Abstract
The processes of attention and working memory are conspicuously interlinked, suggesting that they may involve overlapping neural mechanisms. Working memory (WM) is the ability to maintain information in the absence of sensory input. Attention is the process by which a specific target is selected for further processing, and neural resources directed toward that target. The content of WM can be used to direct attention, and attention can in turn determine which information is encoded into WM. Here we discuss the similarities between attention and WM and the role prefrontal cortex (PFC) plays in each. First, at the theoretical level, we describe how attention and WM can both rely on models based on attractor states. Then we review the evidence for an overlap between the areas involved in both functions, especially the frontal eye field (FEF) portion of the prefrontal cortex. We also discuss similarities between the neural changes in visual areas observed during attention and WM. At the cellular level, we review the literature on the role of prefrontal DA in both attention and WM at the behavioral and neural levels. Finally, we summarize the anatomical evidence for an overlap between prefrontal mechanisms involved in attention and WM. Altogether, a summary of pharmacological, electrophysiological, behavioral, and anatomical evidence for a contribution of the FEF part of prefrontal cortex to attention and WM is provided.
1. Models of Attention and WM
Numerous computational models of WM and attention exist and exploit diverse strategies to recreate the key features of neural activity during these states. One type of computational model often used for both WM and attention is conceptually defined by neural population “attractor states.” WM models primarily focus on the maintenance and evolution of these attractor states, while attention models have characterized how attractor states can be influenced by top-down activity.
Neurons can fire action potentials at specific rates and patterns, contingent on the stimulus. Given that neurons have limited range in firing rates and limited selectivity to stimuli, there is a finite set of firing “states” that neurons and populations of neurons can occupy. Factors such as recurrent inhibition and excitation cause some of these firing rate states to be more stable than others. Those relatively stable states “attract” the firing rate activity of individual neurons and populations, creating “attractor states.” When a stimulus is present, these attractor states can be thought of as neural representations of its qualities. In periods when the stimulus is no longer present, these attractor states represent a memory maintained in the system.
In WM networks, attractor states have been simulated in neural populations using stable firing rates (Compte et al. 2000), time-varying firing rates (Druckmann and Chklovskii 2012), and even “activity-silent” mechanisms that maintain representations synaptically (Goldman 2009). Neural populations can only support a finite number of attractor states at any one moment. These states can thus be thought of as competing with one another for representation (either in discrete slots or for shared resources), with some “winning,” and thus maintained through the delay in WM (Wang 2008).
Various models of attention can be conceived as ways to direct the competition of attractor states or align their transmission from one brain area to another. The sources of such signals are often thought to arise from prefrontal cortex and be directed to other brain areas, such as visual cortex. At the most basic, models of attention selectively increase the activity of an attractor state that represents an “attended” stimulus. That particular state then has a competitive bias relative to others (Ardid et al. 2007). One can also model more complicated experimental findings, such as normalization effects of attention, through a variety of mathematical frameworks (Carandini and Heeger 2012), including network models (Kraynyukova and Tchumatchenko 2018). The reduction in trial-to-trial variability seen with attention has been modeled as a reduction in excursion between various attractor states from one trial to the next, again caused by biased competition (Deco and Hugues 2012). This increased consistency caused by attention can also be applied to population oscillations, which has been used to model the communication of attractor content from one brain area to another (Deco and Kringelbach 2016).
Instantiations of these network models using spiking units or rate units allow one to capture patterns of response, the variability of that response from trial to trial, and more general oscillations in currents. Spiking networks additionally allow one to characterize spike timing and other discrete properties. These and other modeling tools are proving incredibly powerful for elucidating the underlying mechanisms of WM and attention.
2. FEF’s Role in Attention and WM
Psychophysical and electrophysiological studies indicate that the FEF is heavily involved in both covert visual attention and WM. The first evidence suggesting the FEF’s key role in high-level brain cognitive functions comes from its anatomical properties. Through massive reciprocal connections, this area is interconnected with many visual cortical and subcortical brain areas (Anderson et al. 2011; Markov et al. 2014b; Schall et al. 1995; Stanton et al. 1995). Some of these connected brain areas have been suggested as sources of visual attention, including adjacent prefrontal areas (Stanton et al. 1993), lateral intraparietal cortex (LIP) (Anderson et al. 2011; Stanton et al. 1995), and the superior colliculus (SC) (Sommer and Wurtz 2000).
Originally, the FEF was identified as an area involved in making saccadic eye movements (Robinson and Fuchs 1969). Electrical stimulation of a FEF site with microampere-magnitude currents (microstimulation) results in a fixed vector saccade to a specific part of space which is considered as the response field (RF) of that site (Bruce and Goldberg 1985). Neurons in the FEF show diverse responses including any combination of visual, memory-related, or saccade-related activity (Bruce and Goldberg 1985; Lawrence et al. 2005). During the last couple of decades, several studies have examined the role of the FEF in covert attention and WM. Psychophysical studies revealing a tight link between attention and saccadic eye movements (Deubel and Schneider 1996) initially suggested the FEF as a source of visual attention. In the first study showing the causal role of the FEF in the control of visual attention, it was shown that the microstimulation of FEF sites, with the currents less than what is needed to produce a saccadic eye movement (subthreshold microstimulation), improves the animal’s performance in detecting a contrast change for stimuli presented within the area of space represented by the stimulated FEF site (Moore and Fallah 2001, 2004). Thus, stimulating the FEF results in the behavioral benefits of visual attention. Another study showed the link between FEF activation and the neuronal signatures of visual attention: subthreshold microstimulation of FEF is accompanied by an increase in firing rate and selectivity of V4 visual neurons with RFs overlapping with those of the stimulated FEF site (Moore and Armstrong 2003). Neurons in FEF also encode the locus of visual attention (Armstrong et al. 2009). Interestingly, the same neurons in the FEF that carry memory signals during the delay period are more likely to reflect target selection during the attention portion of a task (Armstrong et al. 2009), which will be discussed further in the context of identifying the FEF signal sent to visual areas. FEF inactivation profoundly impairs behavioral performance in both spatial WM (Noudoost et al. 2014) and attention-dependent tasks such as covert visual search and visual discrimination tasks (Wardak et al. 2006). In sum, the FEF portion of PFC appears to play a crucial role in both attention and WM.
3. Signatures of Attention and How WM-Induced Changes Resemble Attention
Similarities between the neural signatures of attention and WM maintenance within sensory areas further support the theory that these two processes have overlapping mechanisms within prefrontal cortex.
The neural signatures of attention at the level of individual neurons include an increase in response magnitude (Green and Swets 1966), reduced neuronal response latency (Galashan et al. 2013; Sundberg et al. 2012; Lee et al. 2005), a shift of RFs toward the locus of attention and shrinking RFs at the attended location (Anton-Erxleben et al. 2009; Connor et al. 1996, 1997; Kusunoki and Goldberg 2003; Womelsdorf et al. 2006; Suzuki and Cavanagh 1997; Anton-Erxleben & Carrasco 2013), reduced burstiness (Anderson et al. 2013), and reduced variability of visual responses (Mitchell et al. 2007). Each of these signatures can contribute to increasing the signal-to-noise ratio and thus lead to better discrimination and perception (Cohen and Maunsell 2009; Ling et al. 2009; Mitchell et al. 2009) or a faster reaction (Albrecht 1995; Bell et al. 2006; Oram et al. 2002; Raiguel et al. 1999). Attention also affects the correlated noise of simultaneously recorded pairs of neurons (Cohen and Kohn 2011; Cohen and Maunsell 2009; Ruff and Cohen 2014). The shared trial-to-trial fluctuations in firing rates in response to the same stimulus are often called the noise correlation. Attention can reduce the noise correlation in V4; interestingly, this reduction in noise correlation enhances the signal-to-noise ratio of the pooled neural activity more than the changes in firing rates do (Mitchell et al. 2009). A study by Cohen and Maunsell reported that over 80% of the attentional-induced enhancement in V4 population sensitivity was due to the reduced noise correlations between the firing rates of neurons (Cohen and Maunsell 2009).
