Abstract
Female Macrobrachium nipponense has the characteristic of short sexual maturity during the breeding season, which can increase breeding risk and lead to prevalent female individual miniaturization. In this study, we characterized micro (mi)RNA-seq data of the eyestalk (E) and cerebral ganglia (B) of female M. nipponense during breeding and non-breeding seasons. A total of 393 and 189 differentially expressed miRNAs (DE miRNAs) were identified in BSE vs. NBSE and BSB vs. NBSB, respectively. The most abundant up- and down-regulated DE miRNAs were miR-124, miR-14, and miR-7. Enrichment analysis showed that DE miRNA target genes were mainly involved in ‘metabolic process’ and ‘binding’, and were associated with ‘neurohormonal regulation’ and ‘photoreceptor activity’ signaling pathways. Integrated analysis of miRNA–mRNA expression showed that the most abundant DE miRNAs were miR-14 and miR-278 in BSE vs. NBSE and BSB vs. NBSB, respectively. Four pairs of DE miRNAs and their corresponding target annotated genes were selected from the DE miRNA–mRNA interaction network (bmo-miR-316-5p/opsin protein, ame-miR-125/skeletal muscle actin 8, dmo-miR-278/sugar transporter, and tca-miR-3885-5p/5-HT1 receptor). Gene expression analysis of these four pairs in different ovary development stages showed their potential regulatory roles in ovary maturation.
Keywords: Macrobrachium nipponense, Integrated analysis, microRNAs, mRNAs, Reproduction
Introduction
Macrobrachium nipponense, also called the oriental river prawn, is widely distributed in Southeast Asia (Cai and Ng 2002; Fu et al. 2012). It is very popular in China, and its culture now plays an important role in increasing agricultural efficiency and in fishermen’s income. The annual output of the oriental river prawn in China has been increasing, and cultured production reached about 272,592 tons in 2016 (Bureau of Fishery 2017). However, female prawns have a short period of sexual maturity and ovaries mature too quickly (Qiao et al. 2017), which results in individual miniaturization, and is a roadblock to basic reproductive research. Therefore, identifying the mechanisms involved in ovarian maturation of M. nipponense has great significance to both science and aquaculture production.
In crustaceans, the eyestalk and cerebral ganglia are the main sites of the neuroendocrine system, and they secrete many hormones that are involved in regulating reproduction (Nagaraju 2010; Suwansa-ard et al. 2015). With recent developments in genomic sequencing and transcriptome mapping, many reproduction-related genes have been identified in the crustacean eyestalk, cerebral ganglia, and gonads at a large scale, and at the genome-wide level, such as crustacean hyperglycemic hormone, molt-inhibiting hormone, vitellogenesis-inhibiting hormone, pigment-dispersing hormone, vasa, vitellogenin, etc (Qiao et al. 2012; Jin et al. 2013; Bao et al. 2015; Veenstra 2015; Xu et al. 2015; Jiang et al. 2016). MicroRNAs (miRNAs), which consist of approximately 18–26 nucleotides (nt), are non-coding RNAs that act as post-transcriptional regulators to inhibit the expression of mRNAs (Bushati and Cohen 2002; Stefani and Slack 2008). Thus, mining miRNA–mRNA regulatory networks could lead to a better understanding of gene expression (Liu et al. 2015).
In previous studies, we compared the transcriptomes of the eyestalk and cerebral ganglia from female M. nipponense in breeding and non-breeding seasons, which revealed many important reproduction-related genes and signaling pathways (Qiao et al. 2017). In this study, we characterized miRNA-seq of the eyestalk and cerebral ganglia from female M. nipponense during breeding and non-breeding seasons. We also conducted co-expression network analyses to identify candidate miRNA–mRNA networks that might regulate reproduction. The data provide valuable information about the molecular regulatory mechanisms of ovary maturation in M. nipponense.
Materials and methods
Female adult M. nipponense prawns, with 2.26 ± 0.59 g were obtained from Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi, China.
Fifty non-breeding season (NBS) prawns captured in January while another 50 captured in June were defined as breeding season group (BS) in 2015. All the prawns were cultured in a re-circulating water aquarium system with some artificial grass and fed with paludina twice per day at room temperature for 3 days before sample collection. The prawns were maintained under a natural photoperiod; the pH was 7.6–8.0; and the dissolved oxygen concentrations were maintained above 5.0 mg/L. The concentration of ammonia nitrogen in the aquaculture water did not exceed 0.05 mg/L and the concentration of nitrite did not exceed 0.05 mg/L. Fresh eyestalk and cerebral ganglia tissues were quickly collected and immediately stored at − 80 °C until processed. Eyestalk and cerebral ganglia from non-breeding season prawns were named as NBSE and NBSB. The tissues from breeding season prawns were named as BSE and BSB.
Total RNA was extracted separately using RNAiso Plus Reagent (Takara, Japan) according to the manufacturer’s protocols and then treated with RNase-free DNase I (Takara, Japan) to remove genomic DNA contamination. The extracted RNA was further treated with RNase-free DNase I (Takara, Japan) to avoid DNA contamination. The RNA integrity and quantity were assessed using an Agilent 2100 Bioanalyzer (Agilent, Shanghai, China) and a NanoDrop 1000 spectrophotometer (Thermo Scientific, USA), respectively. Finally, RNA was stored at − 80 °C until further processing.
Small RNA libraries were constructed from purified RNA of eyestalks and cerebral ganglia of non-breeding and breeding season. sRNA-Seq services were carried out using Illumina HiSeq™ 2500 platform (Illumina Inc., San Diego, CA, USA) provided by Genepioneer Biotechnologies—Nanjing, China. Small RNAs were isolated from the total RNA by size fractionation in a 15% TBE urea polyacrylamide gel, small RNAs were ligated to 3′ adaptor and 5′ adaptor with T4 RNA ligase. Subsequently, PCR reaction was performed using primers complementary to the two adaptors. The amplification products were further purified on 6% polyacrylamide TBE gel and used for sequence analysis. Sequencing libraries were generated using an IlluminaTruSeq. Truseq Small RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) following the manufacturer’s recommendations. Sequencing cycle number is 50 and the read length is 50 nt with single end sequencing pattern. The reads of sRNA-Seq have been deposited in NCBI database with accession number PRJNA395269.
The raw reads were further processed to remove the low-quality reads and the adaptor (5′-GCCTTGGCACCCGAGAATTCCA-3′) by Trimmomatic (Bolger et al. 2014). Then the length of sRNA tags within a certain range of 18–30 nt from clean reads was mapped to the databases of mRNA (http://www.ncbi.nlm.nih.gov/), RFam (http://www.sanger.ac.uk/software/Rfam), and Repbase (http://www.girinst.org/repbase/) to match the known small RNAs of rRNAs, tRNAs, snRNAs, snoRNAs, repeat, and other non-coding RNAs (Langmead et al. 2009). The sequences were used to BLAST search against miRBase database (http://www.mirbase.org/) to identify known miRNAs by Bowtie (http://bowtie-bio.sourceforge.net/index.shtml). Only perfect matches were considered as conserved miRNAs. Novel miRNA prediction was explored by the miRDeep2 (http://mdc.helmholtz.de) software for the secondary structure, the Dicer cleavage site, and the minimum free energy of the former un-annotated small RNA tags that could be mapped to reference sequences (Friedländer et al. 2012).
