruz, Rio de Janeiro
Jociele Malacarne, Fundação Oswaldo Cruz, Rio de Janeiro
Ana Beatriz Reis, Fundação Oswaldo Cruz, Rio de Janeiro
Renata Hydee Hasue, Faculty of Medicine, University of São Paulo, São Paulo.
Carolina Y.P. Aizawa, Faculty of Medicine, University of São Paulo, São Paulo
Fernanda F. Genovesi, Faculty of Medicine, University of São Paulo, São Paulo
Christa Einspieler, Medical University of Graz, Graz
Peter B Marschik, Medical University of Graz, Graz, University Medical Center, Göttingen, Germany
José Paulo Pereira, Jr., Fundação Oswaldo Cruz, Rio de Janeiro
Stephanie L. Gaw, UCSF School of Medicine, San Francisco
Kristina Adachi, David Geffen UCLA School of Medicine, Los Angeles
James D. Cherry, David Geffen UCLA School of Medicine, Los Angeles
Zhiheng Xu, State Key Laboratory of Molecular Developmental Biology, CAS Center for Excellence in Brain Science and Intelligence Technology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences; Parkinson’s Disease Center, Beijing Institute for Brain Disorders, Beijing 100101, China
Genhong Cheng, David Geffen UCLA School of Medicine, Los Angeles
Maria Elisabeth Moreira, Fundação Oswaldo Cruz, Rio de Janeiro
References:
- 1.Brasil P et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro — Preliminary Report. N. Engl. J. Med. Epub 2016, March 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil P et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore CA et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 171, 288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes Moreira ME et al. Neurodevelopment in Infants Exposed to Zika Virus In Utero. N. Engl. J. Med. 379, 2377–2379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapogiannis BG, Chakhtoura N, Hazra R & Spong CY Bridging Knowledge Gaps to Understand How Zika Virus Exposure and Infection Affect Child Development. JAMA Pediatr. 171, 478 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Honein MA et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 317, 59–68 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Rice ME et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection - U.S. Territories and Freely Associated States, 2018. MMWR Morb. Mortal. Wkly. Rep 67, 858–867 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacheco O et al. Zika Virus Disease in Colombia - Preliminary Report. N. Engl. J. Med (2016). doi: 10.1056/NEJMoa1604037 [DOI] [PubMed] [Google Scholar]
- 9.Barcellos C et al. Increased Hospitalizations for Neuropathies as Indicators of Zika Virus Infection, according to Health Information System Data, Brazil. Emerg. Infect. Dis 22, 1894–1899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira ML et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin. Microbiol. Infect 24, 646–652 (2018). [DOI] [PubMed] [Google Scholar]
- 11.do Meneses JA. et al. Lessons Learned at the Epicenter of Brazil’s Congenital Zika Epidemic: Evidence From 87 Confirmed Cases. Clin. Infect. Dis 64, 1302–1308 (2017). [DOI] [PubMed] [Google Scholar]
- 12.de Melo ASO. et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 73, 1407–1416 (2016). [DOI] [PubMed] [Google Scholar]
- 13.van der Linden V et al. Description of 13 Infants Born During October 2015-January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth - Brazil. MMWR Morb. Mortal. Wkly. Rep 65, 1343–1348 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Reynolds MR et al. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb. Mortal. Wkly. Rep 66, 366–373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moura da Silva AA et al. Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg. Infect. Dis 22, 1953–1956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayley N COMPARISONS OF MENTAL AND MOTOR TEST SCORES FOR AGES 1–15 MONTHS BY SEX, BIRTH ORDER, RACE, GEOGRAPHICAL LOCATION, AND EDUCATION OF PARENTS. Child Dev 36, 379–411 (1965). [PubMed] [Google Scholar]
- 17.Ballot DE et al. Use of the Bayley Scales of Infant and Toddler Development, Third Edition, to Assess Developmental Outcome in Infants and Young Children in an Urban Setting in South Africa. Int Sch Res Notices 2017, 1631760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PJ et al. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med 164, 352–356 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Vohr BR et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr 161, 222–228.e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherry J, Demmler-Harrison GJ, Hotez PJ, Kaplan SL & Steinbach WJ Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. (Saunders WB, 2018). [Google Scholar]
- 21.Vianna P et al. Zika Virus as a Possible Risk Factor for Autism Spectrum Disorder: Neuroimmunological Aspects. Neuroimmunomodulation 25, 320–327 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Muenchhoff M & Goulder PJR Sex differences in pediatric infectious diseases. J. Infect. Dis. 209 Suppl 3, S120–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halai U-A et al. Maternal Zika Virus Disease Severity, Virus Load, Prior Dengue Antibodies, and Their Relationship to Birth Outcomes. Clin. Infect. Dis 65, 877–883 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zin AA et al. Screening Criteria for Ophthalmic Manifestations of Congenital Zika Virus Infection. JAMA Pediatr. 171, 847–854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsui I et al. Eye Findings in Infants With Suspected or Confirmed Antenatal Zika Virus Exposure. Pediatrics 142, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossetto JD et al. Visual function assessment in children with congenital Zika virus infection. J. AAPOS 22, e60 (2018). [Google Scholar]
- 27.Ventura CV et al. Risk Factors Associated With the Ophthalmoscopic Findings Identified in Infants With Presumed Zika Virus Congenital Infection. JAMA Ophthalmol. 134, 912–918 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Ventura CV et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq. Bras. Oftalmol 79, (2016). [DOI] [PubMed] [Google Scholar]
- 29.Yepez JB et al. Ophthalmic Manifestations of Congenital Zika Syndrome in Colombia and Venezuela. JAMA Ophthalmol. 135, 440–445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
References (Methods only)
- 30.Lanciotti RS et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 14, 1232–1239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von der Hagen M et al. Diagnostic approach to microcephaly in childhood: a two-center study and review of the literature. Dev. Med. Child Neurol. 56, 732–741 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Bayley N Bayley Scales of Infant and Toddler Development: Administration Manual. (2006). [Google Scholar]
- 33.Madaschi V, Mecca TP, Macedo EC & Paula CS Bayley-III Scales of Infant and Toddler Development: Transcultural Adaptation and Psychometric Properties. Paidéia (Ribeirão Preto) 26, 189–197 (2016). [Google Scholar]
- 34.Souza CT et al. Assessment of global motor performance and gross and fine motor skills of infants attending day care centers. Rev. Bras. Fisioter 14, 309–315 (2010). [PubMed] [Google Scholar]
- 35.da Cunha RDES, Lamy Filho F, Rafael EV, Lamy ZC & de Queiroz ALG Breast milk supplementation and preterm infant development after hospital discharge: a randomized clinical trial. J. Pediatr 92, 136–142 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Johnson S, Moore T & Marlow N Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr. Res 75, 670–674 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Jary S, Kmita G & Whitelaw A Differentiating developmental outcome between infants with severe disability in research studies: the role of Bayley Developmental Quotients. J. Pediatr 159, 211–4.e1 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Haataja L et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J. Pediatr 135, 153–161 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Romeo DMM et al. Neuromotor development in infants with cerebral palsy investigated by the Hammersmith Infant Neurological Examination during the first year of age. Eur. J. Paediatr. Neurol 12, 24–31 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Romeo DM, Ricci D, Brogna C & Mercuri E Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev. Med. Child Neurol 58, 240–245 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.