WM plays an essential role in our daily life, determining how we interact with the world based on our current goals (D’Esposito and Postle 2015). WM enhances the processing of information related to the items held in WM, in part by altering the processing of sensory information (Awh and Jonides 2001; Postle 2005). Finding the neural basis by which WM engages sensory areas and enhances sensory representations in these areas is crucial to understanding the mechanisms underlying goal-directed behavior. Our recent study revealed that sensory areas receive a WM-rich signal from the FEF part of the prefrontal cortex during a memory-guided saccade (MGS) task (Fig. 1a) (Merrikhi et al. 2017). This evidence, together with studies demonstrating the FEF’s role in attention (reviewed in Clark et al. 2014), supports the idea that one important purpose of persistent signals sent from the FEF is to change neural processing within sensory areas (Gazzaley and Nobre 2012; Postle 2006).
Fig. 1.
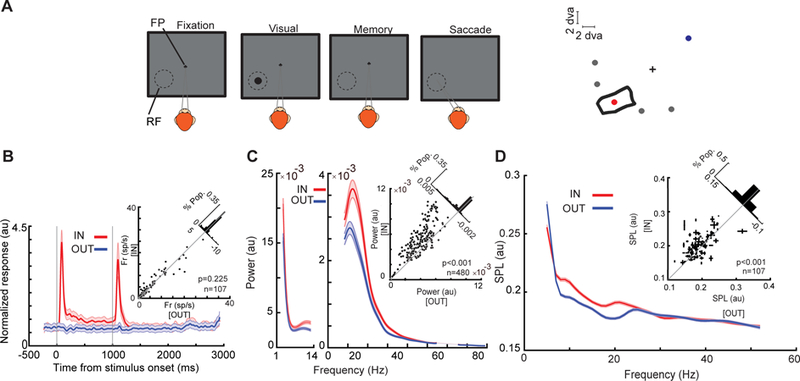
Changes in MT oscillatory power and spike timing during WM. (a) Schematic of the MGS task. The monkey fixates on a central fixation point (FP), and a cue stimulus appears in one of six positions arranged around the neuron’s RF location (right). The cue stimulus disappears, and the monkey maintains fixation throughout a blank delay period. Following the disappearance of the fixation point, the monkey saccades to the remembered location to receive a reward. (b) Firing rate of MT neurons does not change based on WM location. The bottom plot shows the normalized firing rate of 107 MT neurons across the course of the MGS task, when the memorized location is inside (IN, red) and outside (OUT, blue) of the neurons’ RFs. The upper-right scatter plot shows raw firing rates during the last 500 ms of the memory period, and diagonal histogram shows the distribution of firing rate changes. (c) αβ LFP power reflects WM location. The average LFP power spectrum during the memory period across frequencies (n = 480 LFP recordings), for memory IN (red) and memory OUT (blue) condition. The scatter plot shows αβ power (8–25 Hz) during memory IN vs. OUT. The diagonal histogram shows the distribution of differences in αβ power for all LFPs. (d) αβ SPL reflects WM location. The SPL for memory IN (red) and memory OUT (blue), across frequencies for all pairs of neurons and simultaneously recorded LFPs (n = 1,605 neuron-LFP pairs). Inset scatter plot shows the SPL at αβ for memory IN compared to memory OUT, with the SPL values for multiple simultaneously recorded LFPs averaged for each neuron (n = 107 neurons). Shading and error bars show standard error. Adapted from Bahmani et al. (2018)
Surprisingly, despite the WM-rich signal sent to extrastriate visual areas, there is no change in the firing rate of these neurons during the memory period of WM tasks (Fig. 1b) (Bahmani et al. 2018; Lee et al. 2005; Mendoza-Halliday et al. 2014; Zaksas and Pasternak 2006). The behavioral impact of the content of WM on perception in psychological studies (Awh and Jonides 2001), despite the lack of firing rate modulation, suggests that the WM signal sent to visual areas may be exerting a subthreshold effect on neurons in these areas, which in turn renders them more sensitive to incoming visual signals. Oscillatory power can provide a signature of such subthreshold effects. Indeed, the content of WM reflected is reflected in the αβ LFP power in area MT (Fig. 1c) (Bahmani et al. 2018). Moreover, this signature was correlated with memory performance: monkeys had more precise and accurate saccadic responses in trials with higher values of αβ power (Bahmani et al. 2018). This change also affected the temporal pattern of firing in MT neurons: spikes were locked to the phase of αβ ongoing oscillations during the memory period (Fig. 1d). Furthermore, in the presence of a bottom-up sensory signal, evoked visual responses were enhanced (Fig. 2a). WM improved the ability of neurons in visual areas, including V4 and MT, to distinguish between stimuli presented near the memorized location by shifting and expanding their RFs toward the location held in WM (Fig. 2b) (Merrikhi et al. 2017). WM was also able to enhance the discriminability of stimuli based on oscillatory patterns of spikes in the αβ frequency range (Fig. 2c). In fact, when visual information was presented during the delay period of the MGS task, the gain and discriminability of spiking activity increased in a αβ-phase-dependent manner: the enhancement of gain and discriminability was more for the spikes acquired at the preferred phase of ongoing αβ oscillations (Bahmani et al. 2018). Quantitatively, changes in spike timing had a greater effect on information encoding during memory than changes in firing rate (Bahmani et al. 2018). Altogether, these findings suggest that changes in spike timing in visual areas, driven by a WM signal sent from the prefrontal cortex, could form the basis for enhanced sensory representations and the accompanying benefits for visual perception.
Fig. 2.
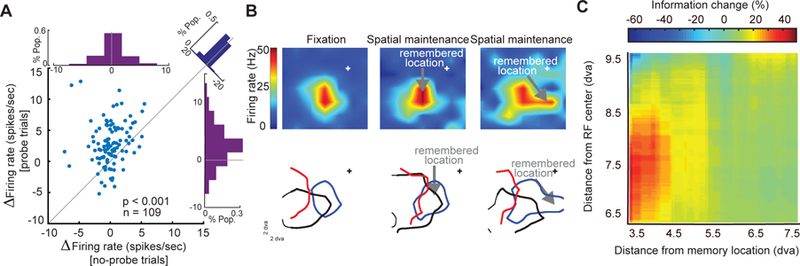
Changes in MT visual sensitivity during WM. (a) During the memory period, visually evoked activity increased, but delay activity in the absence of probes was unchanged. A revised version of MGS task with probe presentation was used; visual probes appeared on 91% of trials, during both the fixation and delay periods of the MGS task. Scatter plot of WM-induced changes in the visually evoked spiking activity (probe trials) against WM-induced changes in the delay period activity (no-probe trials). Top histogram indicates the WM-induced change in delay period activity; right histogram indicates the WM-induced change in visually evoked activity. Diagonal histogram illustrates the difference between the effects of WM on firing rates in the presence and absence of visual probes. (b) RFs shift toward the WM location. Heat map showing the RF of an example MT neuron during fixation (at cross); RF of the same neuron measured while the monkey remembered a location inside of the RF, indicated by the arrow; and RF of the same neuron while the monkey remembered a location to the right of the fixation RF. Lower plots show the RF outlines of three simultaneously recorded MT neurons during fixation (left) and the delay period when the monkey remembered different locations (right). The blue outline is the RF of the neuron shown in upper plot. (c) Visual information encoded in spike phases increases near the memory location. The increase in mutual information during WM depends on the distance between the probes and the RF center or memory location. Color scale shows the change in mutual information (memory – fixation) between the spikes’ phases (αβ) and probe location for pairs of probes. The change is plotted as a function of the probes’ distance from the RF center (y-axis) and distance from the memory location (x-axis). The geometric mean of the two probe positions was used to calculate distances. Adapted from Bahmani et al. (2018) and Merrikhi et al. (2017)
4. Prefrontal Dopamine, a Common Modulator of Attention and WM
Dopamine signaling within PFC has been shown to play a role both in WM activity and behavior, and in generating attention-like modulations of visual signals elsewhere in the brain.