Each identified miRNA read count was normalized to the total number of miRNA reads in each given sample and multiplied by a million (RPKM). The p value could be assigned to each gene and adjusted by the Benjamini and Hochberg’s approach for controlling the false discovery rate. miRNAs with |log2(FC)| ≥ 1 and p value < 0.05 are identified as significantly differentially expressed miRNAs (DEMs).
Miranda (http://www.miRNA.org/miRNA/home.do) was used to predict the target genes, in accordance with the following criteria in seed matches: nucleotides 1–9 from the 5′-end and G–U content < 3. The reference genomes used in the prediction are the eyestalks and cerebral ganglia transcriptomes of M. nipponense (NCBI, PRJNA339889). The functional classification and pathways were based on the six databases: NR (fttp://ftp.ncbi.nih.gov/blast/db/), COG (http://www.ncbi.nlm.nih.gov/COG/), Swissprot (http://www.uniprot.org/), KEGG (http://www.genome.jp/kegg/), GO (http://www.geneontology.org/), and Pfam (http://pfam.xfam.org/) using BLAST with a cutoff E value of 10−5.
To define all the possible miRNA–mRNA interactions, including positive and negative relationships between miRNA and mRNA expression, we used Cytoscape (http://www.cytoscape.org/download.php) to construct the miRNA–mRNA regulatory network. Afterward, integration of miRNA-seq with mRNA-seq was achieved by integrating expression profiles of DE miRNAs and DE mRNAs with the addition of DE miRNA-targeting information. Function and pathway enrichment were analyzed depending on the Gene Ontology and KEGG database. As the enrichment increases, the corresponding GO item or pathway is more specific, this helps us to find GO items or pathways with greater significance in the experiment.
The expression profiles of DE miRNAs and their corresponding target genes among the miRNA–mRNA interaction network were further examined in eyestalk and cerebral ganglia of female M. nipponense during different ovary stages using stem-loop qPCR and qRT-PCR. Total RNA was extracted from the cerebral ganglia and eyestalk using Trizol (Takara, Dalian, China) and treated with RNase-free Dnase I (Promega, USA). For miRNA, 2 μg total RNA was used in a reverse transcription reaction with the Prime-Script RT reagent Kit (Takara, Dalian, China). Reverse transcription was performed according to the previous study (Varkonyi-Gasic et al. 2007). qRT-PCR analysis of the miRNA was performed on a 96-well plate with a 20 μL reaction volume containing 2 μL cDNA, 10 μL 2 × miRcute Plus miRNA Premix (with SYBR&ROX) (TIANGEN, Beijing, China), 0.4 μL 10 μM of primers, and 7.2 μL of ddH2O. The PCR temperature profile was 95 °C for 15 min followed by 40 cycles of 95 °C for 20 s, 60 °C for 34 s. For target genes, reverse transcription and qRT-PCR were performed by following the previous study (Qiao et al. 2017). U6 and β actin were used as the internal controls for miRNA and their targets. All the primers are listed in Table 1. All PCR reactions were performed with three biological replicates and the relative gene expression level was analyzed using the comparative method. SPSS17.0 was used to compute for the Ct mean and standard deviation between replicate samples, and one-way ANOVA was adopted for analysis of significance.
Table 1.
The specific primers used in qRT-PCR
| Primer name | Sequence (5′ → 3′) |
|---|---|
| bmo-miR-316-5p | F: TGTCTTTTTCCGCTTTGCTGCTG |
| ame-miR-125 | F: GCCCCTGAGACCCTAACTTGTG |
| dmo-miR-278 | F: CGGTGGGACTTTCGTCCGTTT |
| tca-miR-3885-5p | F: TAATAAATAAGGCGGCGGCG |
| U6 |
F: CTCGCTTCGGCAGCACA R: AACGCTTCACGAATTTGCGT |
| Opsin protein |
F: TTCGCATTGCTAAGACCG R: GAACTTGGGATGGCTGAT |
| Skeletal muscle actin 8 |
F: CGGAGTTCGTTGTAGAAGGTG R: TGTGATGGTCGGTATGGGTC |
| Sugar transporter |
F: AACGCTCGTAGGGAAGTA R: GCCTCGTCGTCTCACATT |
| 5-HT1 receptor |
F: GGGGTTCAAGGTGGAGTT R: TATGTTGGCTGCCGTTCT |
| β actin |
F: TATGCACTTCCTCATGCCATC R: AGGAGGCGGCAGTGGTCAT |
Results
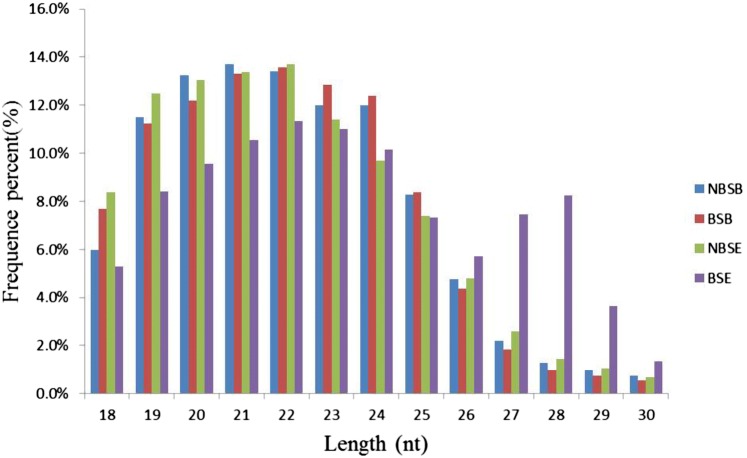
To identify miRNAs involved in reproduction regulation of female M. nipponense, small RNA libraries were constructed for sequencing from NBSE vs. NBSB and BSE vs. BSB. The small RNA libraries produced a dataset ranging from 10 to 13 million reads (Table 2). After filtering out low-quality reads and removing adaptor sequences, the number of clean reads was reduced to 5–10 million. Ultimately, 6,856,476, 10,450,380, 5,028,534, and 8,832,845 total clean reads were obtained from the NBSB, BSB, NBSE, and BSE samples, respectively (Table 2). Different categories of RNA, including rRNA, snRNA, snoRNA, tRNA, and repeat-associated sRNA, were detected in this study (Table 3). The length distributions of the unique sRNA sequences were analyzed, and sRNAs with a sequence length between 18 and 30 nt were selected (Fig. 1). The majority of the unique sRNAs ranged from 20 to 24 nt, and the most abundant distributions were for sequences that were 21 and 22 nt long.
Table 2.