Iontophoretic application of dopaminergic drugs can increase persistent activity within PFC during a spatial WM task (Williams and Goldman-Rakic 1995). This dopamine-modulated change in persistent activity displays an “inverted-U” dose dependency, with the greatest persistent activity at an intermediate level of dopamine signaling (Vijayraghavan et al. 2007). Shifts in the baseline dopaminergic tone within PFC may account for changes in WM observed due to stress or aging (Arnsten et al. 1994, 1995; Arnsten 2000; Gamo and Arnsten 2011). Further work has shown that dopamine alters the probability of glutamate release within PFC (Gao et al. 2001) and increases the coincident firing of pyramidal PFC neurons, consistent with an increase in the synaptic reliability of their inputs (Castner and Williams 2007). More recent work suggests that the effect of prefrontal dopamine on excitatory and inhibitory neurons may differ (Jacob et al. 2013), reducing the activity of inhibitory neurons while increasing the activity and reliability of excitatory neurons.
Different classes of dopamine receptors within PFC have different effects on PFC activity and behavior. Dopamine receptors are generally divided into two classes: D1-like receptors (D1Rs) and D2-like receptors (D2Rs) (Missale et al. 1998; Seamans and Yang 2004); D1Rs are expressed in both superficial and deep cortical layers, but D2R expression is restricted to the deep layers (Lidow et al. 1991). (Expression of dopamine receptors within PFC will be discussed in greater detail in a later section.) While D1R manipulation was found to enhance persistent activity within PFC during a spatial WM task (Williams and Goldman-Rakic 1995), as discussed above, D2R manipulation instead modulates motor-related activity, without altering persistent activity (Wang et al. 2004). Similarly, during a rule-based oculomotor task, D1Rs affected PFC neuronal tuning for rule and memory, while D2Rs only altered motor signals (Vijayraghavan et al. 2016). D1Rs and D2Rs also have different effects on oscillations within PFC, with power in the beta and gamma bands sensitive to D1R and D2R signaling, respectively (Ott et al. 2018); both receptor types altered alpha and theta power. Behaviorally, both D1R and D2R manipulation can bias the animal toward saccading to the retinotopic location corresponding to the infusion site; however, they have differential effects on the effect of experienced reward on subsequent choices (Soltani et al. 2013). It should also be noted that those effects which are similar for the two receptor types are usually obtained using a D1R antagonist and a D2R agonist (Ott et al. 2018; Soltani et al. 2013). Thus, dopamine signaling via D1Rs vs. D2Rs produces distinct and contrasting effects on prefrontal neurophysiology and behavior.
The effects of dopamine signaling within PFC are not limited to the activity of prefrontal neurons. Noudoost and colleagues have shown that pharmacologically manipulating dopamine signaling within the FEF can alter sensory responses in extrastriate visual cortex (Noudoost and Moore 2011b). Local pharmacological infusions into the FEF produced localized biases in saccade target selection in a free-choice task, causing an increased tendency to choose the portion of space represented by neurons near the infusion site (which can be estimated using electrical stimulation, see Fig. 3a). Infusing a D1R antagonist (SCH23390) into the FEF enhances visual responses at a retinotopically corresponding location of the V4 representation. This enhanced visual response included greater peak firing rates, greater reliability, and greater feature selectivity (Fig. 3b, c). The effects observed in V4 following FEF D1R manipulation resemble the reported signatures of spatial attention (reviewed in Clark et al. 2015; Noudoost et al. 2010; Squire et al. 2013). A D2R agonist, in contrast, produced a bias in saccadic target selection but no change in visual responses. Inactivating FEF with a GABA agonist (muscimol) resulted in a decrease in the selectivity of V4 visual responses, consistent with a net excitatory effect of a D1R antagonist within PFC. In combination with the previously discussed iontophoresis experiments showing that D1R but not D2R signaling enhances persistent activity within PFC, these results are consistent with the hypothesis that persistent activity in FEF is a key mediator of the attentional modulation of extrastriate visual responses.
Fig. 3.
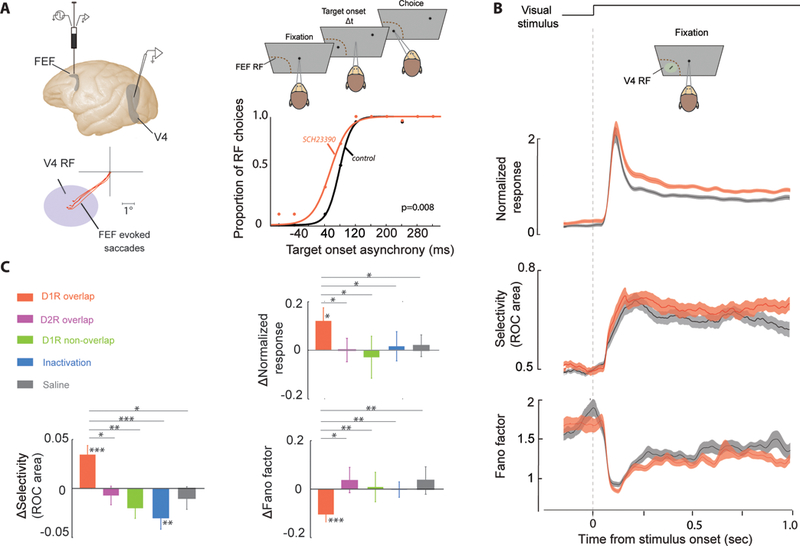
PFC D1R manipulation enhances visual responses in V4. (a) A micro-injectrode (Noudoost and Moore 2011a) was used to deliver a small volume of D1R antagonist into the FEF. Electrical microstimulation of the FEF prior to pharmacological infusion allowed estimation of the affected area of space based on the endpoints of electrically evoked saccades (red traces). D1R antagonist infusion biased the animal’s saccades toward the RF location in a two target free-choice saccade task (right). (b) Visual responses were recorded from V4 neurons during a passive fixation task, both before and after FEF D1R manipulation (gray and red traces, respectively). Results are shown for an example V4 neuron. Following FEF manipulation, normalized responses were greater (top), orientation selectivity increased (middle), and variability decreased (bottom). (c) Summary of effects of FEF D1R manipulation for the population of V4 neurons (n = 37). In the “overlap” condition, V4 RFs corresponded with the endpoints of electrically evoked saccades. Bar graphs to the right show the change in V4 orientation selectivity, normalized response, and variability (Fano Factor) after FEF D1R manipulation compared to baseline (orange). V4 visual response magnitude and selectivity increased, while cross-trial variability decreased, following FEF D1R manipulation. Infusing GABA agonist muscimol into the FEF reduced the selectivity of V4 responses, without altering overall firing rate or variability (blue). No changes in V4 activity, selectivity, or variability were observed when the D1R manipulation occurred at an FEF site not overlapping with the V4 RFs (green) or when a D2R agonist (magenta) or saline (gray) was infused at an overlapping FEF site. In all cases D1R effects were significantly different from all other conditions. Single, double, and triple asterisks denote significance at p < 0.05, p < 0.01, and p < 0.001, respectively. Adapted from Noudoost and Moore (2011b)
5. DA Imbalance and Attention-WM Impairments
The coincident impairment of WM and attention in many neurological disorders associated with disruptions in dopamine signaling also suggests that both processes rely on this pathway for normal function. Here we review findings on the effect of dopamine-related disorders on attention and WM task performance, focusing on Parkinson’s disease (PD).
Visual processing and cognitive functions are considerably altered in populations with dopamine dysfunction such as PD, schizophrenia, and attention deficit hyperactivity disorder (ADHD) (Barkley 1997; Dakin et al. 2005; Gurvich et al. 2007; Karatekin and Asarnow 1998; Seymour et al. 2013). WM and tasks requiring top-down control are remarkably dependent on the integrity of dopamine function in prefrontal cortex (Cools et al. 2002; Miller et al. 1996; Moustafa et al. 2008; Williams and Goldman-Rakic 1995). In PD patients, dopamine dysfunction leads to disruption of frontal cortical networks and explains performance deficits (i.e., slower reaction time and higher error rates) often found in cognitive tasks that involve central planning, WM, motor learning, conflict resolution, and prior information about stimulus uncertainty (Brown et al. 1993; Brown and Marsden 1988; Ghilardi et al. 2003; Herz et al. 2016; Jahanshahi and Frith 1998; Perugini et al. 2016; Stern et al. 1983). Furthermore, visuo-motor abnormalities and visual sensory disturbances in PD have been attributed to dopamine deficiency in striatal circuits of the basal ganglia and the retina (Jankovic 2008; Patel et al. 2014; Rodnitzky 1998). Contrast sensitivity tests in PD patients show a significantly elevated threshold for stimulus detection compared to healthy controls (Bodis-Wollner 1990; Bodis-Wollner et al. 1987; Kupersmith et al. 1982).
The challenge of isolating “attentional deficits” from perceptual and executive control deficits in PD was originally addressed by increasing levels of complexity in choice reaction time tasks, in which subjects were instructed to respond according to stimulus features (i.e., color, shape or tone, or the combination of all three) (Cooper et al. 1994). Longer choice reaction time in PD patients suggested impaired central processing. Moreover, PD patients being slower in tests of WM, such as the London tower task, suggested an increased thinking time rather than deficits in the conception of logical steps toward a solution, given that strategies were comparable with control subjects (Morris et al. 1988). This was also proposed to be an issue with attention switching (Morris et al. 1988). The fact that PD patients exhibited WM deficits and difficulty with internal deployment of “top-down” mechanisms raised the question of whether exogenously driven attention was equally impaired. In a simple cued and non-cued Stroop task (i.e., word and ink cues), patients performed better when a cue relevant to the stimulus attribute was presented before each trial (Brown and Marsden 1988). Further studies confirmed that behavioral performance in PD is highly task dependent, with longer reaction times and more errors when tasks rely on internal processing and relatively normal reaction times when external cues are provided (Jahanshahi et al. 1995; Siegert et al. 2002). This benefit of external cues on task performance offers further insight on the ways that sensory stimuli can facilitate movement initiation, hypothetically explained by the pathophysiology of PD (Praamstra et al. 1998).
Several studies have aimed to establish a relationship between task performance and dopaminergic therapy or its withdrawal, using tests that are sensitive to frontal lobe function (Gurvich et al. 2007; Lange et al. 1992, 1995; Moustafa et al. 2008). Treatment with levodopa, a dopamine precursor, helps alleviate the motor symptoms of PD (Cotzias et al. 1969; Yahr et al. 1969) and has been associated with increased blood flow in PFC (Roshan Cools et al. 2002). Withdrawal of levodopa caused performance impairments in tests such as spatial memory and the tower of London (Lange et al. 1992). Furthermore, levodopa withdrawal could either impair or enhance performance on cognitive tasks, depending on the basal levels of dopamine and the nature of the task, suggesting a more complex link (Cools and D’Esposito 2011). An inverted U-shaped action of dopamine could explain the various effects of dopamine signaling on cognitive control (Cools and D’Esposito 2011; Cools et al. 2001, 2010). These observations support the inverted U-shaped findings in animal studies, although on a behavioral rather than neural level (Vijayraghavan et al. 2007).
Relevant studies in PD, schizophrenia, and ADHD are described with more detail in Table 1.
Table 1).
Studies of attention and WM performance in PD, schizophrenia, and ADHD.
| Author | Task | Disease, treatments | Performance change |
|---|---|---|---|
| (Mathis, Wynn, Breitmeyer, Nuechterlein, & Green, 2011) | Attentional blink | Schizophrenia | Patients show an exaggerated attentional blink effect compared to healthy controls, with significantly lower detection of the second target. Results suggest deficits in both visual processing and general attentional deficits. |
| (Javitt, Doneshka, Grochowski, & Ritter, 1995) | WM, auditory oddball task | Schizophrenia | Impaired mismatch negativity, increased reaction time and decreased number of correct detections |
| (Stablein et al., 2016) | WM, visual motion task | Schizophrenia | Decreased task accuracy, attributed to visual deficits and impaired attention in patients |
| (Clementz, Wang, & Keil, 2008) | Target identification sustained attention | Schizophrenia | Abnormal target identification in patients, attributed to visual deficits in schizophrenia |
| (Fuller et al., 2006) | Visual search tasks, four types | Schizophrenia | Rates of search were slower in patients, especially when the task required precise attentional control. Results suggest primary impairment of attention in schizophrenia |
| (Slagter et al., 2016) | Attentional blink | Parkinson’s disease on and off dopaminergic therapy (levodopa and agonists). | Patients exhibited poorer performance than healthy controls. An optimum level of dopamine for cognitive function is suggested |
| (R Cools & D’Esposito, 2011) | Review, tower of London, WM task, attentional set-shifting | Parkinsons’ disease on and off medication | PD patients had deficits on the Tower of London planning task, WM and attentional set-shifting tests |
| (Rodriguez-Oroz et al., 2009) | Review of tasks in which shifting of attention is impaired in PD | Parkinson’s disease | Issues when switching attention, and double task demands |
| (Bulens, Meerwaldt, Vanderwildt, & Vandeursen, 1987) (Hutton, Morris, & Elias, 1993) |
Contrast sensitivity test | Parkinson’s disease on and off levodopa treatment | Loss of contrast sensitivity in patients. Levodopa improves contrast sensitivity function, close to normal levels |
| (Lange et al., 1992) | Tower of London and spatial WM task | Parkinson’s disease; on and off L-dopa medication | L-dopa withdrawal dramatically impaired performance in the tower of London test, with times slower than baseline condition (on L-dopa). Increase of errors in the spatial WM task during L-dopa withdrawal |
| (Moustafa et al., 2008) | WM task, continuous performance AX-CPT | Parkinson’s disease; on and off dopaminergic medications (L-dopa and agonists) | PD patients showed deficits for ignoring distractors and attentional shifting |
| (Kempton et al., 1999) | Cambridge Neuropsychological Test Automated Battery (CANTAB) | ADHD; stimulant methylphenidate, D-amphetamine | Medicated ADHD children did not show impairment in most of the executive function tasks, except the spatial recognition memory task. These results suggested that stimulant medication improved executive function performance |
| (Karatekin & Asarnow, 1998) | Dot test of visuospatial WM and digit span subtest of the Wechsler intelligence scale (digit recall) | Childhood onset schizophrenia and ADHD | Normal children were able to recall more digits than children with schizophrenia and ADHD. Both schizophrenic and ADHD children had greater distance error on the dot test. |
6. Anatomical Basis of Prefrontal Dopamine’s Contribution to Attention and WM
Anatomical evidence, including patterns of receptor expression and the specific properties of neurons projecting from the PFC, also suggests a unique and important role for dopamine in the prefrontal networks that modulate sensory processing.
FEF neurons exhibit a mixture of visual, motor, and memory-related activity in varying proportions (Bruce and Goldberg 1985; Lawrence et al. 2005; Sommer and Wurtz 2000, 2001; Umeno and Goldberg 2001). FEF neurons also project to multiple cortical and sub-cortical areas (Leichnetz 1982; Markov et al. 2014a; Schnyder et al. 1985; Sommer and Wurtz 2000; Stanton et al. 1995). In order to determine which kinds of FEF activity are sent via these projections, projecting neurons must be identified, e.g., via antidromic stimulation in the target area (Ferraina et al. 2002; Sommer and Wurtz 2000), and their response characteristics assessed. For example, a combination of visual, motor, and delay activity is sent from the FEF to the SC (Sommer and Wurtz 2000, 2001). Recently, the content of FEF’s projection to extrastriate visual cortex was examined using antidromic stimulation (Merrikhi et al. 2017), revealing that memory-related, delay period activity is the defining feature of FEF neurons projecting to extrastriate visual cortex. This memory activity was significantly stronger in the FEF projection to V4 than in the FEF population as a whole (Fig. 4). Motor activity, in contrast, was present in a much smaller fraction of V4-projecting FEF neurons than in the FEF population. Since modulation of FEF activity has been shown to modulate V4 activity in ways that mimic the effects of selective attention (Moore and Armstrong 2003; Noudoost and Moore 2011b), and we know now that the direct projection to V4 contains primarily memory-related activity (Merrikhi et al. 2017), this suggests that neurons exhibiting delay-period activity could be a source of attentional modulation in extrastriate cortex. In fact, the same neurons in FEF that display persistent memory-related activity are more likely to show attentional modulation (Armstrong et al. 2009) – suggesting that the same memory-selective FEF neurons which project to visual areas drive both the modulations of these areas during WM (see Fig. 3) and also attentional modulation of incoming visual signals. Consistent with this is the fact that D1Rs in PFC selectively modulate persistent memory-related activity there (Williams and Goldman-Rakic 1995) and also modulate activity in visual areas (Noudoost and Moore 2011b). In sum, dopamine’s modulation of persistent prefrontal activity (Williams and Goldman-Rakic 1995), which we know to be strongly represented in FEF’s projections to visual areas (Merrikhi et al. 2017), drives attention-like modulations of visual cortex (Noudoost and Moore 2011b).