Statistics of microRNA transcriptome output sequencing
| NBSB | BSB | NBSE | BSE | |
|---|---|---|---|---|
| Total raw reads | 10,904,636 | 13,357,557 | 11,715,163 | 12,600,522 |
| Total nucleotides (nt) | 545,231,800 | 667,877,850 | 585,758,150 | 630,026,100 |
| Reads with adapters | 10,878,639 | 13,351,437 | 11,643,203 | 12,568,787 |
| Total filtered clean readsa | 6,856,476 | 10,450,380 | 5,028,534 | 8,832,845 |
| GC percentage | 53.98% | 52.87% | 53.83% | 54.12% |
| Q30 percentageb | 97.46% | 96.67% | 96.88% | 97.99% |
NBSE and NBSB represent three replications for prawn eyestalks and brains of non-breeding season; BSE and BSB represent three replications for prawn eyestalks and brains of breeding season
aTotal filtered clean reads: the number of paired-end reads in clean data
bQ30 ratio (%): the percentage of the base whose clean data quality value is at least 30
Table 3.
Distribution of small RNAs among different categories
| NBSB | BSB | NBSE | BSE | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Unique (%) | Total | Unique (%) | Total | Unique (%) | Total | Unique (%) | |
| miRNA | 1,301,607 | 11,083 (2.00%) | 3,283,199 | 15,107 (2.32%) | 809,833 | 8566 (3.28%) | 1,496,073 | 9481 (1.77%) |
| rRNA | 3,278,514 | 274,741 (49.65%) | 4,239,281 | 316,414 (48.59%) | 2,935,424 | 139,461 (53.39%) | 2,956,648 | 240,050 (44.86%) |
| tRNA | 108,700 | 19,489 (3.52%) | 128,036 | 19,235 (2.95%) | 43,098 | 8566 (3.28%) | 53,837 | 12,293 (2.30%) |
| snRNA | 10,709 | 2209 (0.40%) | 9030 | 2013 (0.31%) | 4128 | 889 (0.34%) | 6803 | 1816 (0.34%) |
| snoRNA | 10,436 | 1457 (0.26%) | 16,562 | 1738 (0.27%) | 3701 | 554 (0.21%) | 7450 | 1156 (0.22%) |
| Repeat | 233,866 | 23,035 (4.16%) | 308,856 | 29,153 (4.48%) | 126,285 | 10,157 (3.89%) | 202,634 | 15,122 (2.83%) |
| Other | 1,912,644 | 221,362 (40.00%) | 2,465,416 | 267,575 (41.09%) | 1,106,065 | 93,009 (35.61%) | 4,109,400 | 255,199 (47.69%) |
| Total | 6,856,476 | 553,376 (8.07%) | 10,450,380 | 651,235 (6.23%) | 5,028,534 | 261,202 (5.19%) | 8,832,845 | 535,117 (6.06%) |
Fig. 1.
Length distribution of sequencing reads. NBSE and NBSB represent prawn eyestalks and cerebral ganglias of non-breeding season; BSE and BSB represent eyestalks and cerebral ganglias of breeding season
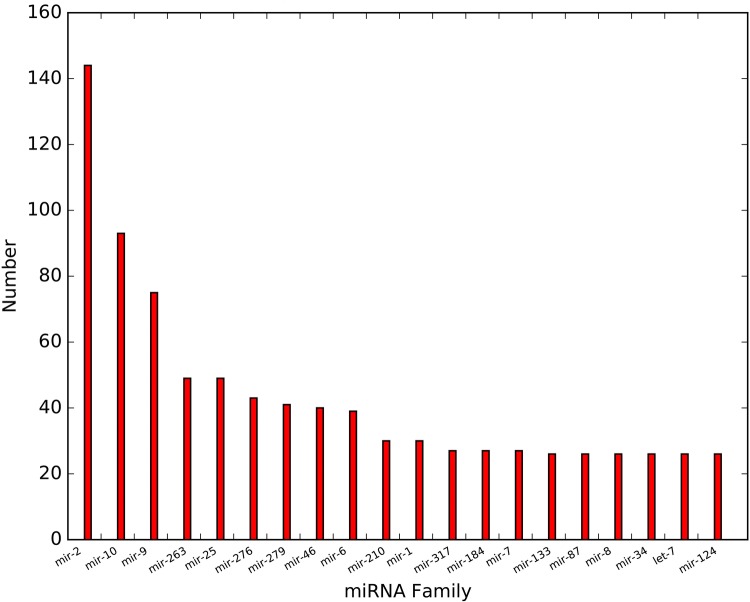
In total, 1944 unique miRNA sequences were identified; of these, 1452 conserved miRNAs belonging to 147 miRNA families and 492 predicted novel miRNAs were identified. Figure 1 shows the top 20 family members of miRNA. A total of 113 miRNA families contained more than 1. Of them, mir-2, mir-10, and mir-9 were universally expressed, and mir-2 showed the highest expression level (Fig. 2).
Fig. 2.
Top 20 miRNA family distribution
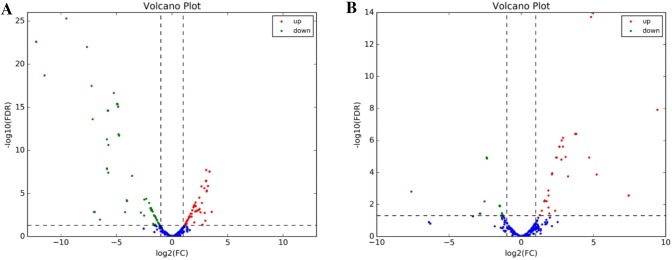
Based on the statistical analysis of BSE vs. NBSE, 393 differentially expressed (DE) miRNAs were identified; of these, 166 were up-regulated and 227 were down-regulated. In BSB vs. NBSB, 189 DE miRNAs were identified, of which 108 were up-regulated and 81 were down-regulated (Table 4). The most abundant up-regulated DE miRNAs were miR-124 and miR-14 in BSE vs. NBSE and BSB vs. NBSB, respectively. Figure 3 presents volcano plots of DE miRNAs in BSE vs. NBSE and BSB vs. NBSB. The most abundant down-regulated DE miRNA was the same (miR-7) in both comparison groups.
Table 4.
Statistics on the number of DE miRNAs in BSE vs. NBSE and BSB vs. NBSB
| DEG set | DEG number | Up-regulated | Down-regulated |
|---|---|---|---|
| BSE vs. NBSE | 393 | 166 | 227 |
| BSB vs. NBSB | 189 | 108 | 81 |
Fig. 3.
The volcano plots of DE miRNAs. a The volcano plots of DE miRNAs in BSE vs. NBSE and b the volcano plots of DE miRNAs in BSB vs. NBSB. Green dots represent down-regulated miRNAs, red dots indicate up-regulated miRNAs. Blue dots represent no significant difference miRNAs
In total, 90,491 transcriptome unigenes of M. nipponense (NCBI, SRX2054321-SRX2054332) were used to identify the DE miRNAs targets (Qiao et al. 2017). The results showed that 1208 miRNAs had 17,610 affiliated target genes. In BSE vs. NBSE, 393 DE miRNAs had 1306 affiliated targets, whereas in BSB vs. NBSB, 189 DE miRNAs had 3254 affiliated target genes. Table 5 shows the results of target gene functional annotation of DE miRNAs in BSE vs. NBSE and BSB vs. NBSB using six different databases.