Fig. 4.
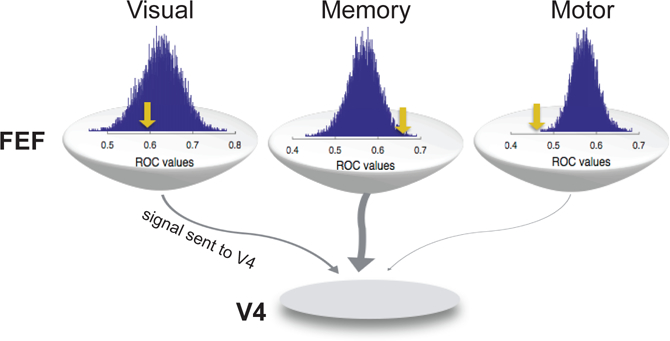
Comparison of visual, memory, and motor activity in V4-projecting FEF neurons and the FEF population as a whole. Visual, memory, and motor selectivity were assessed using a memory-guided saccade task in which the cue appeared inside or opposite the RF of the FEF neurons being recorded (see Fig. 1a). Histograms show the distribution of average visual, memory, and motor selectivity for 1,000 ensembles of 15 FEF neurons chosen at random from the population (n = 307 non-projecting FEF neurons). Yellow arrow shows the mean selectivity for the V4-projecting FEF neurons (n = 15). Selectivity was measured based on the ROC value for trials in which the cue appeared inside vs. outside the FEF RF (during the visual, delay, or motor epochs of the task). Memory selectivity was significantly stronger in the V4-projecting FEF population, and motor selectivity was significantly weaker, compared to the non-projecting FEF population. Modified from (Merrikhi et al. 2017)
Examinations of patterns of dopamine receptor expression suggest a specialized role for dopamine in prefrontal processing. Dopamine receptors are enriched in the prefrontal cortex compared to more caudal regions in both macaque (Lidow et al. 1991) and rodent (Gaspar et al. 1995). Dopamine terminals converge onto pyramidal neurons and a class of fast-spiking interneurons (Sesack et al. 1995; Verney et al. 1990). However, the contacts are more often made onto pyramidal neurons than interneurons (Goldman-Rakic et al. 1989; Sesack et al. 1995; Smiley and Goldman-Rakic 1993; Smiley et al. 1992). Goldman-Rakic and colleagues (Bergson et al. 1995; Smiley and Goldman-Rakic 1993) found that D1Rs were most prominent on pyramidal neurons in macaque dlPFC. Mueller et al. (2018) examined the expression of D5Rs (which are part of the D1 family of receptors) on different classes of neurons in the macaque PFC and found that D5Rs are more prevalent on pyramidal neurons than inhibitory interneurons and are especially prevalent on anatomically defined putative long-range projection neurons. These findings suggest that dopamine will exert a strong influence on the majority of pyramidal neurons through the D1 family of dopamine receptors.
The expression of dopamine receptors also varies across cortical layers. Previous in situ results have shown that within the macaque PFC, mRNA encoding all dopamine receptor subtypes was expressed most strongly in layer V; however, this was not the case for all cortical regions (Lidow 1998). Others (Bergson et al. 1995; Smiley and Goldman-Rakic 1993) found that D1R staining was most prominent in layers II–III and V. Goldman-Rakic and colleagues (Goldman-Rakic et al. 1990; Smiley et al. 1994) used autoradiography and immunofluorescence to show comparatively higher expression of D1Rs in layers I–II, medium expression in layers V and VI, and lower expression in layers IIIb and IV of dlPFC. They found D2R expression was strongest in layer V. Collectively these results suggest dopamine is likely to influence pyramidal neurons through D1 and D5 dopamine receptors in the macaque PFC, particularly in layer II–III and V. Because D1R activation potentiates NMDA receptor-mediated increases in excitability (Gonzalez-Islas and Hablitz 2003; Tseng and O’Donnell 2004), activation of D1Rs on recurrently connected pyramidal neurons could directly influence the increase in sustained activity in attention and WM.
Some interneuron classes in primate dlPFC differentially express D1Rs in their processes (Glausier et al. 2009). However, only very few studies have examined the expression of D1Rs on different neuronal populations in the macaque PFC. Muly et al. (1998) examined the distribution of D1Rs across different interneuron cell types across PFC layers. They found no variation in expression of D1R+/calretinin+ neurons but did for D1R+/calbindin+ and D1R+/parvalbumin+ neurons: there were fewer D1R+/parvalbumin+ neurons in superficial layers (I and II) than other layers, and D1R+/calbindin+ expression peaked in layer III (Muly et al. 1998). This suggests that the expression of D1Rs may also be laminarly specific to facilitate differential processing of sustained signals (WM- or attention-related) or motor signals.
There are also many differences in dopamine neuroanatomy between different species (Fig. 5). In contrast to dopamine receptor expression in the macaque, in the mouse PFC, it was shown that D1R+ neurons are mainly in deep layers and D2R+ neurons are mainly in superficial neurons (Wei et al. 2018). In the rat several studies show D1Rs and D2Rs are both mainly found in deeper layers (Berger et al. 1988, 1991; Davidoff and Benes 1998; Descarries et al. 1987; Gaspar et al. 1995; van Eden et al. 1987). Again in contrast to the macaque, immunological studies in rat mPFC showed that the density of D1R+ neurons was almost one third lower in layers II–III than layers V–VI and also that there was a higher density of D2R+ neurons than D1R+ neurons in layers II–III (Vincent et al. 1993). Further in situ of different classes of neurons in the rat mPFC found that D1R expression was lower on pyramidal neurons compared to inhibitory neurons (11–21% vs 25–52%) (Santana and Artigas 2017) – again a stark contrast to dopamine receptor expression in the macaque. Combined, these very divergent results suggest a different role of neuromodulators in PFC-related cognitive functions across different species.
Fig. 5.
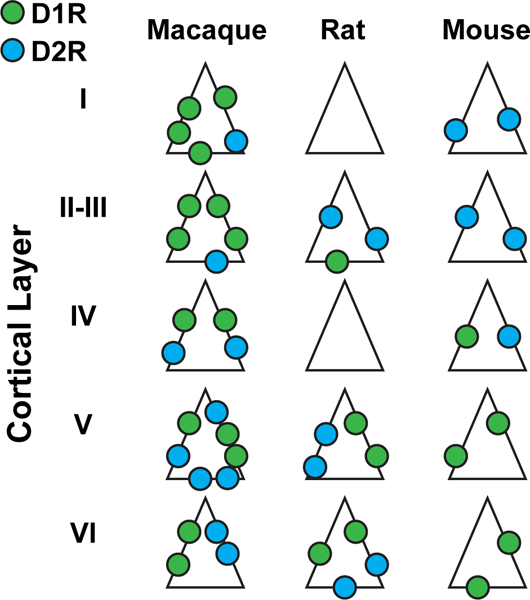
Distribution of D1Rs and D2Rs across cortical layers in different species. D1Rs (green) and D2Rs (blue) are more abundant in macaque than rodent species. In the macaque, D1R expression tends to decrease with cortical depth, while D2R expression increases. The opposite is true in both rodent species
7. Conclusion
Numerous lines of evidence indicate the involvement of the PFC, and specifically the FEF, in both attention and WM. The FEF shows both memory- and attention-related activity, largely in the same neurons, and its activity is tied to performance on both attention and WM tasks. The FEF sends memory-related activity to visual areas, and the modulation of visual responses observed during spatial WM resembles that caused by covert spatial attention. Dopamine signaling and persistent activity within PFC may be the key mechanisms linking these two processes. Patients with altered dopamine signaling, such as PD, show disruptions of WM performance and tasks involving top-down control. Dopamine receptors display specific expression patterns within primate PFC, and manipulating PFC dopamine signaling both selectively modulates memory-related activity within PFC and reproduces the signatures of attention within visual cortex. In sum, dopamine-modulated activity in prefrontal cortex appears to be a critical player in both the behavioral and neural signatures of attention and WM.