Table 5.
Target gene annotation of differentially expressed miRNA in BSE vs. NBSE and BSB vs. NBSB
| DEG set | Total | NR | GO | KEGG | COG | Pfam | Swiss-prot |
|---|---|---|---|---|---|---|---|
| BSE vs. NBSE | 1396 | 1371 | 1078 | 573 | 406 | 963 | 988 |
| BSB vs. NBSB | 3254 | 3194 | 2629 | 1241 | 902 | 2054 | 2072 |
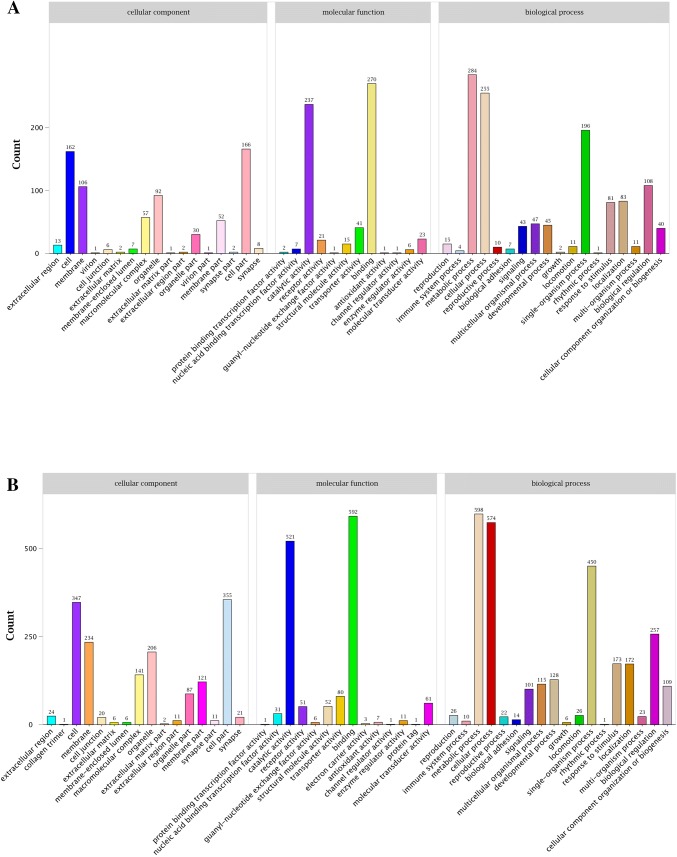
GO enrichment analysis was performed to evaluate significantly overrepresented GO terms in the DE miRNA targeted genes with a p value < 0.05 and FDR < 0.01. ‘Metabolic process’, ‘binding’, and ‘cellular process’ were the most abundant terms in both BSE vs. NBSE and BSB vs. NBSB (Fig. 4). KEGG pathway enrichment analysis also revealed that many miRNAs were involved in different pathways. Interestingly, ‘neuroactive ligand–receptor interaction’ and ‘phototransduction—fly’ were enriched in BSB vs. NBSB and BSE vs. NBSE, respectively, suggesting that some miRNAs were involved in neurohormonal regulation and photoreceptor activity (Fig. 5).
Fig. 4.
The GO classification analyses of DE miRNAs targets in NBSE vs. BSE and NBSB vs. BSB. a NBSE vs. BSE and b NBSB vs. BSB
Fig. 5.
Statistics of KEGG pathway enrichment of DE miRNAs targets in NBSE vs. BSE and NBSB vs. BSB. The abscissa value represented rich factor which means DEG/unigene of pathway term; the ordinate value represented −log 10 (Q value). a NBSE vs. BSE and b NBSB vs. BSB
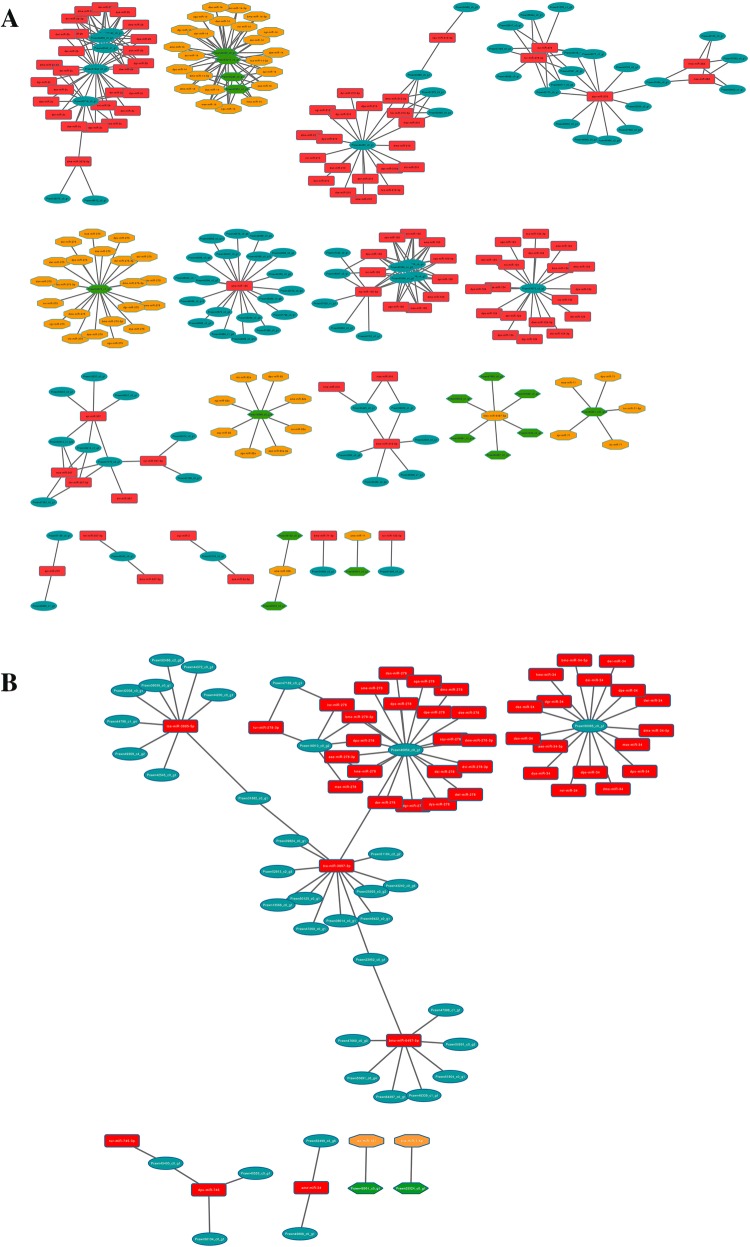
In BSE vs. NBSE, 372 interaction pairs of miRNAs and its corresponding targets were detected, including 105 down-regulated (28.3%) and 267 up-regulated (71.7%) DE miRNAs and their target genes. In BSB vs. NBSB, 85 interactions were found, with 83 DE miRNAs (97.6%) showing significant up-regulated expression (Fig. 6). Table 6 lists the top ten most abundant miRNAs in BSE vs. NBSE and BSB vs. NBSB. The most abundant miRNAs in BSE vs. NBSE were miR-14, miR-2, and miR-100, and those in BSB vs. NBSB were miR-278, miR-34, and miR-3897-3p.
Fig. 6.
DE miRNA–mRNA negative correlation network. For miRNAs: red rectangle represent up-regulation, orange octagon represent down-regulation; for genes: green hexagon represent up-regulation, cyan oval represent down-regulation. a NBSE vs. BSE and b NBSB vs. BSB
Table 6.
Top ten abundant miRNAs by integrated analysis
| BSE vs. NBSE | BSB vs. NBSB | ||
|---|---|---|---|
| miRNA | No. | miRNA | No. |
| miR-14 | 48 | miR-278 | 22 |
| miR-2b | 42 | miR-34 | 16 |
| miR-100 | 38 | miR-3897-3p | 13 |
| miR-278 | 22 | miR-3885-5p | 9 |
| miR-2c | 20 | miR-6497-5p | 8 |
| miR-275 | 20 | miR-278-3p | 7 |
| miR-125 | 20 | miR-745 | 3 |
| miR-210 | 17 | miR-34-5p | 3 |
| miR-124 | 16 | miR-745-3p | 2 |
| miR-100-5p | 13 | miR-7-5p | 1 |
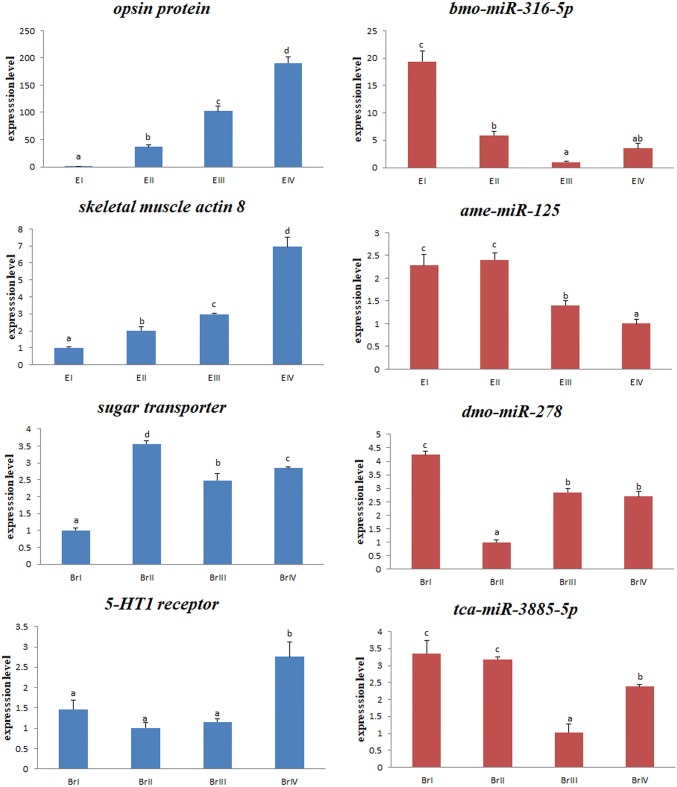
To investigate the potential reproduction-related miRNAs and their targets in M. nipponense, four pairs of DE miRNAs and their corresponding target annotated genes were selected from the DE miRNA–mRNA interaction network (bmo-miR-316-5p/opsin protein, ame-miR-125/skeletal muscle actin 8, dmo-miR-278/sugar transporter, and tca-miR-3885-5p/5-HT1 receptor); these were also included in the most significant enriched GO and KEGG pathways. Their expression levels in the eyestalk and cerebral ganglia of female prawns were determined during four ovarian development stages during breeding season using qRT-PCR (Fig. 7). The ovarian cycle of prawns was classified into different stages based on the previous results (Qiao et al. 2015). The qRT-PCR results revealed that most of these mRNAs/miRNAs shared similar expression tendencies with those found in the mRNA-Seq/miRNASeq data (RPKM/reads-based expression values). As the ovary developed, all four miRNAs showed significant down-regulated expression, whereas their target annotated genes displayed significant up-regulated expression (p < 0.05) in either the eyestalk or cerebral ganglia.
Fig. 7.
Expression analysis of selected miRNAs and their targets during different ovarian development stages using qRT-PCR. Expression changes are expressed as the ratio of gene expression after normalization to U6 and β-actin. E: eyestalk; Br: cerebral ganglia. Stage I (undeveloped stage, oogonium proliferation, transparent), stage II (developing stage, primary vitellogenesis, yellow), stage III (nearly ripe stage, secondary vitellogenesis, light green), and stage IV (ripe stage, vitellogenesis termination, dark green)
Discussion
Recent advances in high-throughput sequencing technologies enabled large-scale studies aimed at the identification of miRNA transcriptome profiles in various crustaceans, such as Triops cancriformis, Scylla paramamosain, Eriocheir sinensis, Daphnia pulex, and Procambarus clarkia (He et al. 2015; Li et al. 2013; Ou et al. 2013; Ikeda et al. 2014; Du et al. 2015). For M. nipponense, some miRNAs have been identified in sex-differentiation and hypoxia stress-related genes (Jin et al. 2015; Sun et al. 2016). Although much progress has been made, little is known about the role of miRNAs in the regulation of ovary maturation in crustaceans.
In this study, we compared the miRNA profiles of the eyestalk and cerebral ganglia from female M. nipponense during the breeding and non-breeding season and identified 1452 conserved miRNAs and 492 predicted novel miRNAs using small RNA high-throughput sequencing. The length distribution results showed that the peak lengths were 21 and 22 nt long, followed by 20, 23, and 24 nt. This was highly consistent with the common size of miRNAs from Dicer digestion products (Cai et al. 2010) and was similar to the results reported for other crustaceans (Tan et al. 2013; Song et al. 2014; Jin et al. 2015; Sun et al. 2016). mir-2 was accumulated to the greatest extent in libraries of M. nipponense, followed by mir-10 and mir-9. As demonstrated by previous studies (Tehler et al. 2011; Marco et al. 2012; Coolen et al. 2013), the mir-2 family is an invertebrate-specific family of miRNAs that likely plays a key role in neural development and maintenance. The miR-10 family is highly conserved, and members act as Hox gene developmental regulators. MiR-9 is reported to play a prominent role in both the developing and mature nervous system of vertebrates and is involved in wing development and embryo segmentation in insects (Coolen et al. 2013; Lucas and Raikhel 2013). Thus, the results of our study suggest that miRNAs from the eyestalk and cerebral ganglia play an important role in coordinated regulation of neural system and segmentation development-related gene expression.