References
- Albrecht DG (1995) Visual cortex neurons in monkey and cat: effect of contrast on the spatial and temporal phase transfer functions. Vis Neurosci 12(6):1191–1210Google Scholar [DOI] [PubMed] [Google Scholar]
- Anderson JC, Kennedy H, Martin KAC (2011) Pathways of attention: synaptic relationships of frontal eye field to V4, lateral intraparietal cortex, and area 46 in macaque monkey. J Neurosci 31(30):10872–10881. 10.1523/JNEUROSCI.0622-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EB, Mitchell JF, Reynolds JH (2013) Attention-dependent reductions in burstiness and action-potential height in macaque area V4. Nat Neurosci 16(8):1125–1131. 10.1038/nn.3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K, Carrasco M (2013) Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat Rev Neurosci 14(3):188–200Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K, Stephan VM, Treue S (2009) Attention reshapes center-surround receptive field structure in macaque cortical area MT. Cereb Cortex 19(10):2466–2478. 10.1093/cercor/bhp002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardid S, Wang X-J, Compte A (2007) An integrated microcircuit model of attentional processing in the neocortex. J Neurosci 27(32):8486–8495. 10.1523/JNEUROSCI.1145-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KM, Chang MH, Moore T (2009) Selection and maintenance of spatial information by frontal eye field neurons. J Neurosci 29(50):15621–15629. 10.1523/JNEUROSCI.4465-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2000) Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res 126:183–192. 10.1016/S0079-6123(00)26014-7 [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS (1994) Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology 116(2):143–151Google Scholar [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS (1995) Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci 15(5 Pt 1):3429–3439Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J (2001) Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci 5(3):119–126Google Scholar [DOI] [PubMed] [Google Scholar]
- Bahmani Z, Daliri MR, Merrikhi Y, Clark K, Noudoost B (2018) Working memory enhances cortical representations via spatially specific coordination of spike times. Neuron 97(4):967–979.e6. 10.1016/j.neuron.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65–94Google Scholar [DOI] [PubMed] [Google Scholar]
- Bell AH, Meredith MA, Van Opstal AJ, Munoz DP (2006) Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Exp Brain Res 174(1):53–59. 10.1007/s00221-006-0420-z [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C (1988) Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol 273(1):99–119. 10.1002/cne.902730109 [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C (1991) Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci 14(1):21–27Google Scholar [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS (1995) Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 15(12):7821–7836Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodis-Wollner I (1990) The visual system in Parkinson’s disease. Res Publ Assoc Res Nerv Ment Dis 67:297–316Google Scholar [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M (1987) Visual dysfunction in Parkinson’s disease – loss in spatiotemporal contrast sensitivity. Brain 110:1675–1698. 10.1093/brain/110.6.1675 [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD (1988) Internal versus external cues and the control of attention in Parkinsons-disease. Brain 111:323–345. 10.1093/brain/111.2.323 [DOI] [PubMed] [Google Scholar]
- Brown RG, Jahanshahi M, Marsden D (1993) Response choice in Parkinsons-disease – the effects of uncertainty and stimulus-response compatibility. Brain 116:869–885. 10.1093/brain/116.4.869 [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME (1985) Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53(3):603–635Google Scholar [DOI] [PubMed] [Google Scholar]
- Bulens C, Meerwaldt JD, Vanderwildt GJ, Vandeursen JBP (1987) Effect of levodopa treatment on contrast sensitivity in Parkinsons-disease. Ann Neurol 22(3):365–369. 10.1002/ana.410220313 [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ (2012) Normalization as a canonical neural computation. Nat Rev Neurosci 13(1):51–62. 10.1038/nrn3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner S a, Williams GV (2007) Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn 63(2):94–122. 10.1016/j.bandc.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Clark KL, Noudoost B, Schafer RJ, Moore T (2014) Neuronal mechanisms of attentional control: frontal cortex. In: Kastner S, Nobre AC (eds) Handbook of attention Oxford University Press, OxfordGoogle Scholar [Google Scholar]
- Clark K, Squire RF, Merrikhi Y, Noudoost B (2015) Visual attention: linking prefrontal sources to neuronal and behavioral correlates. Prog Neurobiol 132:59–80. 10.1016/j.pneurobio.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A (2008) Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci 28(50):13411–13418. 10.1523/jneurosci.4095-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A (2011) Measuring and interpreting neuronal correlations. Nat Neurosci 14(7):811–819. 10.1038/nn.2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH (2009) Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12(12):1594–1600. 10.1038/nn.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang X-J (2000) Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10(9):910–923Google Scholar [DOI] [PubMed] [Google Scholar]
- Connor CE, Gallant JL, Preddie DC, Van Essen DC (1996) Responses in area V4 depend on the spatial relationship between stimulus and attention. J Neurophysiol 75(3):1306–1308Google Scholar [DOI] [PubMed] [Google Scholar]
- Connor CE, Preddie DC, Gallant JL, Van Essen DC (1997) Spatial attention effects in macaque area V4. J Neurosci 17(9):3201–3214Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69(12):E113–E125. 10.1016/j.biopsych.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 11(12):1136–1143Google Scholar [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002) Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain 125(Pt 3):584–594Google Scholar [DOI] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D’Esposito M (2010) Enhanced frontal function in Parkinson’s disease. Brain 133(Pt 1):225–233. 10.1093/brain/awp301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Tidswell P, Jordan N (1994) Slowed central processing in simple and go no-go reaction-time tasks in Parkinson’s disease. Brain 117:517–529. 10.1093/brain/117.3.517 [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Papavasiliou PS, Gellene R (1969) Modification of Parkinsonism – chronic treatment with L-dopa. N Engl J Med 280(7):337–345. 10.1056/NEJM196902132800701 [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR (2015) The cognitive neuroscience of working memory. Annu Rev Psychol 66(1):115–142. 10.1146/annurev-psych-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S, Carlin P, Hemsley D (2005) Weak suppression of visual context in chronic schizophrenia. Curr Biol 15(20):R822–R824. 10.1016/j.cub.2005.10.015 [DOI] [PubMed] [Google Scholar]
- Davidoff SA, Benes FM (1998) High-resolution scatchard analysis shows D1 receptor binding on pyramidal and nonpyramidal neurons. Synapse 28(1):83–90. [DOI] [PubMed] [Google Scholar]
- Deco G, Hugues E (2012) Neural network mechanisms underlying stimulus driven variability reduction. PLoS Comput Biol 8(3):e1002395 10.1371/journal.pcbi.1002395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Kringelbach ML (2016) Metastability and coherence: extending the communication through coherence hypothesis using a whole-brain computational perspective. Trends Neurosci 39(3):125–135Google Scholar [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B (1987) Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience 21(3):807–824Google Scholar [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX (1996) Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis Res 36(12):1827–1837Google Scholar [DOI] [PubMed] [Google Scholar]
- Druckmann S, Chklovskii DB (2012) Neuronal circuits underlying persistent representations despite time varying activity. Curr Biol 22(22):2095–2103Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraina S, Paré M, Wurtz RH (2002) Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J Neurophysiol 87(2):845–858. 10.1152/jn.00317.2001 [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM (2006) Impaired control of visual attention in schizophrenia. J Abnorm Psychol 115(2):266–275. 10.1037/0021-843x.115.2.266 [DOI] [PubMed] [Google Scholar]