miRNA target identification and target enrichment are two critical steps that can help elucidate their biological functions. In our study, GO enrichment analysis showed that the predicted target genes are significantly involved in ‘metabolic process’, ‘binding’, and ‘cellular process’ in both BSE vs. NBSE and BSB vs. NBSB. Organisms generally have the same metabolic strategies when entering into breeding season: an increase in metabolic rate to total reproduction metabolism. These subclasses may be necessary to meet the increased energy demand in the eyestalk and cerebral ganglia for the female in response to regulating ovary maturation and fertilization. KEGG pathway analyses suggested that targets of DE miRNAs were significantly involved in neurohormonal regulation and photoreceptor activity. Our results indicated that partial miRNAs may play vital roles in regulating hormone secretion during breeding and non-breeding seasonal changes. For both vertebrates and invertebrates, light is a crucial source of energy for organisms, and it is also a primary environmental signal used to control reproductive processes (Rüdiger and López-Figueroa 1992). In our previous study (Qiao et al. 2017), ‘Phototransduction—fly’ was the most significant enrichment pathway, which supports the hypothesis that a photoperiodic pathway may be involved in regulating reproduction in crustaceans. In the current study, KEGG pathway analyses produced consistent results and suggested that photoreceptor activity may mediate environmental factors regulating ovarian maturation and play an important role in seasonal reproduction in crustaceans. Further investigations are necessary to clarify the relationship between these photoreceptors (both miRNAs and genes) and reproduction.
We also built a gene co-expression network using the DE miRNA and DE mRNA data. Among these miRNAs, many have been reported to be involved in gonad maturation (miR-124, miR-278) and ecdysis (miR-100, miR-125). Real et al. (2013) reported that knocking down or overexpressing miR-124 in primary gonadal cell cultures could induce repression of both SOX9 translation and transcription in mouse ovarian cells. Yu et al. (2009) found that miR-278 had multiple functions, especially mediated between nutrient availability and egg development. It was also found to play a role in insulin producing cells of the fly brain by regulating insulin production and metabolism (Varghese et al. 2001). In Drosophila, miR-125 exhibited ecdysone-dependent increased expression during initiation of metamorphosis, and the loss of miR-100 and miR-125 resulted in temporal delays in the terminal cell cycle exit in the wing and maturation of neuromuscular junctions in adult abdominal muscles (Caygill and Johnston 2008; Kenny et al. 2013; Rubio and Belles 2013). Adult female prawns can only copulate and spawn after a pubertal molt and ovary maturation during the breeding season. Our results showed that some miRNAs and their targets in the eyestalk and cerebral ganglia, such as miR-124, miR-278, miR-100, and miR-125, may participate in regulating M. nipponense reproduction.
To investigate the potential involvement of miRNAs and their targets in reproduction of M. nipponense, four pairs of DE miRNAs and their corresponding target annotated genes were selected from the DE miRNA–mRNA interaction network (bmo-miR-316-5p/opsin protein, ame-miR-125/skeletal muscle actin 8, dmo-miR-278/sugar transporter, and tca-miR-3885-5p/5-HT1 receptor). These also were included in the most significant enriched GO and KEGG pathways. Some opsins play important roles in reproductive regulation in birds and fishes (García-Fernández et al. 2015). Recent studies found that in birds and mammals, several opsins mediate photoperiodic regulation of seasonal changes of gonadotropin-releasing hormone (e.g., rhodopsin, OPN4, OPN5, and VA-opsin) (Nakane et al. 2010; Yoshimura 2013; García-Fernández et al. 2015). Knockout of Opsin9 by crispr/cas9 technology in the jellyfish Clytia can delay ovary maturation and egg laying (Artigas et al. 2017). In crustaceans, photoperiod has been shown to have a strong induction effect on gonad development (Quackenbush 1994; Matsuda et al. 2002). In our previous study, we evaluated the DE gene in the ‘Phototransduction—fly’ pathway, called Mn-LW opsin (GenBank accession no. MF438043), and found that knockdown of Mn-LW opsin by dsRNA injection significantly reduced vitellogenin expression (Li et al. 2018). In our current study, dramatic up-regulation of the transcript level of opsin protein from the oogonium proliferation stage (stage I) to vitellogenesis termination (stage IV) suggested that it plays a role in mediation between environmental factors and ovarian maturation.
The skeletal muscle actins, which belong to the muscle actin family in vertebrates, play an important role in complex growth and development in vertebrates (Crawford et al. 2002). However, information about its role in vertebrate and invertebrate reproduction is lacking. In crustaceans, ecdysis is an important event that is involved with reproduction (Brown et al. 2009). For female M. nipponense, fertilization is combined with molting during the breeding season. An important GO term identified in our previous study is ‘structural constituent of cuticle’, which includes more than 110 related genes (Qiao et al. 2017). In the current study, significant up-regulated expression of the skeletal muscle actin 8 gene during ovary development suggested a strong link between molting and reproduction; whether it also plays a role in the process of crustacean reproduction requires further investigation.
Sugar transporters transport sugar into cells in the form of carbohydrate in most organisms (Baldwin and Henderson 1989). Ge et al. (2015) recently reported that silencing a sugar transporter gene (Nlst6) of Nilaparvata lugens had profound effects, such as significantly shortening the oviposition period, decreasing the number of eggs deposited, and reducing body weight. Considering the effects of sugar transporters in insects, the significantly increased expression levels of a sugar transporter at the primary vitellogenic stage (stage II) of M. nipponense might facilitate the supply of energy to yolk synthesis, and hence stimulate ovarian maturation.
Serotonin (5-hydroxytryptamine, 5-HT) acts as a neurotransmitter and mediates a series of diverse physiological functions. The potential effect of 5-HT and its receptor to induce oocyte maturation in crustaceans has been examined previously (Ongvarrasopone et al. 2006; Tomy et al. 2016). In the current study, high expression of the 5-HT1 receptor at the vitellogenesis termination stage (stage IV) indicates that these receptors might be involved in promoting yolk accumulation during late vitellogenesis for ovarian maturation. miR-3885-5p exhibited an inverse expression trend compared to the target gene 5-HT1 receptor, which also suggested that it plays a regulatory role in ovarian maturation.
Conclusion
We performed comprehensive transcriptome-wide identification and characterization of female M. nipponense miRNAs and conducted an integration analysis of miRNA/mRNA expression profiles to screen for miRNAs and genes potentially related to reproduction regulation in breeding and non-breeding seasons. Integrated analysis revealed that bmo-miR-316-5p/opsin protein, ame-miR-125/skeletal muscle actin 8, dmo-miR-278/sugar transporter, and tca-miR-3885-5p/5-HT1 receptor play important roles in reproduction regulation. The results of this study provide insight into the regulatory functions of the eyestalk and cerebral ganglia in energy metabolism and the biological processes involved in ovarian maturation in crustaceans. Future research using experimental approaches such as knockdown or overexpression of candidate miRNAs and mRNAs in vitro likely will provide a better understanding of the regulatory mechanisms involved in reproduction in M. nipponense.
Acknowledgements
This research was supported by grants by Central Public-Interest Scientific Institution Basal Research Fund CAFS (2017JBFM05); the National Natural Science Foundation of China (Grant No. 31572617), New varieties creation Major Project in Jiangsu province (PZCZ201745), the Science & Technology Supporting Program of Jiangsu Province (BE2016308), China Agriculture Research System-48 (CARS-48), Jiangsu Fisheries Research System-02 (JFRS-02).
References
- Artigas P, Lapébie P, Leclère L, Takeda N, Deguchi R, Jékely G, Momose T, Houliston E. A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia. eLife. 2018;7:e29555. doi: 10.7554/eLife.29555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Henderson PJF. Homologies between sugar transporters from eukaryotes and prokaryotes. Annu Rev Physiol. 1989;51:459–471. doi: 10.1146/annurev.ph.51.030189.002331. [DOI] [PubMed] [Google Scholar]
- Bao CC, Yang YN, Huang HY, Ye HH. Neuropeptides in the cerebral ganglia of the mud crab, Scylla paramamosain: transcriptomic analysis and expression profiles during vitellogenesis. Sci Rep. 2015;5:17055. doi: 10.1038/srep17055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usade IB. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Sieglaf DH, Rees HH. Gonadal ecdysteroidogenesis in Arthropoda: occurrence and regulation. Annu Rev Entomol. 2009;54:105–125. doi: 10.1146/annurev.ento.53.103106.093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Fishery . China fishery yearbook. Beijing: China Agricultural Press; 2017. Ministry of Agriculture, P.R.C. fisheries economic statistics. [Google Scholar]
- Bushati N, Cohen SM. MicroRNA functions. Annu Rev Cell Dev Biol. 2002;2007(23):175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Cai Y, Ng PKL. The freshwater palaemonid prawns of Myanmar (Crustacea: Decapoda: Caridea) Hydrobiologia. 2002;487:59–83. doi: 10.1023/A:1022991224381. [DOI] [Google Scholar]
- Cai YM, Yu XM, Zhou Q, Yu CX, Hu HY, Liu JC, Lin HB, Yang J, Zhang B, Cui P. Novel microRNAs in silkworm (Bombyx mori) Funct Integr Genom. 2010;10(405–415):1. doi: 10.1007/s10142-010-0162-7. [DOI] [PubMed] [Google Scholar]
- Caygill EE, Johnston LA. Temporal regulation of metamorphic processes in Drosophila by the let-7 and miR-125 heterochronic microRNAs. Curr Biol. 2008;18:943–950. doi: 10.1016/j.cub.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen M, Katz S, Bally-Cuif L. miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci. 2013;7:220. doi: 10.3389/fncel.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford K, Flick R, Close L, Shelly D, Paul R, Bove K, Kumar A, Lessard J. Mice lacking skeletal muscle actin show reduced muscle strength and growth deficits and die during the neonatal period. Mol Cell Biol. 2002;22:5887–5896. doi: 10.1128/MCB.22.16.5887-5896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du JH, Niu XM, Wang Y, Kong LB, Wang RQ, Zhang YG, Zhao SX, Nan YM. MiR-146a-5p suppresses activation and proliferation of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis through directly targeting Wnt1 and Wnt5a. Sci Rep. 2015;5:16163. doi: 10.1038/srep16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HT, Jiang SF, Xiong YW. Current status and prospects of farming the giant river prawn (Macrobrachium rosenbergii) and the oriental river prawn (Macrobrachium nipponense) in China. Aquac Res. 2012;43:993–998. doi: 10.1111/j.1365-2109.2011.03085.x. [DOI] [Google Scholar]
- García-Fernández JM, Cernuda-Cernuda R, Davies WI, Rodgers J, Turton M, Peirson SN, Follett BK, Halforde S, Hughes S, Hankins MW, Foster RG. The hypothalamic photoreceptors regulating seasonal reproduction in birds: a prime role for VA opsin. Front Neuroendocrinol. 2015;37:13–28. doi: 10.1016/j.yfrne.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Ge LQ, Jiang YP, Xia T, Song QS, Stanley D, Kuai P, Lu XL, Yang GQ, Wu JC. Silencing a sugar transporter gene reduces growth and fecundity in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Sci Rep. 2015;5:12194. doi: 10.1038/srep12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YD, Ju CY, Zhang XB. Roles of small RNAs in the immune defense mechanisms of crustaceans. Mol Immunol. 2015;68:399–403. doi: 10.1016/j.molimm.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Ikeda KT, Hirose Y, Hiraoka K, Noro E, Fujishima K, Tomita M, Kanai A. Identification, expression, and molecular evolution of microRNAs in the “living fossil” Triops cancriformis (tadpole shrimp) RNA. 2014;114:1–13. doi: 10.1261/rna.045799.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HX, Li XL, Sun YH, Hou FJ, Zhang YF, Li F, Gu ZM, Liu XL. Insights into sexual precocity of female oriental river prawn Macrobrachium nipponense through transcriptome analysis. PLoS One. 2016;11(6):e0157173. doi: 10.1371/journal.pone.0157173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SB, Fu HT, Zhou Q, Sun SM, Jiang SF, Xiong YW, Gong YS, Qiao H, Zhang WY. Transcriptome analysis of androgenic gland for discovery of novel genes from the oriental river prawn, Macrobrachium nipponense, using Illumina Hiseq 2000. PLoS One. 2013;8:e76840. doi: 10.1371/journal.pone.0076840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SB, Fu HT, Jiang SF, Xiong YW, Qiao H, Zhang WY, Gong YS, Wu Y. Identification of androgenic gland microRNA and their target genes to discover sex-related microRNA in the oriental river prawn, Macrobrachium nipponense. Genet Mol Res. 2015;14:18396–18406. doi: 10.4238/2015.December.23.27. [DOI] [PubMed] [Google Scholar]
- Kenny NJ, Quah S, Holland PW, Tobe SS, Hui JH. How are comparative genomics and the study of microRNAs changing our views on arthropod endocrinology and adaptations to the environment? Gen Comp Endocrinol. 2013;188:16–22. doi: 10.1016/j.ygcen.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SK, Zhu S, Li CB, Zhang Z, Zhou LZ, Wang SJ, Wang SQ, Zhang YL, Wen XB. Characterization of microRNAs in Mud Crab Scylla paramamosain under Vibrio parahaemolyticus Infection. PLoS One. 2013;8:e73392. doi: 10.1371/journal.pone.0073392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Qiao H, Fu HT, Sun SM, Zhang WY, Jin SB, Jiang SF, Gong YS, Xiong YW, Wu Y. Identification and characterization of opsin gene and its role in ovarian maturation in the oriental river prawn Macrobrachium nipponense. Comp Biochem Phys B. 2018;218:1–12. doi: 10.1016/j.cbpb.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Liu W, Lin SK, Sun WJ, Liu T, Li YH, Zhong GH, Zhao DS, Zhang PS, Song JP, Jin XY. Circulating microRNAs correlated with the level of coronary artery calcification in symptomatic patients. Sci Rep. 2015;5:16099. doi: 10.1038/srep16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K, Raikhel AS. Insect microRNAs: biogenesis, expression profiling and biological functions. Insect Biochem Mol. 2013;43:24–38. doi: 10.1016/j.ibmb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco A, Hooks K, Griffiths-Jones S. Evolution and function of the extended miR-2 microRNA family. RNA Biol. 2012;9:242–248. doi: 10.4161/rna.19160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Takenouchi T, Yamakawa T. Effects of photoperiod and temperature on ovarian development and spawning of the Japanese spiny lobster Panulirus japonicus. Aquaculture. 2002;205:385–398. doi: 10.1016/S0044-8486(01)00687-1. [DOI] [Google Scholar]