- Galashan FO, Saßen HC, Kreiter AK, Wegener D (2013) Monkey area MT latencies to speed changes depend on attention and correlate with behavioral reaction times. Neuron 78(4):740–750. 10.1016/j.neuron.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF (2011) Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci 125(3):282–296. 10.1037/a0023165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W-J, Krimer LS, Goldman-Rakic PS (2001) Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci 98(1):295–300. 10.1073/pnas.011524298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C (1995) D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci 7(5):1050–1063Google Scholar [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC (2012) Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci 16(2):129–135. 10.1016/j.tics.2011.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi MF, Eidelberg D, Silvestri G, Ghez C (2003) The differential effect of PD and normal aging on early explicit sequence learning. Neurology 60:1313–1319. 10.1212/01.wnl.0000059545.69089 [DOI] [PubMed] [Google Scholar]
- Glausier JR, Khan ZU, Muly EC (2009) Dopamine D1 and D5 receptors are localized to discrete populations of interneurons in primate prefrontal cortex. Cereb Cortex 19(8):1820–1834. 10.1093/cercor/bhn212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MS (2009) Memory without feedback in a neural network. Neuron 61(4):621–634Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M (1989) Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci 86(22):9015–9019Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW (1990) Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci 10(7):2125–2138Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ (2003) Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 23(3):867–875Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Swets DJ (1966) Signal detection theory and psychophysics Wiley, New York: Google Scholar [Google Scholar]
- Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB (2007) Inhibitory control and spatial working memory in Parkinson’s disease. Mov Disord 22(10):1444–1450. 10.1002/mds.21510 [DOI] [PubMed] [Google Scholar]
- Herz DM, Bogacz R, Brown P (2016) Neuroscience: impaired decision-making in Parkinson’s disease. Curr Biol 26(14):R671–R673. 10.1016/j.cub.2016.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton JT, Morris JL, Elias JW (1993) Levodopa improves spatial contrast sensitivity in Parkinsons-disease. Arch Neurol 50(7):721–724. 10.1001/archneur.1993.00540070041012 [DOI] [PubMed] [Google Scholar]
- Jacob SN, Ott T, Nieder A (2013) Dopamine regulates two classes of primate prefrontal neurons that represent sensory signals. J Neurosci 33(34):13724–13734. 10.1523/JNEUROSCI.0210-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Frith CD (1998) Willed action and its impairments. Cogn Neuropsychol 15(6–8):483–533. 10.1080/026432998381005 [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins H, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. 1. An investigation using measurement of regional cerebral blood-flow with pet and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933. 10.1093/brain/118.4.913 [DOI] [PubMed] [Google Scholar]
- Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiat 79(4):368–376. 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- Javitt DC, Doneshka P, Grochowski S, Ritter W (1995) Impaired mismatch negativity generation reflects widespread dysfunction of working-memory in schizophrenia. Arch Gen Psychiatry 52(7):550–558Google Scholar [DOI] [PubMed] [Google Scholar]
- Karatekin C, Asarnow RF (1998) Working memory in childhood-onset schizophrenia and attention-deficit/hyperactivity disorder. Psychiatry Res 80(2):165–176. 10.1016/s0165-1781(98)00061-4 [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C (1999) Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychol Med 29(3):527–538. 10.1017/s0033291799008338 [DOI] [PubMed] [Google Scholar]
- Kraynyukova N, Tchumatchenko T (2018) Stabilized supralinear network can give rise to bistable, oscillatory, and persistent activity. Proc Natl Acad Sci 115(13):3464–3469. 10.1073/pnas.1700080115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersmith MJ, Shakin E, Siegel IM, Lieberman A (1982) Visual system abnormalities in patients with Parkinson’s disease. Arch Neurol 39(5):284–286Google Scholar [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Goldberg ME (2003) The time course of perisaccadic receptive field shifts in the lateral intraparietal area of the monkey. J Neurophysiol 89(3):1519–1527. 10.1152/jn.00519.2002 [DOI] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM (1992) L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology 107(2–3):394–404Google Scholar [DOI] [PubMed] [Google Scholar]
- Lange KW, Paul GM, Naumann M, Gsell W (1995) Dopaminergic effects on cognitive performance in patients with Parkinson’s disease. J Neural Trans Suppl 46:423–432Google Scholar [PubMed] [Google Scholar]
- Lawrence BM, White RL, Snyder LH (2005) Delay-period activity in visual, visuomovement, and movement neurons in the frontal eye field. J Neurophysiol 94(2):1498–1508. 10.1152/jn.00214.2005 [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G (2005) Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45(1):147–156. 10.1016/j.neuron.2004.12.025 [DOI] [PubMed] [Google Scholar]
- Leichnetz GR (1982) Connections between the frontal eye field and pretectum in the monkey: an anterograde/retrograde study using HRP GEL and TMB neurohistochemistry. J Comp Neurol 207(4):394–402. 10.1002/cne.902070410 [DOI] [PubMed] [Google Scholar]
- Lidow MS (1998) Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann N Y Acad Sci 846:182–193Google Scholar [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P (1991) Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience 40(3):657–671Google Scholar [DOI] [PubMed] [Google Scholar]
- Ling S, Liu T, Carrasco M (2009) How spatial and feature-based attention affect the gain and tuning of population responses. Vis Res 49(10):1194–1204. 10.1016/j.visres.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Ercsey-Ravasz MM, Ribeiro Gomes AR, Lamy C, Magrou L, Vezoli J et al. (2014a) A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb Cortex 24(1):17–36. 10.1093/cercor/bhs270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov NT, Vezoli J, Chameau P, Falchier A, Quilodran R, Huissoud C et al. (2014b) Anatomy of hierarchy: feedforward and feedback pathways in macaque visual cortex. J Comp Neurol 522(1):225–259. 10.1002/cne.23458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis KI, Wynn JK, Breitmeyer B, Nuechterlein KH, Green MF (2011) The attentional blink in schizophrenia: isolating the perception/attention interface. J Psychiatr Res 45(10):1346–1351. 10.1016/j.jpsychires.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Halliday D, Torres S, Martinez-Trujillo JC (2014) Sharp emergence of feature-selective sustained activity along the dorsal visual pathway. Nat Neurosci 17(9):1255–1262. 10.1038/nn.3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikhi Y, Clark KK, Albarran E, Parsa M, Zirnsak M, Moore T, Noudoost B (2017) Spatial working memory alters the efficacy of input to visual cortex. Nat Commun 8:15041 10.1038/ncomms15041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson C a, Desimone R (1996) Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16(16):5154–5167Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78(1):189–225Google Scholar [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH (2007) Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55(1):131–141. 10.1016/j.neuron.2007.06.018 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH (2009) Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63(6):879–888. 10.1016/j.neuron.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM (2003) Selective gating of visual signals by microstimulation of frontal cortex. Nature 421:370–373. 10.1038/nature01285. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M (2001) Control of eye movements and spatial attention. Proc Natl Acad Sci 98(3):1273–1276. 10.1073/pnas.021549498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M (2004) Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol 91(1):152–162. 10.1152/jn.00741.2002 [DOI] [PubMed] [Google Scholar]
- Morris RG, Downes JJ, Sahakian BJ, Evenden JL, Heald A, Robbins TW (1988) Planning and spatial working memory in Parkinson’s disease. J Neurol Neurosurg Psychiatry 51(6):757–766. 10.1136/jnnp.51.6.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ (2008) A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia 46(13):3144–3156. 10.1016/j.neuropsychologia.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Mueller A, Shepard SB, Moore T (2018) Differential expression of dopamine D5 receptors across neuronal subtypes in macaque frontal eye field. Front Neural Circuits 12:12 10.3389/fncir.2018.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Szigeti K, Goldman-Rakic PS (1998) D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci 18(24):10553–10565Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T (2011a) A reliable microinjectrode system for use in behaving monkeys. J Neurosci Methods 194(2):218–223. 10.1016/j.jneumeth.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T (2011b) Control of visual cortical signals by prefrontal dopamine. Nature 474(7351):372–375. 10.1038/nature09995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T (2010) Top-down control of visual attention. Curr Opin Neurobiol 20(2):183–190Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Clark KL, Moore T (2014) A distinct contribution of the frontal eye field to the visual representation of saccadic targets. J Neurosci 34(10):3687–3698. 10.1523/JNEUROSCI.3824-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram MW, Xiao D, Dritschel B, Payne KR (2002) The temporal resolution of neural codes: does response latency have a unique role? Philos Trans R Soc Lond Ser B Biol Sci 357(1424):987–1001. 10.1098/rstb.2002.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott T, Westendorff S, Nieder A (2018) Dopamine receptors influence internally generated oscillations during rule processing in primate prefrontal cortex. J Cogn Neurosci 30:770–784. 10.1162/jocn_a_01248 [DOI] [PubMed] [Google Scholar]
- Patel N, Jankovic J, Hallett M (2014) Sensory aspects of movement disorders. Lancet Neurol 13(1):100–112Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugini A, Ditterich J, Basso MA (2016) Patients with Parkinson’s disease show impaired use of priors in conditions of sensory uncertainty. Curr Biol 26(14):1902–1910. 10.1016/j.cub.2016.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR (2005) Delay-period activity in the prefrontal cortex: one function is sensory gating. J Cogn Neurosci 17(11):1679–1690. 10.1162/089892905774589208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR (2006) Working memory as an emergent property of the mind and brain. Neuroscience 139(1):23–38. 10.1016/j.neuroscience.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Horstink M (1998) Reliance on external cues for movement initiation in Parkinson’s disease – evidence from movement-related potentials. Brain 121:167–177. 10.1093/brain/121.1.167 [DOI] [PubMed] [Google Scholar]
- Raiguel SE, Xiao DK, Marcar VL, Orban GA (1999) Response latency of macaque area MT/V5 neurons and its relationship to stimulus parameters. J Neurophysiol 82(4):1944–1956Google Scholar [DOI] [PubMed] [Google Scholar]
- Robinson DA, Fuchs AF (1969) Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol 32(5):637–648Google Scholar [DOI] [PubMed] [Google Scholar]
- Rodnitzky RL (1998) Visual dysfunction in Parkinson’s disease. Clin Neurosci 5(2):102–106Google Scholar [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8(12):1128–1139. 10.1016/s1474-4422(09)70293-5 [DOI] [PubMed] [Google Scholar]
- Ruff D, Cohen M (2014) Attention can either increase or decrease spike count correlations in visual cortex. Nat Neurosci 17(11):1591–1597Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Artigas F (2017) Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex. Front Neuroanat 11:87 10.3389/fnana.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J (1995) Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci 15(6):4464–4487Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Reisine H, Hepp K, Henn V (1985) Frontal eye field projection to the paramedian pontine reticular formation traced with wheat germ agglutinin in the monkey. Brain Res 329(1–2):151–160Google Scholar [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74(1):1–58. 10.1016/j.pneurobio.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA (1995) Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol 363(2):264–280. 10.1002/cne.903630208 [DOI] [PubMed] [Google Scholar]
- Seymour K, Stein T, Sanders LLO, Guggenmos M, Theophil I, Sterzer P (2013) Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology 38(13):2607–2612. 10.1038/npp.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, Harper DN, Cameron FB, Abernethy D (2002) Self-initiated versus externally cued reaction times in Parkinson’s disease. J Clin Exp Neuropsychol 24(2):146–153. 10.1076/jcen.24.2.146.991 [DOI] [PubMed] [Google Scholar]
- Slagter HA, van Wouwe NC, Kanoff K, Grasman R, Claassen DO, van den Wildenberg WPM, Wylie SA (2016) Dopamine and temporal attention: an attentional blink study in Parkinson’s disease patients on and off medication. Neuropsychologia 91:407–414. 10.1016/j.neuropsychologia.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS (1993) Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereb Cortex 3(3):223–238Google Scholar [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS (1992) Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol 321(3):325–335. 10.1002/cne.903210302 [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS (1994) D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci 91(12):5720–5724Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Noudoost B, Moore T (2013) Dissociable dopaminergic control of saccadic target selection and its implications for reward modulation. Proc Natl Acad Sci 110(9):3579–3584. 10.1073/pnas.1221236110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH (2000) Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83(4):1979–2001Google Scholar [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH (2001) Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. J Neurophysiol 85(4):1673–1685Google Scholar [DOI] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T (2013) Prefrontal contributions to visual selective attention. Annu Rev Neurosci 36(1):451–466. 10.1146/annurev-neuro-062111-150439 [DOI] [PubMed] [Google Scholar]
- Stablein M, Sieprath L, Knochel C, Landertinger A, Schmied C, Ghinea D et al. (2016) Impaired working memory for visual motion direction in schizophrenia: absence of recency effects and association with psychopathology. Neuropsychology 30(6):653–663. 10.1037/neu0000267 [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME (1993) Topography of projections to the frontal lobe from the macaque frontal eye fields. J Comp Neurol 330(2):286–301. 10.1002/cne.903300209 [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME (1995) Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol 353(2):291–305. 10.1002/cne.903530210 [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Rosen J, Ilson J (1983) Perceptual motor dysfunction in Parkinson’s disease: a deficit in sequential and predictive voluntary movement. J Neurol Neurosurg Psychiatry 46(2):145–151. 10.1136/jnnp.46.2.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg KA, Mitchell JF, Gawne TJ, Reynolds JH (2012) Attention influences single unit and local field potential response latencies in visual cortical area V4. J Neurosci 32(45):16040–16050. 10.1523/JNEUROSCI.0489-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Cavanagh P (1997) Focused attention distorts visual space: an attentional repulsion effect. J Exp Psychol Hum Percept Perform 23(2):443–463Google Scholar [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P (2004) Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci 24(22):5131–5139. 10.1523/JNEUROSCI.1021-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME (2001) Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol 86(5):2344–2352Google Scholar [DOI] [PubMed] [Google Scholar]
- van Eden CG, Hoorneman EM, Buijs RM, Matthijssen MA, Geffard M, Uylings HB (1987) Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience 22(3):849–862Google Scholar [DOI] [PubMed] [Google Scholar]
- Verney C, Alvarez C, Geffard M, Berger B (1990) Ultrastructural double-labelling study of dopamine terminals and GABA-containing neurons in rat anteromedial cerebral cortex. Eur J Neurosci 2(11):960–972Google Scholar [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF (2007) Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10(3):376–384. 10.1038/nn1846 [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Major AJ, Everling S (2016) Dopamine D1 and D2 receptors make dissociable contributions to dorsolateral prefrontal cortical regulation of rule-guided oculomotor behavior. Cell Rep 16(3):805–816. 10.1016/j.celrep.2016.06.031 [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM (1993) Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci 13(6):2551–2564Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J (2008) Decision making in recurrent neuronal circuits. Neuron 60(2):215–234. 10.1016/j.neuron.2008.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS (2004) Selective D2 receptor actions on the functional circuitry of working memory. Science 303(5659):853–856. 10.1126/science.1091162 [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel J-R, Olivier E (2006) Contribution of the monkey frontal eye field to covert visual attention. J Neurosci 26(16):4228–4235. 10.1523/JNEUROSCI.3336-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ma T, Cheng Y, Huang CCY, Wang X, Lu J, Wang J (2018) Dopamine D1 or D2 receptor-expressing neurons in the central nervous system. Addict Biol 23(2):569–584. 10.1111/adb.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376(6541):572–575. 10.1038/376572a0 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S (2006) Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nat Neurosci 9(9):1156–1160. 10.1038/nn1748 [DOI] [PubMed] [Google Scholar]
- Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM (1969) Treatment of parkinsonism with levodopa. Arch Neurol 21(4):343–354. 10.1001/archneur.1969.00480160015001 [DOI] [PubMed] [Google Scholar]
- Zaksas D, Pasternak T (2006) Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task. J Neurosci 26(45):11726–11742. 10.1523/JNEUROSCI.3420-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]