- Nagaraju GPC. Reproductive regulators in decapod crustaceans: an overview. J Exp Biol. 2010;214:3–16. doi: 10.1242/jeb.047183. [DOI] [PubMed] [Google Scholar]
- Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Shizufumi Ebihara S, Kubo Y, Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongvarrasopone C, Roshorm Y, Somyong S, Pothiratana C, Petchdee S, Tangkhabuanbutra J, Sophasan S, Panyim S. Molecular cloning and functional expression of the Penaeus monodon 5-HT receptor. BBA Gene Struct Exp. 2006;1759:328–339. doi: 10.1016/j.bbaexp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ou JT, Li Y, Ding ZF, Xiu YZ, Wu T, Du J, Li WZ, Zhu HX, Ren Q, Gu W, Wang W. Transcriptome-wide identification and characterization of the Procambarus clarkii microRNAs potentially related to immunity against Spiroplasma eriocheiris infection. Fish Shellfish Immunol. 2013;35:607–617. doi: 10.1016/j.fsi.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Qiao H, Fu HT, Jin SB, Wu Y, Jiang SF, Gong YS, Xiong YW. Constructing and random sequencing analysis of normalized cDNA library of testis tissue from oriental river prawn (Macrobrachium nipponense) Comp Biochem Phys D. 2012;7:268–276. doi: 10.1016/j.cbd.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Qiao H, Xiong YW, Zhang WY, Fu HT, Jiang SF, Sun SM, Bai HK, Jin SB, Gong YS. Characterization, expression, and function analysis of gonad-inhibiting hormone in oriental river prawn, Macrobrachium nipponense and its induced expression by temperature. Comp Biochem Phys A. 2015;185:1–8. doi: 10.1016/j.cbpa.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Qiao H, Fu HT, Xiong YW, Jiang SF, Zhang WY, Sun SM, Jin SB, Gong YS, Wang YB, Shan DY, Wu Y. Molecular insights into reproduction regulation of female oriental river prawns Macrobrachium nipponense through comparative transcriptomic analysis. Sci Rep. 2017;7:12161. doi: 10.1038/s41598-017-10439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush SL. Lobster reproduction: a review. Crustaceana. 1994;67:82–94. doi: 10.1163/156854094X00323. [DOI] [Google Scholar]
- Real FM, Sekido R, Lupiáñez DG, Lovell-Badge R, Jiménez R, Burgos M. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol Reprod. 2013;89:78–81. doi: 10.1095/biolreprod.113.110957. [DOI] [PubMed] [Google Scholar]
- Rubio M, Belles X. Subtle roles of microRNAs let-7, miR-100 and miR-125 on wing morphogenesis in hemimetabolan metamorphosis. J Insect Physiol. 2013;59:1089–1094. doi: 10.1016/j.jinsphys.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Rüdiger W, López-Figueroa F. Photoreceptors in algae. Annu Rev Photochem Photobiol. 1992;55:949–954. doi: 10.1111/j.1751-1097.1992.tb08542.x. [DOI] [Google Scholar]
- Song YN, Shi LL, Liu ZQ, Qiu GF. Global analysis of the ovarian microRNA transcriptome: implication for miR-2 and miR-133 regulation of oocyte meiosis in the Chinese mitten crab, Eriocheir sinensis (Crustacea: Decapoda) BMC Genom. 2014;15:547. doi: 10.1186/1471-2164-15-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- Sun SM, Fu HT, Ge XP, Zhu J, Gu ZM, Xuan FJ. Identification and comparative analysis of the oriental river prawn (Macrobrachium nipponense) microRNA expression profile during hypoxia using a deep sequencing approach. Comp Biochem Phys D. 2016;17:41–47. doi: 10.1016/j.cbd.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Suwansa-ard S, Thongbuakaew T, Wang T, Zhao M, Elizur A, Hanna PJ, Prapee Sretarugsa P, Cummins SF, Sobhon P. In silico neuropeptidome of female Macrobrachium rosenbergii based on transcriptome and peptide mining of eyestalk, central nervous system and ovary. PLoS One. 2015;10:e0123848. doi: 10.1371/journal.pone.0123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TT, Chen M, Harikrishna JA, Khairuddin N, Shamsudin MIM, Zhang G, Bhassu S. Deep parallel sequencing reveals conserved and novel miRNAs in gill and hepatopancreas of giant fresh water prawn. Fish Shellfish Immunol. 2013;35:1061–1069. doi: 10.1016/j.fsi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Tehler D, Høyland-Kroghsbo NM, Lund AH. The miR-10 microRNA precursor family. RNA Biol. 2011;8:728–734. doi: 10.4161/rna.8.5.16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomy S, Saikrithi P, James N, Balasubramanian CP, Panigrahim A, Otta SK, Subramoniam T, Ponniah AG. Serotonin induced changes in the expression of ovarian gene network in the Indian white shrimp, Penaeus indicus. Aquaculture. 2016;452:239–246. doi: 10.1016/j.aquaculture.2015.11.003. [DOI] [Google Scholar]
- Varghese J, Lim SF, Cohen SM. Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev. 2001;24:2748–2753. doi: 10.1101/gad.1995910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007;3:12. doi: 10.1186/1746-4811-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra JA. The power of next-generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii. Gen Comp Endocrinol. 2015;224:84–95. doi: 10.1016/j.ygcen.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Zhao MZ, Li XG, Lu QP, Li YH, Ge JC, Pan JL. Transcriptome profiling of the eyestalk of precocious juvenile Chinese mitten crab reveals putative neuropeptides and differentially expressed genes. Gene. 2015;569:280–286. doi: 10.1016/j.gene.2015.05.075. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Front Neuroendocrinol. 2013;34:157–166. doi: 10.1016/j.yfrne.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Yu JY, Reynolds SH, Hatfield SD, Shcherbata HR, Fischer KA, Ward EJ, Ruohola-Baker H. Dicer-1-dependent Dacapo suppression acts downstream of Insulin receptor in regulating cell division of Drosophila germline stem cells. Development. 2009;136:1497–1507. doi: 10.1242/dev.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]